Abstract
This study is focused on the optimization and application of an analytical methodology for the determination of 15 selected pesticides in three substrates during the vinification process. QuEChERS extraction was used followed by GC-MS to develop a simple and rapid method for the determination of these compounds. The optimized methodology was validated, providing for all selected pesticides excellent linearity, recoveries ranging between 60.9% and 95.0% and LOQs lower than 50 ng g−1 f for all substrates. The developed methodology was tested on real samples of grapes, must and wine obtained from a winery in the Epirus region. The results indicated the existence of some of the selected pesticides at comparatively low levels in contrast to the valid MRLs.
1. Introduction
Greece’s wine and viticulture industries are vital to the country’s economic growth. As mentioned by the International Organization of Vine and Wine (OIV), Greece has been, for many years, amongst the biggest wine-producing powers in the world. Although its area is small, it ranks among the top twenty wine-producing countries in the world [1].
The application of pesticides to deal with pests and diseases is a widespread practice in vineyards to boost production and minimize possible losses because grapes are susceptible to infections by pathogenic microorganisms throughout their cultivation. These chemicals can not only penetrate the fruit [2,3], but also, throughout the winemaking process, occur in the wine, with a consequent risk to the consumer [4,5,6,7,8,9,10,11,12,13]. Thus far, about 200 pesticides have been found in grapes and wine [14].
The European Union (EU) has established maximum residue levels (MRLs) for pesticides in table and wine grapes. (0.01–10 mg/Kg) [15] (Commission of the European Communities Regulation 396/2005). On the other hand, there are no MRLs established for their processed products, like wine. In this case, must and wine samples should follow the same MRLs as the raw commodities. It is essential to comprehend the fate of these substances throughout the vinification process to ascertain the health risks linked to pesticide residues.
Subprocesses can result in the reduction in pesticide concentrations in wine [16]. Nonetheless, research has shown that grapes and wine both have similar levels of pesticide residues [17,18]. The quantity of steps in the production process is one of the many variables that determine whether pesticide residues are present in wine. According to the literature, the reduction in pesticide residues can vary in different steps, such as maceration, pressing, fermentation and fining [18,19,20,21,22,23], and relies not only on the type of pesticide, but also the physicochemical properties, including vapor pressure, solubility, boiling point and the octanol–water partition coefficient [24,25,26]. An important factor to consider regarding pesticides’ fate is the environmental conditions as well as the grape storage and washing procedure [26].
Significant advancements have been made in extraction techniques recently to identify pesticide residues in a variety of food products. The pesticide residues in grapes [27] and their processed products have been determined using a wide range of extraction methods. Analytical methodologies for the analysis of pesticides in grapes include SPE [28,29], SPME [30,31] and QuEChERS [12,32,33,34,35,36], and for wines, LLE [37], SPE [38], SPME [39,40] and QuEChERS [8,12,41,42,43]. Applying dispersive solid extraction (dSPE) as a clean-up step after acetonitrile extraction, Anastasiades et al. [44] presented a novel technique in 2003 called QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe). This technique is widely applied for multiclass or multiresidue pesticide analysis, mostly in agricultural products. Due to its appropriateness for multiresidue analysis in a variety of matrices, this approach has gained attention [45].
It is necessary to determine a wide variety of chemicals in a single analysis due to the frequent application of many pesticides at the same time. Typical determination techniques comprise liquid chromatography (LC) and gas chromatography (GC) coupled to various detectors. Owing to its high sensitivity, mass spectrometry (MS) is a remarkably successful detection technique [46]. For multiresidue pesticide analysis in food commodities and the environment, GC-MS is arguably the most often used determination technique [47,48,49].
The developed analytical approach for pesticide determination is challenging since the selected substrates are considered complex matrices. Grapes are classified as ‘high acid content’ and ‘high water content’ commodities along with small fruits and berries [50]. Grapes are frequently regarded as medium-acid matrices with a high sugar content [51,52]. It is difficult to analyze pesticide residues in wine because matrices which include alcohols, organic acids, sugars and polyphenols are complex.
The major objective of this study was to optimize and validate a multiresidue method for the simultaneous determination of 15 pesticide residues in grape, must and wine samples by GC–MS. The selected pesticides have different physicochemical properties (e.g., logKow, 2.15–5.5; water solubility, 0.0002–26,000 mg/L), leading to a challenging extraction methodology. The specific goal was to minimize the impact of matrix interferences during extraction to maximize efficiency for the target analytes. The limit of quantification (LOQ), linearity, precision, accuracy and matrix effect were all used to validate the proposed method. Different QuEChERS parameters such as extraction salts and solvents, sample weight and clean-up sorbents were assessed. Finally, after validation, the methodology was applied to real samples from a winery of the Epirus region (northwestern Greece) to provide an understanding of the levels of pesticide residue in four different substrates acquired during the vinification process.
