Three-Dimensionally Printed Agarose Micromold Supports Scaffold-Free Mouse Ex Vivo Follicle Growth, Ovulation, and Luteinization
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Three-Dimensional Design and Generation of Agarose Micromolds
2.3. Ex Vivo Follicle Growth
2.4. Ex Vivo Ovulation and Luteinization Assay
2.5. Timelapse Culture and Analysis
2.6. Whole-Mount Immunocytochemistry and Spindle Analysis
2.7. Progesterone Quantification Immunoassay
2.8. Histologic Analysis
2.9. Statistical Analysis
3. Results
3.1. Rationale Design and Precision Engineering of Agarose Micromolds
3.2. Scaffold-Free Follicle Culture in Agarose Micromolds Supports Comparable Follicle Growth to Alginate Encapsulation
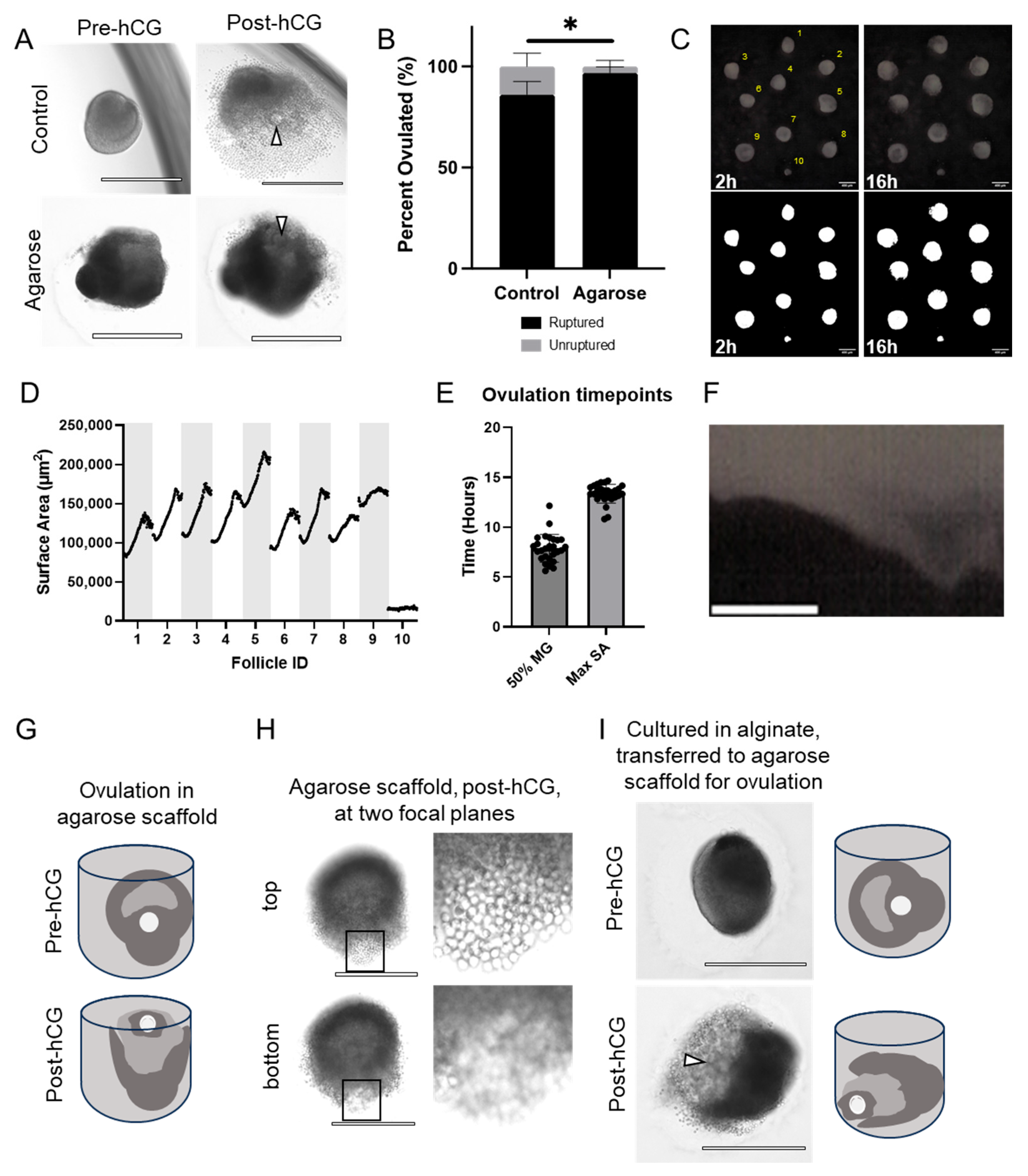
3.3. Follicles Grown in Agarose Micromolds Ovulate COCs in a Consistent Spatial Orientation
3.4. Eggs Collected from Follicles Grown in Agarose Micromolds Are Meiotically Competent and Exhibit Normal Spindle Morphology
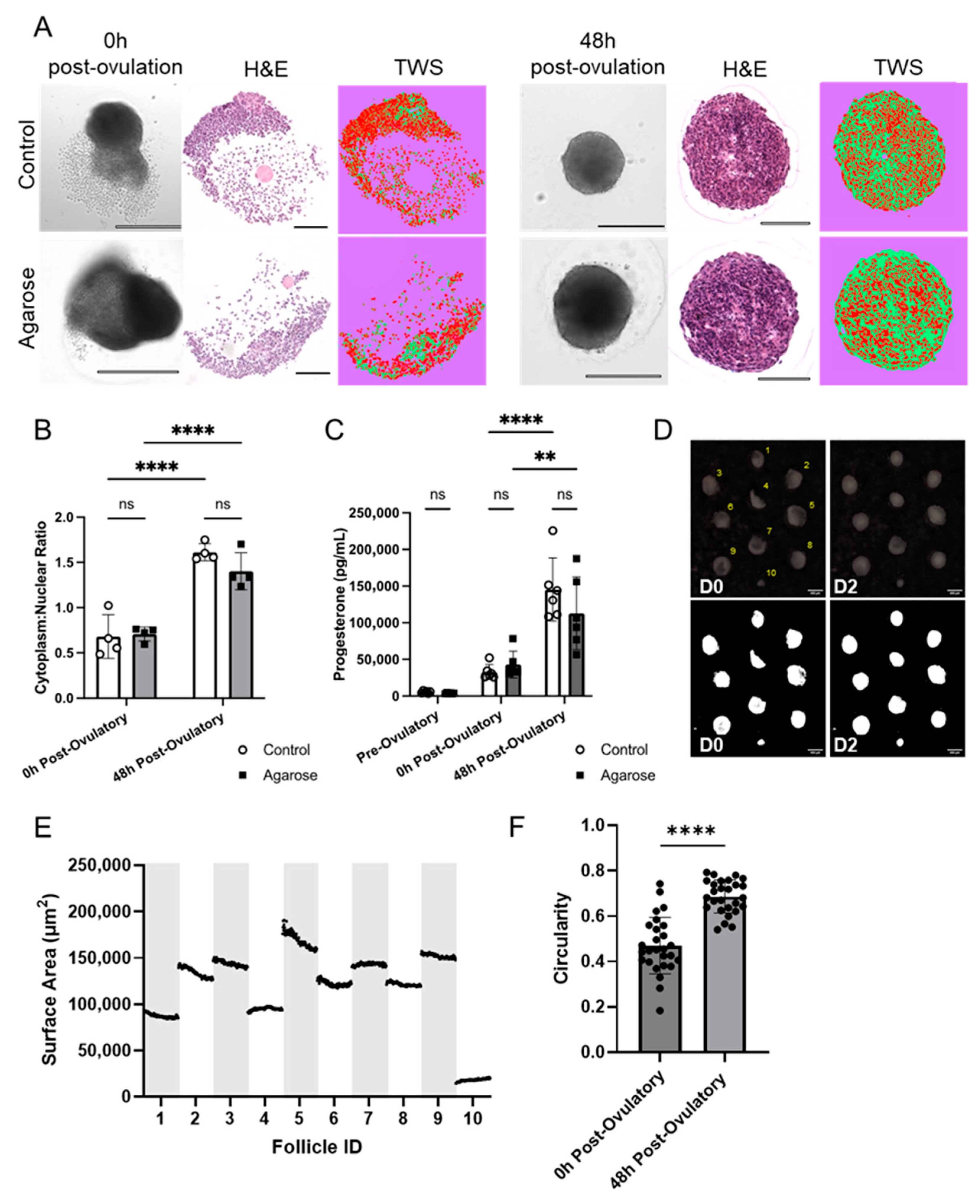
3.5. Agarose Micromolds Sustain Hormonally Active Corpora Lutea following Ex Vivo Ovulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J. Vis. Exp. 2011, 49, e2695. [Google Scholar] [CrossRef]
- Li, L.; Shi, X.; Shi, Y.; Wang, Z. The Signaling Pathways Involved in Ovarian Follicle Development. Front. Physiol. 2021, 12, 730196. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.A.; Amargant, F.; Shvartsman, S.Y.; Duncan, F.E.; Gavis, E.R. Bidirectional communication in oogenesis: A dynamic conversation in mice and Drosophila. Trends Cell Biol. 2022, 32, 311–323. [Google Scholar] [CrossRef]
- El-Hayek, S.; Yang, Q.; Abbassi, L.; FitzHarris, G.; Clarke, H.J. Mammalian Oocytes Locally Remodel Follicular Architecture to Provide the Foundation for Germline-Soma Communication. Curr. Biol. 2018, 28, 1124–1131.e3. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.E. The early stages of follicular development: Activation of primordial follicles and growth of preantral follicles. Anim. Reprod. Sci. 2003, 78, 135–163. [Google Scholar] [CrossRef]
- Griffin, J.; Emery, B.R.; Huang, I.; Peterson, C.M.; Carrell, D.T. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J. Exp. Clin. Assist. Reprod. 2006, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum. Reprod. Update 2012, 18, 73–91. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rimon-Dahari, N.; Yerushalmi-Heinemann, L.; Alyagor, L.; Dekel, N. Ovarian folliculogenesis. In Molecular Mechanisms of Cell Differentiation in Gonad Development; Springer: Cham, Switzerland, 2016; pp. 167–190. [Google Scholar]
- Plant, T.M.; Zeleznik, A.J.; Albertini, D.F. Knobil and Neill’s Physiology of Reproduction, 4th ed.; Elsevier/Academic Press: London, UK, 2015. [Google Scholar]
- Richards, J.S.; Liu, Z.; Shimada, M. Chapter 22—Ovulation. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 997–1021. [Google Scholar]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels With Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Franchimont, F.; Hazee-Hagelstein, M.; Charlet-Renard, B.; Urindts, Y.; Gaspar, S.; Hazout, A.; Salat-Baroux, J. Some basic mechanisms of ovulation. In The Triggering of Ovulation in Stimulated Cycles: HCG or LH; The Parthenon Publishing Group: New York, NY, USA, 1994; pp. 13–20. [Google Scholar]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Richards, J.S. Hormonal Control of Gene Expression in the Ovary. Endocr. Rev. 1994, 15, 725–751. [Google Scholar] [CrossRef]
