High-Precision Multi-Axis Robotic Printing: Optimized Workflow for Complex Tissue Creation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bioinks
2.2. Preparation of the Suspension Bath
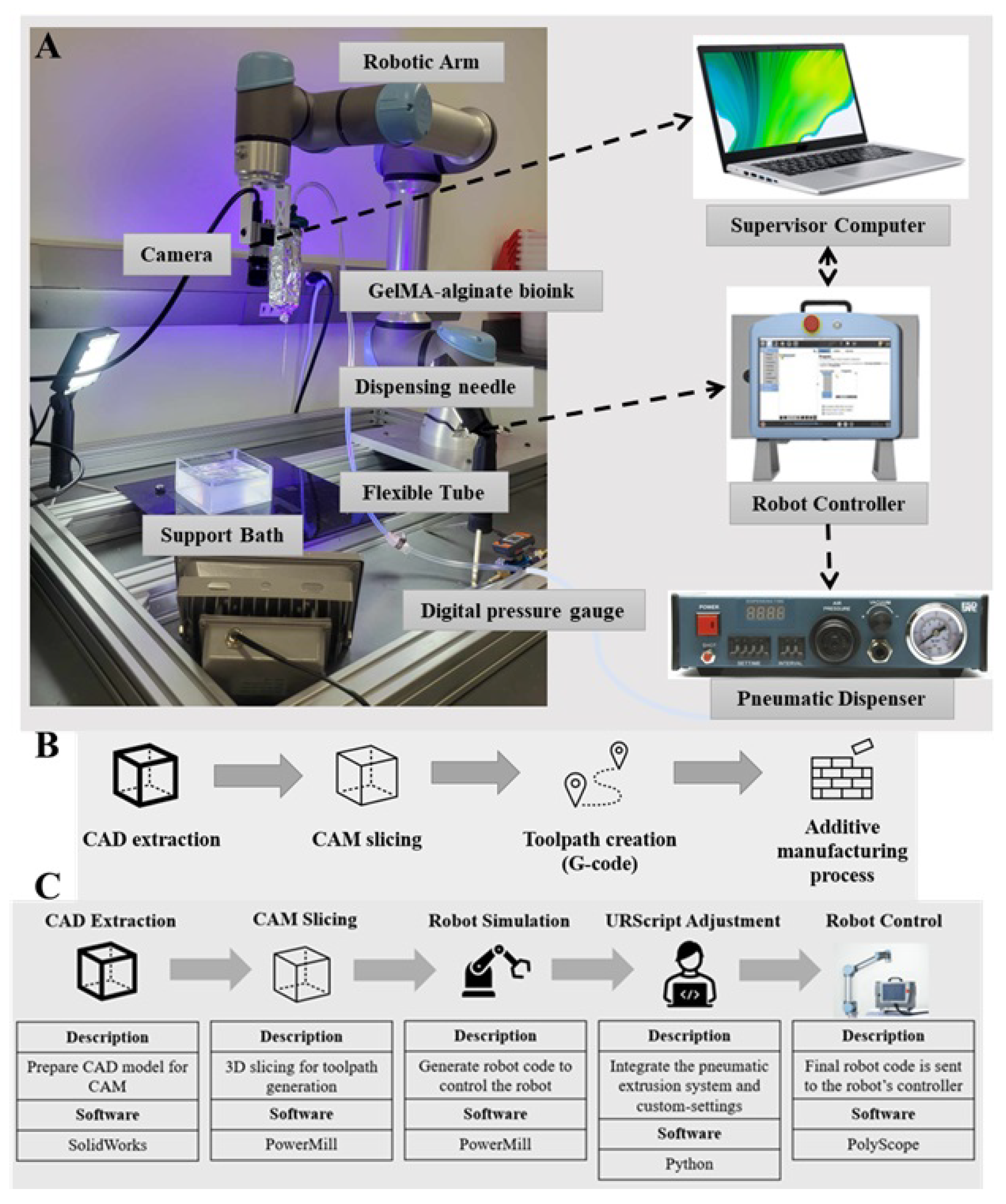
2.3. Development of the Multi-Axis Robotic Bioprinting Platform
2.4. Integrated Control System for Robotic Bioprinting
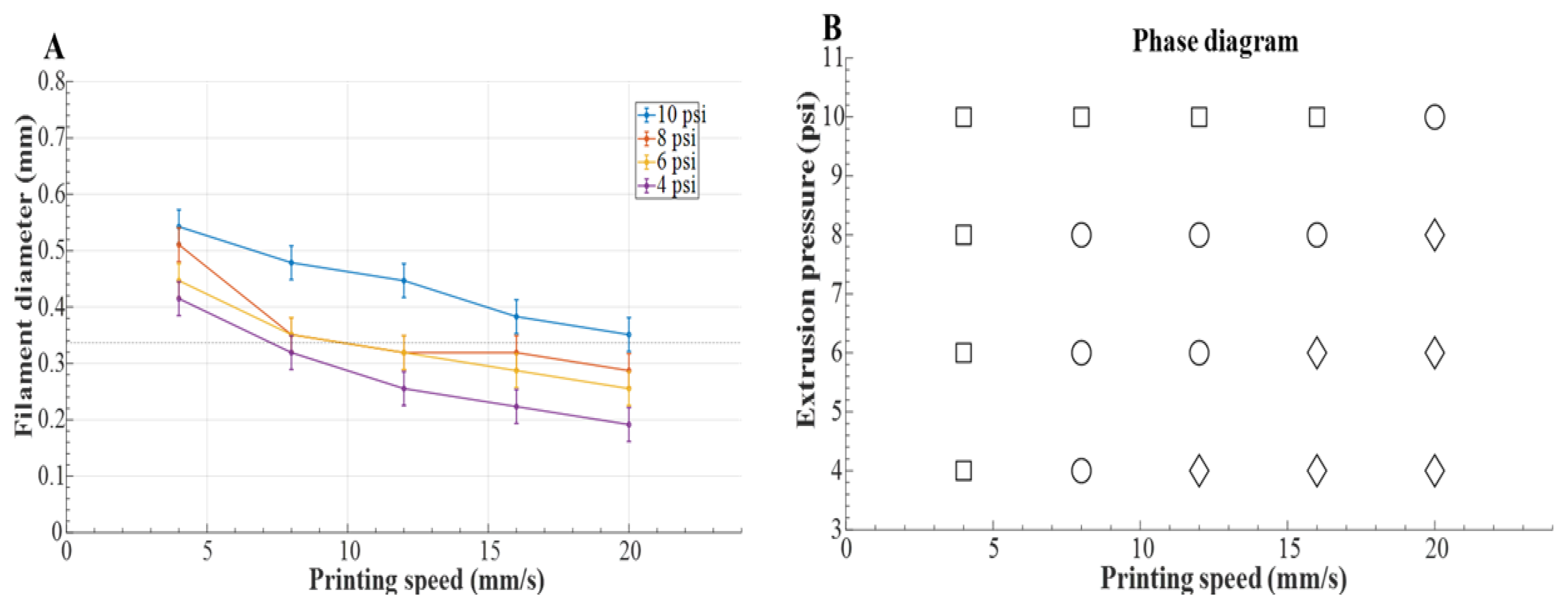
2.5. Optimization of Robotic Bioprinting Workflow and Parameters
3. Case Study
4. Results and Discussion
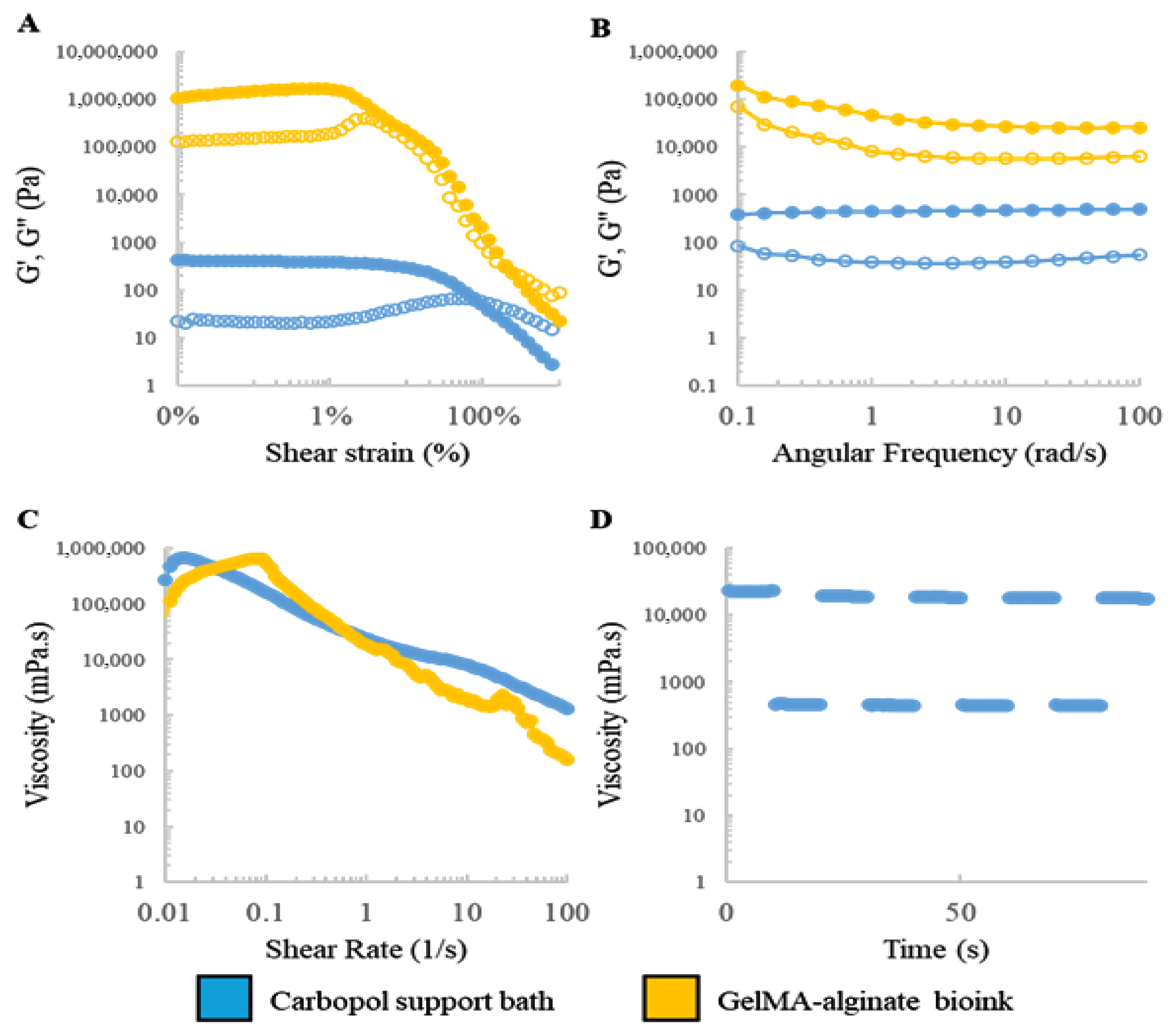
4.1. Rheological Characterization and Optimization of Printing Parameters
4.2. Case Study Implementation
4.2.1. CAD Modeling and Toolpath Generation
4.2.2. Robot Simulation and Control
4.2.3. Fabrication and Evaluation of Bioprinted Constructs
4.2.4. Quantitative Benchmarking Against Other Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| CAD | Computer-Aided Design |
| CAM | Computer-Aided Manufacturing |
| DOF | Degrees of Freedom |
| PBS | Phosphate-Buffered Saline |
| GelMA | Gelatin Methacryloyl |
| TEOA | Triethanolamine |
| UR | Universal Robots |
| URScript | Universal Robots Scripting Language |
| RMS | Root Mean Square |
| STL | Stereolithography (file format) |
References
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Juengst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Liu, J. Bioprinting of 3D tissues/organs combined with microfluidics. RSC Adv. 2018, 8, 21712–21727. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, F.; Barbe, L.; Pohlit, H.; Tenje, M. A perfusable multi-hydrogel vasculature on-chip engineered by 2-photon 3D printing and scaffold molding to improve microfabrication fidelity in hydrogels. Adv. Mater. Technol. 2024, 9, 2300718. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef]