2. Materials and Methods
2.1. Chemicals and Reagents
The pesticide compounds were selected based on their use in local grape cultivation. The pesticide standards (cyprodinil, deltamethrin, difenoconazole, fenhexamid, fludioxonil, folpet, iprovalicarb, kresoxim-methyl, λ-cyhalothrin, metalaxyl-m, myclobutanil, penconazole, pyraclostrobin, pyrimethanil and tebuconazole) were of high purity (>97%) and were purchased from Sigma Aldrich (Steinheim, Germany). In Table S1, the physicochemical properties of the selected pesticides are presented. Concentrated standard solutions (stock solutions) for each compound were prepared separately, at a concentration of 1000 mg L−1. The diluted standard working solutions and their mixtures were prepared at various concentrations with appropriate dilutions in hexane and were stored in amber glassware at 4 °C. High-purity solvents were used, namely acetonitrile, methanol, ethyl acetate (Fisher Scientific, Leicester, UK), n-hexane from Labscan (Dublin, Ireland), acetone from Riedel-de Haën (Hannover, Germany) and acetic acid from Supelco (Bellefonte, PA, USA). To perform QuEChERS extraction, 50 mL and 15 mL polypropylene centrifuge tubes were purchased from Sarsdedt (Nümbrecht, Germany), and the salt-sorbent materials included the following: anhydrous magnesium sulfate (MgSO4), C18 (LiChroprep RP-18 (40–64 μm) and tri-sodium citrate dehydrate (C6H5Na3O7·H2O) purchased from Merck KGaA (Darmstadt, Germany); sodium acetate (NaOAc) and sodium chloride (NaCl) of analytical reagent grade from Riedel de Haën (Hannover, Germany); sodium citrate dibasic sesquihydrate (C6H6Na2O7·1.5H2O) from Sigma–Aldrich (Bellefonte, PA, USA), primary secondary amine (PSA; 40 μm) and graphitized carbon black (GCB) purchased from Agilent Technologies (Waldbronn, Germany). Lastly, Millipore (Cork, Ireland) supplied Millex-HV (PTFE, 0.22 μm) syringe filters.
2.2. Sampling and Sample Preparation
To develop and validate the extraction methods, certified organic samples were obtained from a winery in the region of Epirus, Greece (Figure 1). Samples were gathered either in dark glass bottles (for must and wine) or in plastic bags (for grapes), after which they were transferred in coolers to the laboratory. The grapes were homogenized and kept at −20 °C until analysis. A complete description of the sampling procedure is provided in the Supplementary Material.
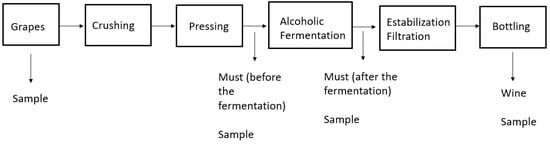
Figure 1.
Sample collection procedure.
2.2.1. Grape
Following grape sample homogenization, five grams were placed into a 50 mL polypropylene tube and fortification with the standard solution was carried out. Subsequently, 10 mL of ACN was added and shaken for 1 min. The next step was the addition of the extraction salts (4 g MgSO4/1 g NaCl/1 g tri-sodium citrate dehydrate/0.5 g sodium citrate dibasic sesquihydrate), followed by vortexing for 1 min. The sample was centrifuged at 4000 rpm (1610× g) for 7 min and 2 mL of the supernatant layer was collected and transferred to a 15 mL polypropylene centrifuge tube containing 300 mg MgSO4, 50 mg PSA and 15 mg C18. The tube was mixed with a vortex mixer and centrifuged at 4000 rpm (1610× g) for 5 min. The next step was the collection of the aliquot, followed by evaporation under a gentle nitrogen stream and reconstitution in 200 μL of n-hexane. The extract was filtered using a polytetrafluoroethylene filter (PTFE, 0.22 μm) and then placed into an autosampler vial as the last step before GC-MS analysis.
2.2.2. Must
The same QuEChERS protocol used for the grape samples was applied for must, with the difference being the addition of 10 mL of purified water to the sample before the extraction process to reduce the interactions between the substrate compounds and the analytes [53]. This water addition results in a better distribution of analytes between the aqueous and organic phases [54]. The water used should be cold, reducing the temperature arising from the heat produced after the addition of MgSO4, so that thermally sensitive compounds are protected [55]. Moreover, in the cleaning step, the PSA quantity was increased to 200 mg for the effective clean-up of the matrix interferences owing to its high concentration of sugars.
2.2.3. Wine
The same extraction protocol followed for the grape samples can be followed for the wine, with the only difference being the quantity of PSA being 100 mg to eliminate matrix interferences.
