Abstract
Recent advancements in the field of room-temperature ferromagnetic metal oxide semiconductors (RTFMOS) have revealed their promising potential for enhancing photocatalytic performance. This review delves into the combined investigation of the photocatalytic and ferromagnetic properties at room temperature, with a particular focus on metal oxides like TiO2, which have emerged as pivotal materials in the fields of magnetism and environmental remediation. Despite extensive research efforts, the precise mechanism governing the interplay between ferromagnetism and photocatalysis in these materials remains only partially understood. Several crucial factors contributing to magnetism, such as oxygen vacancies and various metal dopants, have been identified. Numerous studies have highlighted the significant role of these factors in driving room-temperature ferromagnetism and photocatalytic activity in wide-bandgap metal oxides. However, establishing a direct correlation between magnetism, oxygen vacancies, dopant concentration, and photocatalysis has posed significant challenges. These RTFMOS hold immense potential to significantly boost photocatalytic efficiency, offering promising solutions for diverse environmental- and energy-related applications, including water purification, air pollution control, and solar energy conversion. This review aims to offer a comprehensive overview of recent advancements in understanding the magnetism and photocatalytic behavior of metal oxides. By synthesizing the latest findings, this study sheds light on the considerable promise of RTFMOS as effective photocatalysts, thus contributing to advancements in environmental remediation and related fields.
1. Introduction
In recent years, there has been a heightened pursuit of sustainable and eco-friendly technologies, leading to substantial research endeavors aimed at developing advanced materials with multifunctional capabilities. Among these materials, room-temperature ferromagnetic metal oxide semiconductors (RTFMOS) have emerged as a promising class, demonstrating potential applications in both magnetic and photocatalytic fields [1,2,3,4,5,6]. RTFMOS plays a pivotal role in advancing various technological domains, spanning from spintronics to magnetic data storage. Their significance lies in their ability to manifest ferromagnetism at ambient temperatures, streamlining device fabrication and operational processes. RTFMOS offers a pathway toward energy-efficient electronics by facilitating low power consumption and high-speed processing. Furthermore, their compatibility with existing semiconductor manufacturing processes positions them as promising candidates for next-generation electronic and spin-based devices, thus opening avenues for innovative applications in computing, communication, and sensing technologies.
The global Imperative for sustainable technologies has catalyzed a surge in research focused on materials endowed with multifaceted capabilities, aimed at confronting environmental adversities and amplifying energy conservation efforts. This focus has emphasized the significance of ferromagnetic metal oxide semiconductors (FMOS) as promising candidates for sustainable technology solutions. FMOS exhibit unique characteristics that render them highly desirable for various applications, including their capacity to maintain ferromagnetism at room temperature. This attribute not only simplifies device fabrication and operation but also aligns to reduce energy consumption in electronics. By integrating FMOS into electronic devices, such as spintronics and magnetic data storage systems, we can progress toward a more sustainable future by developing energy-efficient technologies with minimal environmental impact. Additionally, the intriguing combination of ferromagnetism and photocatalysis within metal oxide semiconductors (MOS) has garnered significant interest due to its potential to address pressing environmental issues, particularly related to environmental restoration efforts [7]. The process of photocatalysis—harnessing light energy to drive catalytic transformations—offers an environmentally conscious approach to the breakdown of contaminants in both water and air. Simultaneously, the manifestation of room-temperature ferromagnetism introduces a spectrum of potential technological applications, spanning spintronics and magnetic storage devices [8,9,10,11].
Moreover, the following are also considered:
- The amalgamation of these attributes not only extends the functionality of MOS but also stimulates the development of innovative avenues in multifunctional material design;
- The intersection of the ferromagnetic and photocatalytic properties of MOS necessitates sophisticated characterization techniques to unravel the underlying mechanisms and interactions, fostering deeper insights for optimized applications;
- MOS exhibiting dual ferromagnetism and photocatalysis broaden the horizons of pollutant-removal strategies, enabling simultaneous catalytic activity and pollutant adsorption for enhanced purification efficiency [12];
- The intricate interplay between ferromagnetism and photocatalysis in MOS can be harnessed for advanced water treatment systems in which contaminants can be effectively removed while exploiting the material’s magnetic responsiveness for facile separation and recovery [13,14,15];
- Further research into the fundamental principles governing the coexistence of these phenomena could pave the way for tailored MOS hybrids with tunable functionalities, offering versatile solutions across fields spanning environmental science to electronics [16].
This comprehensive review delves into the recent advancements made in the domain of RTFMOS and their implications for enhanced photocatalytic performance [17]. With a primary focus on metal oxides like titanium dioxide (TiO2) that stand at the forefront of this exciting field, the review aims to shed light on the intriguing interplay between magnetism and photocatalysis in these materials.
Despite decades of research, the exact mechanisms governing the coexistence of ferromagnetism and photocatalytic properties in metal oxides remain only partially understood [18]. Several key factors, such as oxygen vacancies and metal doping, have been identified as crucial contributors to the observed room-temperature ferromagnetism and photocatalytic behavior [19]. However, a clear and direct correlation between these factors and the enhancement of photocatalytic performance has yet to be fully established [20,21].
The synergistic investigation of photocatalytic and ferromagnetic properties at room temperature presents a compelling avenue of research with promising implications across multiple fields. This combined study involves the exploration of materials possessing both photocatalytic capabilities and ferromagnetic behavior without the need for extreme conditions, thus enabling practical applications. The photocatalytic prowess of such materials allows for efficient light-driven catalysis, promoting environmentally friendly energy conversion and pollutant degradation [22]. Concurrently, their ferromagnetic attributes open doors to spintronic applications, including data storage and sensing [23]. The interplay between these two distinct yet interconnected properties holds the potential to revolutionize fields like sustainable energy, environmental remediation, and information technology, paving the way for innovative technologies and a more sustainable future [24,25].
Throughout this review, we delve into the latest findings and key insights that have propelled advancements in understanding the magnetism and photocatalytic attributes of MOS, particularly under visible light illumination. Our objectives encompass a comprehensive analysis of the existing body of knowledge to offer valuable insights into the potential applications of RTFMOS for environmental remediation and related fields. In subsequent sections, we aim to explore the mechanisms underlying the interplay of ferromagnetism and photocatalysis, shed light on the roles of oxygen vacancies and metal doping, and discuss both the challenges and opportunities in harnessing these materials for enhanced photocatalytic performance. Ultimately, this review endeavors to contribute to the ongoing efforts in developing cutting-edge technologies that pave the way for a cleaner and more sustainable future.