- Richards, J.S.; Russell, D.L.; Robker, R.L.; Dajee, M.; Alliston, T.N. Molecular mechanisms of ovulation and luteinization. Mol. Cell. Endocrinol. 1998, 145, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.A.S. Genetics of ovulation. In Proceedings of the Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2007; pp. 235–242. [Google Scholar]
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of Ovulation and Oocyte Maturation: A Good Egg at the Right Time. Endocrinology 2018, 159, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.L.; Robker, R.L. Molecular mechanisms of ovulation: Co-ordination through the cumulus complex. Hum. Reprod. Update 2007, 13, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Turathum, B.; Gao, E.-M.; Chian, R.-C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, M.; Sato, E. Cumulus-oocyte complex interactions during oocyte maturation. Int. Rev. Cytol. 2004, 235, 251–282. [Google Scholar] [PubMed]
- Zhou, H.; Shikanov, A. Three-Dimensional Hydrogel-Based Culture to Study the Effects of Toxicants on Ovarian Follicles. Methods Mol. Biol. 2018, 1758, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Gedye, K.; Craig, Z.R.; Ziv-Gal, A. Propylparaben inhibits mouse cultured antral follicle growth, alters steroidogenesis, and upregulates levels of cell-cycle and apoptosis regulators. Reprod. Toxicol. 2019, 89, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, J.; Liu, M.; Iwahata, H.; Rogers, H.B.; Woodruff, T.K. Doxorubicin Has Dose-Dependent Toxicity on Mouse Ovarian Follicle Development, Hormone Secretion, and Oocyte Maturation. Toxicol. Sci. 2017, 157, 320–329. [Google Scholar] [CrossRef]
- Cortvrindt, R.; Smitz, J. Follicle culture in reproductive toxicology: A tool for in-vitro testing of ovarian function? Hum. Reprod. Update 2002, 8, 243–254. [Google Scholar] [CrossRef]
- Xu, Y.; Duncan, F.E.; Xu, M.; Woodruff, T.K. Use of an organotypic mammalian in vitro follicle growth assay to facilitate female reproductive toxicity screening. Reprod. Fertil. Dev. 2016, 28, 1295–1306. [Google Scholar] [CrossRef]
- Smitz, J.; Dolmans, M.M.; Donnez, J.; Fortune, J.E.; Hovatta, O.; Jewgenow, K.; Picton, H.M.; Plancha, C.; Shea, L.D.; Stouffer, R.L.; et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: Implications for fertility preservation. Hum. Reprod. Update 2010, 16, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Yang, M.; Wang, T. In VitroGrowth of Human Ovarian Follicles for Fertility Preservation. Reprod. Dev. Med. 2018, 2, 230–236. [Google Scholar] [CrossRef]
- Resetkova, N.; Hayashi, M.; Kolp, L.A.; Christianson, M.S. Fertility preservation for prepubertal girls: Update and current challenges. Curr. Obstet. Gynecol. Rep. 2013, 2, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.B.; Kelsey, T.W.; Anderson, R.A. Fertility preservation in pre-pubertal girls with cancer: The role of ovarian tissue cryopreservation. Fertil. Steril. 2016, 105, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.-M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013, 9, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, J.P.; Gambini, A. Advancements and challenges in in vitro reproductive technologies for the conservation of equine species. Theriogenology Wild 2023, 2, 100036. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Hill, A.M.; Songsasen, N. In vitro development of mechanically and enzymatically isolated cat ovarian follicles. Reprod. Fertil. 2021, 2, 35–46. [Google Scholar] [CrossRef]