2.3. GC–MS Analysis
To conduct the GC-MS analysis, a SHIMADZU QP-2020 GC-MS system (Shimadzu Corporation, Kyoto, Japan) equipped with an AOC-20i autosampler (Shimadzu Corporation, Kyoto, Japan) was used. The column was a MEGA–5MS plus column (5% phenyl composition and 95% methyl polysiloxane) (30 m × 0.25 mm, i.d. 0.25 μm). The ion source temperature was 240 °C and the injector temperature was 250 °C. The spitless mode was in the injection port, and the splitter was opened after 2 min. Helium was used as the carrier gas, with a constant flow of 1.2 mL/min. The column oven temperature program started at 110 °C for 1 min and was then heated to 200 °C at a rate of 10 °C/min, where it was held for 2 min. Next, it was heated to 220 °C at a rate of 2 °C/min, where it was held for 1 min, then increased to 270 °C at a rate of 3 °C/min and finally to 320 °C at a rate of 8 °C/min, where it was held for 1 min. The total program time was 46.92 min. The injection volume was 1 μL injected using an autosampler. SIM mode was used, and quantification was performed using matrix-matched multi-level calibration curves. The retention times, target ions and reference ions are shown in Table S2. A chromatogram of a standard solution of the selected pesticides at a concentration of 500 ng mL−1 is also shown in Figure S1.
2.4. Validation Characteristics
The validation was conducted as stated by the EU guidelines [50]. Linearity of the method was examined from the LOQ value of each analyte up to 1000 ng g−1 and was expressed as the determination coefficient (R2). The method recoveries were assessed at three concentration levels: 10 ng g−1, 50 ng g−1 and 100 ng g−1 (n = 5). The precision of the method was expressed as the repeatability %RSDr (intra-day) of five samples in the same day, and within-laboratory reproducibility %RSDR (inter-day) was determined using five replicate samples over five consecutive days.
For the estimation of the matrix effect (ME), a calibration curve of the sample extract was prepared and compared to the calibration curve of the solvent, both in the same concentration range. The calculation was conducted according to the following equation [56]:
3. Results
3.1. Optimization of QuEChERS Method
Grape Samples
The three variations of QuEChERS extraction were tested as part of the extraction optimization process’s initial step. These are Method 1 (“original” QuEChERS) [44], Method 2 (“AOAC 2007.01” QuEChERS) [57] and Method 3 (“Buffered CEN 15662” QuEChERS) [58] European Standard EN 15662). To conduct the experiments for optimization, a spiking level of 100 ng g−1 wet weight was chosen. Method 1 and 2 resulted in lower recoveries compared to Method 3 (Figure 2). Moreover, as it is shown in Figure S2, using Method 3, the majority of the selected compounds provided recoveries between 60 and 120%, as demonstrated by the EU guidelines [50].
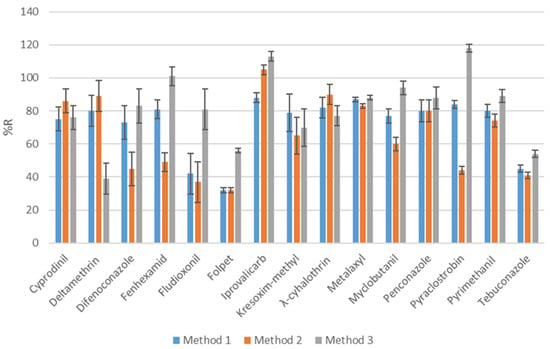
Figure 2.
Recoveries for all selected pesticides at spiking level of 100 ng g−1.
Selection of the extraction solvent was the following stage of the optimization process. Acetonitrile (ACN) is widely used because it is suitable for a variety of analytes [27]. The second solvent used was ethyl acetate, which has been used in other studies [59,60]. The other two solvents evaluated were hexane and a mixture of hexane and acetone at a 4:1 ratio. According to Figure 3, extraction with acetonitrile led to better recoveries compared to that with hexane, ethyl acetate and hexane/acetone.
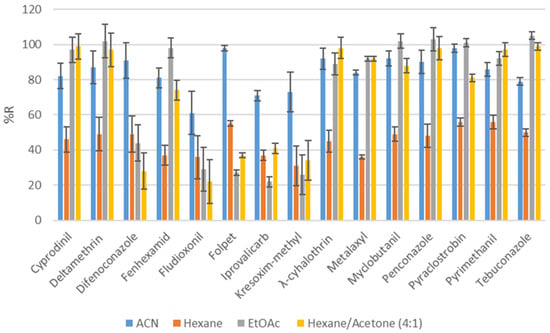
Figure 3.
Recoveries (%) obtained with different extraction solvents at spiking level of 100 ng g−1.
The sample weight was an additional factor to be evaluated during the optimization procedure. The initial experiments to determine the extraction salts and solvent were performed with 2 g of the sample. After determining the optimal values of the above parameters, amounts of 1 g, 5 g and 10 g of the sample were studied, as they are commonly used in similar studies. The 2 g and 5 g sample weights did not show any notable differences in the recoveries, whereas the chromatograms obtained from the 10 g sample were more encumbered. The use of 5 g was considered the optimal choice based on the recoveries and cleaner chromatograms.
