Abstract
Non-Saccharomyces yeasts have recently garnered significant interest in oenology. When co-inoculated with Saccharomyces cerevisiae, they contribute to the improvement of wine quality from a sensory point of view. In the present study, a group of yeasts previously isolated from manna and honey by-products were subjected to a genotypic identification. The D1/D2 variable domains of the 26-sRNA gene and the ITS region of the 5.8S gene were sequenced. Additionally, a differentiation of strains was carried out by RAPD-PCR. All strains underwent in vitro screening. Subsequently, a micro-vinification experiment was conducted, focusing on strains with favourable technological characteristics: Lachancea thermotolerans, Starmerella lactis-condensi, and Candida oleophila. These strains were sequentially inoculated alongside a control strain of Saccharomyces cerevisiae. Technological screening revealed that some strains exhibited limited H2S production, ethanol tolerance (up to 8% v/v), resistance to potassium metabisulphite (200 mg/L), osmotic stress tolerance (up to 320 g/L of glucose), and copper resistance (on average 5 mM). The findings from this study can guide the selection of new starters and co-starters for regional wine production.
1. Introduction
In traditional wine fermentation practices, commercial strains of Saccharomyces cerevisiae are used as starters to ensure consistent fermentation and contribute to the production of well-balanced wines. Since the 20th century, S. cerevisiae active dry yeast has been widely used for its reliability in achieving fast and predictable fermentations. Nevertheless, there exists a diverse array of both Saccharomyces and non-Saccharomyces strains that can persist during fermentation [1]. Non-Saccharomyces yeasts, while of secondary importance in must fermentation, are sometimes considered spoilage microorganisms [2] due to their limited fermentative capacity and tendency to produce off-flavours such as acetaldehyde, acetic acid, ethyl acetate, and acetoin [3]. Additionally, unwanted volatile phenols, such as those produced by Brettanomyces spp. [4], can be associated with these yeasts. Interestingly, strain-dependent studies have revealed that certain non-Saccharomyces yeasts exhibit positive effects. Specifically, they have been contributing to improving wine complexity, texture, and flavour integration in spontaneous fermentations since the 1980s [5].
Non-Saccharomyces yeasts play a crucial role in wine production, contributing unique aromatic complexity and mouthfeel. These effects are closely tied to the concept of terroir [6], which resonates with consumer preference for novel wine styles [7]. During the early stages of alcoholic fermentation, non-Saccharomyces yeasts, predominantly from genera like Hanseniaspora, Candida, Meyerozyma, Zygosaccharomyces, Schizosaccharomyces, Torulaspora, Kluyveromyces, and Metschnikowia, take the lead. However, as fermentation progresses, they yield the stage to S. cerevisiae, which ultimately completes the fermentation process [5,8]. These non-Saccharomyces strains often originate from grape berry surfaces, cellar equipment surfaces, or the winery environment. Initially, their demise was attributed to rising ethanol concentrations and the addition of SO2. However, recent research has revealed a more intricate picture, with strain-specific survival mechanisms. Surprisingly, even in the late stages of fermentation, several non-Saccharomyces species persist and thrive at significant levels [5,9,10,11,12,13].
During the pre-fermentative phase, three primary genera dominate: Hanseniaspora spp., Candida spp., and Metschnikowia spp. Among these, Hanseniaspora uvarum stands out as a key non-Saccharomyces yeast during the initial stages of fermentation. Additionally, Starmerella bacillaris is consistently found in grape must across various wine-producing regions and grape varieties, while Metschnikowia spp. thrive abundantly in grape must [13]. These non-Saccharomyces yeasts can significantly influence wine fermentation. Some impact flavour production directly, while others modulate the growth and metabolism of S. cerevisiae. Metschnikowia pulcherrima and S. bacillaris contribute to the production of 2-phenylethyl alcohol, associated with pleasant flavours at moderate concentrations [14,15]; Hanseniaspora uvarum, on the other hand, produces acetate and fruity esters [16,17]. Several non-Saccharomyces strains, including Torulaspora delbrueckii, Lachancea thermotolerans, M. pulcherrima, and Pichia kluyveri, have recently entered commercial use. These species play specific roles in wine production, aiming to achieve the following objectives: (i) enhancing varietal aromas [18]; (ii) regulating acidity characteristics [19]; (iii) improving colour extraction and mouthfeel characteristics [20]; (iv) reducing ethanol content [2]; (v) in the context of sparkling wines, playing a role in improving effervescence [21]. Despite their valuable contributions, non-Saccharomyces yeasts generally exhibit lower fermentation performance and do not numerically dominate the entire fermentation process due to their limited tolerance to ethanol and SO2 [22,23].
During the initial stages of fermentation, yeasts play a relevant role by significantly impacting the metabolic processes, leading to noticeable changes in the volatile characteristics of wine. This makes them suitable candidates for co-inoculation or sequential inoculation alongside S. cerevisiae [1,24]. Numerous studies indicate that matrices with high sugar content harbour both Saccharomyces and non-Saccharomyces yeasts, which have potential applications in oenology and the production of fermented beverages. For instance, Matraxia et al. [17] explored the use of H. uvarum, isolated from honey by-products (honeycombs and capping waxes) during beer fermentation, in co-inoculation with S. cerevisiae. Alfonzo et al. [25] successfully employed S. cerevisiae strains isolated from honey in winemaking, revealing significant differences compared to S. cerevisiae strains isolated from grapes.
Microbial communities specific to a particular food matrix significantly contribute to its composition and properties for food-related purposes. Guarcello et al. [26] conducted a study analyzing the microbial ecology of Sicilian manna ash (the phloem sap, a cerulean liquid that, on contact with air, quickly thickens and forms a light crystalline whitish layer that represents manna). Their goal was to gain insight into the hygienic quality, shelf- life, and potential applications of this traditional food. The study characterised the microorganisms associated with different products obtained during manna processing. Additionally, Gaglio et al. [27] investigated the microbial biodiversity of honey by-products used in the production of “Spiritu re fascitrari”. They discovered a niche rich in Saccharomyces and non-Saccharomyces yeasts.
Based on the above considerations, the aims of the present study are as follows: (i) identify a group of yeasts isolated from manna and honey; (ii) assess oenology through specific resistance, osmotolerance, and enzymatic activity tests; (iii) determine the fermentation performance of the best strains (such as starter or co-starter) by micro-fermentation.
