Optimization of Extraction Parameters to Enhance the Antioxidant Properties of Pyrus spinosa Fruit Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fruit Collection and Preparation
2.3. Extraction Process
2.4. Response Surface Methodology (RSM) Extraction Optimization and Design of Experiment
2.5. Analyses of PS Fruit Extracts
2.6. HPLC-Based Analysis of the Polyphenolic Compounds
2.7. Statistical Analysis
3. Results and Discussion
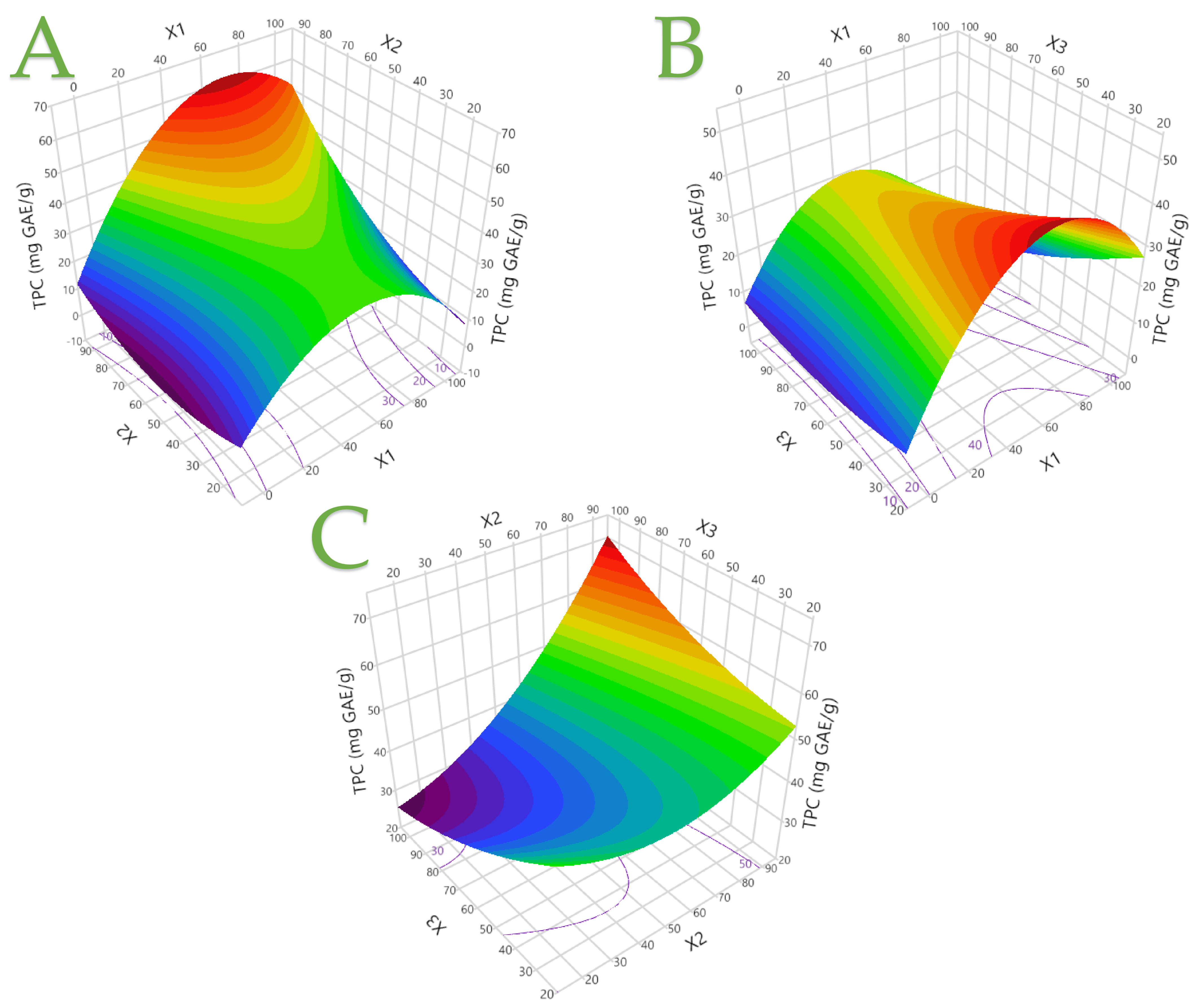
3.1. Extraction Optimization
3.2. Pareto Plot Analysis for the Impact of the Extraction Parameters on Assays
3.3. Analysis of the Extracts and Optimal Extraction Conditions
3.3.1. Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC) of the PS Fruit Extracts
3.3.2. Antioxidant Capacity of the PS Fruit Extracts
3.3.3. Ascorbic Acid Content (AAC) of the PS Fruit Extracts
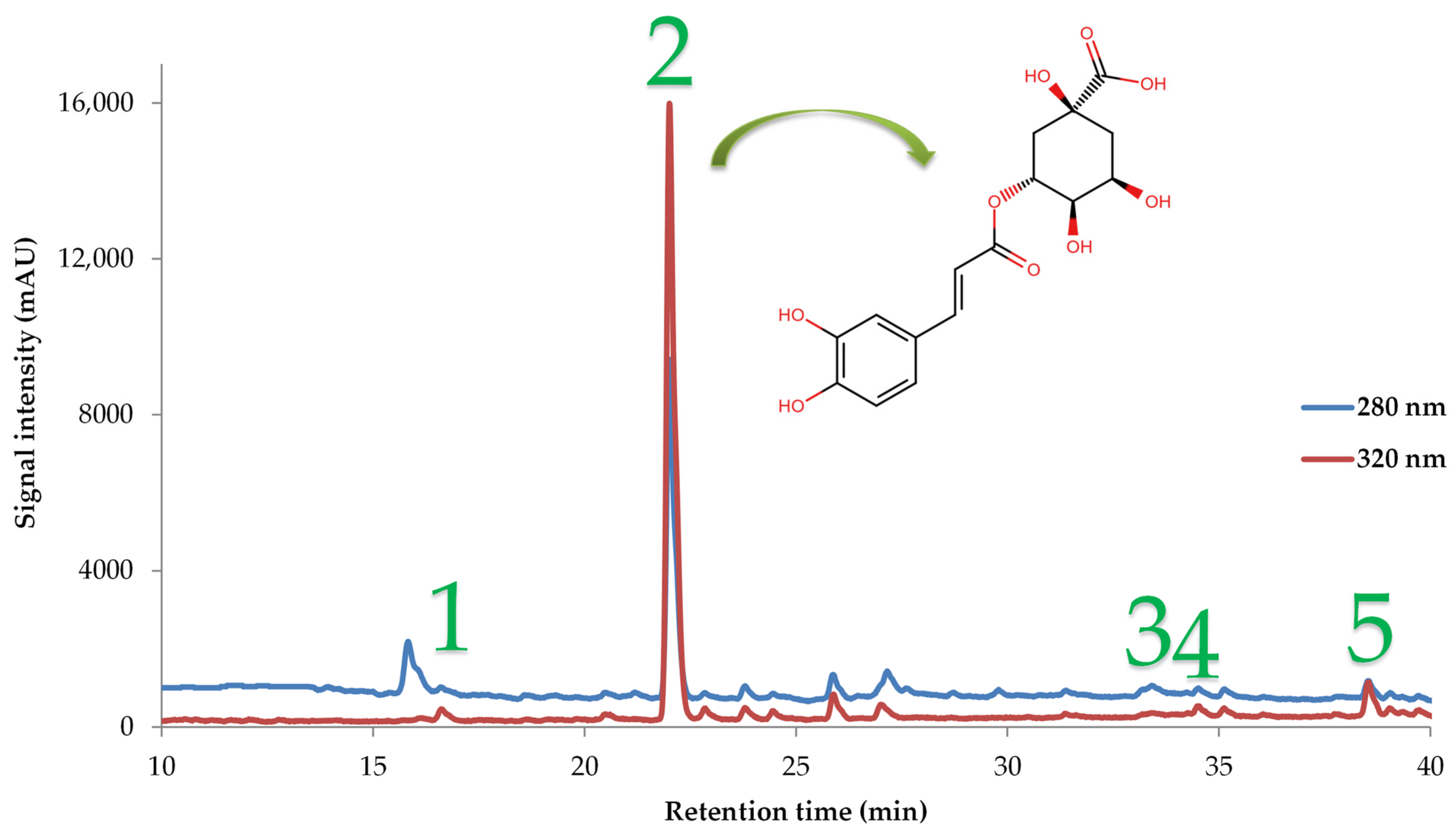
3.3.4. Polyphenolic Compounds of the Optimum PS Fruit Extract
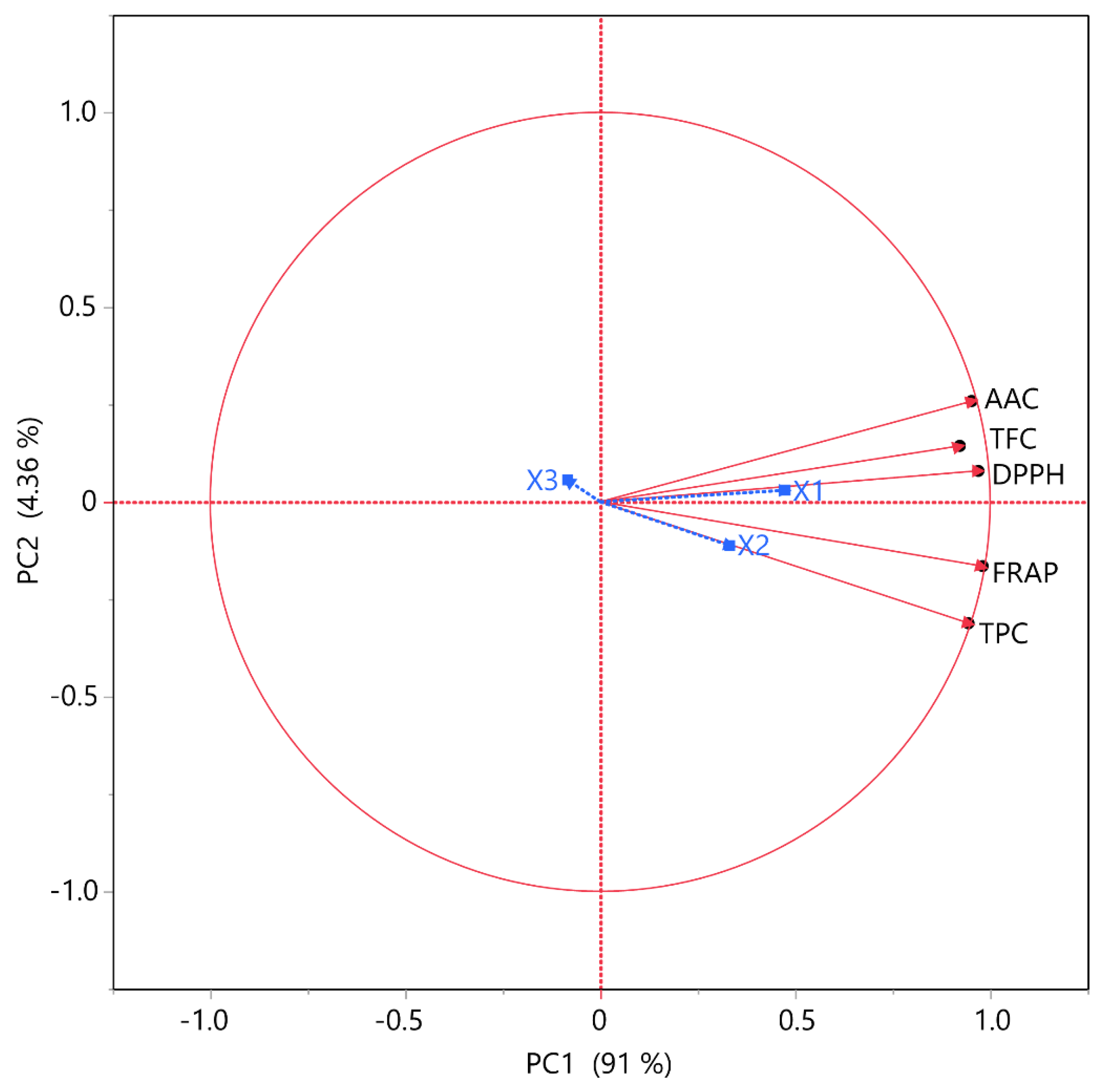
3.4. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
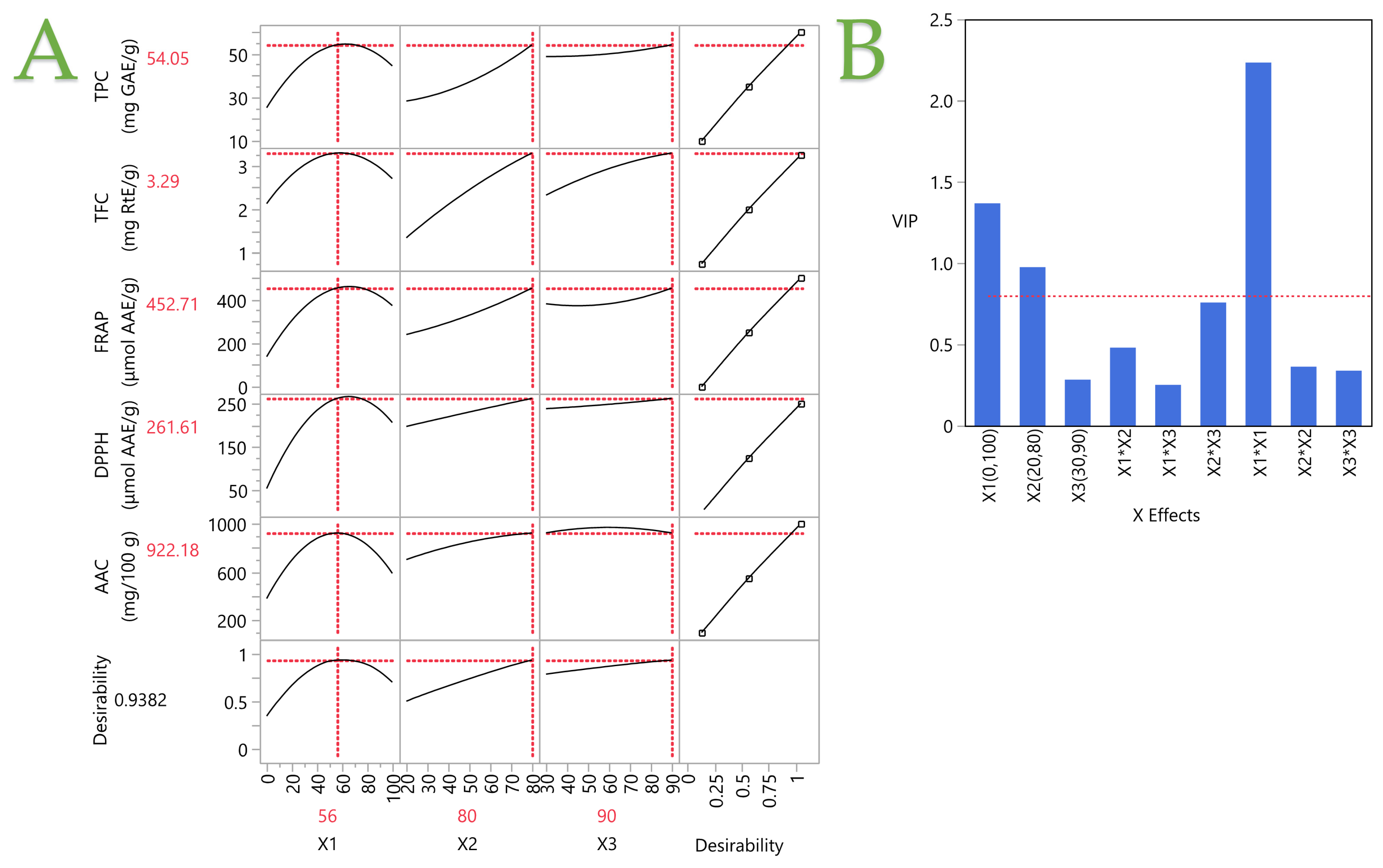
3.5. Partial Least Squares (PLSs) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidaković, A.; Liber, Z.; Šatović, Z.; Idžojtić, M.; Volenec, I.; Zegnal, I.; Pintar, V.; Radunić, M.; Poljak, I. Phenotypic diversity of almond-leaved pear (Pyrus spinosa forssk.) along eastern Adriatic coast. Forests 2021, 12, 1630. [Google Scholar] [CrossRef]
