Human Activities Increased Microplastics Contamination in the Himalaya Mountains
Abstract
1. Introduction
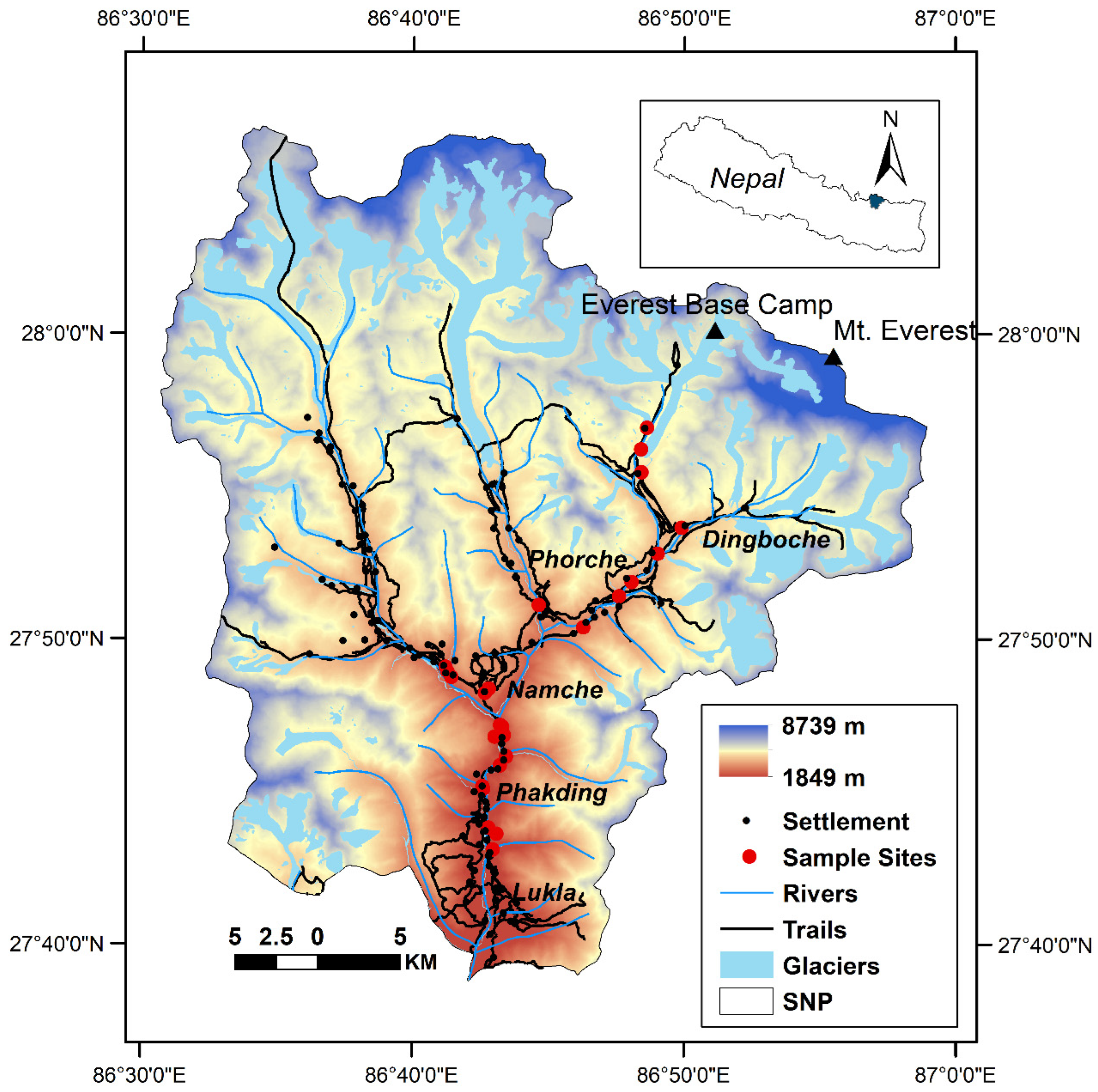
2. Study Area
3. Methods
3.1. Sample Collection
3.2. Laboratory Analysis
3.3. Data Analysis
3.4. Quality Assurance and Quality Control
4. Results and Discussion
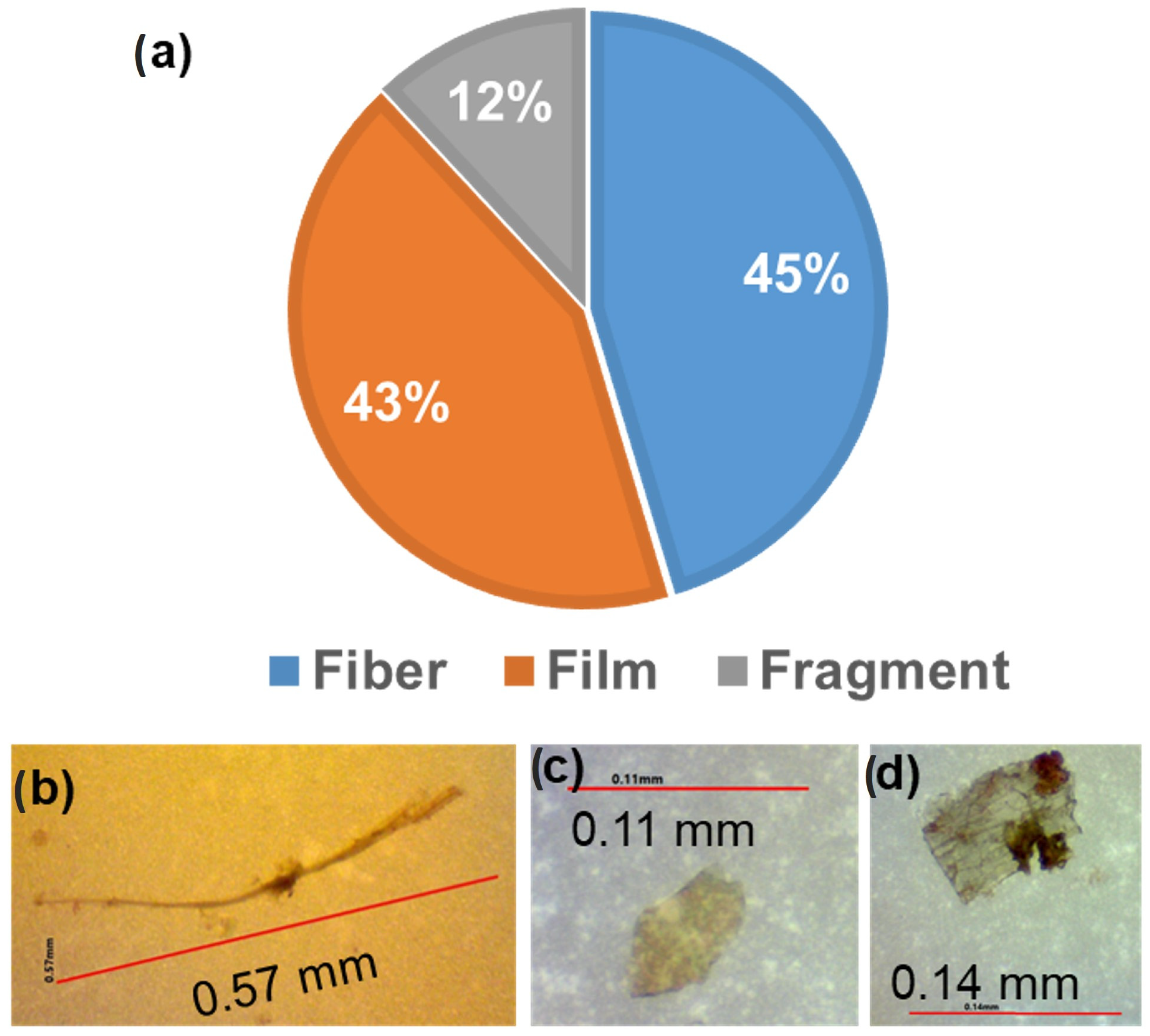
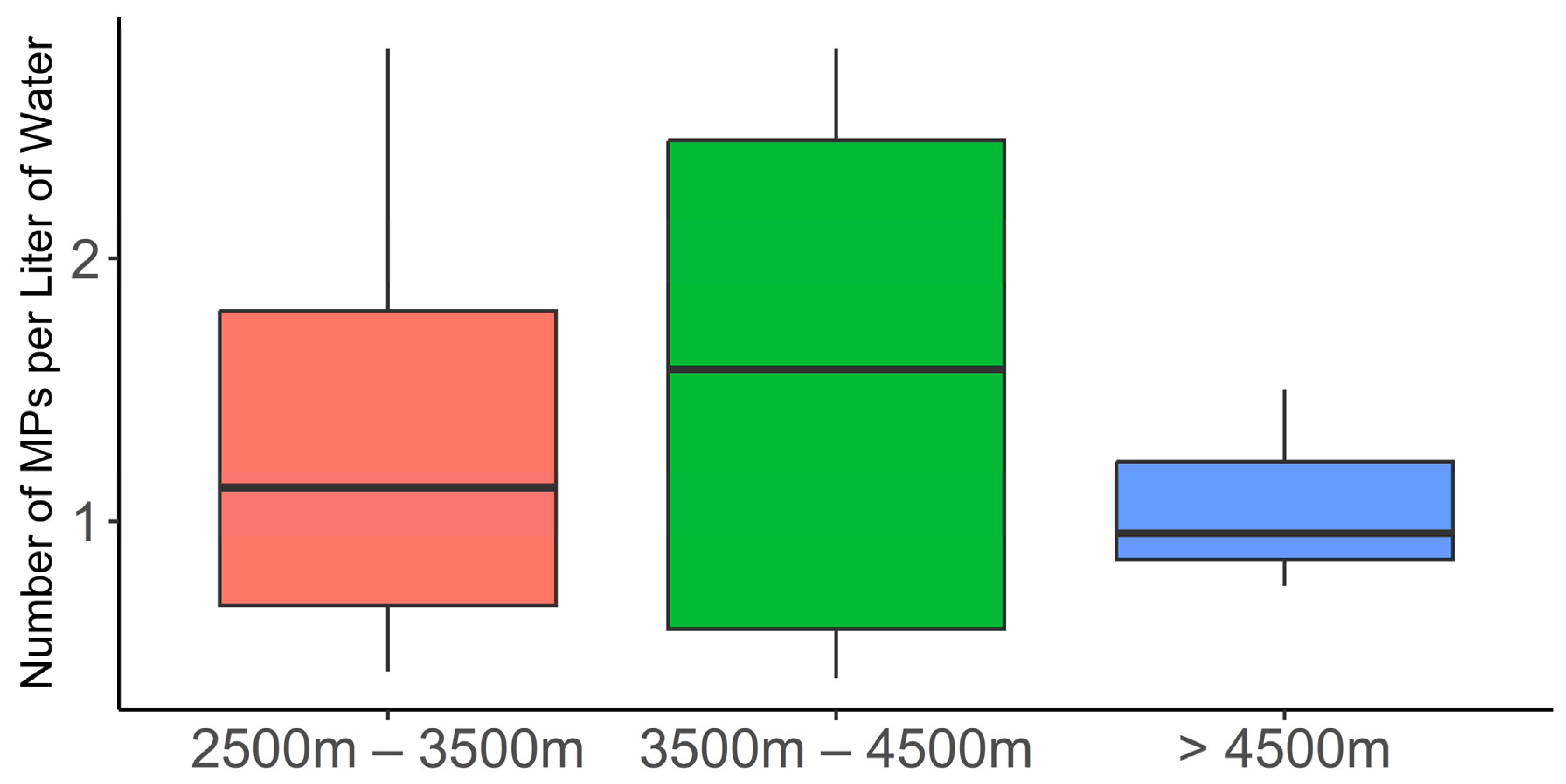
4.1. Abundance of MPs in the SNP Water Bodies
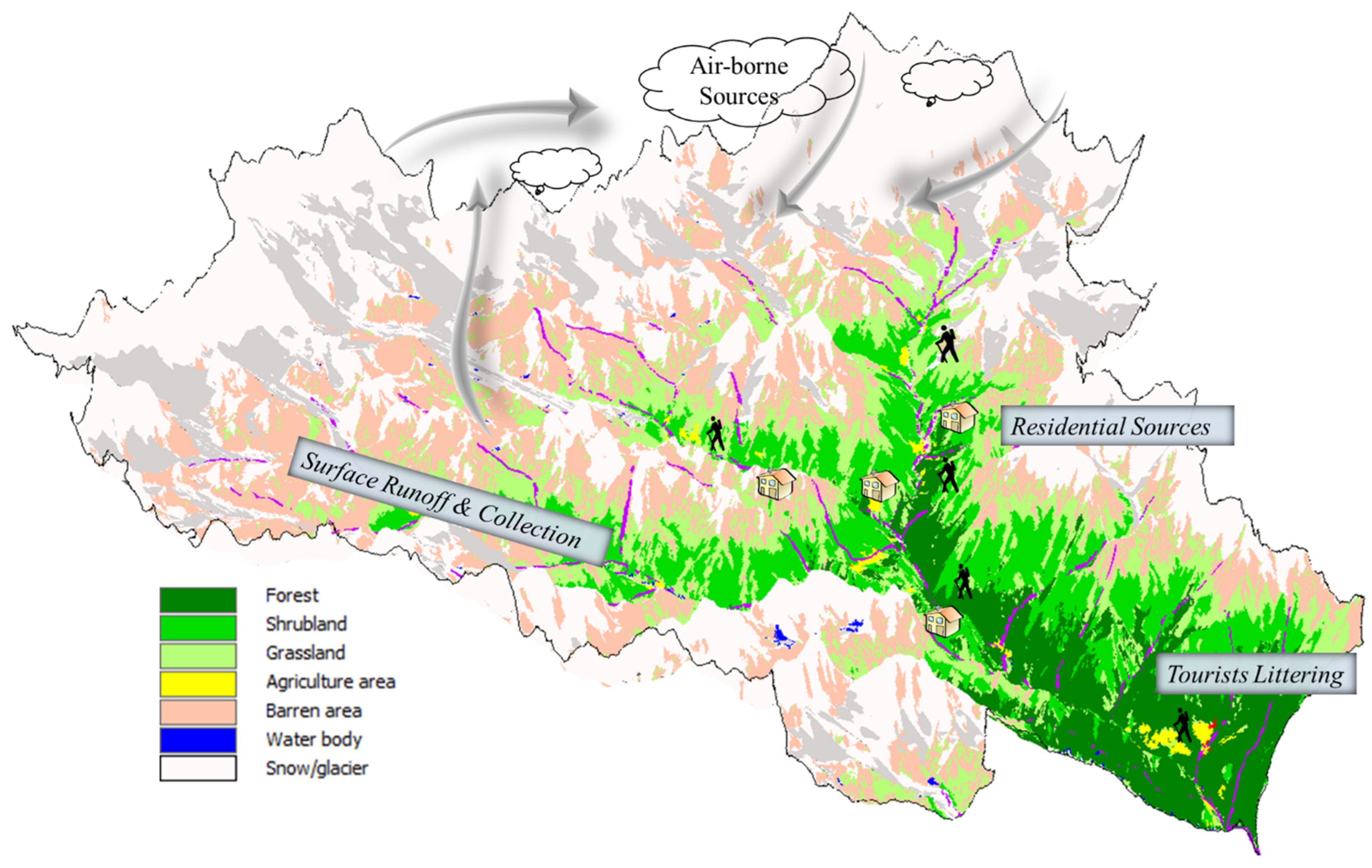
4.2. Spatial Distribution and Potential Sources of MPs in the SNP Water Bodies
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Appendix A
| Sample# | Lat | Lon | Altitude (m) | Volume (L) | Total MPs | MP Density (Pieces/L) | Fiber | Film | Fragment | Site Category |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27.7185 | 86.7161 | 2554 | 19.9 | 19 | 1.0 | 3 | 13 | 3 | Tributary |
| 2 | 27.7531 | 86.7097 | 2735 | 10.0 | 28 | 2.8 | 11 | 8 | 9 | Tributary |
| 3 | 27.7854 | 86.7218 | 2871 | 10.0 | 27 | 2.7 | 5 | 17 | 5 | S. Tributary |
| 4 | 27.7864 | 86.7205 | 2847 | 10.0 | 24 | 2.4 | 13 | 7 | 4 | Main River |
| 5 | 27.8039 | 86.7112 | 3468 | 5.0 | 25 | 5.0 | 13 | 11 | 1 | Settlement |
| 6 | 27.8126 | 86.6904 | 3497 | 5.0 | 32 | 6.4 | 21 | 7 | 4 | Settlement |
| 7 | 27.8181 | 86.6869 | 3450 | 19.9 | 15 | 0.8 | 5 | 8 | 2 | Tributary |
| 8 | 27.8156 | 86.6881 | 3509 | 5.0 | 20 | 4.0 | 3 | 13 | 4 | Settlement |
| 9 | 27.8520 | 86.7442 | 3583 | 49.8 | 20 | 0.4 | 5 | 6 | 9 | Tributary |
| 10 | 27.9488 | 86.8104 | 4908 | 19.9 | 19 | 1.0 | 11 | 8 | 0 | Glacier |
| 11 | 27.9371 | 86.8068 | 4856 | 10.0 | 15 | 1.5 | 9 | 3 | 3 | Glacier |
