Electrospun Polycaprolactone/Collagen Scaffolds Enhance Manipulability and Influence the Composition of Self-Assembled Extracellular Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of PCLCOL Samples Using Electrospinning
2.2. Microstructure Analysis
2.3. Mechanical Strength Analysis
2.4. Fourier-Transform Infrared Spectroscopy (FTIR) Assay
2.5. Degradation Rate Measurement
2.6. Cell Culture
2.7. Cell Adhesion Assay
2.8. Cell Viability Assay
2.9. Production of the Self-Assembled ECM on the PCLCOL
2.10. Production of Self-Assembled ECM on TCP
2.11. Histological Analysis
2.12. Dot Blot Assay
2.13. Growth Factors Secretion Profile Analysis
2.14. Statistical Analysis
3. Results
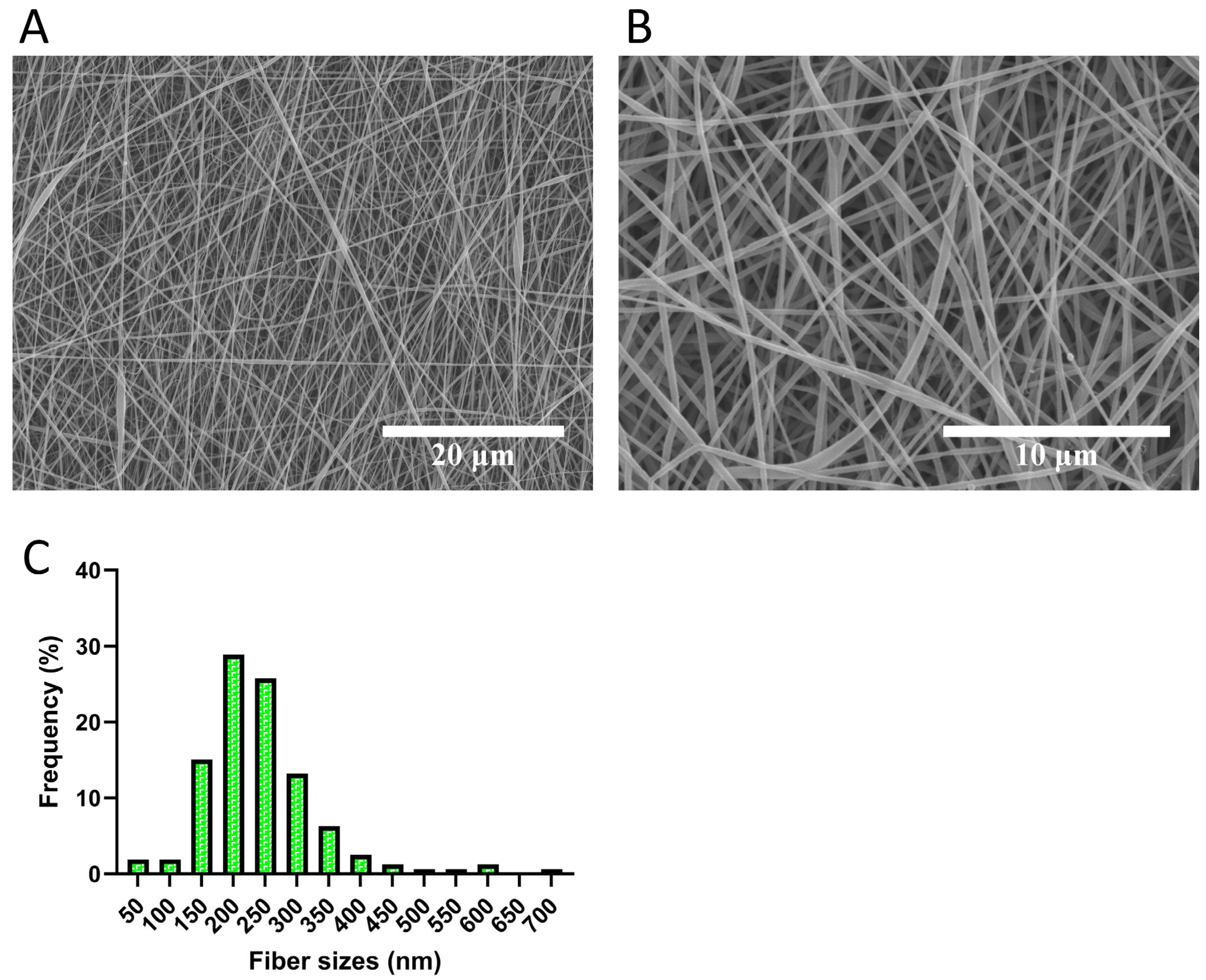
3.1. Microstructure Analysis Revealed Fibrous Structure of the Electrospun Scaffolds
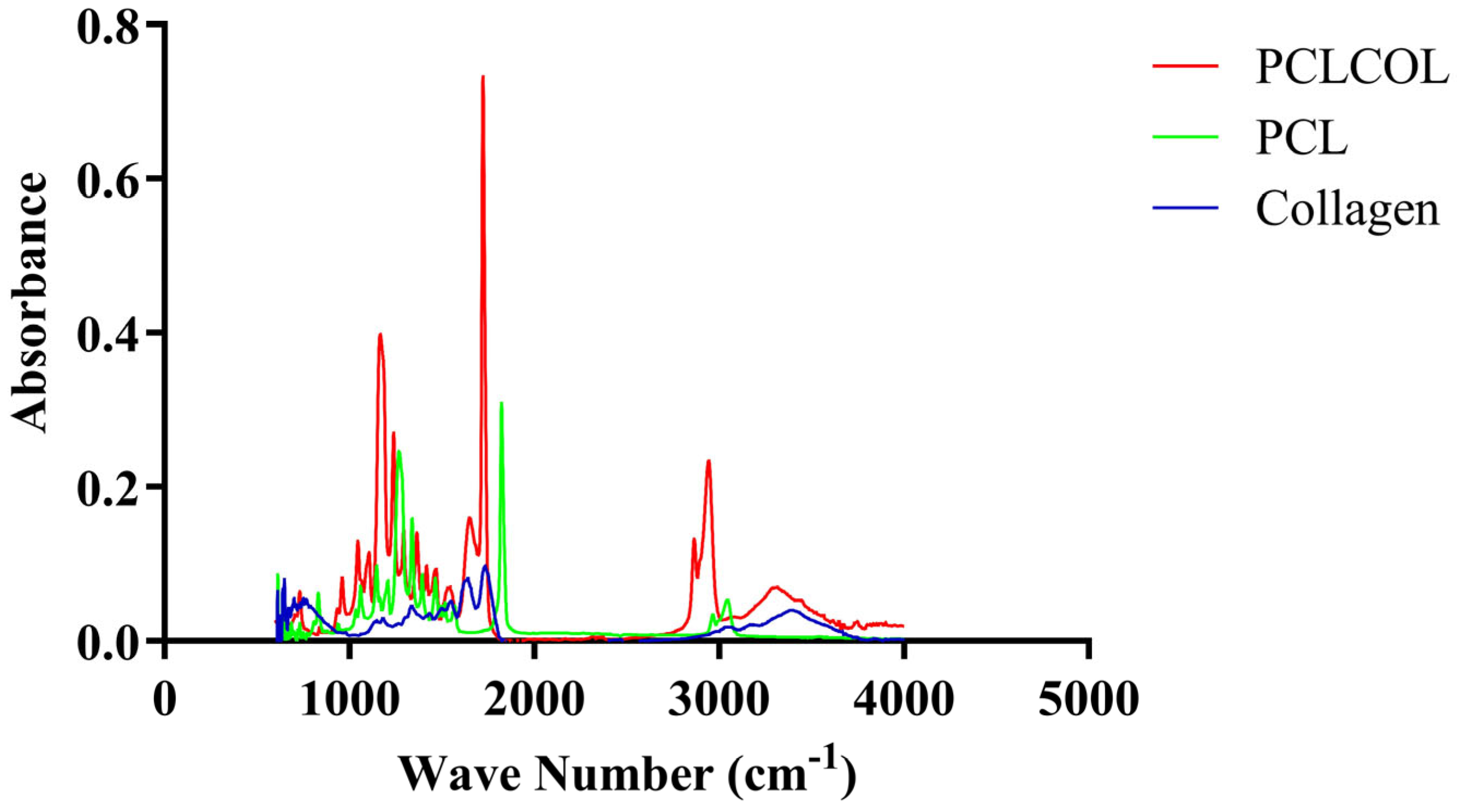
3.2. Confirmation of Collagen Incorporation into PCL Using FTIR Spectroscopy
3.3. Time-Dependent Degradation Behavior of PCLCOL in Culture Media
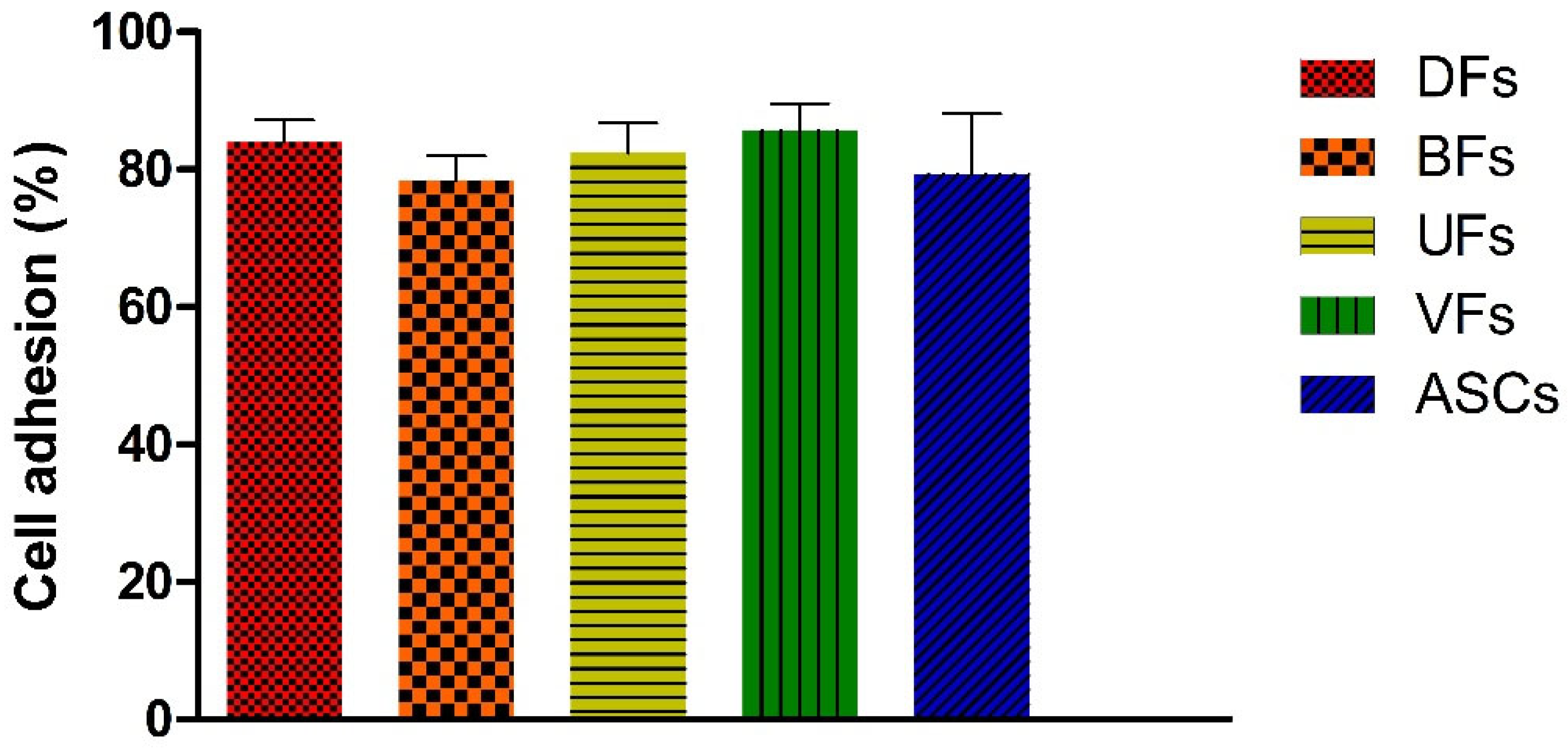
3.4. PCLCOL Supports Strong and Consistent Adhesion Across Stromal Cell Types
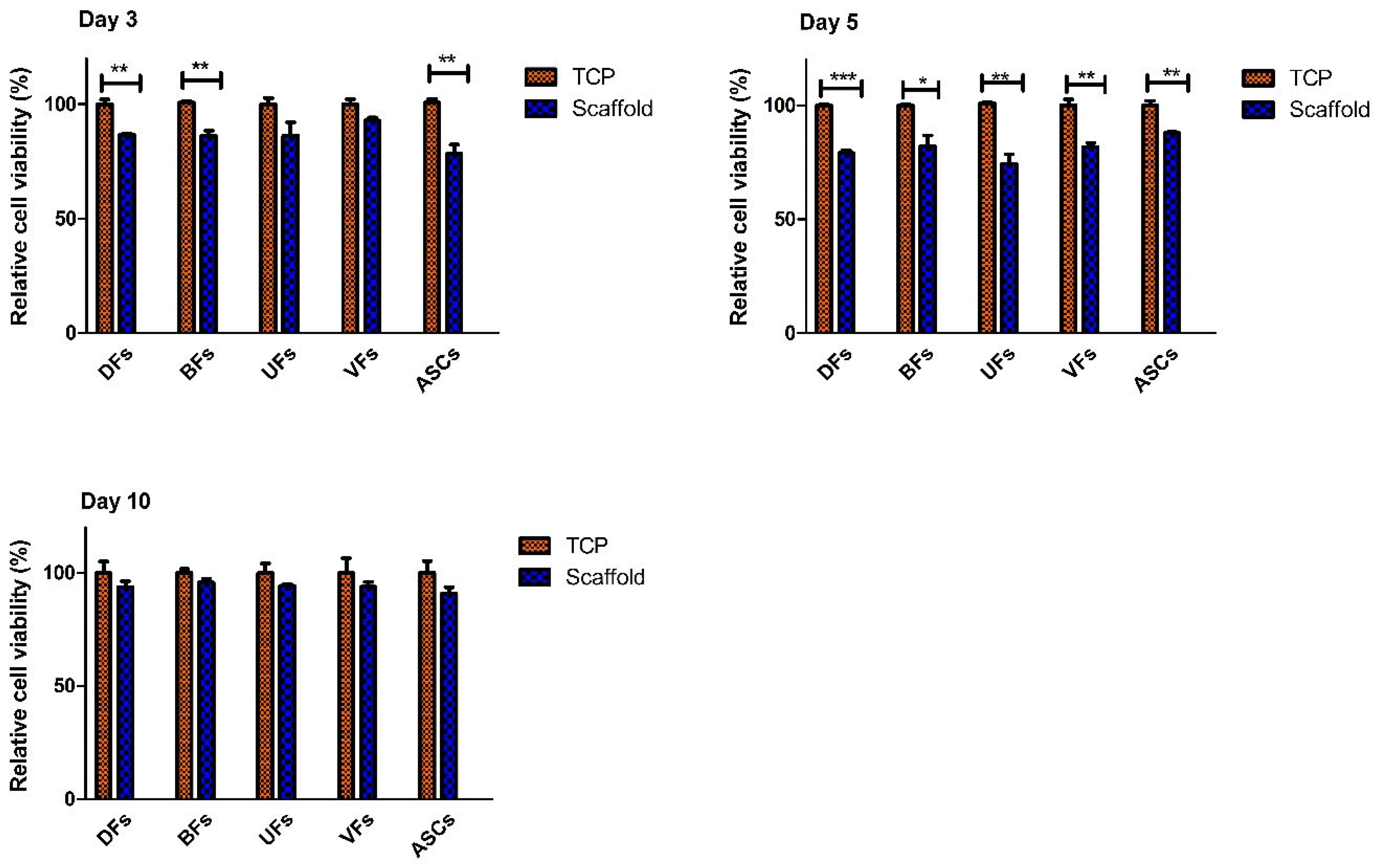
3.5. Initial Decline and Subsequent Stabilization of Stromal Cell’s Viability on PCLCOL
3.6. PCLCOL Promotes Superior ECM Thickness in UFs, VFs, and ASCs Compared to TCP
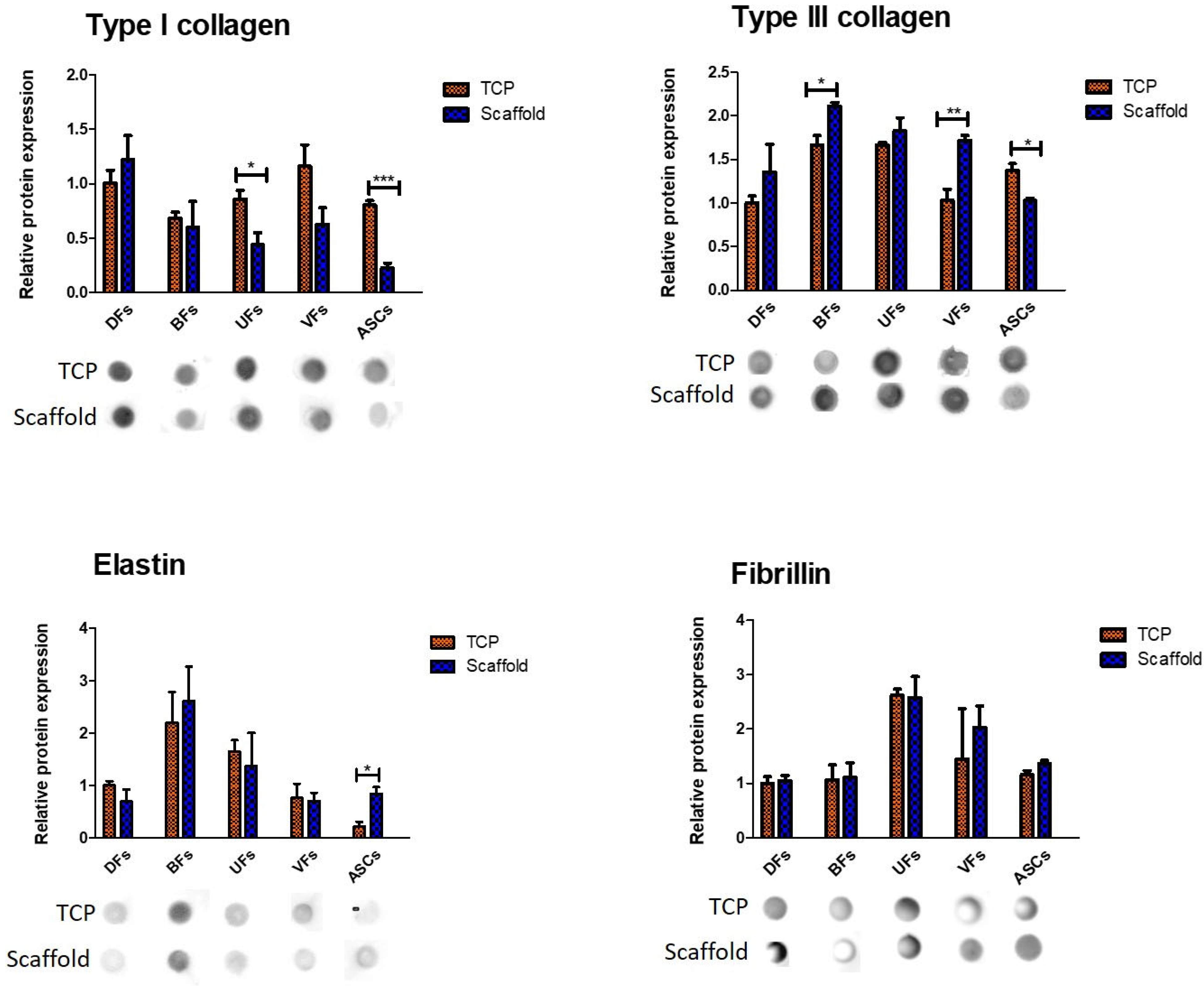
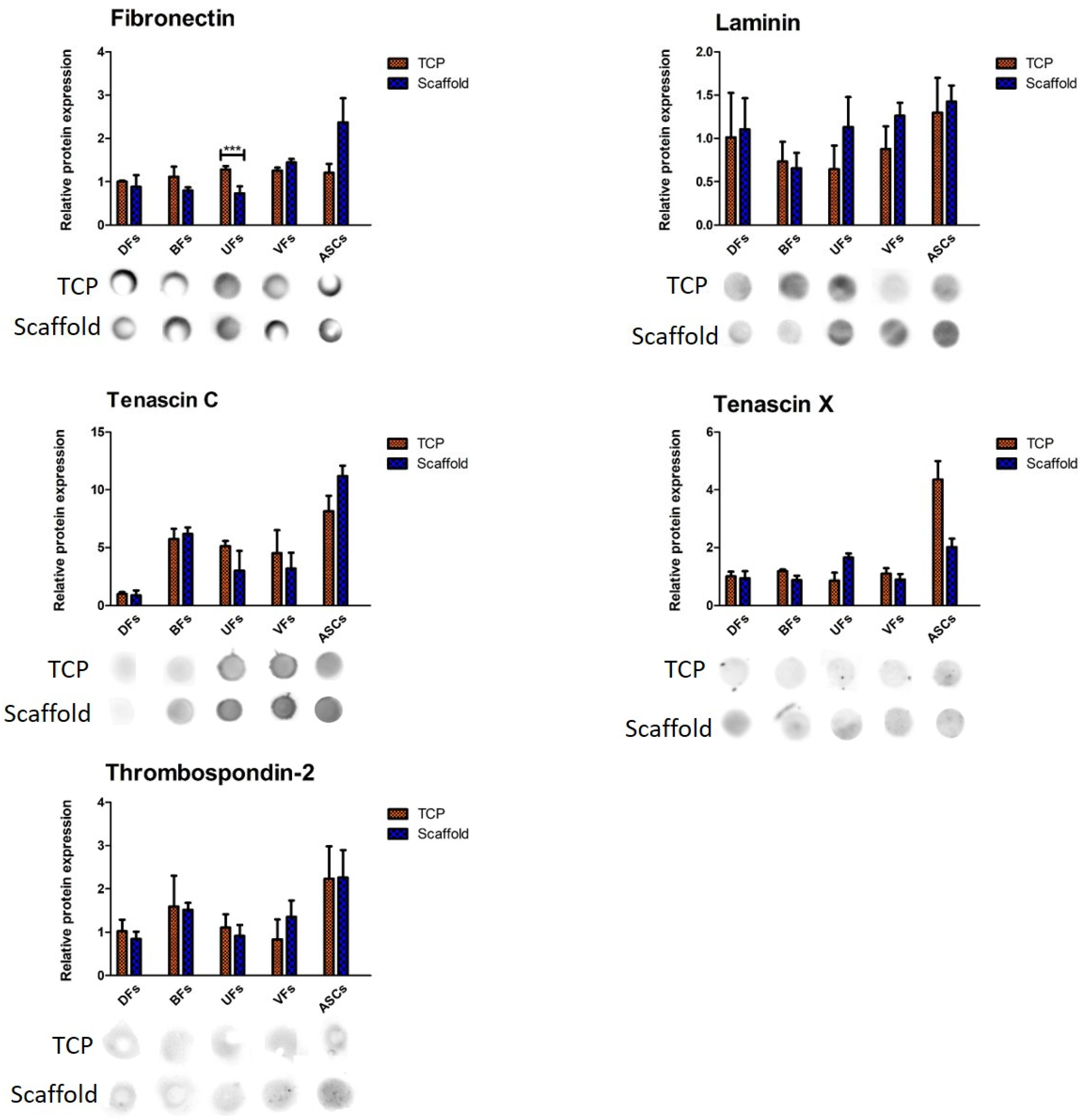
3.7. Stromal Cell-Specific ECM Protein Secretion Profiles on TCP and PCLCOL
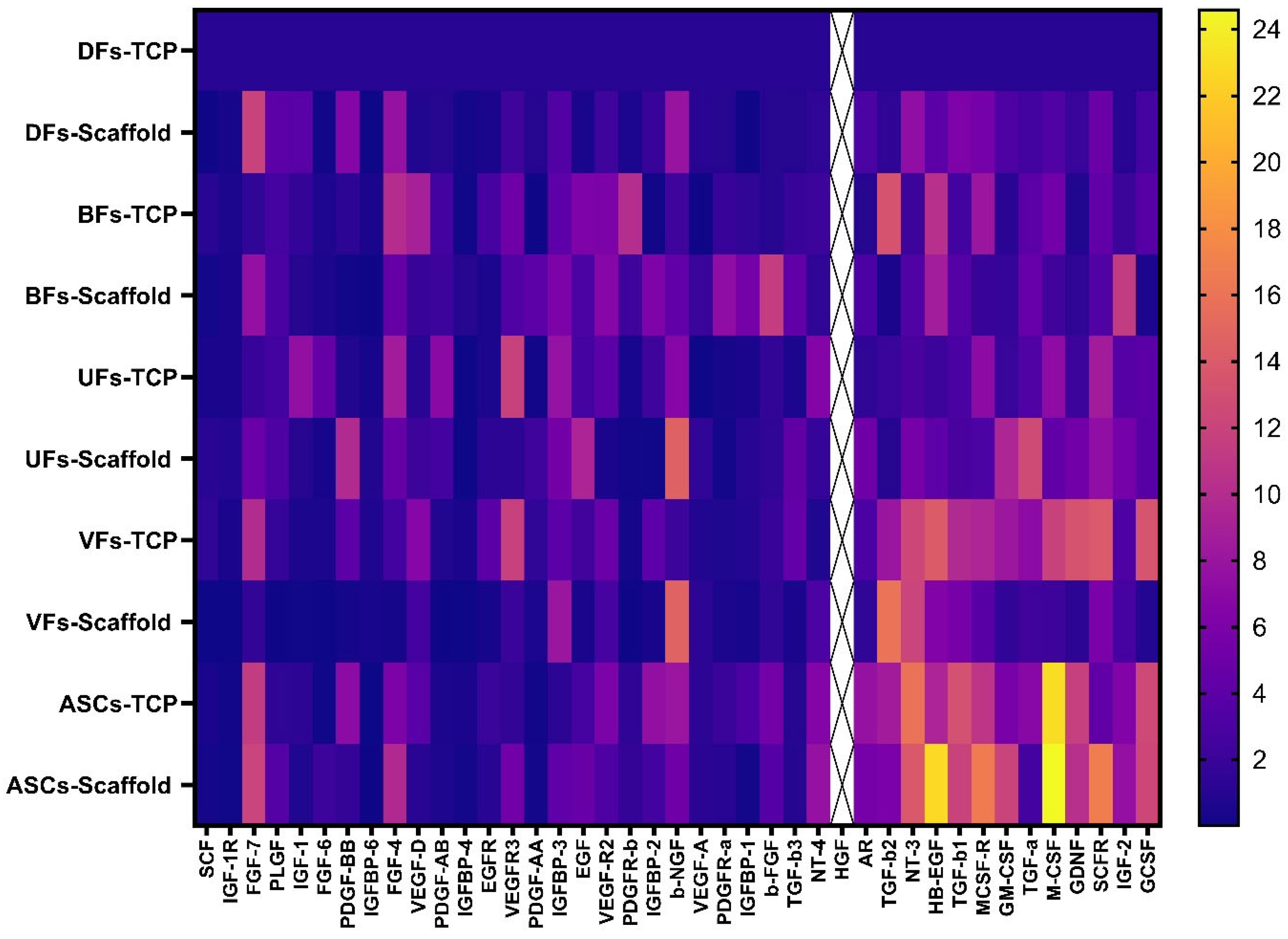
3.8. Stromal Cells Cultured on PCLCOL and TCP Exhibited Distinct, Substrate-Dependent Growth Factor Secretion Profiles
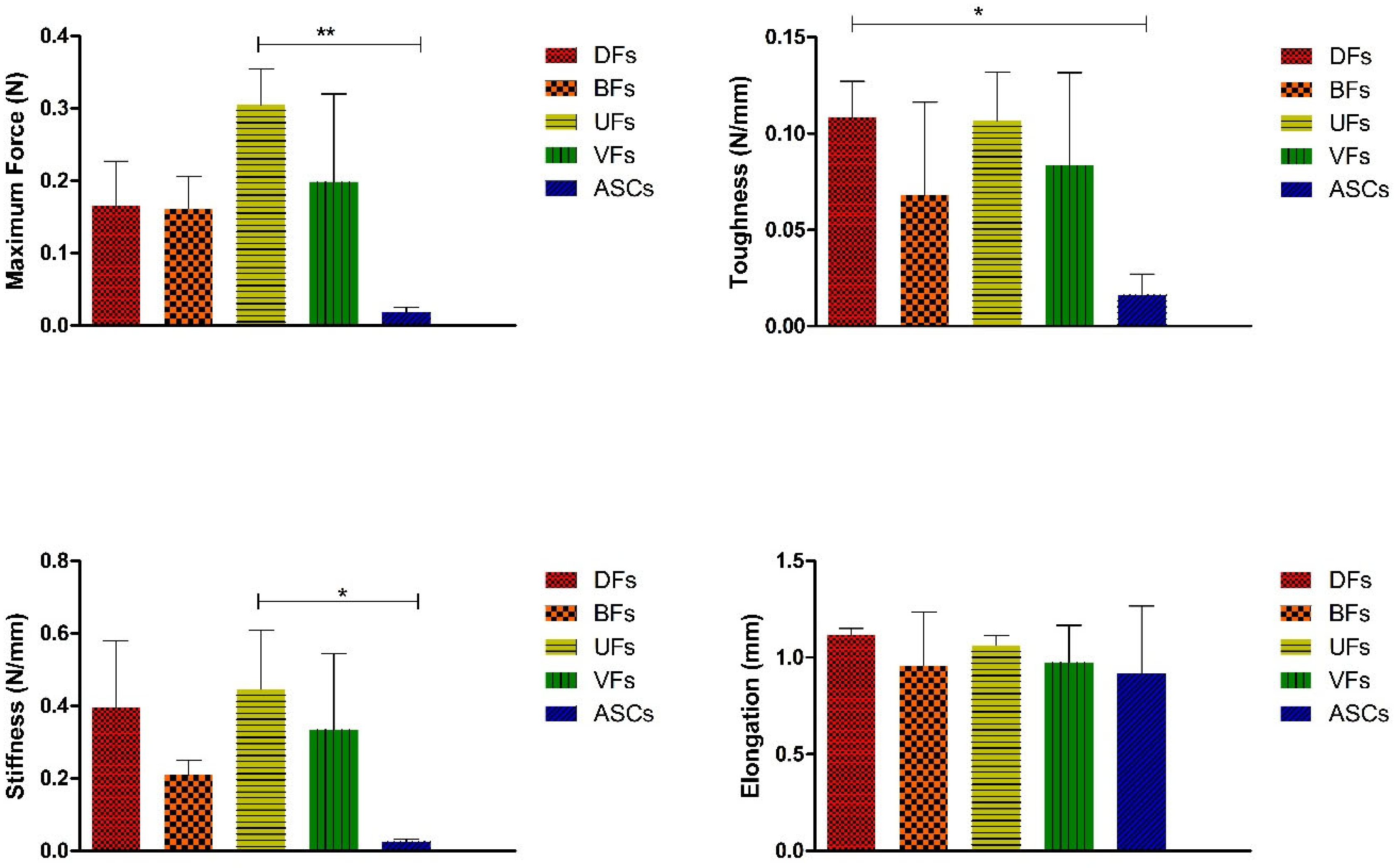
3.9. Stromal Cell Seeding Did Not Alter PCLCOL Scaffold Mechanics, but ECM Constructs on TCP Showed Cell-Dependent Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-derived extracellular matrix for tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, Y.; Zhou, L.; Dan, N.; Min, J.; Chen, Y.; Wang, Y. Evolution of biomimetic ECM scaffolds from decellularized tissue matrix for tissue engineering: A comprehensive review. Int. J. Biol. Macromol. 2023, 246, 125672. [Google Scholar] [CrossRef]