- Tsoulpha, P.; Alexandri, S.; Tsaktsira, M. Critical factors affecting an efficient micropropagation protocol for Pyrus spinosa Forskk. J. Appl. Hortic. 2018, 20, 190–195. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lansky, E.; Kang, S.S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. Ther. 2021, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Saadatian, M.; Mohammad, R.H.; Jumaah, A.A.; Oagaz, J.A. Evaluation of Nutritional Value, Fatty Acids and Polyphenols Profiles of Pyrus amygdaliformis L. Grown in North-East Kurdistan Regional Government, Iraq. J. Oleo Sci. 2022, 71, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Özderin, S. Determination of Some Chemical Properties of Wild Pear (Pyrus spinosa Forsk.). BioResources 2022, 17, 1659–1669. [Google Scholar] [CrossRef]
- Wiersema, J.H.; León, B. World Economic Plants; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429102158. [Google Scholar]
- Pérez-Rodríguez, M.L.; Serrano-Carretero, A.; García-Herrera, P.; Cámara-Hurtado, M.; Sánchez-Mata, M.C. Plant-based beverages as milk alternatives? Nutritional and functional approach through food labelling. Food Res. Int. 2023, 173, 113244. [Google Scholar] [CrossRef] [PubMed]
- Smart Protein Project. Plant-Based Foods in Europe: How Big is the Market? Smart Protein Plant-Based Food Sector Report; European Union’s Horizon 2020 Research and Innovation Programme. 2021, p. 199. Available online: https://smartproteinproject.eu/plant-based-food-sector-report/ (accessed on 1 May 2024).
