The Potential of Isotopic Tracers for Precise and Environmentally Clean Stream Discharge Measurements
Abstract
:1. Introduction
2. Materials and Methods
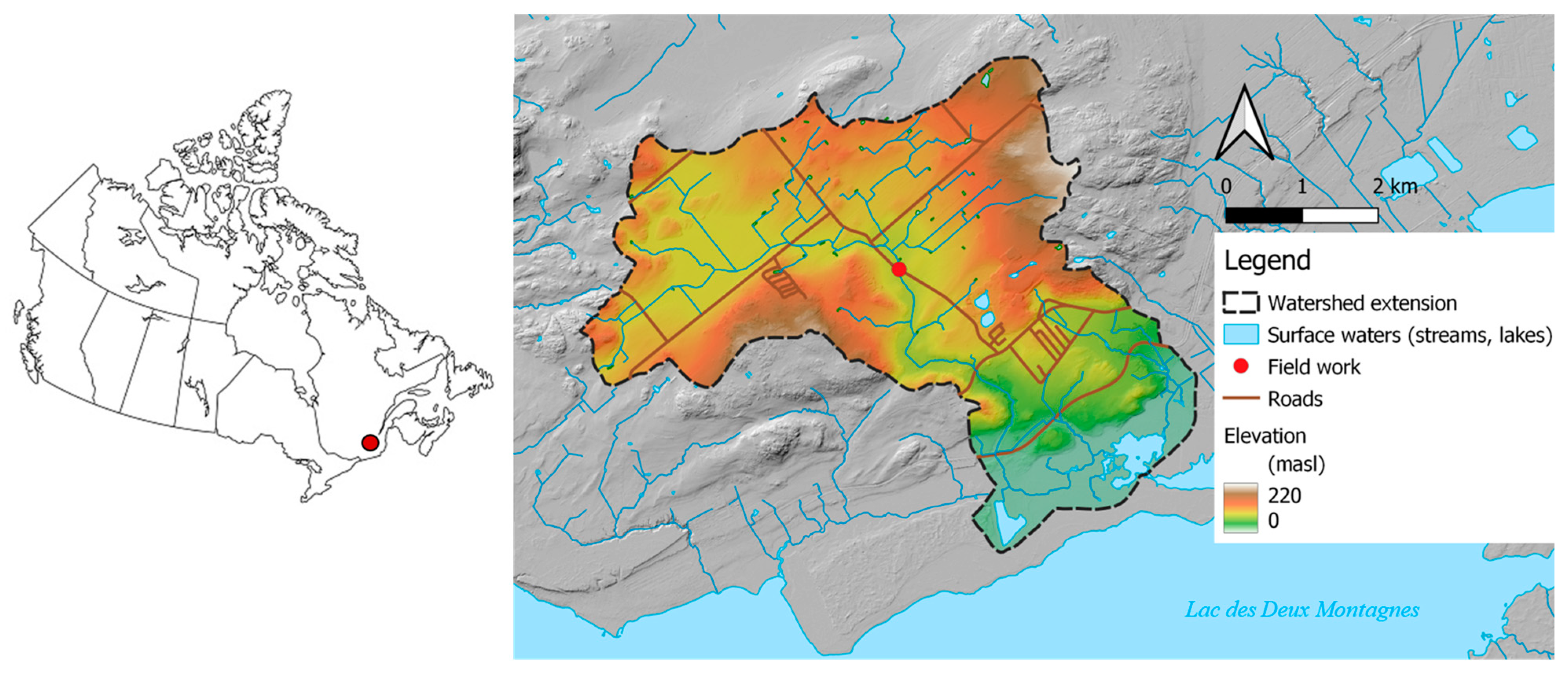
2.1. Description of the Field Site
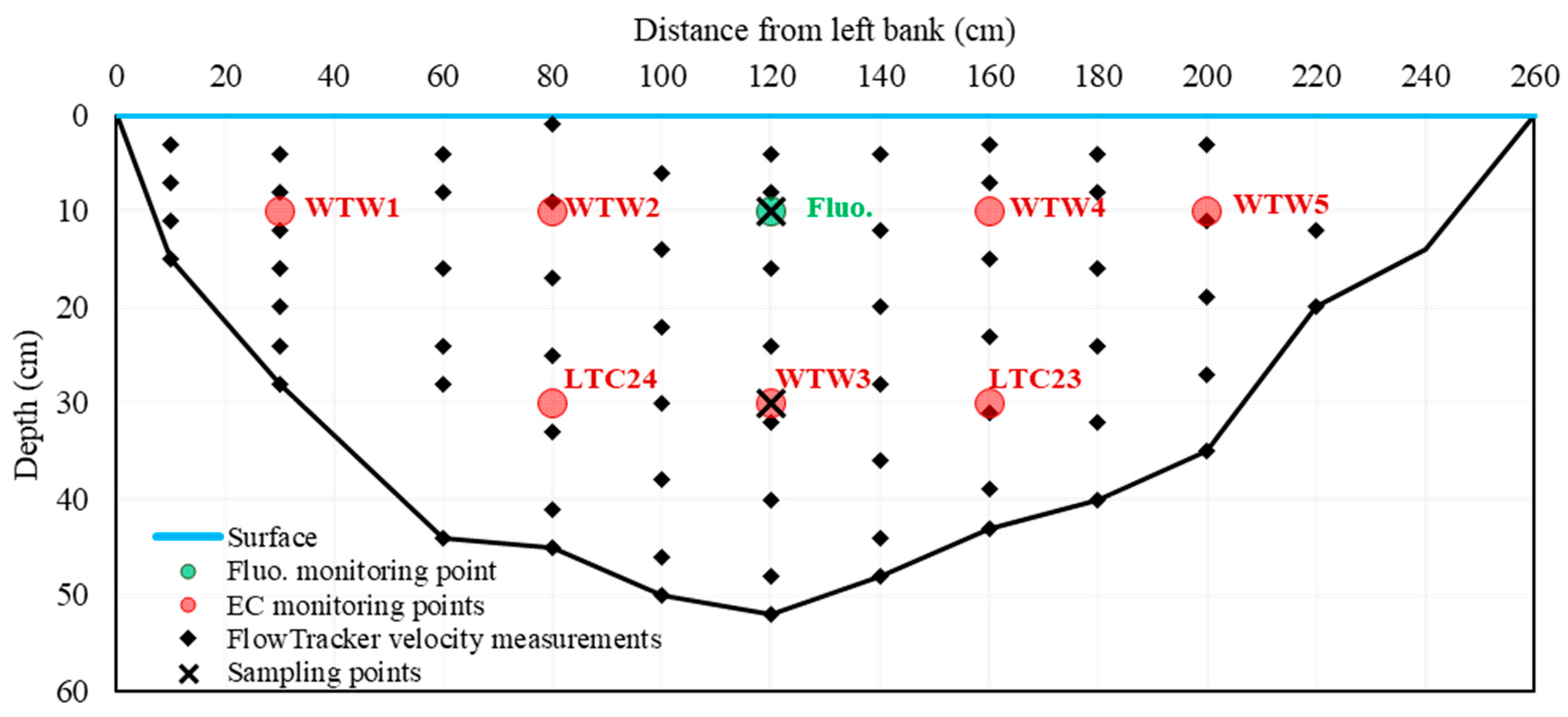
2.2. Field Work
2.3. Analytical Methods
2.4. Data Interpretation
3. Results and Discussion
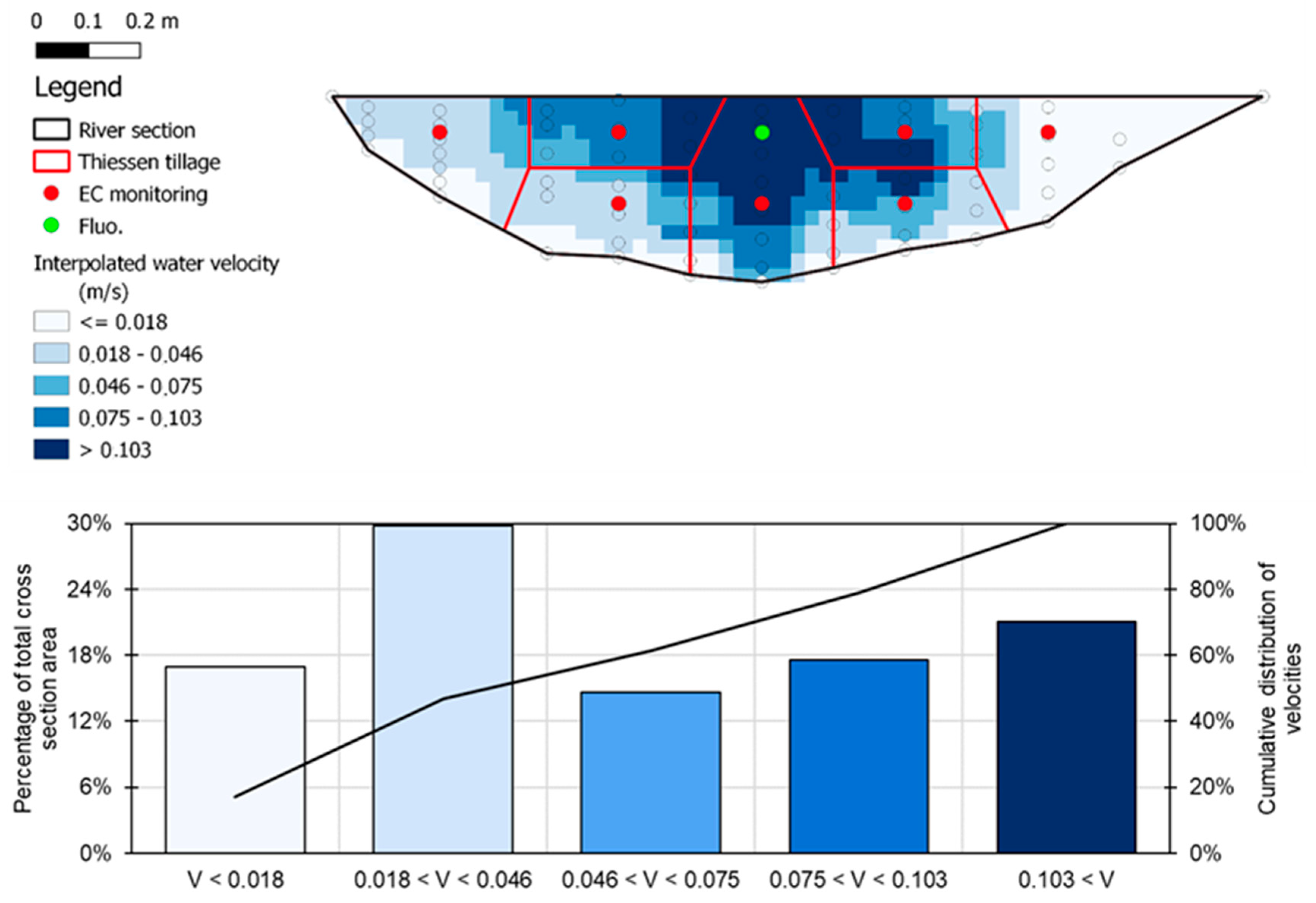
3.1. River Section Water Velocity Distribution