- Farzamfar, S.; Elia, E.; Richer, M.; Chabaud, S.; Naji, M.; Bolduc, S. Extracellular matrix-based and electrospun scaffolding Systems for Vaginal Reconstruction. Bioengineering 2023, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Brownell, D.; Chabaud, S.; Bolduc, S. Genitourinary tissue engineering: Reconstruction and research models. Bioengineering 2021, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Shtilbans, V. Role of stromal-epithelial interaction in the formation and development of cancer cells. Cancer Microenviron. 2013, 6, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Higgins, S.J.; Donjacour, A.A.; Hayashi, N.; Young, P. The Role of Stromal-Epithelial Interactions in the Regulation of Growth and Differentiation in Adult Epithelial Cells. In Autocrine and Paracrine Mechanisms in Reproductive Endocrinology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 67–84. [Google Scholar]
- Ayansola, H.; Mayorga, E.J.; Jin, Y. Subepithelial stromal cells: Their roles and interactions with intestinal epithelial cells during gut mucosal homeostasis and regeneration. Biomedicines 2024, 12, 668. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Leuning, D.G.; Beijer, N.R.; Du Fossé, N.A.; Vermeulen, S.; Lievers, E.; Van Kooten, C.; Rabelink, T.J.; de Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef]
- Carrier, P.; Deschambeault, A.; Audet, C.; Talbot, M.; Gauvin, R.; Giasson, C.J.; Auger, F.A.; Guerin, S.L.; Germain, L. Impact of cell source on human cornea reconstructed by tissue engineering. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2645–2652. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- DeLeon-Pennell, K.Y.; Barker, T.H.; Lindsey, M.L. Fibroblasts: The arbiters of extracellular matrix remodeling. Matrix Biol. 2020, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Anaya Mancipe, J.M.; Boldrini Pereira, L.C.; de Miranda Borchio, P.G.; Dias, M.L.; da Silva Moreira Thire, R.M. Novel polycaprolactone (PCL)-type I collagen core-shell electrospun nanofibers for wound healing applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Salehi, M.; Tavangar, S.M.; Verdi, J.; Mansouri, K.; Ai, A.; Malekshahi, Z.V.; Ai, J. A novel polycaprolactone/carbon nanofiber composite as a conductive neural guidance channel: An in vitro and in vivo study. Prog. Biomater. 2019, 8, 239–248. [Google Scholar] [CrossRef]
- Li, P.; Ruan, L.; Wang, R.; Liu, T.; Song, G.; Gao, X.; Jiang, G.; Liu, X. Electrospun scaffold of collagen and polycaprolactone containing ZnO quantum dots for skin wound regeneration. J. Bionic Eng. 2021, 18, 1378–1390. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Mimpen, J.Y.; Cribbs, A.P.; Stace, E.; Philpott, M.; Dakin, S.G.; Carr, A.J.; Snelling, S.J. Electrospun scaffold micro-architecture induces an activated transcriptional phenotype within tendon fibroblasts. Front. Bioeng. Biotechnol. 2022, 9, 795748. [Google Scholar] [CrossRef]
- Caneparo, C.; Chabaud, S.; Fradette, J.; Bolduc, S. Engineered human organ-specific urethra as a functional substitute. Sci. Rep. 2022, 12, 21346. [Google Scholar] [CrossRef]
- Caneparo, C.; Chabaud, S.; Fradette, J.; Bolduc, S. Evaluation of a serum-free medium for human epithelial and stromal cell culture. Int. J. Mol. Sci. 2022, 23, 10035. [Google Scholar] [CrossRef]
- Orabi, H.; Rousseau, A.; Laterreur, V.; Bolduc, S. Optimization of the current self-assembled urinary bladder model: Organ-specific stroma and smooth muscle inclusion. Can. Urol. Assoc. J. 2015, 9, E599. [Google Scholar] [CrossRef]
- Orabi, H.; Saba, I.; Rousseau, A.; Bolduc, S. Novel three-dimensional autologous tissue-engineered vaginal tissues using the self-assembly technique. Transl. Res. 2017, 180, 22–36. [Google Scholar] [CrossRef]
- Vermette, M.; Trottier, V.; Ménard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860. [Google Scholar] [CrossRef]