- Deziderio, M.A.; de Souza, H.F.; Kamimura, E.S.; Petrus, R.R. Plant-Based Fermented Beverages: Development and Characterization. Foods 2023, 12, 4128. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, W.; Chen, L.; Jiang, T.; Dong, Y.; Wang, L.; Tong, X.; Zhou, H.; Yu, X.; Peng, Y.; et al. A review of natural plant extracts in beverages: Extraction process, nutritional function, and safety evaluation. Food Res. Int. 2023, 172, 113185. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The effect of ultrasonication pretreatment on the production of polyphenol-enriched extracts from Moringa oleifera L. (drumstick tree) using a novel bio-based deep eutectic solvent. Appl. Sci. 2020, 10, 220. [Google Scholar] [CrossRef]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology. Biomass Convers. Biorefinery 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K.; Palaiogiannis, D.; Lalas, S.I. Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology. Foods 2023, 12, 2099. [Google Scholar] [CrossRef]
- Pham, T.N.; Lam, T.D.; Nguyen, M.T.; Le, X.T.; Vo, D.V.N.; Toan, T.Q.; Vo, T.S. Effect of various factors on extraction efficiency of total anthocyanins from Butterfly pea (Clitoria ternatea L. Flowers) in Southern Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544, 012013. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Fatmawati, S.; Asri, A.W. The effect of ethanol concentrations as the extraction solvent on antioxidant activity of Katuk (Sauropus androgynus (L.) Merr.) leaves extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012060. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Díaz Huamaní, K.S.; Palomino Villegas, Y.C.; Allcca-Alca, E.E.; León-Calvo, N.C.; Colque Ayma, E.J.; Zirena Vilca, F.; Mariotti-Celis, M.S. Exploring a Sustainable Process for Polyphenol Extraction from Olive Leaves. Foods 2024, 13, 265. [Google Scholar] [CrossRef]