2. Materials and Methods
2.1. Isolate Origins, DNA Extraction, and RFLP Analysis
The yeasts studied here in this research work belong to the collection of the Department of Agricultural, Food and Forest Sciences (SAAF; University of Palermo, Palermo, Italy); they were isolated from manna and honey by-products (Table S1). Specifically, 27 isolates have already been characterised genotypically at the species level [26], while 38 isolates have been identified using molecular techniques. DNA was extracted using the Quick-DNA Microprep Kit (Zymo Research, Orange, CA, USA) according to the instructions of the manufacturer. For initial discrimination, 38 yeast isolates were analysed by restriction fragment length polymorphism (RFLP) of the region spanning the internal transcribed spacers (ITS1 and ITS2) and the 5.8 S rRNA gene. DNA amplification was performed with the ITS1/ITS4 primer pair in accordance with Esteve-Zarzoso et al. [28]. The resulting amplicons were then digested with CfoI, HaeIII, and HinfI (MBI Fermentas, St. Leon-Rot, Germany) at 37 °C for 8 h. ITS amplicons and the corresponding restriction fragments were analysed on an agarose gel using 1.5% and 3% (w/v) agarose in a 1 × TBE (89 mM Tris-borate, 2 mM EDTA, pH 8) buffer, stained with SYBR safe DNA gel stain (Invitrogen, Waltham, MA, USA), visualised by UV transillumination, and captured on the Gel Doc 1000 video gel documentation system (Bio-Rad, Richmond, CA, USA). The standard DNA ladders used were 1 kb Plus and 50 pb (Invitrogen, Waltham, MA, USA).
2.2. Strain Typing and Species Identification
The intraspecific characterisation of the isolates belonging to the S. cerevisiae strains was carried out by Interdelta analysis with primers delta 12 and delta 21 [29]. The intraspecific characterisation of the isolates belonging to the non-Saccharomyces strains was carried out using different RAPD-PCR assays with primers M13 [30] and XD5 [31]. PCR products were analysed and visualised as described by Settanni et al. [32]. At least one strain per RAPD group was further processed by 26S rRNA gene D1/D2 region sequencing [27]. The data were compared with the sequence published in the GenBank database by means of the BLAST alignment tool http://blast.ncbi.nlm.nih.gov/ (accessed on 22 January 2024).
2.3. Technological Screening
All strains were tested for technological characteristics, H2S production, osmotolerance, and resistance to ethanol, potassium metabisulphite, and copper. In addition, growth tests on lysine were conducted. The ability to produce H2S was tested using a qualitative method performed on Bismuth Sulphite Glucose Glycerin Yeast extract (BiGGY) agar (Oxoid, Milan, Italy) [33]. Hydrogen sulphide was estimated by colony blackening after 3 days of incubation at 28 °C. A four-level scale was used: −, no growth; +, growth and low H2S production; P, growth and medium H2S production; PP, growth and high H2S production. Only strains with low H2S production were subjected to additional tests. The resistance tests were performed in a modified YPD medium containing different doses of each stress agent and according to the selection criteria for non-Saccharomyces yeasts described by Mestre Furlani et al. [34]. Accordingly, the following concentrations were used 4, 8, or 12% (v/v) of ethanol; 220, 270, or 320 g/L of glucose to test osmotolerance; 150 or 200 mg/L of sulphur dioxide (SO2) by addition of potassium metabisulphite (K2S2O5); and 2.5, 5, or 10 mM of copper, supplied as copper sulphate.
2.4. Growth Kinetics on a Single Source of Sugar
The strains were also evaluated for their ability to grow in the presence of single-sugar matrices using the procedure described by Kurtzman et al. [35] with the following modifications: the tests were performed in rimless tubes (16 × 180 mm), each containing 10 mL medium broth (yeast extract, 3 g/L; triptone, 5 g/L; glucose or fructose, 200 g/L) and inoculated with the pure strain cultures as reported by Hall et al. [36].
Growth of pure strain cultures in synthetic media was assessed by measuring optical density (OD) at 600 nm in a 96-well microtitre plate. Measurement was performed every 24 h for 4 days using ScanReady microplate photometer P-800 (Life Real Biotechnology Co., Ltd., Hangzhou, China). The temperature of the incubation was set at 25 °C. A blank measurement was subtracted from each OD reading. All analyses were performed in triplicate. Total growth of the strains was calculated as the integrated area underlying the curve up to 4 days as described by Hall et al. [36].
2.5. Fermentation of Grape Must
The strains with low H2S production, high resistance to ethanol and potassium metabisulphite, and the ability to grow rapidly on glucose and fructose substrates were evaluated for their ability to ferment a grape must.
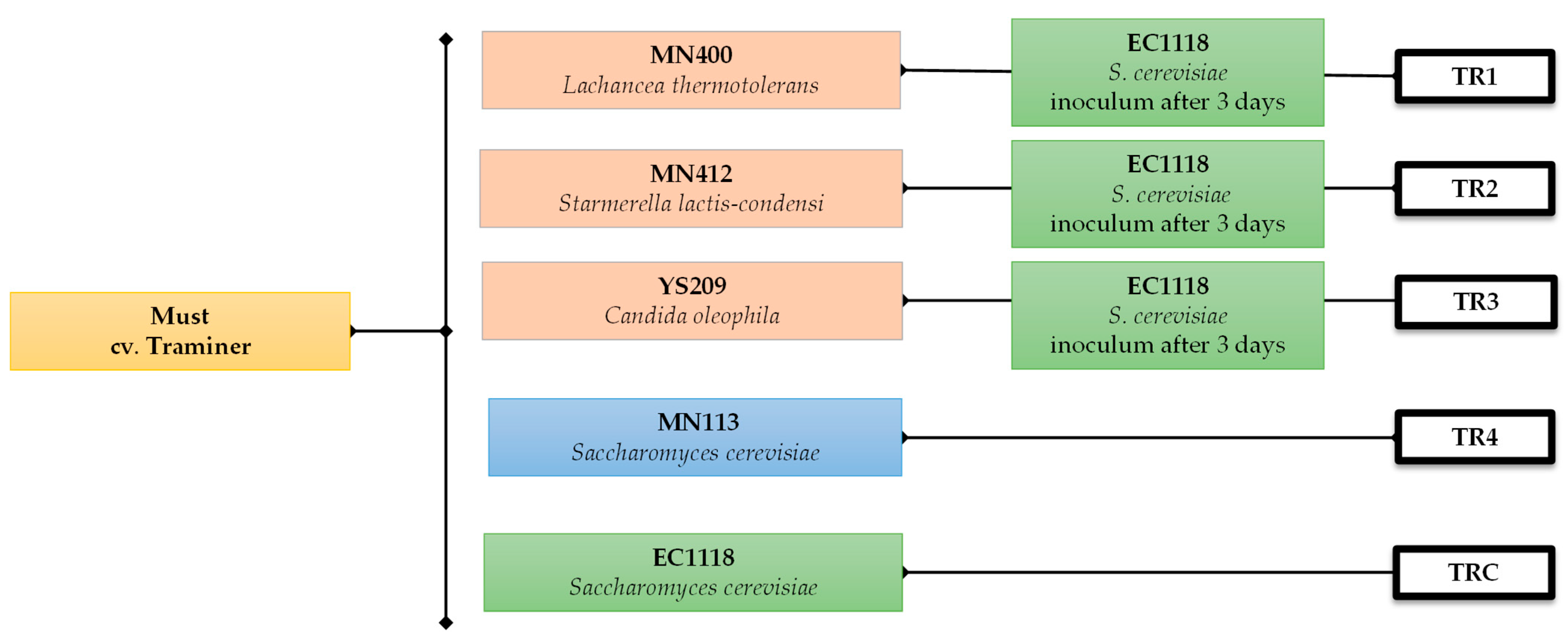
The grapes cv. Traminer were harvested during the 2023 vintage. All the micro-vinifications were carried out in the Department of Agricultural, Food and Forest Sciences (SAAF; University of Palermo, Palermo, Italy). The grapes were harvested, destemmed, and crushed by hand. The must obtained was divided into 21 batches (1 L each) and pasteurised at 72 °C for 15 s. The yeasts were inoculated in liquid concentrated form (approximately 6.0 Log CFU/g) the TR1 to TR3 trials were inoculated with different strains of non-Saccharomyces, each belonging to the species Lachancea thermotolerans MN400, Starmerella lactis-condensi MN412, and Candida oleophila YS209. While experiment TR4 was inoculated with a strain of S. cerevisiae MN113 from manna, TRC was inoculated with a commercial strain of S. cerevisiae EC1118 (Lallemand Inc., Montreal, QC, Canada). The experimental design is shown in Figure 1.