- Bouhout, S.; Perron, É.; Gauvin, R.; Bernard, G.; Ouellet, G.; Cattan, V.; Bolduc, S. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng. Part A 2010, 16, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011, 26, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun scaffolds for tissue engineering: A review. Macromol 2023, 3, 524–553. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of electrospinning for tissue engineering applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Christopherson, G.T.; Song, H.; Mao, H.-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Guzmán-Soria, A.; Moreno-Serna, V.; Canales, D.A.; García-Herrera, C.; Zapata, P.A.; Orihuela, P.A. Effect of electrospun PLGA/collagen scaffolds on cell adhesion, viability, and collagen release: Potential applications in tissue engineering. Polymers 2023, 15, 1079. [Google Scholar] [CrossRef]
- Delaine-Smith, R.M.; Hann, A.J.; Green, N.H.; Reilly, G.C. Electrospun fiber alignment guides osteogenesis and matrix organization differentially in two different osteogenic cell types. Front. Bioeng. Biotechnol. 2021, 9, 672959. [Google Scholar] [CrossRef]
- Hao, D.; Ma, B.; He, C.; Liu, R.; Farmer, D.L.; Lam, K.S.; Wang, A. Surface modification of polymeric electrospun scaffolds via a potent and high-affinity integrin α4β1 ligand improved the adhesion, spreading and survival of human chorionic villus-derived mesenchymal stem cells: A new insight for fetal tissue engineering. J. Mater. Chem. B 2020, 8, 1649–1659. [Google Scholar] [CrossRef]
- Han, P.; Gomez, G.A.; Duda, G.N.; Ivanovski, S.; Poh, P.S. Scaffold geometry modulation of mechanotransduction and its influence on epigenetics. Acta Biomater. 2023, 163, 259–274. [Google Scholar] [CrossRef]
- Wang, S.; Hashemi, S.; Stratton, S.; Arinzeh, T.L. The effect of physical cues of biomaterial scaffolds on stem cell behavior. Adv. Healthc. Mater. 2021, 10, 2001244. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E.H. Adhesion signaling–crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef]