- Agarwal, H.; Gupta, C.; Chauhan, Y.S.; Hu, C. Parameter Extraction. In BSIM-Bulk MOSFET Model for IC Design—Digital, Analog, RF and High-Voltage; Agarwal, H., Gupta, C., Chauhan, Y.S., Hu Analog, R.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 221–243. ISBN 978-0-323-85677-5. [Google Scholar]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Essodolom, P.; Ekpetsi Chantal, B.; Mamatchi, M.; Kousanta, A. Effect of Temperature on the Degradation of Ascorbic Acid (Vitamin C) Contained in Infant Supplement Flours During the Preparation of Porridges. Int. J. Adv. Res. 2020, 8, 116–121. [Google Scholar] [CrossRef]
- Abbas, S.R.; Bano, S. Significance of pyrus communis as a medicinal plant [A Review]. Rev. Pap. Abbas Bano V 2020, 8, 35–40. [Google Scholar]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Cebulak, T.; Oszmiański, J.; Kapusta, I.; Lachowicz, S. Effect of abiotic stress factors on polyphenolic content in the skin and flesh of pear by UPLC-PDA-Q/TOF-MS. Eur. Food Res. Technol. 2019, 245, 2715–2725. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupinska, A.; Kruczynska, D.E. Bioactive compounds and health-promoting properties of pear (Pyrus communis L.) fruits. Molecules 2020, 25, 4444. [Google Scholar] [CrossRef] [PubMed]