2. Advancements in Metal Oxide-Based Semiconductor Manipulation
Recent years have witnessed remarkable advancements in the manipulation of oxide-based semiconductors, leading to a transformative era in electronics and materials science [26]. Oxide-based semiconductors, with their diverse electronic, optical, and magnetic properties, have gained prominence as crucial components in various applications [27]. Through precise engineering at the atomic level, researchers have achieved unprecedented control over the properties of these materials, tailoring their bandgap, conductivity, and even catalytic activity. The emergence of techniques such as epitaxial growth, strain engineering, and doping strategies has enabled the creation of designer interfaces and heterostructures, leading to novel functionalities and enhanced performance [28]. Moreover, the integration of oxide-based semiconductors into flexible and transparent devices has expanded their range of application into wearable electronics and displays. As these advancements continue, the boundaries of what is achievable with oxide-based semiconductors are continuously pushed, promising breakthroughs in fields ranging from energy harvesting and storage to quantum computing and beyond [29].
The use of tiny MOS particles to clean up the environment can be good and bad for nature [30,31]. On the bright side, these particles, especially ones like TiO2 and zinc oxide (ZnO), can break down pollutants well when they are exposed to light [32,33]. This helps in the cleanup of polluted water and air by reducing the levels of pollutants in them, like chemicals, heavy metals, and germs. But there are also concerns about the use of MOS particles [34,35]. One major concern is that if we do not handle them properly, they may build up in the environment [36]. If this happens, they can accumulate in soil, water, and living things, which can cause problems for nature. Also, we are not sure of what may happen over the long run if animals, plants, and tiny organisms are exposed to these particles. We need to conduct more studies to understand the risks better and make sure we use MOS particles safely in cleaning up the environment. Another thing to think about is that making and getting rid of MOS particles can use up large amounts of energy and create waste [37]. If we are not careful, this could add to existing levels of air and water pollution and make climate change worse. So, while MOS particles can help clean up the environment, we need to be really careful about how we use them to make sure we do more good than harm to nature.
2.1. Tunability of Wide-Bandgap MOS Properties by Defect Engineering
The intriguing convergence of ferromagnetism and photocatalysis within MOS has ignited widespread interest due to their potential for addressing environmental challenges and enabling advanced technologies, including the following notable examples.
Defect Engineering for Tuning Properties: The manipulation of defect concentrations offers a versatile means to tailor MOS properties. By controlling defect levels, researchers can exert a profound influence over various aspects, such as photon absorption, emission energies, and even intrinsic magnetism, within MOS compounds [3]. This level of control provides a dynamic platform for customizing material behaviors to suit specific applications, spanning from photocatalysis to magnetics.
Defect-Related Absorption Spectra Tuning: The intricate interplay between defects and the electronic band structure leads to defect-related absorption spectra tuning. This phenomenon has become a cornerstone of innovative applications, including the design of light-emitting diodes (LEDs), opto-magnetic devices, and even tuneable oxide-based materials [4,5,38]. The ability to engineer defect-induced absorption features empowers researchers to craft materials with tailored optical and magnetic functionalities.
Versatility of d0-Magnetism: A distinguishing characteristic of these MOS materials is their d0-magnetism, wherein their lack of partially filled d orbitals challenges conventional magnetic models [36,39]. This unique property enhances their versatility across different applications, ranging from catalysis and sensors to spintronic and optoelectronic devices [40]. The discovery of d0-magnetism has uncovered a new paradigm in materials science and widened the scope of possible applications for these intriguing materials.
Significance of N-Type and P-Type MOS Models: Recent research underscores the significance of the n-type and p-type MOS models in various applications [41]. The distinct electronic characteristics of these models offer diverse avenues for tailoring material responses. N-type MOS materials, which are rich in electrons, are promising candidates for enhanced photocatalytic activity and charge transport, while p-type MOS materials, with electron deficiencies, offer intriguing possibilities for novel magnetic behaviors and spintronic applications. Exploring the capabilities of both models enriches our understanding of their potential roles in the technological landscape [6,42].
Prospects for Advanced Technological Innovations: The capacity to engineer broad bandgap MOS properties by defect engineering is a frontier with vast potential for innovative technological advancements. As researchers delve deeper into the intricate mechanisms that link defects to material behaviors, new avenues for functional materials emerge [43]. The ability to fine tune electronic, optical, and magnetic properties opens up exciting possibilities for applications spanning fields such as energy conversion, environmental remediation, data storage, and beyond. These prospects not only fuel the curiosity of scientific exploration but also inspire the development of transformative technologies that can reshape industries and impact society on a global scale.
The convergence of defect engineering, ferromagnetism, and photocatalysis within wide-bandgap MOS materials presents a captivating arena for exploration and innovation. By harnessing the power of defects to tailor material properties, researchers are poised to unlock a plethora of applications that harness the unique electronic and magnetic behaviors of these materials. This dynamic interplay between defects and properties catalyzes advanced technological innovations, shaping a future where materials are designed with precision to meet the demands of a rapidly evolving world.
2.2. Harnessing MOS Nanoparticles for Unique Properties
MOS nanoparticles (NPs) present a captivating platform for harnessing exceptional properties that span various applications and industries. These nanoparticles, with their distinct characteristics, offer a diverse range of possibilities:
Versatile Property Manipulation: Metal oxide semiconductor NPs allow for the precise tuning of properties, ranging from bandgap to surface chemistry and charge carrier dynamics. This tunability empowers researchers to craft materials that precisely match specific needs, making them invaluable in tailoring materials for desired functionalities across domains [44].
Enhanced Optical Properties: The size and composition-dependent optical behaviors exhibited by metal oxide semiconductor NPs open doors to applications in sensors, displays, and optoelectronics [45]. Through meticulous control of their dimensions, these NPs can be engineered to emit, absorb, or scatter light in unique ways, enabling advancements in technologies such as light-emitting diodes, photodetectors, and optical sensors [46].
Efficient Catalysis: Leveraging their high surface area and tailored reactivity, metal oxide semiconductor NPs emerge as exceptional catalysts. They facilitate a broad spectrum of chemical reactions, from environmental clean-up and pollution mitigation to fuel cell efficiency enhancement. The ability to accelerate reactions at the nanoscale makes these NPs crucial components in addressing global sustainability challenges [47].
Advanced Energy Technologies: Metal oxide semiconductors NPs are instrumental in improving energy storage and conversion devices. Their integration into batteries, supercapacitors, and solar cells enhances overall performance and efficiency. By optimizing charge transport and recombination dynamics, these NPs contribute to the development of more sustainable and powerful energy solutions [48].
Biomaterials and Medicine: Surface engineering of metal oxide semiconductor NPs enables their seamless integration into biomedical applications. They find roles in drug delivery systems, targeted therapies, noninvasive imaging, and diagnostics. Their biocompatibility and tuneable properties open doors to innovative solutions in healthcare and medical technologies [49].