The next step in the optimization procedure was the choice of the clean-up sorbent mixture. Different combinations were evaluated, with the first being 300 mg MgSO4 with 50 mg PSA, and the second being 300 mg MgSO4, 50 mg PSA and 15 mg C18. The third salt mixture consists of 300 mg of MgSO4, 50 mg of PSA and 15 mg of GCB. Finally, a fourth combination of 300 mg MgSO4, 50 mg PSA, 15 mg GCB and 15 mg of C18 was used. The third and fourth combinations were considered unsuitable owing to their significantly reduced recoveries. A combination of PSA and C18 led to increased recoveries, owing to the sufficient removal of interfering substances. As shown in Figure 4, the combination of MgSO4/PSA/C18 resulted in recoveries ranging from 65% to 115%.
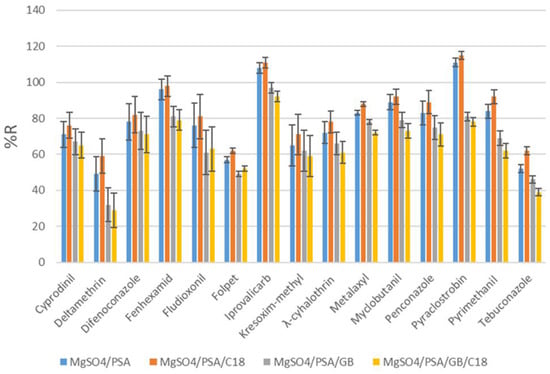
Figure 4.
Recoveries (%) obtained with different dSPE sorbents at spiking level of 100 ng g−1.
3.2. Validation Characteristics
The validation performance characteristics for grape, must and wine are listed in Table 1, Tables S3 and S4, respectively, revealing the excellent linearity of the method for all compounds (R2 > 0.9902). For quantification purposes, matrix-matched calibration curves, for each matrix were used.

Table 1.
Method validation parameters for grape samples.
The recoveries were estimated using fortified grape, must and wine samples at three concentration levels (10, 50 and 100 ng g−1). Precision was evaluated by fortifying five replicates on the same day and on five consecutive days (Table 1, Tables S3 and S4). The recoveries for grape samples were found to be between 68.6% and 95%, those for must samples were between 60.9% and 78.5%, and for wine samples, they were from 63.2% to 80.5%. Relative standard deviation (RSD) values were found to be below 14.8%, 10.2% and 11.7% for grape, must and wine, respectively. In particular, intra-day precision (RSDr) ranged from 1.0% to 14.8% for grapes, from 2.3% to 10.1% for must and from 0.6% to 11.7% for wine. Inter-day precision (RSDR) varied from 1.1% to 12.8%, from 1.9% to 10.2% and from 1.2% to 9.1% for grape, must and wine samples, respectively. The LOQs of the optimized method extended between 1 ng g−1 and 50 ng g−1 and are displayed in detail in Table 1, Tables S3 and S4.
As it is shown in Figures S3–S5, for grape samples, %ME values were found to range between −9.8% and −5.9% for all pesticides. For must samples, the values ranged between −66.8% and 56%, and for wine samples, they ranged between −53.3% and 41.2%.
3.3. Application to Samples Collected from Winery
For the application of the proposed method, real samples from a winery in Epirus, Greece, were collected. Grape samples were collected after harvesting the three different varieties. Must samples were collected before and after fermentation, and the final samples were bottled wine of these varieties. The grape, must and wine samples were from the same varieties but not from the same processing samples. Quantification was conducted using matrix-matched calibration curves for each matrix. The retention time, target and reference ions, and their intensities were used for the identification of the target analytes in real samples. The concentrations of the selected pesticides are presented in Table 2, Table 3, Tables S5 and S6.

Table 2.
Pesticide concentrations in white and red grape samples and their maximum residue levels (MRLs).

Table 3.
Pesticide concentrations in bottled wine samples and their maximum residue levels (MRLs).
4. Discussion
After validating the method, it was used for the analysis of six grape samples, twelve must samples and eight wine samples. The results showed the presence of cyprodinil, fludioxonil, folpet, λ-cyhalothrin, metalaxyl-m and myclobutanil in grape samples. More explicitly, metalaxyl-m was the pesticide residue found at the highest concentration (98.5 μg/kg), whereas λ-cyhalothrin was the most detected pesticide, with a concentration calculated below the LOQ for all samples. As displayed in Table 2, all pesticides were found at values lower than the valid MRLs for vinification grapes. Comparing the results with previous studies, Venkateswarlu et al. (2007) [62] revealed the occurrence of metalaxyl and myclobutanil at concentrations between 0.015 and 0.037 mg/kg and between 0.010 and 0.123 mg/kg, respectively, in grape samples from India. Zhao et al. (2012) [63] detected λ-cyhalothrin at concentrations below 0.020 mg/kg in grape samples from China. Finally, Bakirci et al. (2014) [64] found cyprodinil in 20 samples (at concentrations of 0.03–0.829 mg/kg), fludioxonil in 18 samples (0.014–0.924 mg/kg), λ-cyhalothrin in 12 samples (at concentrations of 0.01–0.134 mg/kg) and metalaxyl-m in 10 grape samples (at concentrations of 0.011–0.162 mg/kg).