- Mandrone, M.; Antognoni, F.; Aloisi, I.; Potente, G.; Poli, F.; Cai, G.; Faleri, C.; Parrotta, L.; Del Duca, S. Compatible and incompatible pollen-styles interaction in Pyrus communis L. Show different transglutaminase features, polyamine pattern and metabolomics profiles. Front. Plant Sci. 2019, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, S.; Tsaktsira, M.; Hatzilazarou, S.; Kostas, S.; Nianiou-Obeidat, I.; Economou, A.; Scaltsoyiannes, A.; Tsoulpha, P. Selection for Sustainable Preservation through In Vitro Propagation of Mature Pyrus spinosa Genotypes Rich in Total Phenolics and Antioxidants. Sustainability 2023, 15, 4511. [Google Scholar] [CrossRef]
- Petridis, A.; Koukourikou, M.; Sotiropoulos, T.; Stylianidis, D. Antioxidant activity of fruits produced in Northern Greece. HortScience 2010, 45, 1341–1344. [Google Scholar] [CrossRef]
- Patricia, V.M.; Syaputri, F.N.; Tugon, T.D.A.; Mardhatillah, A. Antioxidant Properties of Pyrus communis and Pyrus pyrifolia Peel Extracts. Borneo J. Pharm. 2020, 3, 64–70. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Öztürk, A.; Demirsoy, L.; Demirsoy, H.; Asan, A.; Gül, O. Phenolic compounds and chemical characteristics of pears (Pyrus Communis L.). Int. J. Food Prop. 2015, 18, 536–546. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Singh, D.; Kumari, K.; Ahmed, S. Natural herbal products for cancer therapy. In Understanding Cancer: From Basics to Therapeutics; Jain, B., Pandey, S.B.T.-U.C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 257–268. ISBN 9780323998833. [Google Scholar]
- Wang, X.Q.; Wang, W.; Peng, M.; Zhang, X.Z. Free radicals for cancer theranostics. Biomaterials 2021, 266, 120474. [Google Scholar] [CrossRef]

| Independent Variables | Coded Units | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| C (%, v/v) | X1 | 0 | 50 | 100 |