- Mirabella, T.; MacArthur, J.; Cheng, D.; Ozaki, C.; Woo, Y.; Yang, M.; Chen, C. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng. 2017, 1, 0083. [Google Scholar] [CrossRef]
- Ozbolat, I.T. 3D Bioprinting: Fundamentals, Principles and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Castelli, K. Conventional and Multi-Directional Printing Robotic Cell. Ph.D. Thesis, University of Brescia, Brescia, Italy, 2020. [Google Scholar]
- Wulle, F.; Gorke, O.; Schmidt, S.; Nistler, M.; Tovar, G.E.; Riedel, O.; Verl, A.; Weber, A.; Southan, A. Multi-axis 3D printing of gelatin methacryloyl hydrogels on a non-planar surface obtained from magnetic resonance imaging. Addit. Manuf. 2022, 50, 102566. [Google Scholar] [CrossRef]
- Gleadall, A. FullControl GCode Designer: Open-source software for unconstrained design in additive manufacturing. Addit. Manuf. 2021, 46, 102109. [Google Scholar] [CrossRef]
- Edgar, J.; Tint, S. Additive manufacturing technologies: 3D printing, rapid prototyping, and direct digital manufacturing. Johns. Matthey Technol. Rev. 2015, 59, 193–198. [Google Scholar] [CrossRef]
- Adhyapak, H.M.; Gaonkar, S.L.; Namratha, B. Overview of 3D Bioprinting Technology. In Compendium of 3D Bioprinting Technology; CRC Press: Boca Raton, FL, USA, 2025; pp. 27–46. [Google Scholar]