- Simon, L.E.; Kumar, T.R.; Duncan, F.E. In vitro ovarian follicle growth: A comprehensive analysis of key protocol variablesdagger. Biol. Reprod. 2020, 103, 455–470. [Google Scholar] [CrossRef]
- West, E.R.; Shea, L.D.; Woodruff, T.K. Engineering the follicle microenvironment. In Proceedings of the Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2007; pp. 287–299. [Google Scholar]
- Green, L.J.; Shikanov, A. In vitro culture methods of preantral follicles. Theriogenology 2016, 86, 229–238. [Google Scholar] [CrossRef]
- Xu, M.; West-Farrell, E.R.; Stouffer, R.L.; Shea, L.D.; Woodruff, T.K.; Zelinski, M.B. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol. Reprod. 2009, 81, 587–594. [Google Scholar] [CrossRef]
- Xu, M.; Fazleabas, A.T.; Shikanov, A.; Jackson, E.; Barrett, S.L.; Hirshfeld-Cytron, J.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T.K. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol. Reprod. 2011, 84, 689–697. [Google Scholar] [CrossRef]
- Hornick, J.E.; Duncan, F.E.; Shea, L.D.; Woodruff, T.K. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum. Reprod. 2012, 27, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, J.; Romero, M.M.; Smith, K.N.; Shea, L.D.; Woodruff, T.K. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 2015, 5, 17323. [Google Scholar] [CrossRef] [PubMed]
- Skory, R.M.; Xu, Y.; Shea, L.D.; Woodruff, T.K. Engineering the ovarian cycle using in vitro follicle culture. Hum. Reprod. 2015, 30, 1386–1395. [Google Scholar] [CrossRef]
- Xu, M.; Barrett, S.L.; West-Farrell, E.; Kondapalli, L.A.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T.K. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 2009, 24, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S. Oocyte Biology in Fertility Preservation; Springer: New York, NY, USA, 2013. [Google Scholar]
- Kreeger, P.K.; Fernandes, N.N.; Woodruff, T.K.; Shea, L.D. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol. Reprod. 2005, 73, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Kreeger, P.K.; Deck, J.W.; Woodruff, T.K.; Shea, L.D. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006, 27, 714–723. [Google Scholar] [CrossRef]
- Xu, M.; Kreeger, P.K.; Shea, L.D.; Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006, 12, 2739–2746. [Google Scholar] [CrossRef]
- Pangas, S.A.; Saudye, H.; Shea, L.D.; Woodruff, T.K. Novel approach for the three-dimensional culture of granulosa cell–oocyte complexes. Tissue Eng. 2003, 9, 1013–1021. [Google Scholar] [CrossRef]
- Parrish, E.M.; Siletz, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction 2011, 142, 309. [Google Scholar] [CrossRef]