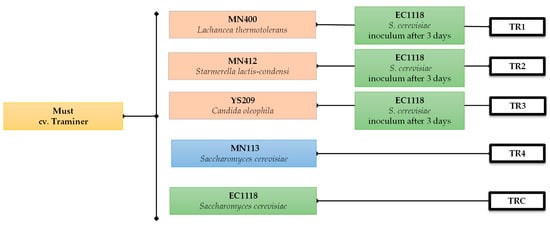
Figure 1.
Experimental plan of micro-vinification.
After 3 days, each experiment from TR1 to TR3 was inoculated with S. cerevisiae EC1118. The alcoholic fermentation of all experiments was carried out at 20 °C for 30 days.
At the end of the alcoholic fermentation, potassium metabisulphite (8 g/hL) was added to all experiments. Samples were collected at different stages of vinification: at the time of non-Saccharomyces strain inoculation (0 days), after the inoculation of S. cerevisiae (3 days of alcoholic fermentation), after 8 days of fermentation, and at the end of alcoholic fermentation. The collected samples were immediately analysed. All analyses were performed in triplicate. To remove CO2, the flasks were sealed with a Müller valve [37], and the weight loss was monitored until it fell below 0.01 g per day, indicating the end of fermentation.
2.6. Microbiological and Oenological Parameters
All samples collected during alcoholic fermentation were analysed for yeast populations. Musts samples were diluted in Ringer’s solution (Sigma-Aldrich, Milan, Italy) and analysed in triplicate for presumptive Saccharomyces spp. yeasts on Wallerstein Laboratory (WL) nutrient agar [38], and non-Saccharomyces were counted on lysine agar [39]. All media and supplements were purchased from Oxoid (Thermofisher, Milan, Italy).
The wines obtained were analysed by means of WineScan (FOSS, Hillerød, Denmark) to determine volatile acidity (VA), reducing sugars, ethanol, glycerol, malic acid, and lactic acid. The instrument was calibrated according to the EEC 2676 standard procedure [40]. pH was determined according to the OIV-MA-AS313-15 method [41] and total acidity (TA) according to the method described in OIV-MA-AS313-01 [42]. All chemical analyses were performed in triplicate.
2.7. Statistical Analysis
The ANOVA test was employed to ascertain the statistical significance between the microbial loads (presumed Saccharomyces and non-Saccharomyces) and the chemical parameters observed during the winemaking process (residual sugar, ethanol, glycerol, malic acid, lactic acid and volatile acidity and total acidity). The post-hoc Tukey method was used for pairwise comparison of all data. Statistical significance was set at p < 0.05 [43].
An exploratory multivariate approach using Agglomerative Hierarchical Clustering (AHC) was used to investigate the relationships between the data obtained at the end of the alcoholic fermentation (ethanol, residual sugar, glycerol, malic acid, lactic acid, pH, total acidity, and volatile acidity) from the different treatments [25]. The software used for agglomerative statistical data processing was XLStat ver. 2019.2.2 (Addinsoft, New York, NY, USA).
3. Results and discussion
3.1. Isolation, Identification, and Strain Typing of Yeasts
Out of a total of 65 yeast isolates, 38 were subjected to genotypic characterisation. The restriction analysis of the ITS1-5.8S-ITS2 region led to the separation of these isolates into five distinct profiles (Table 1).

Table 1.
Molecular identification of yeast species isolated from manna and honey samples.
Preliminary species identification was performed by comparing the restriction profiles with those reported in the literature [28,30,40]. Specifically, the isolates were identified as Citeromyces matritensis, L. thermotolerans, Meyerozyma guillermondii, Starmerella magnoliae, and S. cerevisiae. To further confirm the species identification, genotypic analysis involved pairwise alignment of D1/D2 sequences (Table 1). Strain typing allowed the 65 isolates to be grouped into 21 strains, distributed across eight yeast species (Table 2). Among these, L. thermotolerans had the highest number of strains (n = 7), while S. magnoliae, C. oleophila, and S. cerevisiae were each represented by a single strain.

Table 2.
Technological screening of 21 yeast strains.
3.2. Technological Characteristics of Yeast Strains
Table 2 reports the results of technological screening.