| 12 | 27.9245 | 86.8071 | 4626 | 19.9 | 15 | 0.8 | 6 | 7 | 2 | Main River |
| 13 | 27.8942 | 86.8317 | 4349 | 5.0 | 29 | 5.8 | 16 | 11 | 2 | Settlement |
| 14 | 27.8800 | 86.8171 | 4163 | 10.0 | 20 | 2.0 | 11 | 9 | 0 | Tributary |
| 15 | 27.8646 | 86.8011 | 4000 | 5.0 | 13 | 2.6 | 7 | 5 | 1 | S. Tributary |
| 16 | 27.8568 | 86.7934 | 3946 | 19.9 | 23 | 1.2 | 10 | 13 | 0 | Tributary |
| 17 | 27.8398 | 86.7714 | 3739 | 5.0 | 14 | 2.8 | 8 | 6 | 0 | S. Tributary |
| 18 | 27.8062 | 86.7135 | 3553 | 29.9 | 12 | 0.4 | 4 | 8 | 0 | S. Tributary |
| 19 | 27.7800 | 86.7171 | 3220 | 19.9 | 14 | 0.7 | 8 | 3 | 3 | Tributary |
| 20 | 27.7808 | 86.7228 | 2821 | 10.0 | 16 | 1.6 | 8 | 7 | 1 | Main River |
| 21 | 27.7693 | 86.7242 | 2816 | 19.9 | 10 | 0.5 | 2 | 5 | 3 | Main River |
| 22 | 27.7643 | 86.7205 | 2742 | 10.0 | 15 | 1.5 | 9 | 6 | 0 | S. Tributary |
| 23 | 27.7517 | 86.7102 | 2679 | 39.9 | 17 | 0.4 | 10 | 7 | 0 | Tributary |
| 24 | 27.7304 | 86.7135 | 2578 | 10.0 | 13 | 1.3 | 10 | 3 | 0 | S. Tributary |
| 25 | 27.7271 | 86.7184 | 2913 | 19.9 | 12 | 0.6 | 4 | 8 | 0 | Main River |
References
- Felismino, M.E.L.; Helm, P.A.; Rochman, C.M. Microplastic and other anthropogenic microparticles in water and sediments of Lake Simcoe. J. Great Lakes Res. 2021, 47, 180–189. [Google Scholar] [CrossRef]
- Kershaw, P.; Rochman, C. Sources, Fate and Effects of Microplastics in the Marine Environment: Part 2 of a Global Assessment; Reports and Studies-IMO/FAO/Unesco-IOC/WMO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP) Eng No. 93; IMO/FAO/UNESCO-IOC/ÚNIDO/WMO/IAEA/UN/UNEP/UNDP: New York, NY, USA, 2015. [Google Scholar]