- Xu, M.; West, E.; Shea, L.D.; Woodruff, T.K. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol. Reprod. 2006, 75, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Banc, A.; Woodruff, T.K.; Shea, L.D. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng. 2009, 103, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, M.; Bernuci, M.P.; Fisher, T.E.; Shea, L.D.; Woodruff, T.K.; Zelinski, M.B.; Stouffer, R.L. Primate follicular development and oocyte maturation in vitro. Adv. Exp. Med. Biol. 2013, 761, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Van Langendonckt, A.; David, A.; Dolmans, M.M.; Donnez, J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum. Reprod. 2009, 24, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Babayev, E.; Xu, M.; Shea, L.D.; Woodruff, T.K.; Duncan, F.E. Follicle isolation methods reveal plasticity of granulosa cell steroidogenic capacity during mouse in vitro follicle growth. Mol. Hum. Reprod. 2022, 28, gaac033. [Google Scholar] [CrossRef] [PubMed]
- Converse, A.; Zaniker, E.J.; Amargant, F.; Duncan, F.E. Recapitulating folliculogenesis and oogenesis outside the body: Encapsulated in vitro follicle growth†. Biol. Reprod. 2023, 108, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.R.; Silva, C.M.; Duarte, A.B.; Lima, I.M.; Rodrigues, G.Q.; Rossetto, R.; Sales, A.D.; Lobo, C.H.; Bernuci, M.P.; Rosa, E.S.A.C.; et al. Alginate hydrogel matrix stiffness influences the in vitro development of caprine preantral follicles. Mol. Reprod. Dev. 2014, 81, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.R.; Lima, I.M.; Xu, M.; Shea, L.D.; Woodruff, T.K.; Figueiredo, J.R. Three-dimensional systems for in vitro follicular culture: Overview of alginate-based matrices. Reprod. Fertil. Dev. 2014, 26, 915–930. [Google Scholar] [CrossRef] [PubMed]
- West, E.R.; Xu, M.; Woodruff, T.K.; Shea, L.D. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007, 28, 4439–4448. [Google Scholar] [CrossRef]
- Zhang, J.; Goods, B.A.; Pattarawat, P.; Wang, Y.; Haining, T.; Zhang, Q.; Shalek, A.K.; Duncan, F.E.; Woodruff, T.K.; Xiao, S. An ex vivo ovulation system enables the discovery of novel ovulatory pathways and nonhormonal contraceptive candidates †. Biol. Reprod. 2023, 108, 629–644. [Google Scholar] [CrossRef]
- Campo, H.; Zha, D.; Pattarawat, P.; Colina, J.; Zhang, D.; Murphy, A.; Yoon, J.; Russo, A.; Rogers, H.B.; Lee, H.C. A new tissue-agnostic microfluidic device to model physiology and disease: The lattice platform. Lab Chip 2023, 23, 4821–4833. [Google Scholar] [CrossRef] [PubMed]
- Malo, C.; Oliván, S.; Ochoa, I.; Shikanov, A. In Vitro Growth of Human Follicles: Current and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, F.; Tahir, H.M.; Ali, S.; Ali, A.; Khan, H.A.; Muzamil, A.; Manzoor, H.H.; Qayyum, K.A. Biomolecules based hydrogels and their potential biomedical applications: A comprehensive review. Int. J. Biol. Macromol. 2023, 253, 127362. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Donoughe, S.; Kim, C.; Extavour, C.G. High-throughput live-imaging of embryos in microwell arrays using a modular specimen mounting system. Biol. Open 2018, 7, bio031260. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, D.S.; Lecaudey, V. Standardized mounting method of (zebrafish) embryos using a 3D-printed stamp for high-content, semi-automated confocal imaging. BMC Biotechnol. 