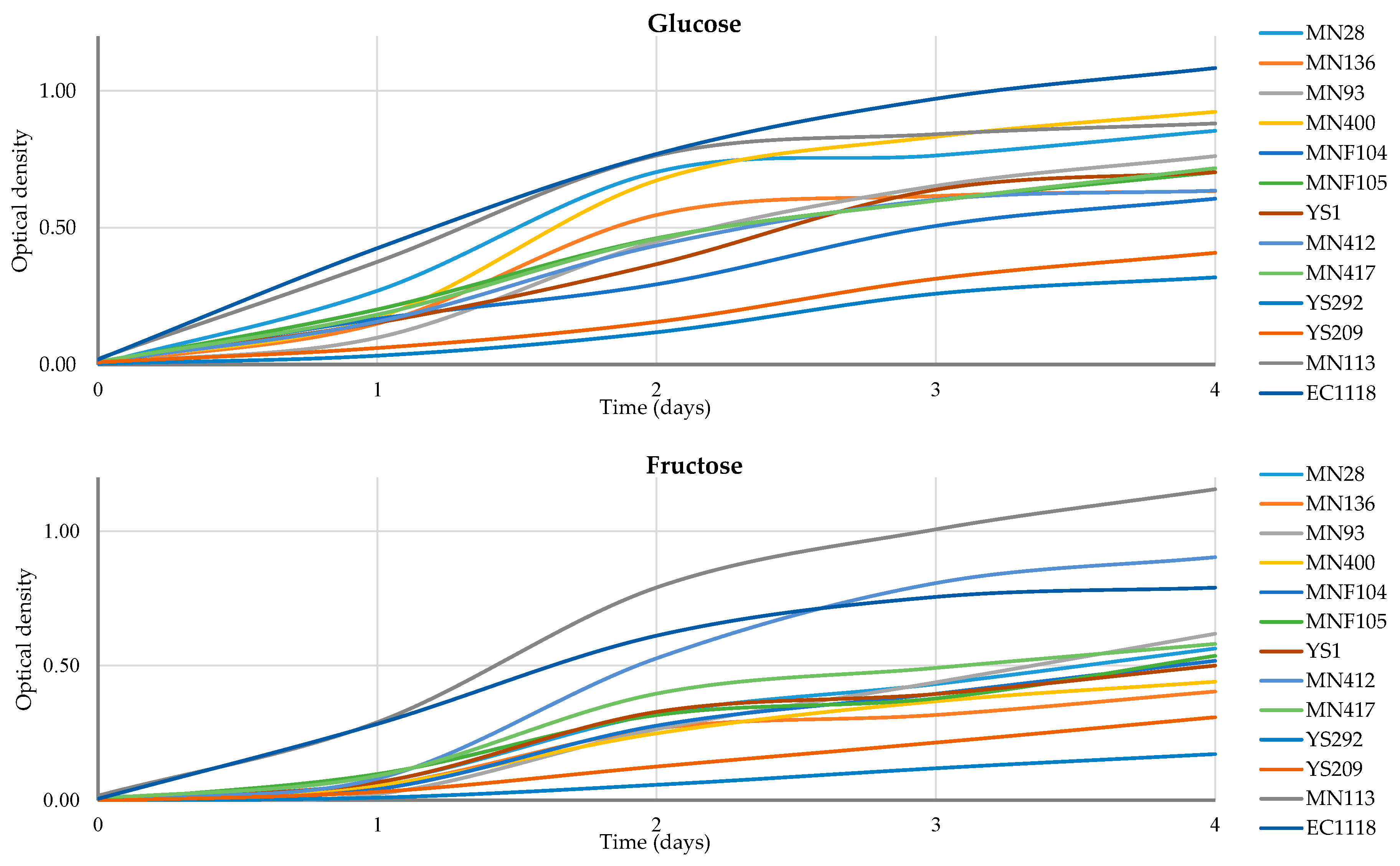
Several differences were observed between different yeast species. All strains underwent assessment for their ability to produce H2S [44]. However, only strains from the species C. matritensis, C. aaseri, and M. guillermondii showed high H2S production and were therefore excluded from subsequent resistance tests. In ethanol resistance tests, strains belonging to the species L. thermotolerans and S. lactis-condensi demonstrated resistance to 8% (v/v) ethanol. Meanwhile, strains from the species S. magnoliae and C. oleophila exhibited resistance to 4% (v/v) ethanol, whereas S. cerevisiae displayed resistance to 12% (v/v) ethanol. Moreover, all strains showed growth in the presence of 200 mg/L of potassium metabisulphite. Regarding copper resistance, there was significant variability among strains of the species L. thermotolerans. However, only strains from the species S. magnoliae and C. oleophila resisted the highest copper concentrations (10 mM). Based on the results of previous technological tests, all 12 strains were selected for further investigation of their growth kinetics on fructose and glucose media (Figure 2).
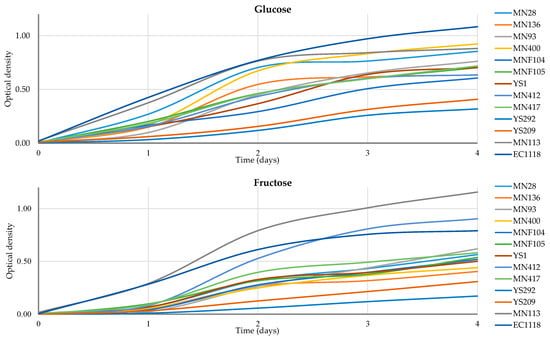
Figure 2.
Growth of different strains in a single-sugar matrix of glucose or fructose. The growth was measured using OD values at 600 nm in triplicate. Values of standard deviation ranged between 0 and 0.16 but are not shown for a better graphical visualisation of the figures.
During fructose fermentation, the highest OD value on the 4th day of fructose fermentation was observed for S. cerevisiae MN113, reaching 1.15. Among non-Saccharomyces, strain MN400 L. thermotolerans showed the best growth, with an OD value of 0.92 after 4 days of glucose fermentation. Notably, during fructose fermentation, S. lactis-condensi MN412 showed OD values (0.90) higher than the control strain S. cerevisiae EC1118 (0.79) after 4 days of incubation. This characteristic suggests potential fructophilic activity of strain MN412. The complete growth values are summarised in Table 3.

Table 3.
Total growth of the strains on a synthetic medium containing exclusively glucose or fructose as a sugar source. Total growth was calculated as the integral area under the curve determined after 4 days of incubation.
In terms of overall growth on glucose, among the non-Saccharomyces strains, L. thermotolerans MN400 showed the highest value (2.14). On the other hand, for fructose growth, S. lactis-condensi MN412 achieved the highest value (1.87). Additionally, when it comes to fructose, S. cerevisiae MN113, isolated from manna, displayed greater growth than the EC1118 strain (control).