Regarding the must samples before fermentation, iprovalicarb, λ-cyhalothrin, metalaxyl-m and myclobunatil were the pesticides detected. After fermentation, λ-cyhalothrin was mostly detected at concentrations below the quantification level and metalaxyl-m was also calculated at concentrations below 6.1 μg/kg.
Concerning the wine samples, iprovalicarb, λ-cyhalothrin and metalaxyl-m were the detected pesticides. Their concentrations ranged between <LOQ for λ-cyhalothrin and 7.8 μg/Κg for iprovalicarb. However, the pesticide with the most frequent detection was metalaxyl-m, which was detected in seven out of the eight total samples, but in low concentrations not exceeding 7.2 μg/Κg, showing that the results correspond with the recent literature [14,43,65,66].
It should be mentioned that the grape samples, the must samples before and after fermentation, as well as the wine samples were from the same variety, but not from the same processing sample. Consequently, the correlation between them could not be determined, and the processing factor could not be estimated.
5. Conclusions
QuEChERS extraction was optimized and validated for the determination of 15 pesticides (13 fungicides and 2 insecticides) in 3 substrates. The method was validated, providing satisfactory results. Recovery studies were conducted at three different concentration levels for all matrices, with the recoveries spanning from 60.9% to 95% for all substrates. In addition to being simple and fast, the suggested method can be utilized for the routine analysis of more than one substrate. This is of great importance, because there can be an estimation of the fate of these pesticides during the winemaking procedure. The final purpose was the application of this method for the analysis of six grape samples, twelve must samples and eight wine samples, with the results revealing the presence of some of those pesticides, but in low levels, leading to the fact that the consumption of these commodities is safe for the consumer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/beverages10030053/s1, Figure S1: GC-MS total ion current (TIC) chromatogram of a standard solution of the selected pesticides at a concentration of 500 ng mL−1. Figure S2: Number of pesticides obtained for different QuEChERS methods; (a) Method 1 (“Original”), (b) Method 2 (“AOAC 2007.01”) and (c) Method 3 (“Buffered CEN 15662”). Figure S3: Matrix effect (%ME) in grape samples. Figure S4: Matrix effect (%ME) in must samples. Figure S5: Matrix effect (%ME) in wine samples; Table S1: Selected pesticides with structure, molecular weight, action, logKow and molecular weight. Table S2: GC-MS parameters of the selected pesticides. Table S3: Method Validation parameters for must samples. Table S4: Method Validation parameters for wine samples. Table S5: Pesticide concentrations in must samples before the fermentation. Table S6: Pesticide concentrations in must samples after the fermentation Sampling procedure.
Author Contributions
Conceptualization, D.L.S. and T.A.A.; methodology, D.L.S. and E.P.T.; software, D.L.S. and E.P.T.; validation, D.L.S., E.P.T. and C.S.T.; formal analysis, D.L.S. and E.P.T.; investigation, D.L.S.; resources, T.A.A.; data curation, D.L.S. and E.P.T.; writing—original draft preparation, D.L.S.; writing—review and editing, T.A.A. and C.S.T.; visualization, D.L.S., E.P.T., C.S.T. and T.A.A.; supervision, C.S.T. and T.A.A.; project administration, C.S.T. and T.A.A.; funding acquisition, T.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the support of this study provided by the project “Development of research infrastructure for the design, production, development of quality characteristics and safety of agrofoods and functional foods (RI-Agrofoods)” (MIS 5047235) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme: Competitiveness, Entrepreneurship and Innovation (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Organisation, I. International Organisation of Vine and Wine 2022. World Wine Production Outlook OIV First Estimates. November 1924, pp. 1–8. 2022. Available online: https://www.oiv.int/what-we-do/statistics (accessed on 25 June 2024).