Nanoelectronics: Metal oxide semiconductor NPs Bridge the gap between traditional semiconductors and the nanoscale realm. This convergence facilitates innovations in nanoelectronics, enabling the development of novel electronic devices, memory technologies, and quantum computing components [27].
Environmental Remediation: One of the standout features of metal oxide semiconductor NPs is their photocatalytic prowess. These NPs can harness solar energy to drive pollutant degradation processes, offering a sustainable solution for ecofriendly water and air purification. This capability is poised to transform how we approach environmental remediation and tackle pollution challenges [50].
The manipulation of metal oxide semiconductor NPs unlocks a treasure trove of unique properties with immense potential across industries. Their adaptability, coupled with continuous research, promises transformative breakthroughs in technology, energy, healthcare, and sustainability. As our understanding of their behavior deepens and our engineering capabilities expand, the applications of these nanoparticles are poised to shape the trajectory of technological advancements in the years to come.
2.2.1. MOS Nanoparticles and Their Multifaceted Attributes
MOS nanoparticles (NPs), which include materials like TiO2, ZnO, and SnO2, have garnered significant attention for their intriguing blend of magnetic and charge transport properties [9,10], and the following are notable examples.
TiO2’s Special Significance: Among MOS NPs, titanium dioxide (TiO2) holds a distinctive position due to its solid photocatalytic behavior and a plethora of advantages. These include affordability, exceptional chemical stability, and a high refractive index. The remarkable photocatalytic activity of TiO2 has led to its widespread use in environmental remediation and self-cleaning surfaces.
Addressing UV Limitations: A significant challenge associated with TiO2 is its reliance on ultraviolet (UV) light for photoexcitation, limiting its effectiveness under visible light. To overcome this limitation, researchers have devised strategies such as doping, co-doping, and surface grafting to enhance TiO2’s photo-absorption capability. These modifications extend the photocatalytic activity of TiO2 to the visible light spectrum, unlocking new possibilities for solar-driven applications [9,10,11,12,51].
Structural Diversity: The versatility of MOS composite nanomaterial structures adds a new dimension to their properties and applications. Configurations like core–shell, matrix-dispersed, Janus, and shell–core–shell arrangements (Figure 1) provide opportunities to enhance specific attributes. These engineered structures enable the fine-tuning of properties such as charge separation efficiency, catalytic activity, and even magnetic behavior [18,19,26,27,51]. This diversity in structure offers a playground for tailoring materials to meet specific requirements, driving innovation across multiple disciplines.
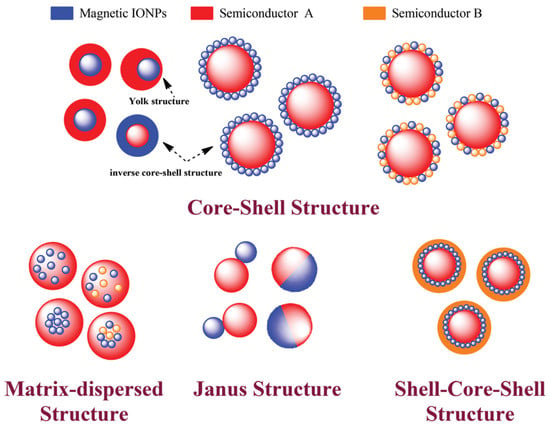
Figure 1.
The diverse architectures of magnetic MOS composite materials, highlighting the presence of magnetic MOS NPs (blue spheres) embedded within nonmagnetic matrices and secondary materials [8].
Exploring the Magnetic–Transport Interplay: The amalgamation of magnetic and charge transport properties within MOS NPs fuels novel scientific inquiries and applications. This interplay paves the way for multifunctional materials that can simultaneously respond to external stimuli, exhibit unique magnetic behaviors, and partake in energy conversion processes. The convergence of these attributes opens up innovative avenues for energy-efficient technologies, catalysis, and sensing.
Beyond Photocatalysis: While photocatalysis is a prominent domain of application for MOS NPs, their magnetic and electronic attributes extend their utility to diverse realms. These nanoparticles hold promise in spintronics, magnetic sensors, data storage, and even as building blocks for quantum technologies. The ability to manipulate both charge and magnetic properties introduces a level of versatility that widens their scope far beyond their traditional roles.
Future Frontiers: As the field of MOS nanoparticles advances, researchers continue to explore uncharted territories. The multifaceted attributes of these nanoparticles create a rich landscape for interdisciplinary research, driving collaborations between materials scientists, chemists, physicists, and engineers. The ongoing exploration of novel structures, enhanced properties, and multifunctional applications ensures that the journey of MOS nanoparticles remains a captivating and transformative one.
2.2.2. Impact of Sn Doping on MOS Properties
Bandgap Modification through Doping: The introduction of metal dopants into TiO2 nanoparticles can exert a profound impact on their electronic properties. Sn doping, in particular, offers a means to modify the bandgap of TiO2, leading to enhanced charge migration and shifts in photo-absorption spectra. This bandgap engineering opens pathways for improved photocatalytic performance and increased efficiency in energy conversion processes.
SnO2-TiO2 Hybrid System: The hybridization of TiO2 with another metal oxide, such as SnO2, presents an intriguing opportunity to enhance the photocatalytic activity. In the case of [SnxTi1−xO2] hybrid nanocomposites, the synergistic interaction between SnO2 and TiO2 creates a platform where charge separation and catalytic processes are optimized. This enhancement can result in improved solar energy utilization and more efficient degradation of pollutants [52,53].
Synthesis Challenges and Influences: The successful synthesis of SnO2-TiO2 nanocomposites hinges on precise control over experimental parameters. Hydrothermal methods are often employed to fabricate these nanocomposites; however, challenges arise in preventing the formation of undesirable secondary phases. Achieving a well-defined SnO2-TiO2 hybrid structure requires careful manipulation of precursor concentrations, reaction temperatures, and growth times [54,55]. The intricacies of the synthesis underscore the importance of mastering materials engineering for tailoring desired properties.
Applications of Sn-Doped TiO2 NPs: Sn-doped TiO2 nanoparticles have exhibited improvements across diverse applications, solidifying their role as versatile materials. In the realm of photocatalysis, their enhanced charge carrier dynamics and modified band structures contribute to more efficient pollutant degradation and hydrogen generation [56,57]. Additionally, Sn-doped TiO2 NPs find utility in energy storage technologies, including batteries and supercapacitors, in which their improved charge transport properties enhance the overall performance [58,59]. Furthermore, their application extends to solar cells, in which the modified bandgap facilitates better light absorption and electron–hole separation, leading to enhanced photovoltaic efficiency [60,61].