3.3. Micro-Fermentation
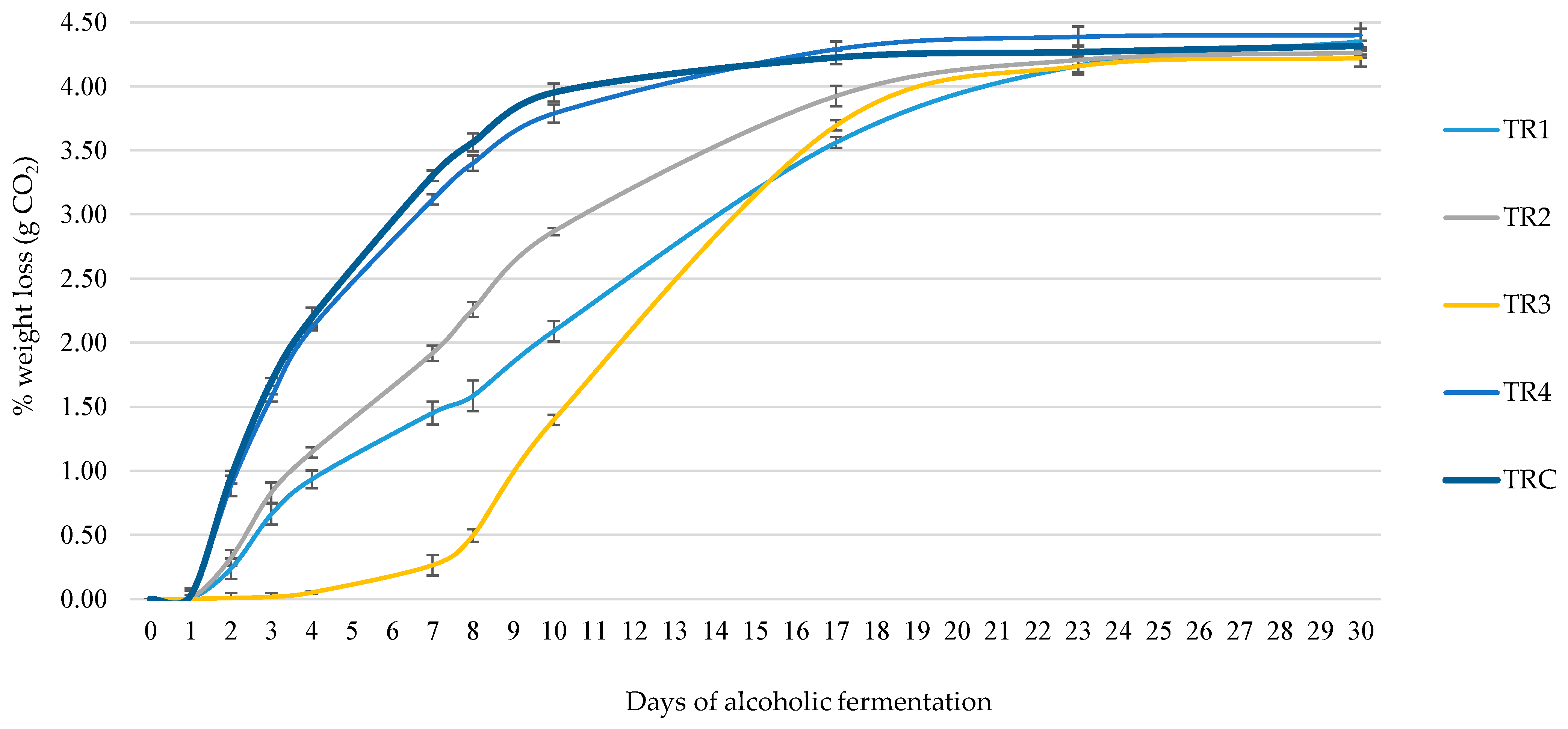
For the TR1 trial, L. thermotolerans MN400 was chosen due to its robust copper resistance and optimal growth dynamics on both glucose and fructose. In the TR2 experiment, S. lactis-condensi MN412 served as a co-starter, exhibiting vigorous growth, specifically on fructose. In the TR4 fermentation experiment, S. cerevisiae MN113 acted as the primary starter. Throughout the micro-fermentations, daily weight loss (CO2 emitted) was monitored over a period of 30 days, spanning the completion of alcoholic fermentation. The results from the fermentation kinetics (Figure 3) demonstrated that 3 days after inoculation the non-Saccharomyces species with the most substantial weight loss were S. lactis-condensi (TR2) and the L. thermotolerans strain (TR1), while among Saccharomyces, the commercial strain EC1118 (TRC) and manna-isolated MN113 strain (TR4) showed the highest fermentation rates. Other strains exhibited minimal fermentation activity.
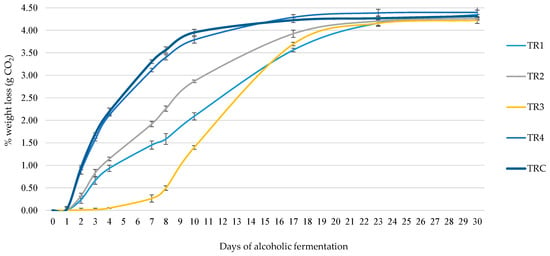
Figure 3.
Weight loss during micro-vinification.
3.4. Microbiological Counts
Table 4 presents the microbial yeast counts during fermentation.

Table 4.
Monitoring of yeast populations during experimental micro-fermentation.
Microbiological monitoring involved presumptive counts of both Saccharomyces and non-Saccharomyces at various stages: T0 (inoculation of the starter/co-starter), day 3 (inoculation of S. cerevisiae in TR1, TR2, and TR3 trials), day 8, and at the end of alcoholic fermentation. The non-Saccharomyces strains were initially inoculated within the range of 5.9 to 6.2 log (CFU/mL), whereas Saccharomyces strains (MN113 and EC1118) were inoculated at a density around 6.5–6.8 log (CFU/mL). By day 3 of alcoholic fermentation across all treatments, yeast populations exhibited growth, reaching values of 6.5 and 7.4 Log CFU/mL. On the third day, Saccharomyces was inoculated at approximately 6.5 Log CFU/mL for each of the experiments (TR1, TR2, and TR3). After 8 days of alcoholic fermentation, TR1 and TR2 trials showed non-Saccharomyces counts approximately 0.5 logarithmic cycle higher than presumptive Saccharomyces. In TR3, the non-Saccharomyces counts fell below the detection limit due to their low resistance to ethanol. This trend aligns with findings by Binati et al. [2] who studied three different non-Saccharomyces species combined with S. cerevisiae. At the end of alcoholic fermentation, TR1 and TR2 exhibited non-Saccharomyces counts of 4.2–4.3 Log CFU/mL, while Saccharomyces counts were approximately 6.4–6.7 Log CFU/mL. Several authors agree that co-inoculating S. cerevisiae and non-Saccharomyces yeast species can lead to the demise or reduced variability of non-Saccharomyces once S. cerevisiae dominates the fermentation and becomes stress-resistant to inhibitory ethanol.
Furthermore, the secretion of inhibitory substances has been identified as a potential cause of inhibition in non-Saccharomyces yeasts [46]. Therefore, researchers recommend a sequential inoculation approach (non-Saccharomyces followed by S. cerevisiae) over a mixed culture. This technique allows for greater expression of non-Saccharomyces yeast metabolism [47]. In trials where single-culture MN113 S. cerevisiae (isolated from manna) was used (TR4), similar trends were observed compared to control trials (TRC) inoculated with grape yeasts. Alfonzo et al. [25] used S. cerevisiae isolated from honey by-products in wine production and found its microbiological behaviour to be comparable to that of S. cerevisiae isolated from grapes.
3.5. Physico-Chemical Analysis
The influence of manna yeasts (both Saccharomyces and non-Saccharomyces) on the chemical composition of wines was evaluated by quantifying key analytical components at the end of alcoholic fermentation. The summarised results of these chemical analyses are presented in Table 5.

Table 5.
Chemical parameters determined during the micro-vinification process.
In terms of glycerol content, the highest values were observed in trials inoculated with L. thermotolerans (7.4 g/L, TR1), followed by TR2 (6.20 g/L), TR3 (5.30 g/L), and TRC (5.10 g/L). Lastly, the trial involving the use of manna-isolated strain MN113 showed the lowest value (4.80 g/L). This trend aligns with findings reported by Hranilovic et al. [48]. Volatile acidity showed the highest value in the TRC trial (0.38 g/L acetic acid), while the lowest value was recorded in the TR2 trial (0.18 g/L acetic acid). Across the other treatments, VA values remained below 0.80 g/L, which is the threshold beyond which wine quality is compromised [49].