2019, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Alkali, I.M.; Colombo, M.; Luciano, A.M.; Nizanski, W.; Ali Hassan, H.; Dziegiel, P.; Luvoni, G.C. Culture of vitrified bovine ovarian tissue on agarose gel inserts maintains follicle integrity. Reproduction 2023, 166, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wandji, S.A.; Eppig, J.J.; Fortune, J.E. FSH and growth factors affect the growth and endocrine function in vitro of granulosa cells of bovine preantral follicles. Theriogenology 1996, 45, 817–832. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, J.; Lee, S.T.; Lee, E. In vitro maturation on ovarian granulosa cells encapsulated in agarose matrix improves developmental competence of porcine oocytes. Theriogenology 2021, 164, 42–50. [Google Scholar] [CrossRef]
- Musgrove, H.B.; Catterton, M.A.; Pompano, R.R. Applied tutorial for the design and fabrication of biomicrofluidic devices by resin 3D printing. Anal. Chim. Acta 2022, 1209, 339842. [Google Scholar] [CrossRef] [PubMed]
- Milton, L.A.; Viglione, M.S.; Ong, L.J.Y.; Nordin, G.P.; Toh, Y.C. Vat photopolymerization 3D printed microfluidic devices for organ-on-a-chip applications. Lab Chip 2023, 23, 3537–3560. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, H.B.; Cook, S.R.; Pompano, R.R. Parylene-C Coating Protects Resin-3D-Printed Devices from Material Erosion and Prevents Cytotoxicity toward Primary Cells. ACS Appl. Bio Mater. 2023, 6, 3079–3083. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, N.P.; Zhu, F.; Hall, C.J.; Reboud, J.; Crosier, P.S.; Patton, E.E.; Wlodkowic, D.; Cooper, J.M. Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab Chip 2016, 16, 291–297. [Google Scholar] [CrossRef]
- Rogers, H.B.; Zhou, L.T.; Kusuhara, A.; Zaniker, E.; Shafaie, S.; Owen, B.C.; Duncan, F.E.; Woodruff, T.K. Dental resins used in 3D printing technologies release ovo-toxic leachates. Chemosphere 2021, 270, 129003. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Hoehnel, S.; Kuttler, F.; Homicsko, K.; Ceroni, C.; Ringel, T.; Gjorevski, N.; Schwank, G.; Coukos, G.; Turcatti, G.; et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020, 4, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Duncan, F.E.; Bai, L.; Nguyen, C.T.; Shea, L.D.; Woodruff, T.K. Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction 2015, 150, 183–192. [Google Scholar] [CrossRef]
- Suebthawinkul, C.; Babayev, E.; Zhou, L.T.; Lee, H.C.; Duncan, F.E. Quantitative morphokinetic parameters identify novel dynamics of oocyte meiotic maturation and cumulus expansiondagger. Biol. Reprod. 2022, 107, 1097–1112. [Google Scholar] [CrossRef]
- Zaniker, E.J.; Babayev, E.; Duncan, F.E. Common mechanisms of physiological and pathological rupture events in biology: Novel insights into mammalian ovulation and beyond. Biol. Rev. 2023, 98, 1648–1667. [Google Scholar] [CrossRef]
- Espey, L.L. Ovarian contractility and its relationship to ovulation: A review. Biol. Reprod. 1978, 19, 540–551. [Google Scholar] [CrossRef]
- Thibault, C.; Levasseur, M.C. Ovulation. Hum. Reprod. 1988, 3, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.M.; Jaffe, L.A. Luteinizing hormone stimulates ingression of mural granulosa cells within the mouse preovulatory follicledagger. Biol. Reprod. 2024, 110, 288–299. [Google Scholar] [CrossRef]