- Zeng, E.Y. Microplastic Contamination in Aquatic Environments: An Emerging Matter of Environmental Urgency; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhang, D.; Liu, X.; Huang, W.; Li, J.; Wang, C.; Zhang, D.; Zhang, C. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut. 2020, 259, 113948. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of plastic debris by rivers into the sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; van der Plaats, R.Q.; van der Wielen, P.W.; Bauerlein, P.S.; de Roda Husman, A.M. Riverine microplastic and microbial community compositions: A field study in the Netherlands. Water Res. 2021, 192, 116852. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Browne, M.A. Sources and pathways of microplastics to habitats. In Marine Anthropogenic Litter; Springer Nature: Berlin, Germany, 2015; pp. 229–244. [Google Scholar]
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric transport of microplastic fibres influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Allen, D.; Allen, S.; Abbasi, S.; Baker, A.; Bergmann, M.; Brahney, J.; Butler, T.; Duce, R.A.; Eckhardt, S.; Evangeliou, N. Microplastics and nanoplastics in the marine-atmosphere environment. Nat. Rev. Earth Environ. 2022, 3, 393–405. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Wang, Q.; Liu, N.; Li, M. Seasonal variations and feedback from microplastics and cadmium on soil organisms in agricultural fields. Environ. Int. 2022, 161, 107096. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kang, S.; Wang, Z.; Luo, X.; Guo, J.; Gao, T.; Chen, P.; Yang, C.; Zhang, Y. Microplastic characteristic in the soil across the Tibetan Plateau. Sci. Total Environ. 2022, 828, 154518. [Google Scholar] [CrossRef] [PubMed]