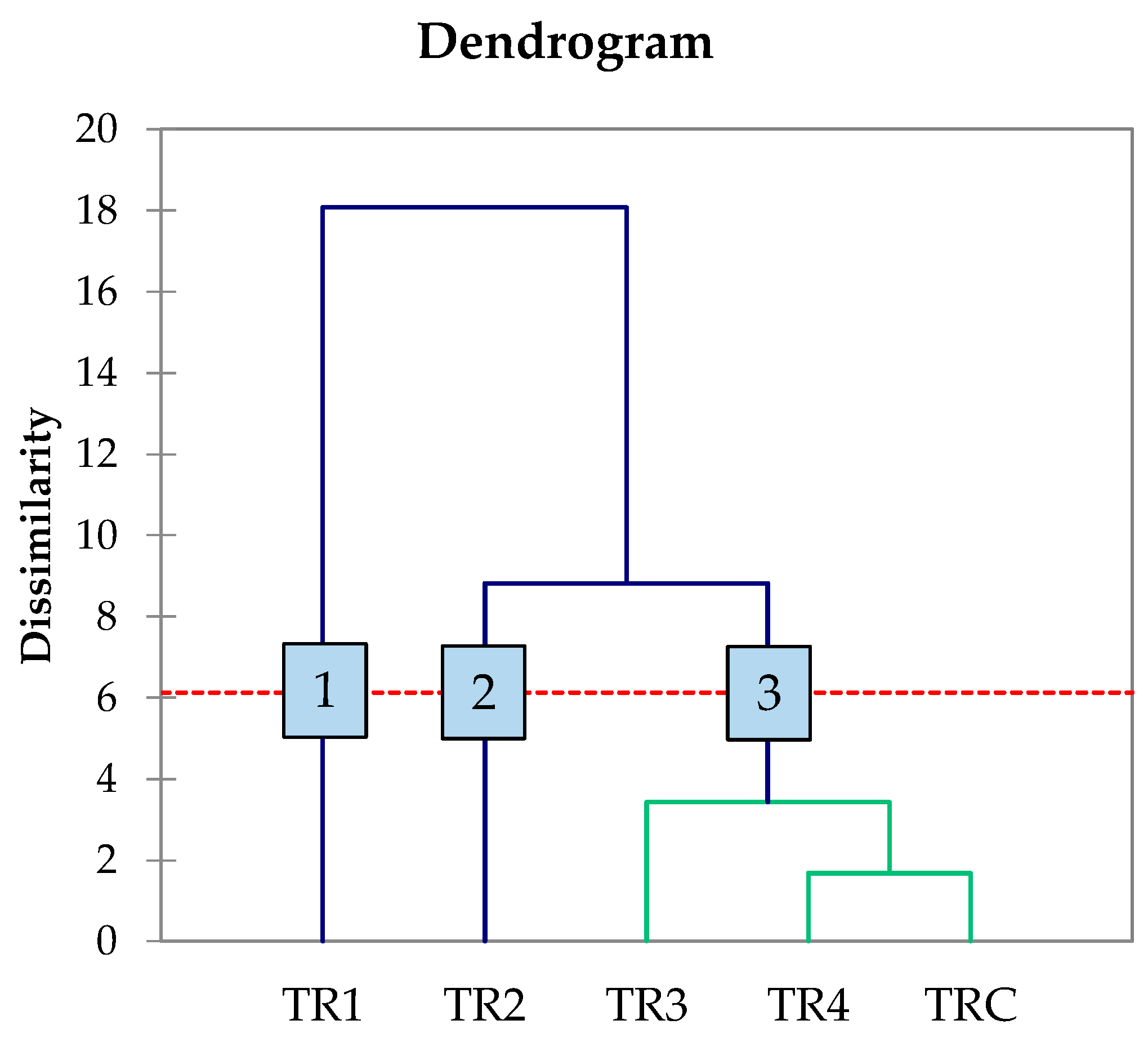
At the conclusion of alcoholic fermentation, many of the obtained wines displayed low residual sugar content (<0.5 g/L), a common characteristic of dry wines [50]. This confirms the successful completion of fermentation by the yeasts. The ethanol content in trials T2, T3, and T4 was comparable to the TRC control trial. However, trial T1 showed lower ethanol values than TRC. Additionally, an AHC analysis was conducted on the primary chemical data from the wines to better visualize the technological variability introduced by the strains used (Figure 4).
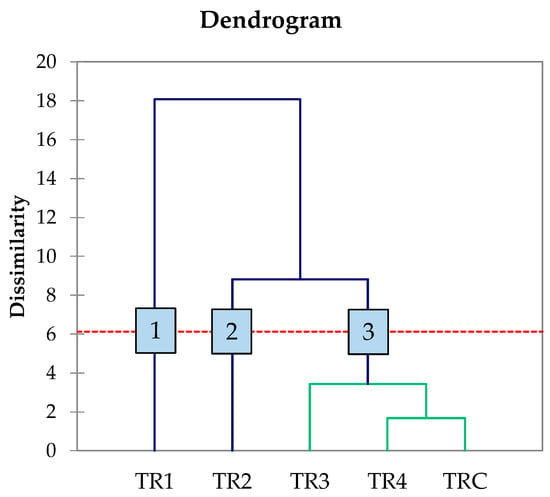
Figure 4.
Dendrogram generated by AHC analysis of principal component analysis of the main oenological parameters at the end of alcoholic fermentation. Numbers indicate the different clusters emerged throught AHC analysis. The red dotted line represents the level of significance.
Through data processing, the five treatments were categorised into three distinct groups. Group 1 was represented by trial TR1, which differed from the others due to variations in glycerol and TA content. Group 2, associated with trial TR2, exhibited distinct volatile acidity compared to the remaining trials. Group 3 consisted of trials TR3, TR4, and TRC. In terms of chemical parameters, strains L. thermotolerans MN400 and S. lactis-condensi MN412 yielded different wine profiles. Interestingly, S. cerevisiae strain MN113 showed a fermentation performance similar to that of the commercial strain, suggesting its potential as a starter for winemaking. However, a comprehensive evaluation of the aromatic and sensory impact of these strains on wines produced at both medium and large scales remains necessary.
4. Conclusions
In this research, both culture-dependent and molecular techniques were used to explore the diversity of yeasts in high-sugar matrices such as manna and honey. The primary focus was on yeasts, aiming to investigate their potential application in oenology. The study revealed a rich variety of non-Saccharomyces and Saccharomyces yeasts present in manna and honey by-products. In order to ascertain their suitability for use as starters or co-starters in winemaking, an extensive technological characterisation was conducted on the pasteurised grape juice. The objective of this characterisation was to facilitate more precise control over the yeast population’s development. In particular, strains with limited H2S production and enhanced tolerance to ethanol, osmotic stress, and copper were carefully selected.