Revealing New Horizons: The impact of Sn doping on MOS properties extends beyond the mere modification of the electronic structure. This deliberate introduction of Sn into TiO2 nanocomposites opens doors to multifunctionality and a tailored performance. As researchers continue to delve into the intricacies of Sn-doped systems, opportunities arise for optimizing synthetic approaches, elucidating fundamental mechanisms, and discovering novel applications. These nanocomposites exemplify the marriage of material design and functional outcomes, propelling the exploration of advanced materials with unprecedented attributes.
2.3. Unlocking Dual Properties: Ferromagnetism and Photocatalysis
The intriguing convergence of ferromagnetism and photocatalysis within metal oxide materials offers an interesting avenue for multifaceted applications, merging magnetic responsiveness and light-driven catalysis. This dualistic interplay presents compelling opportunities across various fields, as follows.
Synergistic Potential: The coexistence of ferromagnetic and photocatalytic properties bestows materials with the capacity to serve diverse functions simultaneously. This transcendence of conventional capabilities opens doors to innovative solutions that harness the strengths of both properties in synergy [62].
Advanced Functionalities: The fusion of ferromagnetism and photocatalysis creates materials with enhanced functionalities that extend beyond traditional single-domain materials. This convergence fosters innovation in domains ranging from environmental remediation and energy conversion to advanced sensing and information storage technologies [62].
Environmental Remediation: Magnetic photocatalysts have emerged as promising candidates for tackling water and air pollution challenges. These materials harness sunlight-driven reactions for pollutant degradation while also allowing for efficient magnetic separation. This dual approach offers an ecofriendly and effective solution for cleaning up environmental contaminants [63].
Energy Conversion: The intrinsic magnetic properties of these materials introduce new dimensions to energy conversion and storage applications. The coupling of magnetic behavior with photocatalysis holds potential for advancements in renewable energy technologies, such as solar-driven hydrogen production and efficient energy storage [64].
Magnetic Manipulation: The presence of ferromagnetism in these materials adds a novel layer of functionality—magnetic manipulation. External magnetic fields can be harnessed to control and modulate material behavior, enabling applications like remote switching and controlled release in drug delivery systems [65].
Tailored Synergy: The interaction between ferromagnetism and photocatalysis can be finely tuned to achieve tailored synergies. By adjusting the material composition, structure, and magnetic properties, researchers can amplify performance in specific applications. This customization empowers materials to address targeted challenges with heightened efficiency [66].
The integration of ferromagnetism and photocatalysis in metal oxide materials marks a pioneering step toward the development of versatile materials capable of addressing multifaceted challenges and pioneering novel technological frontiers. This convergence sparks curiosity and collaboration across scientific disciplines, driving researchers to explore uncharted territories and redefine the possibilities of materials with dual functionalities. This journey of harnessing the interplay between ferromagnetism and photocatalysis holds the promise of having transformative impacts on technology, industry, and our efforts to build a sustainable future. Novel Synthesis Approach for Enhanced Nanocrystals: Innovative synthesis approaches are pivotal in bolstering the synergistic interplay between magnetic and semiconductor properties by adeptly amalgamating and refining their characteristics [67]. These methodologies involve tailoring the composition, structure, and morphology of materials at the nanoscale to attain the desired magnetic and semiconductor functionalities [68]. By finely adjusting parameters such as the particle size and shape, doping, and surface chemistry, researchers can craft materials endowed with superior magnetic and semiconductor properties [69].
For instance, in the realm of FMOS, innovative synthesis techniques, like the sol-gel, hydrothermal, and chemical vapor deposition methods, empower the creation of nanostructured materials with tailored magnetic and semiconductor attributes [70,71].
These techniques afford precise control over the crystalline structure and defect density, which are pivotal factors influencing the magnetic and semiconductor behaviors.
Moreover, innovative approaches to synthesis facilitate the amalgamation of magnetic and semiconductor constituents into hybrid nanostructures, such as core–shell nanoparticles or heterostructures [72,73]. These composite architectures offer unparalleled prospects for harnessing the synergistic interactions between the magnetic and semiconductor phases, culminating in augmented functionalities for applications spanning spintronics, magnetic sensing, and catalysis.
One notable instance of such an approach has yielded remarkable outcomes in the production of Sn-TiO2 nanocrystals, showcasing the synergy between the ferromagnetism and the exceptional photocatalytic activity. This hydrothermal method introduces controlled oxygen vacancies into the nanocrystal structure, inducing ferromagnetic behavior while retaining their photocatalytic prowess [74,75].
This hydrothermal method introduces controlled oxygen vacancies into the nanocrystal structure, inducing ferromagnetic behavior while retaining their photocatalytic prowess.
Optical Shift in Sn-Doped TiO2: A noteworthy observation in Sn-doped and Sn-Fe-co-doped TiO2 systems is the optical absorption spectrum red-shift. This phenomenon results from the incorporation of Sn and Fe dopants, altering the electronic band structure of the nanocrystals. This shift holds the potential to enhance the efficiency of light absorption, enabling applications that require an extended photo-response in the visible light spectrum [76]. This optical modification broadens the utility of these materials in light-driven technologies.
Multifunctional Potential: The tunability of metal oxide semiconductor nanoparticles, particularly by Sn doping, presents a gateway toward the enhancement of both the magnetic and photocatalytic properties. This multifunctional potential holds promise for advancing the scientific understanding and technological applications across a diverse range of fields. From environmental remediation and energy conversion to information technology and biomedicine, the ability to tailor MOS nanoparticles with specific functionalities opens doors to innovative solutions that can reshape industries and improve quality of life [77,78,79,80,81,82,83,84,85].
Harnessing this novel synthesis approach not only contributes to our understanding of materials’ behavior at the nanoscale but also paves the way for pioneering applications. The ability to engineer nanocrystals with tailored magnetic and a photocatalytic property broadens the scope of what these materials can achieve. As researchers continue to explore and refine these fabrication techniques, the world of nanotechnology stands poised to witness transformative advancements with far-reaching implications.
2.4. Elevating Visible-Light Activity via the Co-Doping of MOS
The co-doping of MOS has emerged as a powerful strategy to significantly amplify their visible-light activity, ushering in a new era of enhanced photocatalytic performance and versatile applications.
Synergistic Effects: Co-doping involves introducing multiple dopants that work in synergy to manipulate electronic structures and bandgaps, enhancing light absorption and utilization.
Expanded Photo-Responsive Range: By tuning the co-doping ratios and combinations, MOS can effectively extend their light absorption spectrum into the visible range, unlocking previously untapped energy sources.
Efficient Charge Separation: co-doping creates unique energy levels, facilitating the separation of photo-generated electron–hole pairs and, thus, elevating the catalytic efficiency.
Reduced Bandgap: nonmetal co-dopants introduce additional valence bands, while nontransition metal co-dopants introduce charge carrier traps, collectively narrowing the bandgap for visible-light utilization.
Enhanced Catalytic Performance: the improved charge carrier mobility and suppressed recombination rate achieved through co-doping lead to superior photocatalytic activity.