- Fantke, P.; Juraske, R. Variability of Pesticide Dissipation Half-Lives in Plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef]
- Ryberg, M.W.; Rosenbaum, R.K.; Mosqueron, L.; Fantke, P. Addressing bystander exposure to agricultural pesticides in life cycle impact assessment. Chemosphere 2018, 197, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V.; Gregorčič, A. Pesticide residues in grapes and during vinification process. Food Control 2010, 21, 1512–1518. [Google Scholar] [CrossRef]
- Fernández, M.J.; Oliva, J.; Barba, A.; Cámara, M.A. Fungicide dissipation curves in winemaking processes with and without maceration step. J. Agric. Food Chem. 2005, 53, 804–811. [Google Scholar] [CrossRef]
- Flori, P.; Frabboni, B.; Cesari, A. Pesticide decay models in the wine-making process and wine storage. Ital. J. Food Sci. 2000, 12, 279–289. [Google Scholar]
- He, Z.; Xu, Y.; Wang, L.; Peng, Y.; Luo, M.; Cheng, H.; Liu, X. Wide-scope screening and quantification of 50 pesticides in wine by liquid chromatography/quadrupole time-of-flight mass spectrometry combined with liquid chromatography/quadrupole linear ion trap mass spectrometry. Food Chem. 2016, 196, 1248–1255. [Google Scholar] [CrossRef]
- Kosma, C.I.; Koloka, O.L.; Albanis, T.A.; Konstantinou, I.K. Accurate mass screening of pesticide residues in wine by modified QuEChERS and LC-hybrid LTQ/Orbitrap-MS. Food Chem. 2021, 360, 130008. [Google Scholar] [CrossRef]
- Navarro, S.; Barba, A.; Oliva, J.; Navarro, G.; Pardo, F. Evolution of residual levels of six pesticides during elaboration of red wines. Effect of wine-making procedures in their disappearance. J. Agric. Food Chem. 1999, 47, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; García, B.; Navarro, G.; Oliva, J.; Barba, A. Effect of Wine-Making Practices on the Concentrations of Fenarimol and Penconazole in Rosé Wines. J. Food Prot. 1997, 60, 1120–1124. [Google Scholar] [CrossRef]
- Pelajić, M.; Peček, G.; Pavlović, D.M.; Čepo, D.V. Novel multiresidue method for determination of pesticides in red wine using gas chromatography–mass spectrometry and solid phase extraction. Food Chem. 2016, 200, 98–106. [Google Scholar] [CrossRef]
- Schusterova, D.; Hajslova, J.; Kocourek, V.; Pulkrabova, J. Pesticide residues and their metabolites in grapes and wines from conventional and organic farming system. Foods 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Čepo, D.V.; Pelajić, M.; Vrček, I.V.; Krivohlavek, A.; Žuntar, I.; Karoglan, M. Differences in the levels of pesticides, metals, sulphites and ochratoxin A between organically and conventionally produced wines. Food Chem. 2018, 246, 394–403. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, B.; Zhang, H.; Hao, L.L.; Ma, T.Z.; Wang, J.; Han, S.Y. Monitoring of 49 Pesticides and 17 Mycotoxins in Wine by QuEChERS and UHPLC–MS/MS Analysis. J. Food Sci. 2019, 84, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Regulation (EC) No 396/2005, Maximum residue levels of pesticides in/on food and feed of plant and animal. Off. J. Eur. Union 2005, L70, 1–16. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32005R0396 (accessed on 28 June 2024).
- Corrias, F.; Taddeo, R.; Arru, N.; Angioni, A. Effect of the technological process from vine to wine on pesticide residues in vernaccia di oristano cultivar. Foods 2021, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef]
- Martins, J.; Esteves, C.; Simões, T.; Correia, M.; Delerue-Matos, C. Determination of 24 pesticide residues in fortified wines by solid-phase microextraction and gas chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6847–6855. [Google Scholar] [CrossRef]
- Doulia, D.S.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Removal of pesticides from white and red wines by microfiltration. J. Hazard. Mater. 2016, 317, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Doulia, D.S.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Effect of clarification process on the removal of pesticide residues in white wine. Food Control 2017, 72, 134–144. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Simal-Gándara, J. Decay of fungicide residues during vinification of white grapes harvested after the application of some new active substances against downy mildew. Food Chem. 2011, 125, 549–560. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Bernal, J.L.; del Nozal, M.J.; Bernal, J.; Toribio, L. Persistence and degradation of metalaxyl, lindane, fenvalerate and deltamethrin during the wine making process. Food Chem. 2007, 104, 216–223. [Google Scholar] [CrossRef]
- Sen, K.; Cabaroglu, T.; Yilmaz, H. The influence of fining agents on the removal of some pesticides from white wine of Vitis vinifera L. cv. Emir. Food Chem. Toxicol. 2012, 50, 3990–3995. [Google Scholar] [CrossRef]
- Kittelmann, A.; Müller, C.; Rohn, S.; Michalski, B. Transfer of Pesticide Residues from Grapes (Vitis vinifera) into Wine—Correlation with Selected Physicochemical Properties of the Active Substances. Toxics 2022, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.-D.; Teodosiu, C.; Cotea, V.V. Management of Pesticides from Vineyard to Wines: Focus on Wine Safety and Pesticides Removal by Emerging Technologies. In Grapes and Wine; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Grimalt, S.; Dehouck, P. Review of analytical methods for the determination of pesticide residues in grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sivaperumal, P.; Anand, P.; Riddhi, L. Rapid determination of pesticide residues in fruits and vegetables, using ultra-high-performance liquid chromatography/time-of-flight mass spectrometry. Food Chem. 2015, 168, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Velkoska-Markovska, L.; Petanovska-Ilievska, B.; Jankulovska, M.S.; Ilievski, U. Development and validation of high-performance liquid chromatography method for determination of some pesticide residues in table grape. Acta Chromatogr. 2018, 30, 250–254. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, W.; Chen, H.; Ding, Q.; Li, Q.; Zhang, L. In situ fabrication of nitrogen doped graphitic carbon networks coating for high-performance extraction of pyrethroid pesticides. Talanta 2021, 233, 122542. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, A.; Pawliszyn, J. Multiresidue pesticide quantitation in multiple fruit matrices via automated coated blade spray and liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. 2021, 339, 127815. [Google Scholar] [CrossRef]