- Igor, Z.; Alexey, L.; Artem, B.; Alexander, K.; Svetlana, P.; Anfisa, B.; Natalia, F.; Ekaterina, K.; Andrey, L.; Xinhong, W. Assessment of seasonal variability of input of microplastics from the Northern Dvina River to the Arctic Ocean. Mar. Pollut. Bull. 2022, 175, 113370. [Google Scholar]
- Wang, Z.; Zhang, Y.; Kang, S.; Yang, L.; Luo, X.; Chen, P.; Guo, J.; Hu, Z.; Yang, C.; Yang, Z. Long-range transport of atmospheric microplastics deposited onto glacier in southeast Tibetan Plateau. Environ. Pollut. 2022, 306, 119415. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, C.; Zhang, Q.; Yao, T. Securing water quality of the Asian Water Tower. Nat. Rev. Earth Environ. 2022, 3, 611–612. [Google Scholar] [CrossRef]
- Barnett, T.P.; Adam, J.C.; Lettenmaier, D.P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 2005, 438, 303. [Google Scholar] [CrossRef]
- Ives, J.; Messerli, B. The Himalayan Dilemma: Reconciling Conservation and Development; Routledge: London, UK; New York, NY, USA, 1989. [Google Scholar]
- Solomon, S.; Qin, D.; Manning, M.; Averyt, K.; Marquis, M. Climate Change 2007-the Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Yang, L.; Luo, W.; Zhao, P.; Zhang, Y.; Kang, S.; Giesy, J.P.; Zhang, F. Microplastics in the Koshi River, a remote alpine river crossing the Himalayas from China to Nepal. Environ. Pollut. 2021, 290, 118121. [Google Scholar] [CrossRef]