| T (°C) | X2 | 20 | 50 | 80 |
| t (min) | X3 | 30 | 60 | 90 |
| Design Point | Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 (C, %) | X2 (T, °C) | X3 (t, min) | TPC 1 | TFC 2 | FRAP 3 | DPPH 4 | AAC 5 | |
| 1 | 1 (100) | 1 (80) | 0 (60) | 40.26 | 2.3 | 315.92 | 184.04 | 585.14 |
| 2 | 0 (50) | 1 (80) | 1 (90) | 57.37 | 3.14 | 454.38 | 242.1 | 896.08 |
| 3 | 0 (50) | 0 (50) | 0 (60) | 34.47 | 2.64 | 275.28 | 231.83 | 927.76 |
| 4 | −1 (0) | 0 (50) | −1 (30) | 13.72 | 1.44 | 81.09 | 36.58 | 302.06 |
| 5 | 1 (100) | 0 (50) | −1 (30) | 32.94 | 2.06 | 282.17 | 150.32 | 565.9 |
| 6 | −1 (0) | 1 (80) | 0 (60) | 12.88 | 1.95 | 66.75 | 65.08 | 490.18 |
| 7 | 0 (50) | 1 (80) | −1 (30) | 50.26 | 2.3 | 336.26 | 231.78 | 933.34 |
| 8 | 0 (50) | 0 (50) | 0 (60) | 34.65 | 2.57 | 274.9 | 211.23 | 887.53 |
| 9 | 0 (50) | −1 (20) | −1 (30) | 39.51 | 2.91 | 345.74 | 223.77 | 791.31 |
| 10 | 1 (100) | 0 (50) | 1 (90) | 20.52 | 2.06 | 193.54 | 183.51 | 621.9 |
| 11 | 0 (50) | −1 (20) | 1 (90) | 25.66 | 1.31 | 268.28 | 192.02 | 668.4 |
| 12 | 0 (50) | 0 (50) | 0 (60) | 34.4 | 2.8 | 272.81 | 221.25 | 897.21 |
| 13 | −1 (0) | 0 (50) | 1 (90) | 15.39 | 1.18 | 30.47 | 26.44 | 212.86 |
| 14 | 1 (100) | −1 (20) | 0 (60) | 20.93 | 1.63 | 115.7 | 122.74 | 540.5 |
| 15 | −1 (0) | −1 (20) | 0 (60) | 19.5 | 0.84 | 18.5 | 14.04 | 123.89 |
| Responses | Second-Order Polynomial Equations (Models) | R2 Predicted | R2 Adjusted | p-Value | Equation |
|---|---|---|---|---|---|
| TPC | Y = 44.25 + 0.79X1 − 0.81X2 − 0.44X3 − 0.007X12 + 0.005X22 + 0.002X32 + 0.004X1X2 − 0.002X1X3 + 0.006X2X3 | 0.9353 | 0.8188 | 0.0168 | (1) |