- Dong, B.; Yang, Y.; Pang, N.; Hu, J. Residue dissipation and risk assessment of tebuconazole, thiophanate-methyl and its metabolite in table grape by liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 260, 66–72. [Google Scholar] [CrossRef]
- Hou, X.; Xu, Z.; Zhao, Y.; Liu, D. Rapid analysis and residue evaluation of six fungicides in grape wine-making and drying. J. Food Compos. Anal. 2020, 89, 103465. [Google Scholar] [CrossRef]
- Qin, G.; Chen, Y.; He, F.; Yang, B.; Zou, K.; Shen, N.; Zuo, B.; Liu, R.; Zhang, W.; Li, Y. Risk assessment of fungicide pesticide residues in vegetables and fruits in the mid-western region of China. J. Food Compos. Anal. 2021, 95, 103663. [Google Scholar] [CrossRef]
- Valera-Tarifa, N.M.; Santiago-Valverde, R.; Hernández-Torres, E.; Martínez-Vidal, J.L.; Garrido-Frenich, A. Development and full validation of a multiresidue method for the analysis of a wide range of pesticides in processed fruit by UHPLC-MS/MS. Food Chem. 2020, 315, 126304. [Google Scholar] [CrossRef] [PubMed]
- Volpatto, F.; Wastowski, A.D.; Bernardi, G.; Prestes, O.D.; Zanella, R.; Adaime, M.B. Evaluation of QuEChERS sample preparation and gas chromatography coupled to mass spectrometry for the determination of pesticide residues in grapes. J. Braz. Chem. Soc. 2016, 27, 1533–1540. [Google Scholar] [CrossRef]
- de Melo Abreu, S.; Caboni, P.; Cabras, P.; Garau, V.L.; Alves, A. Validation and global uncertainty of a liquid chromatographic with diode array detection method for the screening of azoxystrobin, kresoxim-methyl, trifloxystrobin, famoxadone, pyraclostrobin and fenamidone in grapes and wine. Anal. Chim. Acta 2006, 573–574, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.; Botitsi, H.; Antoniou, S.; Tsipi, D. Determination of multi-class pesticides in wines by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 5856–5867. [Google Scholar] [CrossRef]
- Wong, J.W.; Webster, M.G.; Halverson, C.A.; Hengel, M.J.; Ngim, K.K.; Ebeler, S.E. Multiresidue Pesticide Analysis in Wines by Solid-Phase Extraction and Capillary Gas Chromatography−Mass Spectrometric Detection with Selective Ion Monitoring. J. Agric. Food Chem. 2003, 51, 1148–1161. [Google Scholar] [CrossRef]
- Zambonin, C.G.; Quinto, M.; De Vietro, N.; Palmisano, F. Solid-phase microextraction—Gas chromatography mass spectrometry: A fast and simple screening method for the assessment of organophosphorus pesticides residues in wine and fruit juices. Food Chem. 2004, 86, 269–274. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Xu, J.; Pan, C.; Zhang, J.; Niu, W. Multiresidue method for the determination of 77 pesticides in wine using QuEChERS sample preparation and gas chromatography with mass spectrometry. Food Addit. Contam. Part A 2009, 26, 859–866. [Google Scholar] [CrossRef]
- Pelajić, M.; Pelajić, I.; Pavlović, D.M.; Čepo, D.V. GC-MS Modified Quechers Method for Multiresidue Pesticide Determination in Red Wine. Croat. Chem. Acta 2019, 92, 419–428. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Díaz-Romero, C.; Rodríguez-Delgado, M.Á. Comparison of Pesticide Residue Levels in Red Wines from Canary Islands, Iberian Peninsula, and Cape Verde. Foods 2020, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘dispersive solid-phase extraction’ for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Camino-Sánchez, F.J.; Zafra-Gómez, A.; Ruiz-García, J.; Bermúdez-Peinado, R.; Ballesteros, O.; Navalon, A.; Vílchez, J.L. UNE-EN ISO/IEC 17025:2005 accredited method for the determination of 121 pesticide residues in fruits and vegetables by gas chromatography–tandem mass spectrometry. J. Food Compos. Anal. 2011, 24, 427–440. [Google Scholar] [CrossRef]
- Afify, A.S.; Abdallah, M.; Ismail, S.A.; Ataalla, M.; Abourehab, M.A.; Al-Rashood, S.T.; Ali, M.A. Development of GC–MS/MS method for environmental monitoring of 49 pesticide residues in food commodities in Al-Rass, Al-Qassim region, Saudi Arabia. Arab. J. Chem. 2022, 15, 104199. [Google Scholar] [CrossRef]
- Huertas-Pérez, J.F.; Baslé, Q.; Dubois, M.; Theurillat, X. Multi-residue pesticides determination in complex food matrices by gas chromatography tandem mass spectrometry. Food Chem. 2024, 436, 137687. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, S.; Vivier, M.; Zaghouani, W.; De Sloovere, A.; Agasse-Peulon, V.; Cardinael, P. Comparison of new approach of GC-HRMS (Q-Orbitrap) to GC–MS/MS (triple-quadrupole) in analyzing the pesticide residues and contaminants in complex food matrices. Food Chem. 2021, 359, 129932. [Google Scholar] [CrossRef]
- Pihlström, T.; Fernández-Alba, A.R.; Amate, C.F.; Poulsen, M.E.; Hardebusch, B.; Anastassiades, M.; Lozano, A.; Alba, F. Analytical Quality Control and Method Validation Procedures for Pestice Residues Analysis in Food and Feed. Sante/11312/2021, p. 42, 2022, [Online]. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11813_2017-fin.pdf (accessed on 28 June 2024).