Versatile Applications: co-doped MOS finds applications in diverse fields, from environmental remediation to solar energy conversion, where efficient visible-light photocatalysis is crucial.
Tailored Designs: the flexibility of co-doping allows for tailoring the properties of MOS according to specific requirements, leading to advancements in materials engineering [78,79,80,81].
The co-doping of MOS is ground breaking opportunity to unlock their untapped potential, revolutionizing their role in harnessing visible light for various sustainable applications and paving the way for a greener and more energy-efficient future.
2.4.1. Synergistic Effects of Nonmetal and Nontransition Metal Co-Doping
Boosting Photo-Electron Separation: the co-doping of nonmetals and nontransition metals enhances the separation of electron–hole pairs, broadening the photo-absorption’s limits [82,83].
Potential of Nitrogen Doping: Nitrogen doping, which is particularly effective, modifies charge transport properties and induces oxygen-defect sites, improving photocatalytic performance [60,61]. Nitrogen atom substitution in the TiO2 lattice reduces bandgap width in Figure 2a, leading to promising visible-light photocatalysis [86].
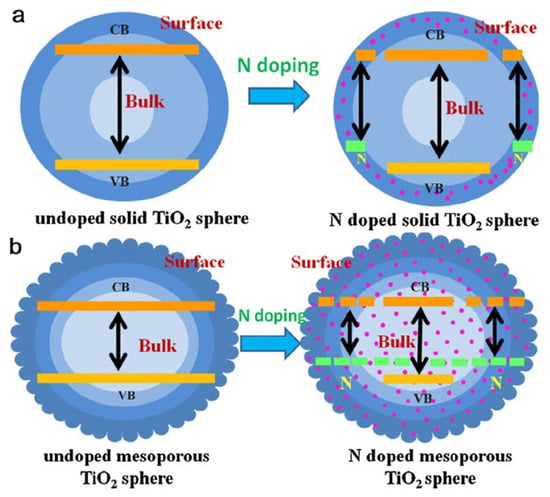
Figure 2.
Schematic diagrams elucidating the band structures of solid and mesoporous TiO2, showcasing the effects of nitrogen doping [86,87]. (a) Undoped and N doped solid TiO2 sphere, and (b) Undoped and N doped mesoporous TiO2 sphere. Orange line indicates that the band gap width of undoped solid and mesoporous TiO2 sphere, and pale green line indicate the reduced band gap width of N doped solid and mesoporous TiO2 sphere.
2.4.2. Unlocking Nitrogen Doping Potential
Challenges of Bulk Nitrogen Doping: nitrogen doping in solid TiO2 structures is hindered because of compact packing in Figure 2b [87].
Advantages of Mesoporous Nitrogen Doping: mesoporous TiO2 with nitrogen doping exhibits uniform energy levels, enhancing the visible-light photocatalytic activity [87].
2.4.3. Advancements in Sn and N Co-Doping
Sol-Gel Preparation: The integration of Sn and N co-doping into TiO2 photocatalysts has demonstrated remarkable improvements in performance, particularly under visible light or simulated solar light irradiation [88]. The sol-gel preparation method has proven to be effective in introducing these dopants into the TiO2 lattice, leading to enhanced photocatalytic activity through improved light absorption and charge separation.
Remaining Frontiers: While significant progress has been made in harnessing Sn and N co-doping for enhanced photocatalysis, there remain frontiers to be explored. Comprehensive research is needed to unravel the full extent of doping effects on the physical, chemical, and catalytic properties of co-doped microspheres. Understanding the intricate interplay between dopants, defects, and material behaviors is crucial for optimizing the design and fabrication of these advanced materials [89].
The strategic manipulation of oxide-based semiconductor properties through defect engineering, co-doping, and novel synthesis techniques holds immense promise across a wide spectrum of scientific and technological advancements. From the remediation of environmental pollutants to the creation of multifunctional materials with tailored properties, these approaches have the potential to reshape industries and drive innovation. Within this dynamic realm of materials engineering, the exploration of Sn and N co-doping has emerged as a noteworthy avenue for achieving enhanced photocatalytic performance and expanding the applications of oxide-based nanocomposites. As research continues to progress, these advancements will contribute to a more sustainable and technologically advanced future.
3. Uncover Diluted Magnetic Semiconductors
Diluted magnetic semiconductors (DMS) are a notable class of materials that resonates with the pursuit of unconventional material functionalities. In recent years, the intersection of magnetic and semiconducting properties within DMS has sparked significant interest. DMS materials ingeniously integrate magnetic impurities—typically transition metal ions—into semiconductor matrices. This deliberate infusion of magnetic dopants within semiconductors engenders localized magnetic moments, effectually transforming otherwise nonmagnetic semiconductor lattices. This intriguing synergy of magnetism and semiconductivity opens pathways to innovative applications spanning diverse scientific fields and technologies. As such, DMS materials stand as a testament to the boundless potential that arises from synergistically marrying distinct material attributes.
Recent strides in the realm of DMS research have uncovered intriguing phenomena and promising avenues that unfold across scientific disciplines and technological horizons. Within this evolving landscape, several distinct facets call exploration.
3.1. Exploring Spintronics Potential
A Glimpse into Spintronics: The field of spintronics, fueled by the intriguing spin degree of freedom in electrons, has ignited significant interest due to its promise of revolutionary advances in electronics. Unlike traditional electronics that rely solely on the charge of electrons, spintronics harnesses the intrinsic spin property of electrons, opening up new horizons for efficient information storage, processing, and transmission [90]. This burgeoning field is characterized by its potential to revolutionize computing, memory, and sensor technologies by offering enhanced speed, lower power consumption, and increased data storage density.
DMS materials are emerging as critical players in spintronics, enabling precise control and manipulation of electron spins through external magnetic fields or electrical triggers. DMS are semiconductor materials doped with magnetic elements, introducing localized magnetic moments in the semiconductor lattice. This controlled introduction of magnetism into a semiconducting host material lays the foundation for creating spin-polarized currents and enabling efficient spin manipulation. The integration of DMS materials into spintronic devices introduces the exciting possibility of designing components that can simultaneously process and store information based on the spin state of electrons [91].
Pioneering Spintronic Devices: DMS materials hold the potential to underpin the development of various spintronic devices, thereby adding momentum to the spin-based information storage and manipulation arena. Spintronic devices, such as spin valves and magnetic tunnel junctions, leverage the ability to manipulate electron spins to encode and retrieve information. These devices are promising candidates for next-generation memory technologies, offering nonvolatile storage with high speed and energy efficiency. Furthermore, the utilization of DMS materials in spintronic devices could potentially lead to the creation of more compact and power-efficient devices, revolutionizing not only the information technology sector but also advancing fields like quantum computing and advanced sensors [92].