| TFC | Y = 2.23 + 0.04X1 − 0.01X2 − 0.02X3 − 0.0003X12 − 0.0001X22 − 0.0001X32 − 0.0001X1X2 + 0.0001X1X3 + 0.0007X2X3 | 0.9825 | 0.9511 | 0.0007 | (2) |
| FRAP | Y = 360.79 + 8.33X1 − 4.81X2 − 7.62X3 − 0.07X12 + 0.02X22 + 0.04X32 + 0.03X1X2 − 0.006X1X3 + 0.05X2X3 | 0.9689 | 0.9128 | 0.0029 | (3) |
| DPPH | Y = 65.13 + 5.69X1 + 0.02X2 − 1.19X3 − 0.05X12 − 0.001X22 + 0.002X32 + 0.002X1X2 + 0.007X1X3 + 0.01X2X3 | 0.9888 | 0.9685 | 0.0002 | (4) |
| AAC | Y = −64.3 + 21.51X1 + 8.54X2 + 2.87X3 − 0.17X12 − 0.04X22 − 0.05X32 − 0.05X1X2 + 0.02X1X3 + 0.02X2X3 | 0.9936 | 0.9820 | <0.0001 | (5) |
| Responses | Optimal Conditions | |||
|---|---|---|---|---|
| Maximum Predicted Response | C (%, v/v) | T (°C) | t (min) | |
| TPC (mg GAE/g dw) | 54.40 ± 13.24 | 62 | 80 | 90 |
| TFC (mg RtE/g dw) | 3.30 ± 0.34 | 57 | 80 | 90 |
| FRAP (μmol AAE/g dw) | 458.81 ± 89.49 | 63 | 80 | 90 |
| DPPH (μmol AAE/g dw) | 265.91 ± 33.08 | 64 | 80 | 90 |
| AAC (mg/100 g dw) | 968.07 ± 57.73 | 54 | 80 | 60 |
| Polyphenolic Compound | Optimal Extract (mg/g dw) | Quantity (%) |
|---|---|---|
| Neochlorogenic acid | 0.54 ± 0.03 | 2.28 |
| Chlorogenic acid | 19.22 ± 0.86 | 80.83 |
| Ferulic acid | 0.20 ± 0.01 | 0.85 |
| Rutin | 0.14 ± 0.01 | 0.60 |
| Kaempferol-3-glucoside | 3.67 ± 0.19 | 15.44 |
| Total identified | 23.77 ± 1.10 |
| Responses | TPC | TFC | FRAP | DPPH | AAC |
|---|---|---|---|---|---|
| TPC | – | 0.8375 | 0.9666 | 0.8745 | 0.8237 |
| TFC | – | 0.8634 | 0.8332 | 0.8684 | |
| FRAP | – | 0.9540 | 0.8895 | ||
| DPPH | – | 0.9581 | |||