- Davis, J.S.; LaVoie, H.A. Chapter 15—Molecular Regulation of Progesterone Production in the Corpus Luteum. In The Ovary, 3rd ed.; Leung, P.C.K., Adashi, E.Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 237–253. [Google Scholar]
- Wycherley, G.; Downey, D.; Kane, M.T.; Hynes, A.C. A novel follicle culture system markedly increases follicle volume, cell number and oestradiol secretion. Reproduction 2004, 127, 669–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nation, A.; Selwood, L. The production of mature oocytes from adult ovaries following primary follicle culture in a marsupial. Reproduction 2009, 138, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Zackrisson, U.; Löfman, C.O.; Janson, P.O.; Wallin, A.; Mikuni, M.; Brännström, M. Alterations of follicular microcirculation and apex structure during ovulation in the rat. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Migone, F.F.; Cowan, R.G.; Williams, R.M.; Gorse, K.J.; Zipfel, W.R.; Quirk, S.M. In vivo imaging reveals an essential role of vasoconstriction in rupture of the ovarian follicle at ovulation. Proc. Natl. Acad. Sci. USA 2016, 113, 2294–2299. [Google Scholar] [CrossRef]
- Brito, I.R.; Silva, G.M.; Sales, A.D.; Lobo, C.H.; Rodrigues, G.Q.; Sousa, R.F.; Moura, A.; Calderón, C.; Bertolini, M.; Campello, C.C.; et al. Fibrin-alginate hydrogel supports steroidogenesis, in vitro maturation of oocytes and parthenotes production from caprine preantral follicles cultured in group. Reprod. Domest. Anim. 2016, 51, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Martin, L.F.; Hubscher, T.; Bowler, A.D.; Broguiere, N.; Langer, J.; Tillard, L.; Nikolaev, M.; Radtke, F.; Lutolf, M.P. Spatiotemporally resolved colorectal oncogenesis in mini-colons ex vivo. Nature 2024, 629, 450–457. [Google Scholar] [CrossRef]
- Girgin, M.U.; Broguiere, N.; Hoehnel, S.; Brandenberg, N.; Mercier, B.; Arias, A.M.; Lutolf, M.P. Bioengineered embryoids mimic post-implantation development in vitro. Nat. Commun. 2021, 12, 5140. [Google Scholar] [CrossRef]
- Nelson, C.M.; Vanduijn, M.M.; Inman, J.L.; Fletcher, D.A.; Bissell, M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 2006, 314, 298–300. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Moghassemi, S.; Peaucelle, A.; Lucci, C.M.; Amorim, C.A. Mind the mechanical strength: Tailoring a 3D matrix to encapsulate isolated human preantral follicles. Hum. Reprod. Open 2023, 2023, hoad004. [Google Scholar] [CrossRef] [PubMed]
- Jamalzaei, P.; Valojerdi, M.R.; Montazeri, L.; Baharvand, H. Effects of Alginate Concentration and Ovarian Cells on In Vitro Development of Mouse Preantral Follicles: A Factorial Study. Int. J. Fertil. Steril. 2020, 13, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; Shea, L.D. A new hypothesis regarding ovarian follicle development: Ovarian rigidity as a regulator of selection and health. J. Assist. Reprod. Genet. 2011, 28, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.M.; Orellana, R.; Soares, M.; Paulini, F.; Donnez, J.; Amorim, C.A. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum. Reprod. 2016, 31, 427–435. [Google Scholar] [CrossRef]