- Yaseri, R.; Fadaie, M.; Mirzaei, E.; Samadian, H.; Ebrahiminezhad, A. Surface modification of polycaprolactone nanofibers through hydrolysis and aminolysis: A comparative study on structural characteristics, mechanical properties, and cellular performance. Sci. Rep. 2023, 13, 9434. [Google Scholar] [CrossRef]
- Sadeghzadeh, H.; Mehdipour, A.; Dianat-Moghadam, H.; Salehi, R.; Khoshfetrat, A.B.; Hassani, A.; Mohammadnejad, D. PCL/Col I-based magnetic nanocomposite scaffold provides an osteoinductive environment for ADSCs in osteogenic cues-free media conditions. Stem Cell Res. Ther. 2022, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.; Mendes, A.; Pereira, R.F.; Bártolo, P.J. Collagen surface modified poly (ε-caprolactone) scaffolds with improved hydrophilicity and cell adhesion properties. Mater. Lett. 2014, 134, 263–267. [Google Scholar] [CrossRef]
- Ferreira, J.; Gloria, A.; Cometa, S.; Coelho, J.F.; Domingos, M. Effect of in vitro enzymatic degradation on 3D printed poly (ε-caprolactone) scaffolds: Morphological, chemical and mechanical properties. J. Appl. Biomater. Funct. Mater. 2017, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, J.; Paz, R.; Alemán-Domínguez, M.E.; Monzón, M.; Donate, R.; Winter, G. Experimental analysis of the enzymatic degradation of polycaprolactone: Microcrystalline cellulose composites and numerical method for the prediction of the degraded geometry. Materials 2021, 14, 2460. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Downes, S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Wang, H.; Haeger, S.M.; Kloxin, A.M.; Leinwand, L.A.; Anseth, K.S. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS ONE 2012, 7, e39969. [Google Scholar] [CrossRef]
- Valcourt, J.R.; Lemons, J.M.; Haley, E.M.; Kojima, M.; Demuren, O.O.; Coller, H.A. Staying alive: Metabolic adaptations to quiescence. Cell Cycle 2012, 11, 1680–1696. [Google Scholar] [CrossRef]