3.2. Using Salt Tracers to Build a Representative Restitution Curve for the Whole Section
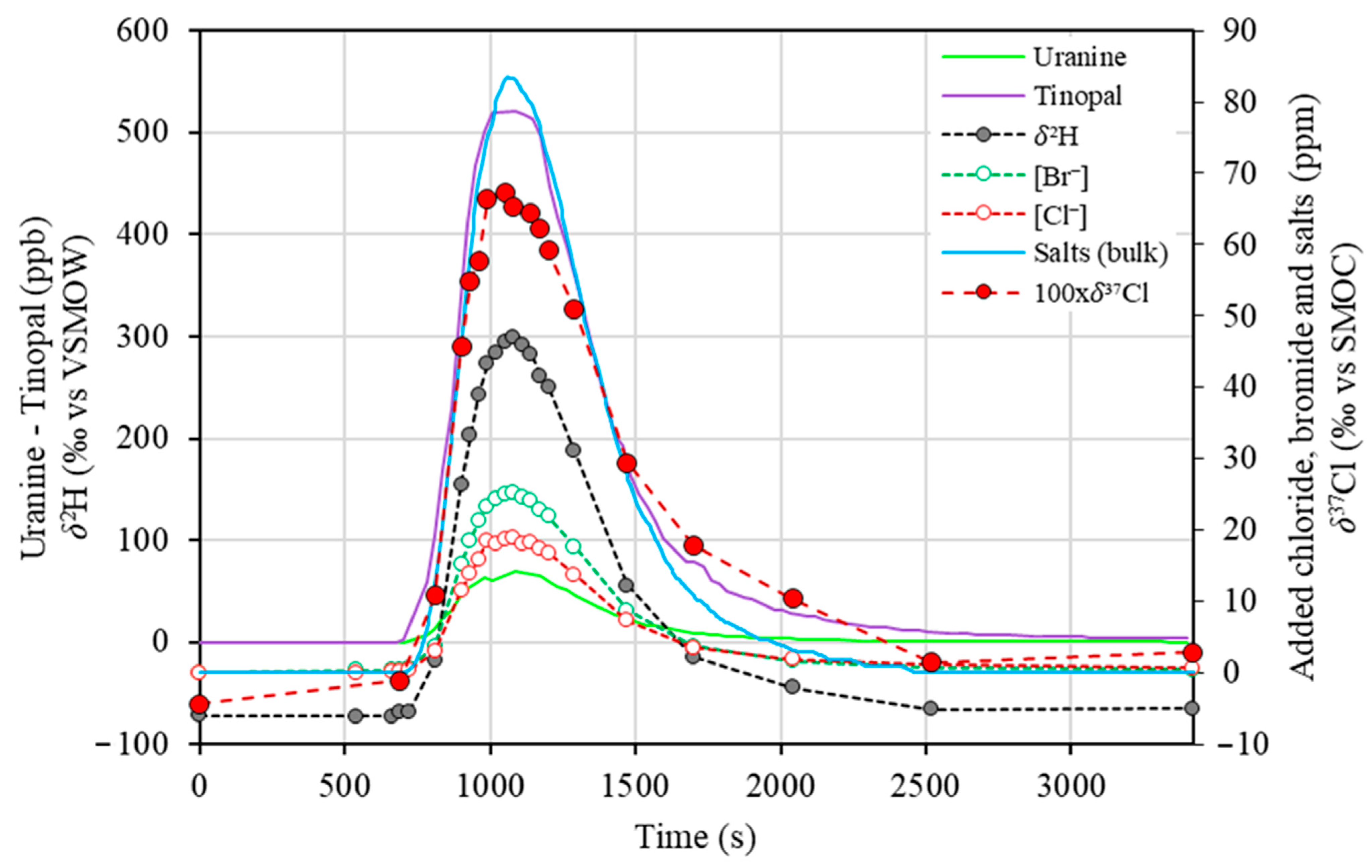
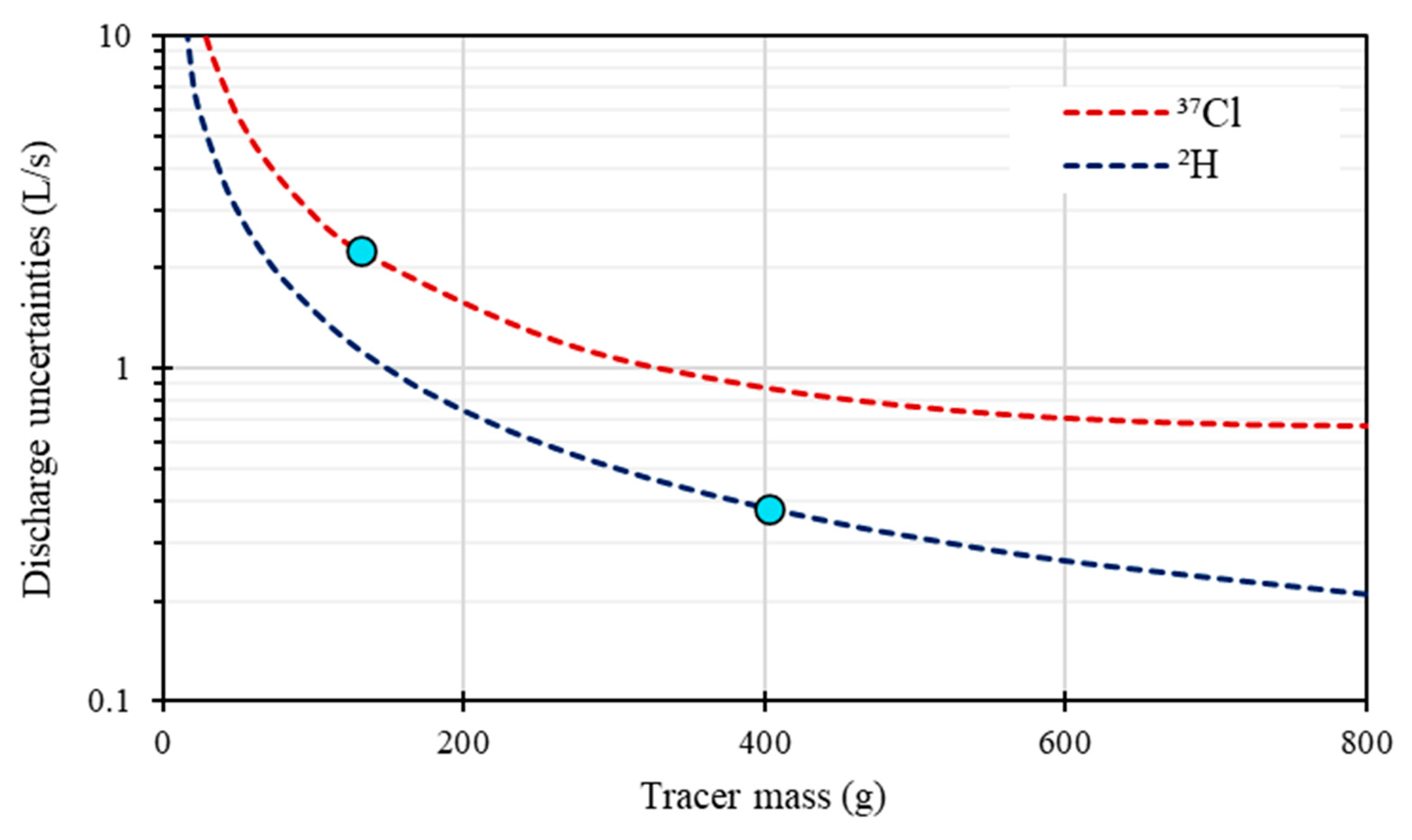
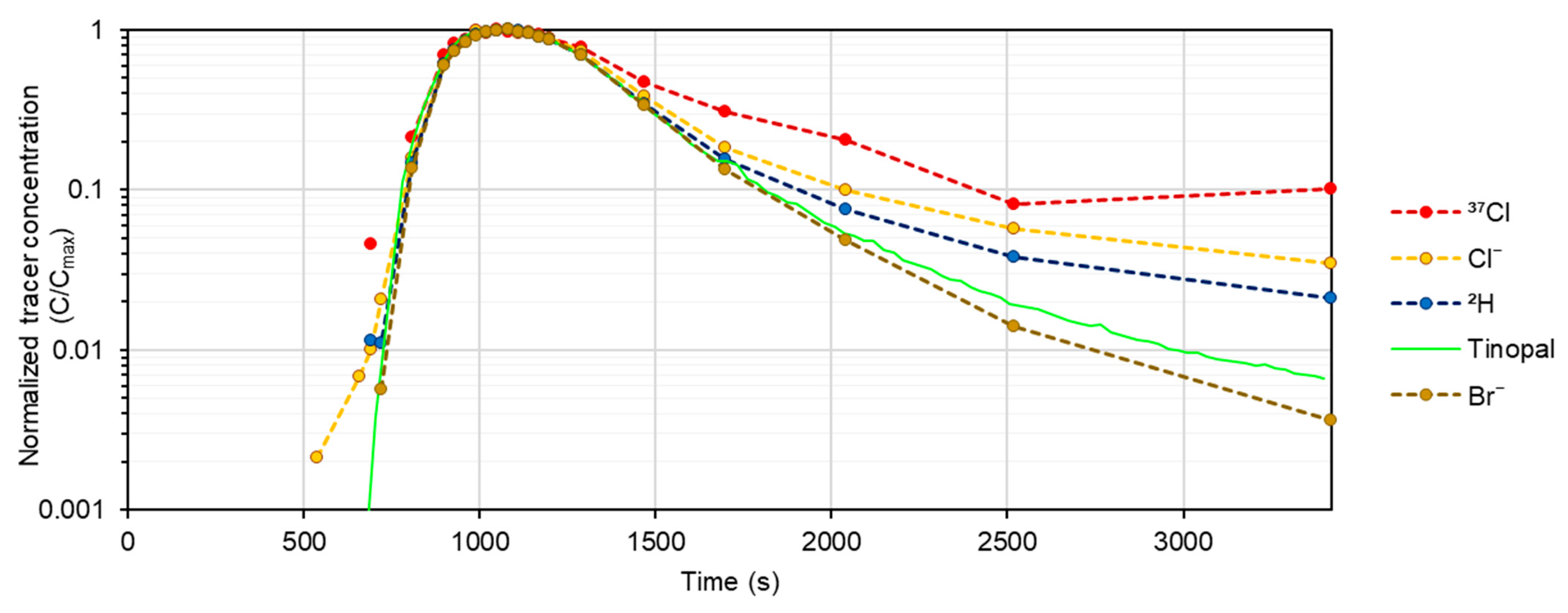
3.3. Isotopic Tracers as New Tracers for Discrete and Clean Discharge Measurements—Comparison with Other Tracers
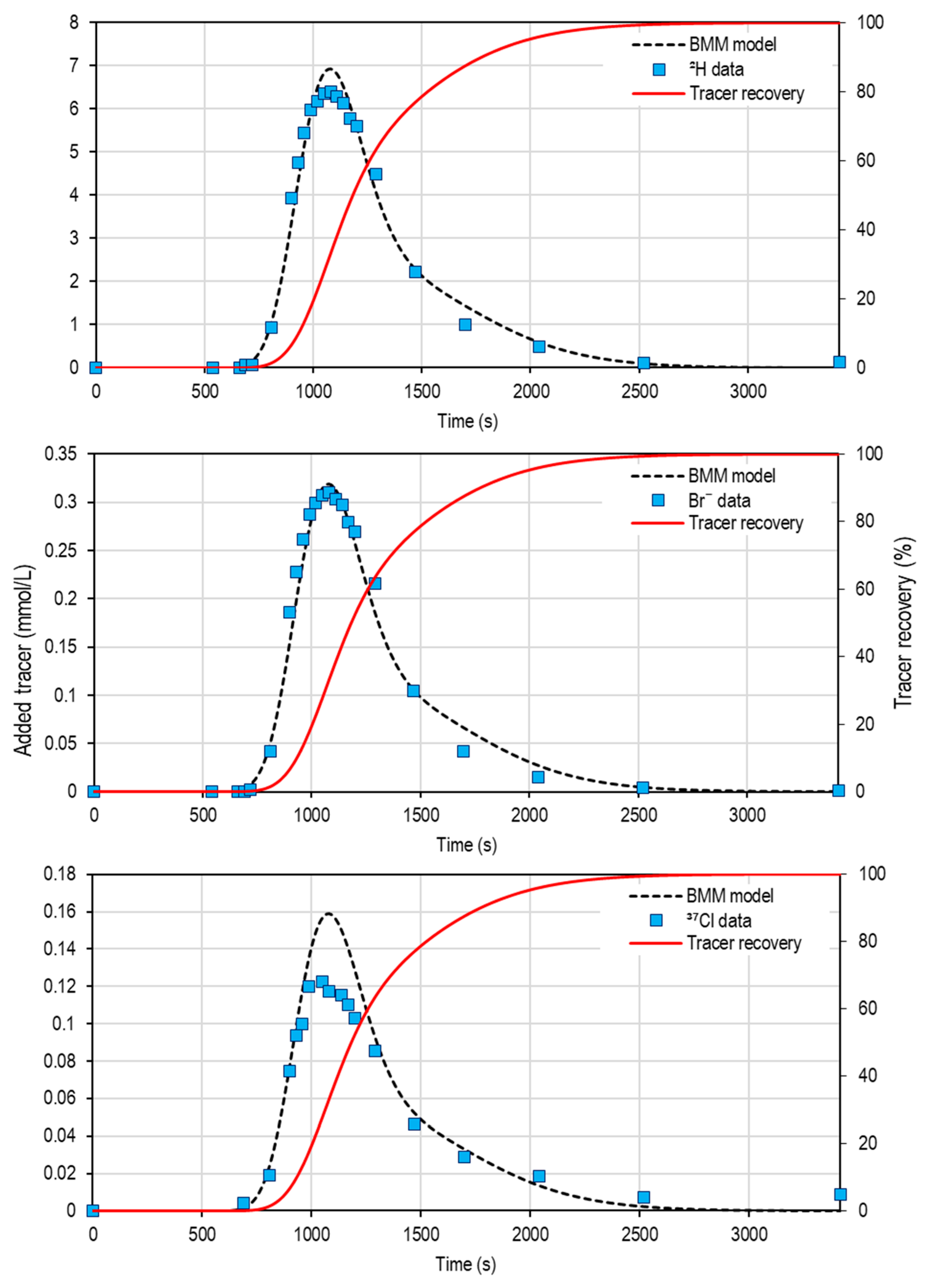
3.4. Transient Storage Zone Exchanges Inferred from Multi-Tracer Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. 2H Calculations