- Chiti, M.C.; Vanacker, J.; Ouni, E.; Tatic, N.; Viswanath, A.; des Rieux, A.; Dolmans, M.M.; White, L.J.; Amorim, C.A. Ovarian extracellular matrix-based hydrogel for human ovarian follicle survival in vivo: A pilot work. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1012–1022. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef]
- Grubliauskaitė, M.; Vlieghe, H.; Moghassemi, S.; Dadashzadeh, A.; Camboni, A.; Gudlevičienė, Ž.; Amorim, C.A. Influence of ovarian stromal cells on human ovarian follicle growth in a 3D environment. Hum. Reprod. Open 2024, 2024, hoad052. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.; Dolmans, M.M.; Vanacker, J.; Scalercio, S.R.; Donnez, J.; Amorim, C.A. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J. Ovarian Res. 2013, 6, 83. [Google Scholar] [CrossRef]
- Sadr, S.Z.; Fatehi, R.; Maroufizadeh, S.; Amorim, C.A.; Ebrahimi, B. Utilizing Fibrin-Alginate and Matrigel-Alginate for Mouse Follicle Development in Three-Dimensional Culture Systems. Biopreserv. Biobank. 2018, 16, 120–127. [Google Scholar] [CrossRef]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef]
- Shikanov, A.; Smith, R.M.; Xu, M.; Woodruff, T.K.; Shea, L.D. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 2011, 32, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.Y.; Yeoman, R.R.; Lawson, M.S.; Zelinski, M.B. Synthetic polymers improve vitrification outcomes of macaque ovarian tissue as assessed by histological integrity and the in vitro development of secondary follicles. Cryobiology 2012, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nason-Tomaszewski, C.E.; Thomas, E.E.; Matera, D.L.; Baker, B.M.; Shikanov, A. Extracellular matrix-templating fibrous hydrogels promote ovarian tissue remodeling and oocyte growth. Bioact. Mater. 2024, 32, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, C.E.; DiLillo, K.M.; Baker, B.M.; Arnold, K.B.; Shikanov, A. Sequestered cell-secreted extracellular matrix proteins improve murine folliculogenesis and oocyte maturation for fertility preservation. Acta Biomater. 2021, 132, 313–324. [Google Scholar] [CrossRef]
- Xu, J.; Lawson, M.S.; Yeoman, R.R.; Molskness, T.A.; Ting, A.Y.; Stouffer, R.L.; Zelinski, M.B. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum. Reprod. 2013, 28, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Y.; Lei, L.; Shikanov, A.; Shea, L.D.; Woodruff, T.K. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil. Steril. 2010, 93, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the Ovarian Follicle Microenvironment. Annu. Rev. Biomed. Eng. 2014, 16, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P. Biotechnologies for wildlife fertility preservation. Anim. Front. 2015, 5, 73–78. [Google Scholar] [CrossRef][Green Version]
- Comizzoli, P.; Songsasen, N.; Wildt, D.E. Protecting and Extending Fertility for Females of Wild and Endangered Mammals. In Oncofertility: Ethical, Legal, Social, and Medical Perspectives; Woodruff, T.K., Zoloth, L., Campo-Engelstein, L., Rodriguez, S., Eds.; Springer: Boston, MA, USA, 2010; pp. 87–100. [Google Scholar]