- Smithmyer, M.; Sawicki, L.; Kloxin, A. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater. Sci. 2014, 2, 634–650. [Google Scholar] [CrossRef]
- Malakpour-Permlid, A.; Buzzi, I.; Hegardt, C.; Johansson, F.; Oredsson, S. Identification of extracellular matrix proteins secreted by human dermal fibroblasts cultured in 3D electrospun scaffolds. Sci. Rep. 2021, 11, 6655. [Google Scholar] [CrossRef]
- Clément, V.; Roy, V.; Paré, B.; Goulet, C.R.; Deschênes, L.T.; Berthod, F.; Bolduc, S.; Gros-Louis, F. Tridimensional cell culture of dermal fibroblasts promotes exosome-mediated secretion of extracellular matrix proteins. Sci. Rep. 2022, 12, 19786. [Google Scholar] [CrossRef] [PubMed]
- Blache, U.; Stevens, M.M.; Gentleman, E. Harnessing the secreted extracellular matrix to engineer tissues. Nat. Biomed. Eng. 2020, 4, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, X.; Yuan, T.; Yang, X.; Fan, Y.; Zhang, X. Regulation of the secretion of immunoregulatory factors of mesenchymal stem cells (MSCs) by collagen-based scaffolds during chondrogenesis. Mater. Sci. Eng. C 2017, 70, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Domingos, M.; Scenini, F. Advanced mechanical and thermal characterization of 3D bioextruded poly (e-caprolactone)-based composites. Rapid Prototyp. J. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent advances in polycaprolactones for anticancer drug delivery. Pharmaceutics 2023, 15, 1977. [Google Scholar] [CrossRef]
- Barilani, M.; Palorini, R.; Votta, G.; Piras, R.; Buono, G.; Grassi, M.; Bollati, V.; Chiaradonna, F.; Lazzari, L. Central metabolism of functionally heterogeneous mesenchymal stromal cells. Sci. Rep. 2019, 9, 15420. [Google Scholar] [CrossRef]
- Chance, T.C.; Herzig, M.C.; Christy, B.A.; Delavan, C.; Rathbone, C.R.; Cap, A.P.; Bynum, J.A. Human mesenchymal stromal cell source and culture conditions influence extracellular vesicle angiogenic and metabolic effects on human endothelial cells in vitro. J. Trauma Acute Care Surg. 2020, 89, S100–S108. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195. [Google Scholar] [CrossRef]