- Keating, S.; Oxman, N. Compound fabrication: A multi-functional robotic platform for digital design and fabrication. Robot. Comput.-Integr. Manuf. 2013, 29, 439–448. [Google Scholar] [CrossRef]
- Efendi, A.; Shao, Y.H.; Huang, C.Y. Technological development and optimization of pushing and grasping functions in robot arms: A review. Measurement 2025, 242, 115729. [Google Scholar] [CrossRef]
- Barjuei, E.S.; Courteille, E.; Rangeard, D.; Marie, F.; Perrot, A. Real-time vision-based control of industrial manipulators for layer-width setting in concrete 3D printing applications. Adv. Ind. Manuf. Eng. 2022, 5, 100094. [Google Scholar]
- Li, L.; Shi, J.; Ma, K.; Jin, J.; Wang, P.; Liang, H.; Cao, Y.; Wang, X.; Jiang, Q. Robotic in situ 3D bio-printing technology for repairing large segmental bone defects. J. Adv. Res. 2021, 30, 75–84. [Google Scholar] [CrossRef]
- Barjuei, E.S.; Shin, J.; Kim, K.; Lee, J. Precision improvement of robotic bioprinting via vision-based tool path compensation. Sci. Rep. 2024, 14, 17764. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Dai, C.; Shi, Q.; Fang, G.; Xie, D.; Zhao, X.; Liu, Y.J.; Wang, C.C.; Wang, X.J. A multi-axis robot-based bioprinting system supporting natural cell function preservation and cardiac tissue fabrication. Bioact. Mater. 2022, 18, 138–150. [Google Scholar] [CrossRef]
- Pedroso, A.F.; Sebbe, N.P.; Silva, F.J.; Campilho, R.D.; Sales-Contini, R.C.; Costa, R.D.; Sánchez, I.I. An overview on the recent advances in robot-assisted compensation methods used in machining lightweight materials. Robot. Comput.-Integr. Manuf. 2025, 91, 102844. [Google Scholar] [CrossRef]