| AAC | – |
| Variables | PLSs Model Values | Experimental Values |
|---|---|---|
| TPC (mg GAE/g dw) | 54.05 | 50.97 ± 3.24 |
| TFC (mg RtE/g dw) | 3.29 | 3.15 ± 0.72 |
| FRAP (μmol AAE/g dw) | 452.71 | 411.72 ± 9.45 |
| DPPH (μmol AAE/g dw) | 261.61 | 271.91 ± 2.74 |
| AAC (mg/100 g dw) | 922.18 | 879.12 ± 11.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsou, K.; Papagiannoula, A.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Sfougaris, A.I.; Lalas, S.I. Optimization of Extraction Parameters to Enhance the Antioxidant Properties of Pyrus spinosa Fruit Extract. Beverages 2024, 10, 56. https://doi.org/10.3390/beverages10030056
Kotsou K, Papagiannoula A, Chatzimitakos T, Athanasiadis V, Bozinou E, Sfougaris AI, Lalas SI. Optimization of Extraction Parameters to Enhance the Antioxidant Properties of Pyrus spinosa Fruit Extract. Beverages. 2024; 10(3):56. https://doi.org/10.3390/beverages10030056
Chicago/Turabian StyleKotsou, Konstantina, Anna Papagiannoula, Theodoros Chatzimitakos, Vassilis Athanasiadis, Eleni Bozinou, Athanassios I. Sfougaris, and Stavros I. Lalas. 2024. "Optimization of Extraction Parameters to Enhance the Antioxidant Properties of Pyrus spinosa Fruit Extract" Beverages 10, no. 3: 56. https://doi.org/10.3390/beverages10030056
APA StyleKotsou, K., Papagiannoula, A., Chatzimitakos, T., Athanasiadis, V., Bozinou, E., Sfougaris, A. I., & Lalas, S. I. (2024). Optimization of Extraction Parameters to Enhance the Antioxidant Properties of Pyrus spinosa Fruit Extract. Beverages, 10(3), 56. https://doi.org/10.3390/beverages10030056