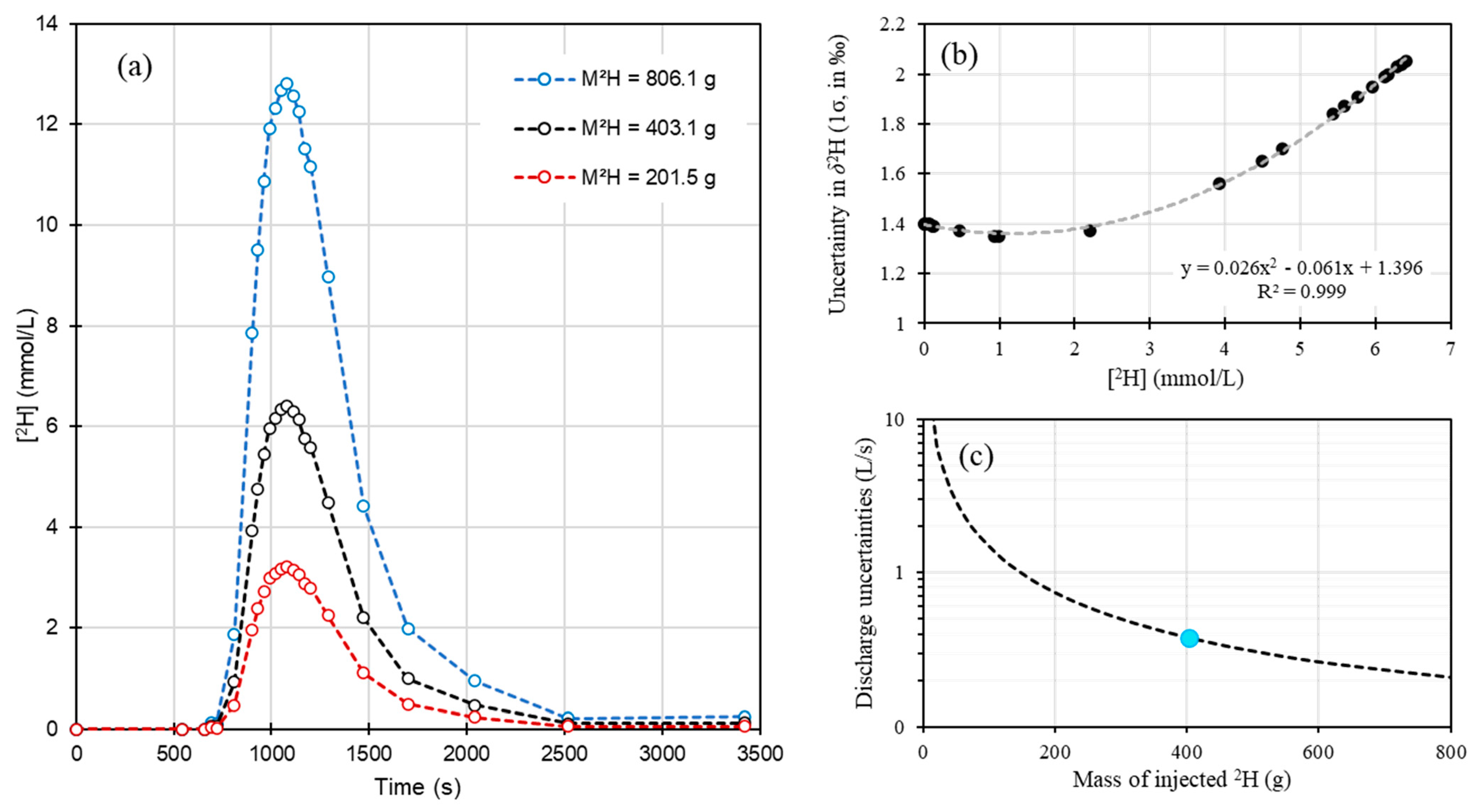
Appendix A.2. 37Cl Calculations
References
- Herschy, R.W. Streamflow Measuremen; Francis, T., Ed.; CRC Press: Boca Raton, FL, USA, 2008; 534p. [Google Scholar]
- WMO. Guide to Hydrological Practice Volume I—From Measurement to Hydrological Information; WMO: Geneva, Switzerland, 2008. [Google Scholar]
- Jensen, C.R.; Genereux, D.P.; Gilmore, T.E.; Solomon, D.K.; Mittelstet, A.R.; Humphrey, C.E.; MacNamara, M.R.; Zeyrek, C.; Zlotnik, V.A. Estimating