- Barjuei, E.S.; Capitanelli, A.; Bertolucci, R.; Courteille, E.; Mastrogiovanni, F.; Maratea, M. Digital workflow for printability checking and prefabrication in robotic construction 3D printing based on Artificial Intelligence planning. Eng. Appl. Artif. Intell. 2024, 133, 108254. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Porcza, L.M.; Leslie, N.R.; Melchels, F.P. Gellan gum-based granular gels as suspension media for biofabrication. PLoS ONE 2024, 19, e0312726. [Google Scholar] [CrossRef]
- Zadorozhnyi, A.; Chovnyuk, Y.; Stakhovsky, O.; Kovrevski, A.; Buhaievskyi, S. Modeling the flow of a Bingham plastic fluid through a circular pipeline with different wall properties. AIP Conf. Proc. 2023, 2490, 050028. [Google Scholar] [CrossRef]
- Ning, L.; Mehta, R.; Cao, C.; Theus, A.; Tomov, M.; Zhu, N.; Weeks, E.R.; Bauser-Heaton, H.; Serpooshan, V. Embedded 3D bioprinting of gelatin methacryloyl-based constructs with highly tunable structural fidelity. ACS Appl. Mater. Interfaces 2020, 12, 44563–44577. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.; Liu, H.; Han, X.; Wang, Z.; Li, S.; Huang, J.; Wang, Z. 3D bioprinting of complex biological structures with tunable elastic modulus and porosity using freeform reversible embedding of suspended hydrogels. Bio-Des. Manuf. 2023, 6, 550–562. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Bliley, J.; Tashman, J.; Stang, M.; Coffin, B.; Shiwarski, D.; Lee, A.; Hinton, T.; Feinberg, A. FRESH 3D bioprinting a contractile heart tube using human stem cell-derived cardiomyocytes. Biofabrication 2022, 14, 024106. [Google Scholar] [CrossRef]