- Schoenenberger, A.D.; Foolen, J.; Moor, P.; Silvan, U.; Snedeker, J.G. Substrate fiber alignment mediates tendon cell response to inflammatory signaling. Acta Biomater. 2018, 71, 306–317. [Google Scholar] [CrossRef]
- Guimberteau, J.C.; Armstrong, C. Architecture of Human Living Fascia: The Extracellular Matrix and Cells Revealed Through Endoscopy; Jessica Kingsley Publishers: London, UK, 2024. [Google Scholar]
- Kolliopoulos, V.; Tiffany, A.; Polanek, M.; Harley, B.; Harley, B. Donor Variability in Human Mesenchymal Stem Cell Osteogenic Response As a Function of Passage Conditions and Donor Sex. bioRxiv 2023. [Google Scholar] [CrossRef]
- Turlo, A.J.; Hammond, D.E.; Ramsbottom, K.A.; Soul, J.; Gillen, A.; McDonald, K.; Peffers, M.J. Mesenchymal stromal cell secretome is affected by tissue source and donor age. Stem Cells 2023, 41, 1047–1059. [Google Scholar] [CrossRef]



| Cell Type | Lab ID | Organ | Age | Sex | Ethic Approval (Laval University) |
|---|---|---|---|---|---|
| ASC | LA35 | Fat (Lipoaspirate) | 35 | Female | DR-002-1117 |
| BF | FHu5 | Bladder | 6 | Male | 2012-1341, DR-002-1190 |
| DF | FD34 | Skin (Dermis) | 34 | Female | 2012-1341, DR-002-1190 |
| UF | FU28 | Urethra (proximal part) | 28 | Male | 2012-1341, DR-002-1190 |
| VF | FVa32 | Vagina | 32 | Female | 2012-1341, DR-002-1190 |
| Antibody | Host Species | Manufacturer | Catalogue Number | Dilution |
|---|---|---|---|---|
| Anti-Collagen I | Rabbit | Rockland Immunochemicals, Inc. (Burlington, VT, Canada) | 600-401-105-0.1 | 1:5000 |
| Anti-Collagen III | Rabbit | Cedarlane (Burlington, VT, Canada) | CL50311AP-1 | 1:5000 |
| Anti-Elastin | Rabbit | Institute Pasteur, (Lyon, France) | Custom-made (lot 25011) | 1:3000 |
| Anti-Laminin | Rabbit | Abcam (Cambridge, UK) | Ab14509 | 1:2000 |
| Anti-Fibronectin | Rabbit | Abcam | ab32419 | 1:3000 |
| Anti-Fibrillin | Rabbit | Elastin Products Company, Inc. (Owensville, MO, USA) | PR225 | 1:2000 |