- Nicholson, K.; Hayes, E.; Neumann, K.; Dowling, C.; Sharma, S. Drinking Water Quality in the Sagarmatha National Park, Nepal. J. Geosci. Environ. Prot. 2016, 4, 43. [Google Scholar] [CrossRef]
- Nicholson, K.N.; Neumann, K.; Dowling, C.; Sharma, S. E. coli and Coliform Bacteria as Indicators for Drinking Water Quality and Handling of Drinking Water in the Sagarmatha National Park, Nepal. Environ. Manag. Sustain. Dev. 2017, 6, 411–428. [Google Scholar] [CrossRef]
- Gruver, J.; Nicholson, K.; Neumann, K.; Sharma, S.; Dowling, C. Water Quality in the Sagarmatha National Park, Nepal: A Modification of Viable Field-based Testing Methods. Environ. Manag. Sustain. Dev. 2017, 6, 361–372. [Google Scholar] [CrossRef][Green Version]
- Krishnan, R.; Shrestha, A.B.; Ren, G.; Rajbhandari, R.; Saeed, S.; Sanjay, J.; Syed, M.A.; Vellore, R.; Xu, Y.; You, Q. Unravelling climate change in the Hindu Kush Himalaya: Rapid warming in the mountains and increasing extremes. In The Hindu Kush Himalaya Assessment: Mountains, Climate Change, Sustainability and People; Spring: Berlin/Heidelberg, Germany, 2019; pp. 57–97. [Google Scholar]
- Devkota, L.P.; Gyawali, D.R. Impacts of climate change on hydrological regime and water resources management of the Koshi River Basin, Nepal. J. Hydrol. Reg. Stud. 2015, 4, 502–515. [Google Scholar] [CrossRef]
- Paudyal, R.; Kang, S.; Sharma, C.M.; Tripathee, L.; Huang, J.; Rupakheti, D.; Sillanpää, M. Major ions and trace elements of two selected rivers near Everest region, southern Himalayas, Nepal. Environ. Earth Sci. 2016, 75, 46. [Google Scholar] [CrossRef]