- Leucht, A.; Volz, A.C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci. Rep. 2020, 10, 5330. [Google Scholar] [CrossRef] [PubMed]
- Aldana, A.A.; Valente, F.; Dilley, R.; Doyle, B. Development of 3D bioprinted GelMA-alginate hydrogels with tunable mechanical properties. Bioprinting 2021, 21, e00105. [Google Scholar] [CrossRef]
- Datta, S. Advantage of alginate bioinks in biofabrication for various tissue engineering applications. Int. J. Polym. Sci. 2023, 2023, 6661452. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Zhang, Z.; Xu, C. Biofabrication of three-dimensional cellular structures based on gelatin methacrylate–alginate interpenetrating network hydrogel. J. Biomater. Appl. 2019, 33, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Sakthivel, K.; Mohamed, M.G.; Boras, E.; Shin, S.R.; Kim, K. Designing gelatin methacryloyl (GelMA)-based bioinks for visible light stereolithographic 3D biofabrication. Macromol. Biosci. 2021, 21, 2000317. [Google Scholar] [CrossRef]
- Zakzak, K.; Semenescu, A.D.; Moacă, E.A.; Predescu, I.; Drăghici, G.; Vlaia, L.; Vlaia, V.; Borcan, F.; Dehelean, C.A. Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives. Gels 2024, 10, 477. [Google Scholar] [CrossRef]
- Dano, M.E.L.; dos Santos, R.S.; da Silva, J.B.; Junqueira, M.V.; de Souza Ferreira, S.B.; Bruschi, M.L. Design of emulgel platforms for local propolis delivery: The influence of type and concentration of carbomer. J. Mol. Liq. 2021, 334, 116025. [Google Scholar] [CrossRef]
- Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–gelatin hydrogel scaffolds; an optimization of post-printing treatment for enhanced degradation and swelling behavior. Gels 2023, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yu, K.; Sun, Y.; Shao, L.; Nie, J.; Gao, Q.; Qiu, J.; Fu, J.; Chen, Z.; He, Y. Protocols of 3D Bioprinting of Gelatin Methacryloyl Hydrogel Based Bioinks; MyJoVE Corporation: Cambridge, MA, USA, 2016. [Google Scholar]
- Kassab, G.S. Scaling laws of vascular trees: Of form and function. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H894–H903. [Google Scholar] [CrossRef] [PubMed]
- Barjuei, E.S.; Boscariol, P.; Gasparetto, A.; Giovagnoni, M.; Vidoni, R. Control design for 3D flexible link mechanisms using linearized models. In Proceedings of the Advances on Theory and Practice of Robots and Manipulators: Proceedings of Romansy 2014 XX CISM-IFToMM Symposium on Theory and Practice of Robots and Manipulators, Moscow, Russia, 23–26 June 2014; Springer: Berlin/Heidelberg, Germany, 2014; pp. 181–188. [Google Scholar]
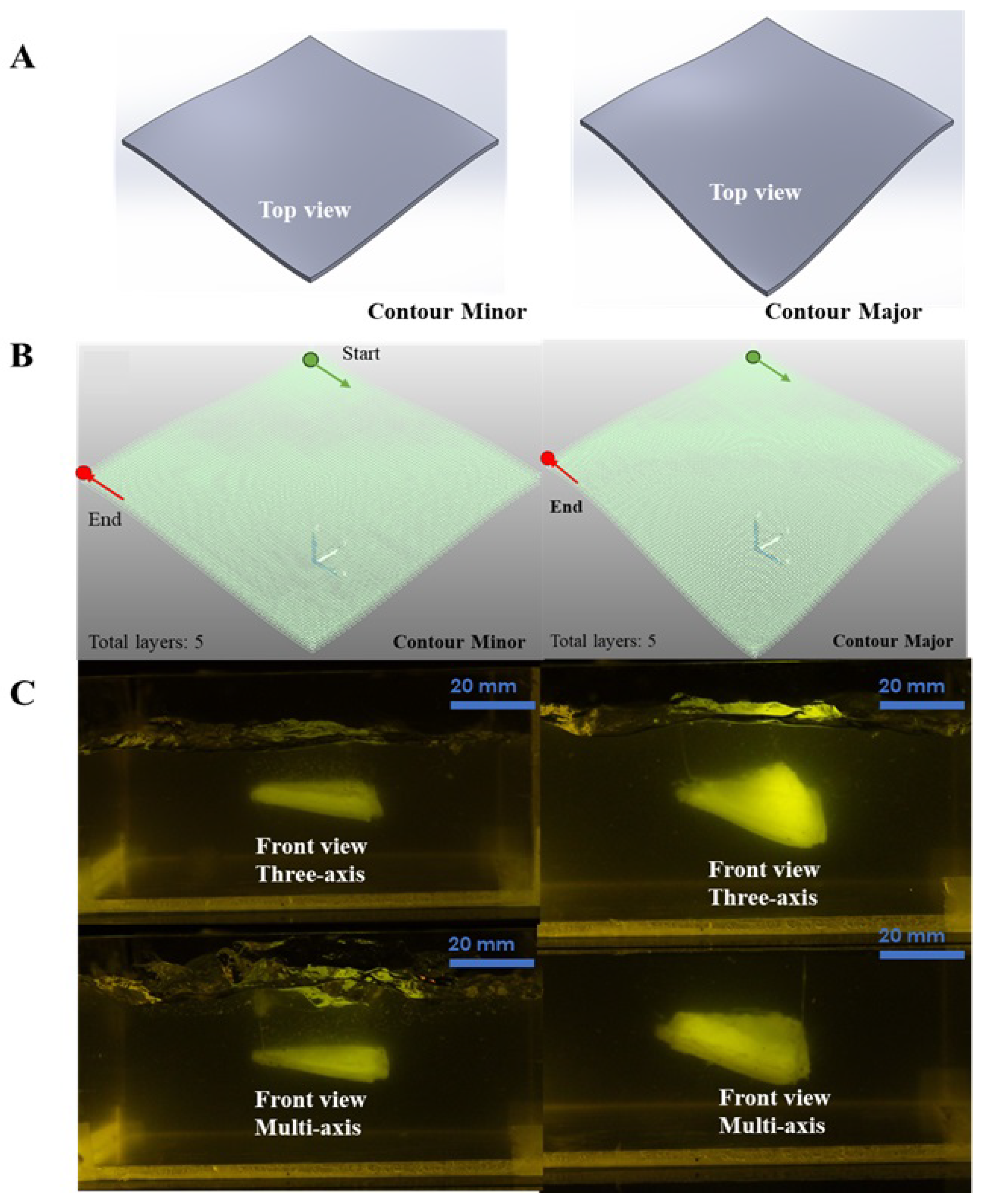

| Printed Surface | Max Orientation Error (°) | Mean Orientation Error (°) |
|---|---|---|
| Contour Minor | 4.3 | 2.1 |
| Contour Major | 4.8 | 2.5 |
| Tubular Structure | 4.9 | 2.3 |
| System | Positional Accuracy | Max Workspace | Feature Resolution | RMS Surface Roughness () |
|---|---|---|---|---|
| Curved-layer three-axis | 300 mm | 150–200 | 35.8–63.4 | |