groundwater mean transit time from SF6 in stream water: Field example and planning metrics for a reach mass-balance approach. Hydrogeol. J. 2022, 30, 479–494. [Google Scholar] [CrossRef]
- Cook, P.G.; Lamontagne, S.; Berhane, D.; Clark, J.F. Quantifying groundwater discharge to Cockburn River, southeastern Australia, using dissolved gas tracers 222Rn and SF6. Water Resour. Res. 2006, 42, 12. [Google Scholar] [CrossRef]
- Le Coz, J.; Camenen, B.; Dramais, G.; Ribot-Bruno, J.; Ferry, M.; Rosique, J.L. Guide Technique Pour le Contrôle des Débits Réglementaires; ONEMA, Cemagref: Vincennes, France, 2011; 132p. [Google Scholar]
- WMO. Manual on Stream Gauging Volume I—Fieldwork; WMO: Geneva, Switzerland, 2010. [Google Scholar]
- Pelletier, P.M. Uncertainties in the single determination of river discharge: A literature review. Can. J. Civ. Eng. 1988, 15, 834–850. [Google Scholar] [CrossRef]
- Tazioli, A. Experimental methods for river discharge measurements: Comparison among tracers and current meter. Hydrol. Sci. J. 2011, 56, 1314–1324. [Google Scholar] [CrossRef]
- Hudson, R.; Fraser, J. The Mass Balance (or Dry Injection) Method. Watershed Manag. Bull. 2005, 9, 6–12. [Google Scholar]
- Winter, T.C.; Harvey, J.W.; Franke, O.L.; Alley, W.M. Ground Water and Surface Water, a Single Resource; Circular 1139; USGS: Reston, VA, USA, 1998; 87p.