- Tripathee, L.; Kang, S.; Sharma, C.M.; Rupakheti, D.; Paudyal, R.; Huang, J.; Sillanpää, M. Preliminary health risk assessment of potentially toxic metals in surface water of the Himalayan Rivers, Nepal. Bull. Environ. Contam. Toxicol. 2016, 97, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef] [PubMed]
- Hylton, L.; Ghezzi, J.; Han, B. Microplastic Pollution in Indiana’s White River: An Exploratory Study. Proc. Indiana Acad.Sci. 2018, 127, 72–81. [Google Scholar]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, M.S.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of sample preparation methods for microplastic analysis in wastewater matrices—Reproducibility and standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and nanoplastics identification: Classic methods and innovative detection techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Neelavannan, K.; Sen, I.S.; Sinha, N.; Thakur, A.K.; Misra, S. Microplastics in the Ganga-Brahmaputra delta: Sources and Pathways to the Sundarbans Biosphere Reserve-an UNESCO World Heritage Centre. Environ. Adv. 2023, 11, 100350. [Google Scholar] [CrossRef]
- Napper, I.E.; Baroth, A.; Barrett, A.C.; Bhola, S.; Chowdhury, G.W.; Davies, B.F.; Duncan, E.M.; Kumar, S.; Nelms, S.E.; Niloy, M.N.H. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021, 274, 116348. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Sillanpää, M. Importance of atmospheric transport for microplastics deposited in remote areas. Environ. Pollut. 2019, 254, 112953. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of microplastics in wastewater samples by means of polarized light optical microscopy. Environ. Sci. Pollut. Res. 2020, 27, 7409–7419. [Google Scholar] [CrossRef]