The synergy between DMS materials and spintronics presents an exciting frontier in materials science and electronics. As researchers delve deeper into understanding the intricate interactions of electron spins within these materials, the realization of novel spintronic devices with unprecedented capabilities draws ever closer. The fusion of materials innovation, theoretical exploration, and device engineering in this domain is poised to reshape the technological landscape and fuel a new era of transformative electronics.
Spin injection, transport, and detection pose significant challenges in spintronics, hindering device development [93]. Efficiently injecting spin-polarized carriers from a ferromagnet into a nonmagnetic material is difficult due to high resistance and spin loss at interfaces [94]. Spin coherence can be lost during injection, especially at higher temperatures [95]. Preserving spin coherence during transport is crucial, but relaxation processes limit spin transport distances. Achieving long spin diffusion lengths requires materials with low scattering rates and high carrier mobilities, which can be hindered by defects [96]. Interfaces between materials with different spin properties can introduce scattering, affecting spin transport [97]. Detecting spin-polarized carriers with sensitivity is challenging, requiring complex setups and facing interference from external noise. Minimizing measurement-induced perturbations is crucial for accurate spin characterization [98]. Overcoming these challenges demands interdisciplinary efforts and the development of novel materials and techniques for efficient spin manipulation and detection in spin-based devices.
3.2. Augmenting Properties through Innovative Synthesis
An effective method for tailoring DMS characteristics involves coprecipitation methods paired with postsynthesis treatments [99,100,101]. Coprecipitation combines magnetic dopant precursor solutions (e.g., Mn, Fe, Co) with the host semiconductor solution (e.g., TiO2, SnO2, ZnO, GaN), allowing precise control over dopant integration into the semiconductor lattice through adjustments in concentration and synthesis parameters like temperature and pH [102,103,104]. Subsequent annealing in controlled atmospheres (e.g., vacuum, inert gas) enhances crystallinity, reduces defects, and optimizes dopant distribution, fostering the formation of magnetic clusters and promoting stronger ferromagnetic behavior at room temperature [105,106,107]. This approach facilitates the customization of semiconductor characteristics, including bandgap engineering and carrier concentration, crucial for diverse electronic and optoelectronic applications.
Hydrothermal synthesis, a method for DMS material preparation, entails growing semiconductor nanomaterials under high-pressure, high-temperature aqueous conditions [108,109,110]. This technique allows for the precise incorporation of magnetic dopants into the semiconductor lattice, controlling both dopant positioning and concentration. Furthermore, ion implantation supplements this process by implanting high-energy ions of desired dopant species into the material, refining dopant distribution and concentration to create well-defined profiles within the semiconductor. This synergy enhances the magnetic coupling among dopant ions, fostering stronger ferromagnetic behavior [111,112]. By combining hydrothermal synthesis with ion implantation, a versatile platform is created for fine-tuning magnetic and semiconductor characteristics according to specific application requirements [113,114,115,116,117]. These synthesis methods showcase the effectiveness of combining diverse techniques and postsynthesis treatments, driving advancements in spintronics, magnetic memory devices, and sensors.
Engineering Magnetic-Semiconductor Synergy: The pursuit of enhancing the synergistic interplay between magnetic and semiconductor properties within DMS materials has propelled the exploration of innovative synthesis methodologies and composite formations. These endeavors are rooted in the understanding that the manipulation of material composition, crystalline structure, and doping profiles can intricately modulate the magnetic and electronic characteristics of DMS materials. By engineering these factors, researchers aim to achieve enhanced control over spin interactions and electronic band structure, paving the way for novel functionalities in spintronic and electronic devices [118].
Tailored DMS Compounds: At the heart of this pursuit lies the discovery and design of tailored DMS compounds, where meticulous tuning of material properties holds the potential to unlock entirely new avenues for technological applications. The deliberate manipulation of DMS materials at the atomic and nano-structural scales allows for the creation of customized materials with properties optimized for specific tasks. This tailored approach enables the exploration of previously inaccessible parameter spaces, facilitating the emergence of unprecedented DMS materials with precisely engineered magnetic and electronic attributes. Such advancements carry profound implications for the development of cutting-edge magnetic sensors, spin-based logic devices, and energy-efficient memory technologies [119,120].
Unveiling new DMS compounds with precisely tailored properties has emerged as a beacon of progress in the field, signifying prospects for the realization of innovative magnetic and electronic devices. These endeavors not only deepen our fundamental understanding of the intricate interplay between magnetism and semiconductivity but also invigorate the exploration of uncharted territories within materials design. The fusion of innovative synthesis strategies with precise property tailoring promises to reshape the landscape of materials science, thrusting DMS materials to the forefront of next-generation electronics and spintronic technologies [121]. As researchers delve further into these transformative approaches, they open doors to a realm of possibilities where the boundaries of traditional materials limitations are redefined, ushering in a new era of functional materials and advanced device architectures.
3.3. Unveiling Magneto-Optical Frontiers
Magneto-optical effects, whereby a material’s magnetic properties affect its interaction with light, hold significance for DMS materials, finding applications in magneto-optical data storage and quantum information processing [122,123,124]. For instance, the Faraday effect in DMS materials allows for data storage by encoding information in the rotation angle of polarized light, whereas the Kerr effect can be utilized for sensing and imaging applications, probing magnetic properties with high sensitivity [125,126,127]. Moreover, in quantum information processing, magneto-optical effects enable the manipulation of electron spins for quantum computation and communication, exemplified by the control of the spin orientation in DMS quantum dots [128,129,130]. These effects offer versatile tools for advancing technologies such as data storage and quantum computing.
Magneto-Optical Enigma: The recent strides in materials research have unveiled a realm of unique magneto-optical effects within DMS materials, heralding a new era of exploration and innovation [131]. These intriguing phenomena have transformed DMS materials into a fertile ground for the convergence of magnetism and optics, with profound implications for the development of next-generation optoelectronic devices. Through the interaction between external magnetic fields and light, researchers have uncovered fascinating magneto-optical properties that can be harnessed for a variety of applications.
A Nexus of Light and Magnetism: The coupling of the magneto-optical attributes with the inherent properties of DMS materials offers an intriguing glimpse into the creation of novel optoelectronic devices. The ability to modulate light properties, such as polarization, reflectance, and transmission, through the manipulation of magnetic characteristics presents unprecedented opportunities. By controlling the interaction between photons and spin-polarized carriers, DMS materials hold the potential to revolutionize fields such as magneto-optical data storage, quantum information processing, and advanced sensing technologies [132].