Given that the characteristics analysed are influenced by the yeast species and strain, the results underscore the importance of characterising a diverse range of isolates. These selected starters and co-starters can be employed in monoculture or mixed fermentations, ultimately enhancing wine quality and imparting distinct characteristics to the final product. However, additional research is necessary to assess how these chosen strains impact the volatile organic components when subjected to sequential inoculation with S. cerevisiae. The use of pasteurised must allowed an initial screening of strains of oenological interest. Further studies will be carried out in real fermentations to verify the suitability of the selected strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/beverages10030048/s1, Table S1: List of yeast isolates from manna and honey subjected to strain typing.
Author Contributions
Conceptualisation, N.F., G.M. and A.A.; methodology, D.O. and R.G.; software, R.P. and V.N.; validation, N.F., A.A. and G.M.; formal analysis, V.C., E.V., A.V. and A.P.; investigation, V.C., R.P., F.A. and G.N.; resources, N.F., F.A. and G.N.; data curation, E.V., V.C., R.P. and A.A.; writing—original draft preparation, V.C., R.P., A.A. and L.S.; writing—review and editing, N.F., A.A., G.M. and L.S.; visualisation, A.A. and N.F.; supervision, A.A., N.F. and G.M.; project administration, N.F.; funding acquisition, N.F. All authors have read and agreed to the published version of the manuscript.
Funding
The support for this research was provided through different funding that were used to support and develop different and separate parts of total research. There was no overlap in the use of funds for the same activity. The funding sources were: (i) Project PRIMA MEDIET4ALL: “Transnational Movement to Support the Sustainable Transition towards a Healthy and Eco-friendly Agri-Food System through the Promotion of MEDIET and its Lifestyle in Modern Society”; transnational call, PRIMA Partnership for Research and Innovation in the Mediterranean Area, call 2022, Thematic Area 3—Food Value Chain: Topic 2.3.1–2022 (RIA) Enabling the transition to healthy and sustainable dietary behaviour. Grant number: B73C23000060001; (ii) PSR—Rural Development Program Sicily 2014/2022-Sub-measure 16.01 “Support for the establishment and management of operational groups of the EIP on agricultural productivity and sustainability”—DDG. no. 5428 of 29/12/2021, project title: “Aromaticity and Longevity of Catarratto: Innovation in Field and Cellar based on Biodiversity, Subproducts and High Sustainability Biotechnologies”. Grant number: 24250128113; (iii) Supply Chain and District Contracts (5th call) project, “White Wine Identity: New Horizons for an Integrated and Sustainable Development of the Italian White Wine Supply Chain”, which was funded by the Ministry of Agriculture, Food Sovereignty, and Forests. Grant number: PRI-1058 (iii) (iv) Research project named “Biotecnologie in ambito Viticolo ed Enologico”, CON 0406, with Milazzo Terre della Baronia srl wine company (Campobello di Licata, Sicily, Italy). Grant number: B75F21001890007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Regional Institute of Wine and Oil (IRVO) for the financing of scholarships for the PhD in Agro-Food and Mediterranean Forestry Systems, DM n.352/2022-PNRR-Mission 4-Component 2-Investment 3.3.
Conflicts of Interest
Author Filippo Amato was employed by the company HTS enologia, he contributed to the definition of potential technological profiles of yeast strains, as well as providing some instrumentation and materials for chemical analysis of fermented musts; Author Giuseppe Notarbartolo was employed by the company Az. Agr. G. Milazzo—Terre Della Baronia S.r.l., he was responsible for selecting the grapes and producing the must used for micro-vinifications, selecting the optimal grapes and taking into account the chemical profile of the starting must. The grapes selected for microvinification come from vineyards on the vineyard at which he works; Author Raffaele Guzzon was employed by the Fondazione Edmund Mach, his role as a co-author in the manuscript was to decide and perform together with the other co-authors the technological tests to be carried out on yeast strains and in vitro technological screening; The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Binati, R.L.; Junior, W.J.L.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Esteves, M.; Barbosa, C.; Vasconcelos, I.; Tavares, M.J.; Mendes-Faia, A.; Pereira Mira, N.; Mendes-Ferreira, A. Characterizing the Potential of the Non-Conventional Yeast Saccharomycodes ludwigii UTAD17 in Winemaking. Microorganisms 2019, 7, 478. [Google Scholar] [CrossRef]
- Kheir, J.; Salameh, D.; Strehaiano, P.; Brandam, C.; Lteif, R. Impact of volatile phenols and their precursors on wine quality and control measures of Brettanomyces/Dekkera yeasts. Eur. Food Res. Technol. 2013, 237, 655–671. [Google Scholar] [CrossRef]
- Gschaedler, A. Contribution of non-conventional yeasts in alcoholic beverages. Curr. Opin. Food Sci. 2017, 13, 73–77. [Google Scholar] [CrossRef]
- Binati, R.L.; Innocente, G.; Gatto, V.; Celebrin, A.; Polo, M.; Felis, G.E.; Torriani, S. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res. Int. 2019, 122, 432–442. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Fermentation performances and aroma production of non-conventional wine yeasts are influenced by nitrogen preferences. FEMS Yeast Res. 2018, 18, foy055. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Andorra, I.; Monteiro, M.; Esteve-Zarzoso, B.; Albergaria, H.; Mas, A. Analysis and direct quantification of Saccharomyces cerevisiae and Hanseniaspora guilliermondii populations during alcoholic fermentation by fluorescence in situ hybridization, flow cytometry and quantitative PCR. Food Microbiol. 2011, 28, 1483–1491. [Google Scholar] [CrossRef]
- David, V.; Terrat, S.; Herzine, K.; Claisse, O.; Rousseaux, S.; Tourdot-Maréchal, R.; Masneuf-Pomarede, I.; Ranjard, L.; Alexandre, H. High-throughput sequencing of amplicons for monitoring yeast biodiversity in must and during alcoholic fermentation. J. Ind. Microbiol. Biotechnol. 2014, 41, 811–821. [Google Scholar] [CrossRef]
- Wang, C.; Esteve-Zarzoso, B.; Mas, A. Monitoring of Saccharomyces cerevisiae, Hanseniaspora uvarum, and Starmerella bacillaris (synonym Candida zemplinina) populations during alcoholic fermentation by fluorescence in situ hybridization. Int. J. Food Microbiol. 2014, 191, 1–9. [Google Scholar] [CrossRef]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef]