- Santos, R.R.; Amorim, C.; Cecconi, S.; Fassbender, M.; Imhof, M.; Lornage, J.; Paris, M.; Schoenfeldt, V.; Martinez-Madrid, B. Cryopreservation of ovarian tissue: An emerging technology for female germline preservation of endangered species and breeds. Anim. Reprod. Sci. 2010, 122, 151–163. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Aziz, A.U.R.; Yu, X.; Jiang, Q.; Zhao, Y.; Deng, S.; Qin, K.; Wang, H.; Liu, B. Doxorubicin-induced toxicity to 3D-cultured rat ovarian follicles on a microfluidic chip. Toxicol. Vitr. 2020, 62, 104677. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.U.R.; Fu, M.; Deng, J.; Geng, C.; Luo, Y.; Lin, B.; Yu, X.; Liu, B. A Microfluidic Device for Culturing an Encapsulated Ovarian Follicle. Micromachines 2017, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef] [PubMed]
- Roberto de Barros, N.; Wang, C.; Maity, S.; Peirsman, A.; Nasiri, R.; Herland, A.; Ermis, M.; Kawakita, S.; Gregatti Carvalho, B.; Hosseinzadeh Kouchehbaghi, N.; et al. Engineered organoids for biomedical applications. Adv. Drug Deliv. Rev. 2023, 203, 115142. [Google Scholar] [CrossRef] [PubMed]
- Gritti, N.; Lim, J.L.; Anlas, K.; Pandya, M.; Aalderink, G.; Martinez-Ara, G.; Trivedi, V. MOrgAna: Accessible quantitative analysis of organoids with machine learning. Development 2021, 148, dev199611. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; van Blitterswijk, C.; Vrij, E.; Giselbrecht, S. Automated, High-Throughput Phenotypic Screening and Analysis Platform to Study Pre- and Post-Implantation Morphogenesis in Stem Cell-Derived Embryo-Like Structures. Adv. Sci. 2024, 11, e2304987. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.M.; Schuster, B.; Kashaf, S.S.; Liu, P.; Ben-Yishay, R.; Ishay-Ronen, D.; Izumchenko, E.; Shen, L.; Weber, C.R.; Bielski, M.; et al. OrganoID: A versatile deep learning platform for tracking and analysis of single-organoid dynamics. PLoS Comput. Biol. 2022, 18, e1010584. [Google Scholar] [CrossRef]
- Barnes, J.; Brendel, M.; Gao, V.R.; Rajendran, S.; Kim, J.; Li, Q.; Malmsten, J.E.; Sierra, J.T.; Zisimopoulos, P.; Sigaras, A.; et al. A non-invasive artificial intelligence approach for the prediction of human blastocyst ploidy: A retrospective model development and validation study. Lancet Digit. Health 2023, 5, e28–e40. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaniker, E.J.; Hashim, P.H.; Gauthier, S.; Ankrum, J.A.; Campo, H.; Duncan, F.E. Three-Dimensionally Printed Agarose Micromold Supports Scaffold-Free Mouse Ex Vivo Follicle Growth, Ovulation, and Luteinization. Bioengineering 2024, 11, 719. https://doi.org/10.3390/bioengineering11070719
Zaniker EJ, Hashim PH, Gauthier S, Ankrum JA, Campo H, Duncan FE. Three-Dimensionally Printed Agarose Micromold Supports Scaffold-Free Mouse Ex Vivo Follicle Growth, Ovulation, and Luteinization. Bioengineering. 2024; 11(7):719. https://doi.org/10.3390/bioengineering11070719
Chicago/Turabian StyleZaniker, Emily J., Prianka H. Hashim, Samuel Gauthier, James A. Ankrum, Hannes Campo, and Francesca E. Duncan. 2024. "Three-Dimensionally Printed Agarose Micromold Supports Scaffold-Free Mouse Ex Vivo Follicle Growth, Ovulation, and Luteinization" Bioengineering 11, no. 7: 719. https://doi.org/10.3390/bioengineering11070719
APA StyleZaniker, E. J., Hashim, P. H., Gauthier, S., Ankrum, J. A., Campo, H., & Duncan, F. E. (2024). Three-Dimensionally Printed Agarose Micromold Supports Scaffold-Free Mouse Ex Vivo Follicle Growth, Ovulation, and Luteinization. Bioengineering, 11(7), 719. https://doi.org/10.3390/bioengineering11070719