- Bakırcı, G.T.; Hışıl, Y. Fast and simple extraction of pesticide residues in selected fruits and vegetables using tetrafluoroethane and toluene followed by ultrahigh-performance liquid chromatography/tandem mass spectrometry. Food Chem. 2012, 135, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Payá, P.; Anastassiades, M.; Mack, D.; Sigalova, I.; Tasdelen, B.; Oliva, J.; Barba, A. Analysis of pesticide residues using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1697–1714. [Google Scholar] [CrossRef]
- Rutkowska, E.; Łozowicka, B.; Kaczyński, P. Three approaches to minimize matrix effects in residue analysis of multiclass pesticides in dried complex matrices using gas chromatography tandem mass spectrometry. Food Chem. 2019, 279, 20–29. [Google Scholar] [CrossRef]
- Bragança, I.; Plácido, A.; Paíga, P.; Domingues, V.F.; Delerue-Matos, C. QuEChERS: A new sample preparation approach for the determination of ibuprofen and its metabolites in soils. Sci. Total Environ. 2012, 433, 281–289. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef]
- Nannou, C.I.; Boti, V.I.; Albanis, T.A. A modified QuEChERS approach for the analysis of pharmaceuticals in sediments by LC-Orbitrap HRMS. Anal. Bioanal. Chem. 2019, 411, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef] [PubMed]
- EN 15662; Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE—QuEChERSmethod. Austrian Standards Institute: Vienna, Austria, 2009.
- Jadhav, M.R.; Oulkar, D.P.; Shabeer, T.A.; Banerjee, K. Quantitative Screening of Agrochemical Residues in Fruits and Vegetables by Buffered Ethyl Acetate Extraction and LC-MS/MS Analysis. J. Agric. Food Chem. 2015, 63, 4449–4456. [Google Scholar] [CrossRef]
- Shabeer, T.P.A.; Jadhav, M.; Girame, R.; Hingmire, S.; Bhongale, A.; Pudale, A.; Banerjee, K. Targeted screening and safety evaluation of 276 agrochemical residues in raisins using buffered ethyl acetate extraction and liquid chromatography–tandem mass spectrometry analysis. Chemosphere 2017, 184, 1036–1042. [Google Scholar] [CrossRef]
- Commission, E. Pesticide Residues. [Online]. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls/searchpr (accessed on 6 June 2024).
- Venkateswarlu, P.; Mohan, K.R.; Kumar, C.R.; Seshaiah, K. Monitoring of multi-class pesticide residues in fresh grape samples using liquid chromatography with electrospray tandem mass spectrometry. Food Chem. 2007, 105, 1760–1766. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Zhou, L.; Zhang, F.; Kang, S.; Pan, C. Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J. Chromatogr. A 2012, 1225, 17–25. [Google Scholar] [CrossRef]
- Bakırcı, G.T.; Acay, D.B.Y.; Bakırcı, F.; Ötleş, S. Pesticide residues in fruits and vegetables from the Aegean region, Turkey. Food Chem. 2014, 160, 379–392. [Google Scholar] [CrossRef]
- Castro, G.; Pérez-Mayán, L.; Carpinteiro, I.; Ramil, M.; Cela, R.; Rodríguez, I. Residues of anilinopyrimidine fungicides and suspected metabolites in wine samples. J. Chromatogr. A 2020, 1622, 461104. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhu, J.; Li, H.; Li, D.; Liu, Z.; Sun, X.; Wang, B.; Wang, Q.; Gao, Y. Disposable Pipette Extraction (DPX) Coupled with Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Determination of Pesticide Residues in Wine Samples. Food Anal. Methods 2019, 12, 2262–2272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).