| UR5e 6-DOF Robotic Arm (this work) | 850 mm | 100–150 | 19.2–28.7 | |
| CNC-based Robotic Systems [13] | 500 mm | 50–100 | 15–25 | |
| Other Robotic Bioprinters [15] | 600 mm | 100 | 20–30 |
| System | Positional Accuracy | Max Workspace | Feature Resolution | RMS Surface Roughness () |
|---|---|---|---|---|
| Curved-layer three-axis | ±100 | 300 mm | 150–200 | 35.8–63.4 |
| UR5e 6-DOF Robotic Arm (this work) | ±30 | 850 mm | 100–150 | 19.2–28.7 |
| CNC-based Robotic Systems [13] | ±10 | 500 mm | 50–100 | 15–25 |
| Other Robotic Bioprinters [15] | ±50 | 600 mm | 100 | 20–30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shojaei Barjuei, E.; Shin, J.; Kim, K.; Lee, J. High-Precision Multi-Axis Robotic Printing: Optimized Workflow for Complex Tissue Creation. Bioengineering 2025, 12, 949. https://doi.org/10.3390/bioengineering12090949
Shojaei Barjuei E, Shin J, Kim K, Lee J. High-Precision Multi-Axis Robotic Printing: Optimized Workflow for Complex Tissue Creation. Bioengineering. 2025; 12(9):949. https://doi.org/10.3390/bioengineering12090949
Chicago/Turabian StyleShojaei Barjuei, Erfan, Joonhwan Shin, Keekyoung Kim, and Jihyun Lee. 2025. "High-Precision Multi-Axis Robotic Printing: Optimized Workflow for Complex Tissue Creation" Bioengineering 12, no. 9: 949. https://doi.org/10.3390/bioengineering12090949
APA StyleShojaei Barjuei, E., Shin, J., Kim, K., & Lee, J. (2025). High-Precision Multi-Axis Robotic Printing: Optimized Workflow for Complex Tissue Creation. Bioengineering, 12(9), 949. https://doi.org/10.3390/bioengineering12090949