Tunable Magnetic and Optical Functionalities: The allure of DMS materials lies in their inherent tunability—allowing for the manipulation of both magnetic and optical properties through external stimuli. This tunability opens pathways for the creation of dynamic and adaptive devices where magnetic and optical functionalities can be tailored in real-time. From magneto-optical modulators to spintronic-based light sources, the synergistic integration of magneto-optical effects within DMS materials empowers engineers and researchers to envisage and fabricate devices that harness the full spectrum of light-matter interactions [133].
As magneto-optical research continues to unravel the complex interplay between magnetic ordering and optical behavior within DMS materials, the stage is set for a vibrant and transformative chapter in optoelectronics. The magneto-optical enigma, once shrouded in mystery, is now being harnessed to drive innovation, pushing the boundaries of what is achievable in the realm of light manipulation and magnetic control. This evolving landscape not only deepens our understanding of fundamental physics but also enriches the potential for breakthrough technologies that will shape the future of information processing, communication, and sensing.
3.4. Surmounting Challenges
Room-Temperature Ferromagnetism: Despite significant progress, achieving robust and stable ferromagnetic order at room temperature within DMS materials remains a formidable challenge. The susceptibility of magnetic properties to temperature fluctuations necessitates innovative strategies to enhance and maintain ferromagnetic behavior. Researchers are actively exploring avenues such as precise doping profiles, defect engineering, and nano-structuring to surmount these challenges and enable consistent ferromagnetic properties at practical operating temperatures [134].
Achieving room-temperature ferromagnetism and effectively manipulating spin in materials pose significant challenges, primarily due to the intricate balance required for stable magnetic properties and spin control [135,136]. Many materials only exhibit ferromagnetism at extremely low temperatures due to thermal fluctuations disrupting ordered magnetic alignment, necessitating materials with robust magnetic interactions capable of withstanding thermal agitation [137,138]. Moreover, maintaining magnetic order while controlling carrier concentration and mobility presents challenges, particularly at room temperature where thermal effects are pronounced [139,140]. Additionally, manipulating spin states, vital for spin-based technologies like spintronics and quantum computing, faces hurdles such as rapid decoherence and information loss due to environmental interactions [141,142]. Addressing these challenges requires interdisciplinary efforts integrating materials science, condensed matter physics, and device engineering to explore novel materials, phenomena, and techniques.
Prospective solutions and current research endeavors to address these challenges include: Developing novel materials with tailored crystal structures and electronic configurations to enhance magnetic stability and promote ferromagnetism at higher temperatures [143,144,145,146]. For instance, exploring spinel structures or multiferroic materials with coupled magnetic and ferroelectric order may offer new avenues for room-temperature ferromagnetism [147]. Introducing magnetic dopants or alloying elements into semiconductor or insulator matrices can modify electronic and magnetic properties, potentially stabilizing ferromagnetic phases at room temperature [100,148]. Research focuses on precise control of dopant concentration, distribution, and coupling to optimize magnetic behavior [149,150]. Manipulating interfaces between different materials or heterostructures can influence spin transport and magnetic properties, aiming to engineer interfaces with controlled spin polarization and reduced spin scattering, thus enhancing spin coherence and manipulation efficiency [151,152]. Advancements in spintronics devices and quantum technologies explore innovative approaches for spin manipulation and information processing, concentrating on developing efficient spintronic devices, spin-based logic gates, and quantum bits (qubits) with long coherence times, paving the way for practical applications in information storage and processing [153,154]. Overall, interdisciplinary research efforts combining materials science, condensed matter physics, and device engineering are crucial for overcoming the challenges of achieving room-temperature ferromagnetism and effectively manipulating spin. Collaboration between experimentalists and theorists facilitates the exploration of new materials, phenomena, and techniques, driving progress toward realizing advanced spin-based technologies.
Efficient Spin Manipulation: The efficacy of spin injection, transport, and detection is a cornerstone of successful DMS research, directly influencing the viability of spintronic applications [155]. Maximizing the efficiency of spin-related functionalities requires intricate control over spin polarization, carrier lifetimes, and spin relaxation mechanisms. Scientists are delving into techniques like spin injection from ferromagnetic electrodes, as well as tailoring interfaces between DMS and nonmagnetic materials, to optimize the interaction between spins and carriers, with created highly efficient spintronic devices [156].
The Evolving Landscape of DMS: The dynamic evolution of DMS research has opened up vistas that extend toward the realm of next-generation spintronic and magneto-optical devices. As we navigate these uncharted territories, the marriage of magnetic and semiconductor properties within DMS materials presents an unprecedented opportunity to craft advanced functionalities. With continued research and development, DMS materials will leave an indelible mark on modern technology, shaping the contours of magnetic and semiconductor domains alike. This trajectory promises to lead to transformative devices that harness the unique interplay of spins and charge carriers, bridging the gap between conventional electronics and the future of spin-based technologies. As the boundaries of what is possible with DMS materials expand, they are set to reshape the technological landscape and catalyze the emergence of a new era of multifunctional, high-performance devices [157].
3.5. Exploring Ferromagnetism in DMS Intricacies of Ferromagnetism: Diluted
Magnetic semiconductors, often dubbed “semi-magnetic semiconductors”, have inspired rigorous exploration because of their unique blend of semiconductor properties and ferromagnetic behavior. The intricate interplay co electron charge and spin has captured the attention of researchers, driving them to delve deeper into the fascinating world of DMS materials [158].
Unraveling the Phenomenon: The coexistence of semiconductor and ferromagnetic properties within DMS materials presents a complex puzzle that scientists are striving to solve. The controlled introduction of magnetic ions into a semiconductor matrix creates a system in which the interaction of colocalized magnetic moments and mobile charge carriers results in novel physics and functionalities [159]. Understanding the mechanisms that govern the emergence and manipulation of ferromagnetism in these materials is essential for harnessing their potential in various applications.
Ferromagnetic Fascination: The tantalizing prospect of achieving room-temperature ferromagnetism in DMS, especially oxide-based variants, has generated a wave of excitement within the scientific community. The ability to achieve and control ferromagnetism at temperatures that are practical for everyday applications opens up a plethora of possibilities.
Cross-Disciplinary Applications: The allure of DMS materials with ferromagnetic properties extends far beyond the realm of fundamental research. The integration of these materials into practical technologies holds promise for numerous fields. For instance, the development of magnetic fluids using DMS could revolutionize industries ranging from transportation to robotics by enabling efficient and controllable fluid manipulation using external magnetic fields [160].
Biomedical Innovations: The intersection of DMS and biomedicine showcases another facet of the potential impact. The magnetic properties of DMS could be harnessed to develop targeted drug delivery systems, in which externally applied magnetic fields guide drug-loaded DMS particles to specific locations within the body. This level of precision could minimize side effects and enhance the efficacy of therapeutic treatments [160].
Catalysis and Environmental Remediation: DMS materials with ferromagnetic behavior have shown promise in catalytic applications and environmental remediation efforts. These materials could be employed as catalysts in various chemical reactions, and their magnetic properties may facilitate separation and recovery processes, reducing waste, and improving the efficiency of resource utilization [160].