| Anti- Thrombospondin 1 | Mouse | Novus Biologicals (Centennial, CO, USA) | NB-100-2059 | 1:2000 |
| Anti-Tenascin C | Mouse | Abcam (Cambridge, UK) | Clone BC-24 | 1:3000 |
| Anti-Tenascin X | Rabbit | Santa Cruz B. (California, CA, USA) | Clone H-90 | 1:3000 |
| Anti-Rabbit Horseradish Peroxidase (HRP) | Goat | Invitrogen (Waltham, MA, USA) | WC320195 | 1:1000 |
| Anti-Mouse HRP | Goat | Invitrogen (Waltham, MA, USA) | WB322805A | 1:1000 |
| Sample | Wavenumber (cm−1) | Assignment |
|---|---|---|
| PCLCOL | ~3300 | Amide A |
| PCLCOL | ~2940 | CH2 asymmetric/symmetric stretching |
| PCLCOL | ~1650 | Amide I |
| PCLCOL | ~1550 | Amide II |
| PCLCOL | ~1470 | CH2 bending |
| PCLCOL | ~1365 | CH bending |
| PCLCOL | ~1290 | C–O stretching |
| PCLCOL | ~1240 | Amide III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farzamfar, S.; Chabaud, S.; Fradette, J.; Rioux, Y.; Bolduc, S. Electrospun Polycaprolactone/Collagen Scaffolds Enhance Manipulability and Influence the Composition of Self-Assembled Extracellular Matrix. Bioengineering 2025, 12, 1077. https://doi.org/10.3390/bioengineering12101077
Farzamfar S, Chabaud S, Fradette J, Rioux Y, Bolduc S. Electrospun Polycaprolactone/Collagen Scaffolds Enhance Manipulability and Influence the Composition of Self-Assembled Extracellular Matrix. Bioengineering. 2025; 12(10):1077. https://doi.org/10.3390/bioengineering12101077
Chicago/Turabian StyleFarzamfar, Saeed, Stéphane Chabaud, Julie Fradette, Yannick Rioux, and Stéphane Bolduc. 2025. "Electrospun Polycaprolactone/Collagen Scaffolds Enhance Manipulability and Influence the Composition of Self-Assembled Extracellular Matrix" Bioengineering 12, no. 10: 1077. https://doi.org/10.3390/bioengineering12101077
APA StyleFarzamfar, S., Chabaud, S., Fradette, J., Rioux, Y., & Bolduc, S. (2025). Electrospun Polycaprolactone/Collagen Scaffolds Enhance Manipulability and Influence the Composition of Self-Assembled Extracellular Matrix. Bioengineering, 12(10), 1077. https://doi.org/10.3390/bioengineering12101077