- Albertin, W.; Zimmer, A.; Miot-Sertier, C.; Bernard, M.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Bely, M.; Marullo, P.; Masneuf-Pomarede, I. Combined effect of the Saccharomyces cerevisiae lag phase and the non-Saccharomyces consortium to enhance wine fruitiness and complexity. Appl. Microbiol. Biotechnol. 2017, 101, 7603–7620. [Google Scholar] [CrossRef]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Andorra, I.; Landi, S.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of fermentation temperature on microbial population evolution using culture-independent and dependent techniques. Food Res. Int. 2010, 43, 773–779. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and oenological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Matraxia, M.; Alfonzo, A.; Prestianni, R.; Francesca, N.; Gaglio, R.; Todaro, A.; Alfeo, V.; Perretti, G.; Columba, P.; Settanni, L.; et al. Non-conventional yeasts from fermented honey by-products: Focus on Hanseniaspora uvarum strains for craft beer production. Food Microbiol. 2021, 99, 103806. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Belda, I.; Conchillo, L.B.; Ruiz, J.; Navascués, E.; Marquina, D.; Santos, A. Selection and use of pectinolytic yeasts for improving clarification and phenolic extraction in winemaking. Int. J. Food Microbiol. 2016, 223, 1–8. [Google Scholar] [CrossRef]
- Medina-Trujillo, L.; González-Royo, E.; Sieczkowski, N.; Heras, J.; Canals, J.M.; Zamora, F. Effect of sequential inoculation (Torulaspora delbrueckii/Saccharomyces cerevisiae) in the first fermentation on the foaming properties of sparkling wine. Eur. Food Res. Technol. 2017, 243, 681–688. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.C.; Coulon, J.; Thibon, C.; Masneuf-Pomarede, I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT 2021, 149, 111781. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef]
- Pandilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine Aroma complexity. Front. Microbiol. 2016, 7, e00411. [Google Scholar] [CrossRef]
- Alfonzo, A.; Prestianni, R.; Gaglio, R.; Matraxia, M.; Maggio, A.; Naselli, V.; Craparo, V.; Badalamenti, N.; Bruno, M.; Vagnoli, P.; et al. Effects of different yeast strains, nutrients, and glutathione-rich inactivated yeast addition on the aroma characteristics of Catarratto wines. Int. J. Food Microbiol. 2021, 360, 109325. [Google Scholar] [CrossRef]
- Guarcello, R.; Gaglio, R.; Todaro, A.; Alfonzo, A.; Schicchi, R.; Cirlincione, F.; Moschetti, G.; Francesca, N. Insights into the cultivable microbial ecology of “Manna” ash products extracted from Fraxinus angustifolia (Oleaceae) trees in Sicily, Italy. Front. Microbiol. 2019, 10, 984. [Google Scholar] [CrossRef]
- Gaglio, R.; Alfonzo, A.; Francesca, N.; Corona, O.; Di Gerlando, R.; Columba, P.; Moschetti, G. Production of the Sicilian distillate “Spiritu re fascitrari” from honey by-products: An interesting source of yeast diversity. Int. J. Food Microbiol. 2017, 261, 62–72. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8 S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Evol. Micr. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Legras, J.L.; Karst, F. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterization. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef]
- Francesca, N.; Sannino, C.; Settanni, L.; Corona, O.; Barone, E.; Moschetti, G. Microbiological and chemical monitoring of Marsala base wine obtained by spontaneous fermentation during large-scale production. Ann. Microbiol. 2014, 64, 1643–1657. [Google Scholar] [CrossRef]
- Di Maro, E.; Ercolini, D.; Coppola, S. Yeast dynamics during spontaneous wine fermentation of the Catalanesca grape. Int. J. Food Microbiol. 2007, 117, 201–210. [Google Scholar] [CrossRef]
- Settanni, L.; Sannino, C.; Francesca, N.; Guarcello, R.; Moschetti, G. Yeast ecology of vineyards within Marsala wine area (western Sicily) in two consecutive vintages and selection of autochthonous Saccharomyces cerevisiae strains. J. Biosci. Bioeng. 2012, 114, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Validation of bismuth-containing indicator media for predicting H2S producing potential of Saccharomyces cerevisiae wine yeasts under oenological conditions. Am. J. Enol. Vitic. 1995, 46, 269–273. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017, 17, fox010. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.M.; Robert, V. Methods for isolation, phenotypic characterization and maintenance of yeasts. In The Yeasts, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 87–110. [Google Scholar] [CrossRef]
- Hall, B.G.; Acar, H.; Nandipati, A.; Barlow, M. Growth rates made easy. Mol. Biol. Evol. 2014, 31, 232–238. [Google Scholar] [CrossRef]
- Vaquero, C.; Escott, C.; Loira, I.; Guamis, B.; del Fresno, J.M.; Quevedo, J.M.; Gervilla, R.; de Lamo, S.; Ferrer-Gallego, R.; González, C.; et al. Cabernet Sauvignon Red Must Processing by UHPH to Produce Wine Without SO2: The Colloidal Structure, Microbial and Oxidation Control, Colour Protection and Sensory Quality of the Wine. Food Bioproc. Technol. 2022, 15, 620–634. [Google Scholar] [CrossRef]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL medium to profile native flora fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—A review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Sannino, C.; Francesca, N.; Corona, O.; Settanni, L.; Cruciata, M.; Moschetti, G. Effect of the natural winemaking process applied at industrial level on the microbiological and chemical characteristics of wine. J. Biosci. Bioeng. 2013, 116, 347–356. [Google Scholar] [CrossRef]
- OIV-MA-AS313-15; Compendium of International Methods of Wine and Must Analysis. OIV (International Organisation of Vine and Wine): France, Paris, 2011. Available online: https://www.oiv.int/it/node/2011/download/pdf (accessed on 25 January 2024).
- OIV-MA-AS313-01; Compendium of International Methods of Wine and Must Analysis. OIV (International Organisation of Vine and Wine): France, Paris, 1995. Available online: https://www.oiv.int/it/node/1995/download/pdf (accessed on 25 January 2024).
- Mazzei, P.; Francesca, N.; Moschetti, G.; Piccolo, A. NMR spectroscopy evaluation of direct relationship between soils and molecular composition of red wines from Aglianico grapes. Anal. Chim. Acta. 2010, 673, 167–172. [Google Scholar] [CrossRef]
- Alonso, A.; Belda, I.; Santos, A.; Navascués, E.; Marquina, D. Advances in the control of the spoilage caused by Zygosaccharomyces species on sweet wines and concentrated grape musts. Food Control 2015, 51, 129–134. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-wesley: Reading, MA, USA, 1977; Volume 2, pp. 131–160. [Google Scholar]
- Englezos, V.; Pollon, M.; Rantsiou, K.; Ortiz-Julien, A.; Botto, R.; Segade, S.R.; Giacosa, S.; Rolle, L.; Cocolin, L. Saccharomyces cerevisiae-Starmerella bacillaris strains interaction modulates chemical and volatile profile in red wine mixed fermentations. Food Res. Int. 2019, 122, 392–401. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Bañuelos, M.A.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT-Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M.; Diako, C.; Ross, C.F. Sensory and chemical characteristics of ‘dry’ wines awarded gold medals in an international wine competition. J. Wine Res. 2019, 30, 204–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).