- Sophocleous, M. Interactions between groundwater and surface water: The state of the science. Hydrogeol. J. 2002, 10, 52–67. [Google Scholar] [CrossRef]
- Gooseff, M.N. Defining Hyporheic Zones—Advancing Our Conceptual and Operational Definitions of Where Stream Water and Groundwater Meet. Geogr. Compass 2010, 4, 945–955. [Google Scholar] [CrossRef]
- Engelhardt, I.; Piepenbrink, M.; Trauth, N.; Stadler, S.; Kludt, C.; Schulz, M.; Schüth, C.; Ternes, T. Comparison of tracer methods to quantify hydrodynamic exchange within the hyporheic zone. J. Hydrol. 2011, 400, 255–266. [Google Scholar] [CrossRef]
- Beck, H.E.; van Dijk, A.I.J.M.; Miralles, D.G.; de Jeu, R.A.M.; Bruijnzeel, L.A.; McVicar, T.R.; Schellekens, J. Global patterns in base flow index and recession based on streamflow observations from 3394 catchments. Water Resour. Res. 2013, 49, 7843–7863. [Google Scholar] [CrossRef]
- Boano, F.; Harvey, J.W.; Marion, A.; Packman, A.I.; Revelli, R.; Ridolfi, L.; Wörman, A. Hyporheic flow and transport processes: Mechanisms, models, and biogeochemical implications. Rev. Geophys. 2014, 52, 603–679. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Newbold, J.D.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2008, 1, 95–101. [Google Scholar] [CrossRef]
- Runkel, R.L. One-Dimensional Transport with Inflow and Storage (OTIS): A Solute Transport Model for Streams and Rivers; USGS: Denver, CO, USA, 1998.
- van Genuchten, M.T.; Leij, F.J.; Skaggs, T.H.; Toride, N.; Bradford, S.A.; Pontedeiro, E.M. Exact Analytical Solutions for Contaminant Transport in Rivers. 2. Transient storage and decay chain solutions. J. Hydrol. Hydromech. 2013, 61, 250–259. [Google Scholar] [CrossRef]
- van Genuchten, M.T.; Leij, F.J.; Skaggs, T.H.; Toride, N.; Bradford, S.A.; Pontedeiro, E.M. Exact analytical solutions for contaminant transport in rivers. 1. The equilibrium advection-dispersion equation. J. Hydrol. Hydromech. 2013, 61, 146–160. [Google Scholar] [CrossRef]
- De Smedt, F. Analytical solution and analysis of solute transport in rivers affected by diffusive transfer in the hyporheic zone. J. Hydrol. 2007, 339, 29–38. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E. Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. J. Appl. Ecol. 2007, 44, 1089–1094. [Google Scholar] [CrossRef]
- Verhoeven, J.T.; Arheimer, B.; Yin, C.; Hefting, M. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol. 2006, 21, 96–103. [Google Scholar] [CrossRef]
- Barlow, P.M.; Leake, S.A. Streamflow Depletion by Wells—Understanding and Managing the Effects of Groundwater Pumping on Streamflow; USGS: Reston, VA, USA, 2012; 95p.
- Wood, P.J.; Dykes, A.P. The use of salt dilution gauging techniques: Ecological considerations and insights. Water Res. 2002, 36, 3054–3062. [Google Scholar] [CrossRef]
- Leibundgut, C.; Maloszewski, P.; Külls, C. Tracers in Hydrology; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- SIGEOM. Available online: https://sigeom.mines.gouv.qc.ca/ (accessed on 23 October 2023).
- Kaufman, R.S.; Long, A.; Bentley, H.; Davis, S. Natural chlorine isotope variations. Nature 1984, 209, 338–340. [Google Scholar] [CrossRef]
- Eggenkamp, H.G.M. The Geochemistry of Stable Chlorine and Bromine Isotopes; Utrecht University: Utrecht, The Netherlands, 1994. [Google Scholar]
- Godon, A.; Jendrzejewski, N.; Eggenkamp, H.G.; Banks, D.A.; Ader, M.; Coleman, M.L.; Pineau, F. A cross-calibration of chlorine isotopic measurements and suitability of seawater as the international reference material. Chem. Geol. 2004, 207, 1–12. [Google Scholar] [CrossRef]
- Jurgens, B.C.; Böhlke, J.K.; Eberts, S.M. TracerLPM (Version 1): An Excel Workbook for Interpreting Groundwater Age Distributions from Environmental Tracer Data; USGS: Reston, VA, USA, 2012; 72p.