- Kotar, S.; McNeish, R.; Murphy-Hagan, C.; Renick, V.; Lee, C.-F.T.; Steele, C.; Lusher, A.; Moore, C.; Minor, E.; Schroeder, J. Quantitative assessment of visual microscopy as a tool for microplastic research: Recommendations for improving methods and reporting. Chemosphere 2022, 308, 136449. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Olson, N.E.; Xiao, Y.; Lei, Z.; Ault, A.P. Simultaneous optical photothermal infrared (O-PTIR) and Raman spectroscopy of submicrometer atmospheric particles. Anal. Chem. 2020, 92, 9932–9939. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Yacoub, M.; Li, A.; Nicholson, K.; Gruver, J.; Neumann, K.; Sharma, S. Human Activities Increased Microplastics Contamination in the Himalaya Mountains. Hydrology 2024, 11, 4. https://doi.org/10.3390/hydrology11010004
Han B, Yacoub M, Li A, Nicholson K, Gruver J, Neumann K, Sharma S. Human Activities Increased Microplastics Contamination in the Himalaya Mountains. Hydrology. 2024; 11(1):4. https://doi.org/10.3390/hydrology11010004
Chicago/Turabian StyleHan, Bangshuai, Moayad Yacoub, Aihua Li, Kirsten Nicholson, Joshua Gruver, Klaus Neumann, and Subodh Sharma. 2024. "Human Activities Increased Microplastics Contamination in the Himalaya Mountains" Hydrology 11, no. 1: 4. https://doi.org/10.3390/hydrology11010004
APA StyleHan, B., Yacoub, M., Li, A., Nicholson, K., Gruver, J., Neumann, K., & Sharma, S. (2024). Human Activities Increased Microplastics Contamination in the Himalaya Mountains. Hydrology, 11(1), 4. https://doi.org/10.3390/hydrology11010004