- Runkel, R.L.; Chapra, S.C. An efficient numerical solution of the transient storage equations for solute transport in small streams. Water Resour. Res. 1993, 29, 211–215. [Google Scholar] [CrossRef]
- Briggs, M.A.; Gooseff, M.N.; Arp, C.D.; Baker, M.A. A method for estimating surface transient storage parameters for streams with concurrent hyporheic storage. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef]
- Cook, P.G.; Herczeg, A.L. Environmental Tracers in Subsurface Hydrology; Springer Science: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Clark, I.D. Groundwater Geochemistry and Isotopes; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Licha, T.; Niedbala, A.; Bozau, E.; Geyer, T. An assessment of selected properties of the fluorescent tracer, Tinopal CBS-X related to conservative behavior, and suggested improvements. J. Hydrol. 2013, 484, 38–44. [Google Scholar] [CrossRef]
- Schiperski, F.; Oertwich, M.; Scheytt, T.; Licha, T. Solubility of TINOPAL CBS-X fluorescent dye at different EDTA concentrations and pH values: Implications regarding its applicability in field tracer tests. J. Hydrol. 2019, 578, 124025. [Google Scholar] [CrossRef]


| Measuring Point | Tracer Used | Discharge (L/s) | Uncertainties (L/s—%) | f (% of Total Flow) |
|---|---|---|---|---|
| FlowTracker | Velocity method | 50.2 | 3.6–7.2 | Not applicable |
| WTW1 | Electrical conductivity | 56.5 | 1.8–3.2 | 8.2 |
| WTW2 | Electrical conductivity | 56.9 | 1.3–2.3 | 17.4 |
| WTW3 | Electrical conductivity | 55.3 | 1.3–2.3 | 34.2 |
| WTW4 | Electrical conductivity | 52.6 | 1.8–3.4 | 16.5 |
| WTW5 | Electrical conductivity | 53.8 | 1.4–2.6 | 3.3 |
| LTC 23 | Electrical conductivity | 49.8 | 1.1–2.2 | 11.4 |
| LTC 24 | Electrical conductivity | 51.5 | 1.2–2.2 | 9.1 |
| Composite restitution | Electrical conductivity | 53.3 | 6.5–12.2 | Not applicable |
| WTW3 | 2H | 51.1 | 0.4–0.7 | Not applicable |
| Fluo. | Tinopal | 53.0 | 0.5–1.0 | Not applicable |
| Fluo. | Uranine | 60.6 | 0.8–1.4 | Not applicable |
| Fluo. | 37Cl | 39.4 | 2.3–5.7 | Not applicable |
| Fluo. | Cl− | 41.3 | Unknown | Not applicable |
| Fluo. | Br− | 54.7 | Unknown | Not applicable |
| g1 Parameters | g2 Parameters | |||||
|---|---|---|---|---|---|---|
| Tracer | f1 | D1 (m2/s) | v1 (m/s) | D2 (m2/s) | v2 (m/s) | χ2 (mol/L) |
| 2H | 0.10 | 0.097 | 0.20 | 0.068 | 9.5 × 10−4 | |
| 37Cl | 0.58 | 0.13 | 0.097 | 0.39 | 0.053 | 7.5 × 10−5 |
| Br− | 0.10 | 0.097 | 0.26 | 0.070 | 6.2 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picard, A.; Barbecot, F.; Bardoux, G.; Agrinier, P.; Gillon, M.; Corcho Alvarado, J.A.; Schneider, V.; Hélie, J.-F.; de Oliveira, F. The Potential of Isotopic Tracers for Precise and Environmentally Clean Stream Discharge Measurements. Hydrology 2024, 11, 1. https://doi.org/10.3390/hydrology11010001
Picard A, Barbecot F, Bardoux G, Agrinier P, Gillon M, Corcho Alvarado JA, Schneider V, Hélie J-F, de Oliveira F. The Potential of Isotopic Tracers for Precise and Environmentally Clean Stream Discharge Measurements. Hydrology. 2024; 11(1):1. https://doi.org/10.3390/hydrology11010001
Chicago/Turabian StylePicard, Antoine, Florent Barbecot, Gérard Bardoux, Pierre Agrinier, Marina Gillon, José A. Corcho Alvarado, Vincent Schneider, Jean-François Hélie, and Frédérick de Oliveira. 2024. "The Potential of Isotopic Tracers for Precise and Environmentally Clean Stream Discharge Measurements" Hydrology 11, no. 1: 1. https://doi.org/10.3390/hydrology11010001
APA StylePicard, A., Barbecot, F., Bardoux, G., Agrinier, P., Gillon, M., Corcho Alvarado, J. A., Schneider, V., Hélie, J.-F., & de Oliveira, F. (2024). The Potential of Isotopic Tracers for Precise and Environmentally Clean Stream Discharge Measurements. Hydrology, 11(1), 1. https://doi.org/10.3390/hydrology11010001