The unraveling of the mysteries of ferromagnetism in DMS continues to be the focus of researchers. As our understanding of the underlying physics deepens and our ability to engineer these materials advances, we stand on the cusp of transformative breakthroughs across multiple scientific disciplines and industries [159,160].
3.6. Novel Synthesis Unveils Potential
Synthesis’s Impressive Yield: The journey of synthesizing DMS materials has been marked by remarkable breakthroughs, each contributing to the expansion of our understanding and capabilities. One exemplary feat in this field comes from the work of Wang et al., who devised an ingenious method to synthesize ZnO crystals enriched with Zn vacancies. This novel approach not only resulted in materials with unique properties but also opened up exciting avenues for applications across various domains [161].
Beyond Conventional Boundaries: The synthesis methods used to engineer DMS materials have evolved significantly, breaking away from conventional strategies and venturing into innovative territories. Wang et al.’s approach exemplifies this trend, whereby the intentional introduction of Zn vacancies within ZnO crystals led to the emergence of unexpected characteristics. These advancements highlight the power of unconventional thinking in materials synthesis, enabling the tailoring of properties that were previously considered elusive [162].
Pioneering Photo-Induced Ferromagnetism: A captivating advancement in the field of DMS synthesis revolves around photo-induced ferromagnetism in transition metal-doped TiO2 nanoparticles. This pioneering discovery challenges conventional notions of temperature-dependent ferromagnetism by demonstrating that controlled defect creation induced by light can lead to ferromagnetic ordering even at room temperature [42]. This phenomenon introduces a new dimension to our understanding of magnetism and lays the groundwork for innovative approaches to engineering magnetic materials.
Unraveling the Mystery: While the emergence of room-temperature ferromagnetism in transition metal-doped TiO2 nanoparticles is a significant stride, the precise mechanisms underlying this phenomenon continue to intrigue researchers. The role of transition metals in inducing ferromagnetic ordering within TiO2 remains an enigma that scientists are diligently working to solve. Unraveling this mystery holds the potential to not only deepen our fundamental understanding of magnetism but also pave the way for tailored synthesis strategies to harness this unique behavior [163].
Synergy of Synthesis and Exploration: The evolving landscape of DMS synthesis exemplifies the symbiotic relationship co materials engineering and scientific exploration. As researchers push the boundaries of what is possible in synthesis techniques, they simultaneously unravel new properties and behaviors in DMS materials. This synergy underscores the dynamic nature of scientific progress, where advancements in synthesis methodologies continually inform and guide our quest to understand and harness the potential of novel materials.
The impressive achievements in DMS synthesis, as exemplified by the innovative work of Wang et al. [162] and the ground-breaking photo-induced ferromagnetism in transition metal-doped TiO2 nanoparticles, reflect the relentless pursuit of scientific discovery and technological innovation. These strides not only contribute to the expansion of our knowledge but also inspire novel applications that could reshape industries and enhance our daily lives.
3.7. Unlocking Magnetic-Photocatalyst Synergy
Magnetic-Photocatalyst Nexus: The pursuit of merging the seemingly disparate realms of ferromagnetism and photocatalytic activity within wide-bandgap metal oxide-based nanocomposites has catalyzed the development of innovative models and approaches [74]. Researchers recognize that the intersection of these two properties holds immense promise for applications spanning environmental remediation, energy generation, and beyond. Central to these investigations is the exploration of how surface oxygen vacancies and heightened charge carrier concentration synergistically influence both magnetism and photocatalytic performance [164,165].
Harnessing Synergistic Effects: The convergence of room-temperature ferromagnetism and enhanced photocatalytic efficiency in nanocomposites has unveiled a remarkable synergy. These materials exhibit the capacity to harness visible light irradiation, a crucial aspect for practical applications, and convert it into efficient photocatalytic processes. This ability to simultaneously manipulate charge carriers for magnetic responses and facilitate photocatalytic reactions highlights the power of engineered nanomaterials in achieving multifunctional capabilities [100,166].
A New Era in Photocatalysis: The emergence of magnetic photocatalysts signifies a paradigm shift in the field of photocatalysis. Traditional diamagnetic photocatalysts often face limitations in efficiently utilizing visible light due to their band structures. The introduction of room-temperature ferromagnetism not only extends the spectral range for photocatalysis but also provides an avenue for fine-tuning catalytic properties through magnetic manipulation [167]. This breakthrough is particularly significant in the quest for sustainable energy solutions and pollution mitigation.
Doping and Co-Doping Strategies: The development of magnetic photocatalysts underscores the pivotal role of controlled doping and co-doping in semiconductor nanocomposites. By judiciously introducing magnetic ions into the semiconductor matrix, researchers can tailor the electronic band structure and modulate charge carrier dynamics [168].
This strategic manipulation empowers materials to exhibit both ferromagnetic behavior and enhanced photocatalytic performance simultaneously [169,170]. Such insights into the synergy co doping strategies and multifunctionality hold promise for the design of next-generation functional materials.
Innovation at the Interface: The convergence of magnetism and photocatalysis at the nanoscale interface exemplifies the power of interdisciplinary research. This fusion necessitates expertise in materials science, solid-state physics, chemistry, and engineering, highlighting the collaborative nature of scientific advancements. As researchers continue to unravel the intricate mechanisms governing magnetic-photocatalyst synergy, they pave the way for transformative technologies with applications that extend from clean energy production to pollutant degradation [167,168,169,170].
The magnetic-photocatalyst synergy exemplifies how harnessing multiple functionalities within a single nanocomposite can lead to ground-breaking advancements. This emergent field not only expands our fundamental understanding of materials but also presents innovative solutions to pressing global challenges. The journey to unlock the full potential of magnetic-photocatalyst nanocomposites is a testament to human ingenuity and the limitless possibilities that interdisciplinary research can unfold.
3.8. DMS for Technological Evolution
DMS’s Technological Relevance: The advent of DMS materials exhibiting room-temperature ferromagnetism has ignited a technological revolution with profound implications. These materials have rapidly transitioned from theoretical curiosities to pivotal players in various technological domains. Their unique combination of semiconductor behavior and ferromagnetic properties holds immense promise for innovations in spintronics, optoelectronics, and memory devices, paving the way for a new era of electronic technologies [171,172].
Reshaping Electronics: DMS materials are set to revolutionize the electronics landscape by enabling the development of spin-based field-effect transistors (spin-FETs) and spin-based light-emitting diodes (spin-LEDs). These advancements are underpinned by the ability to manipulate and control the spin of the charge carriers, offering the potential for low-power, high-speed devices that can surpass the limitations of conventional transistor technology [173,174]. The marriage of ferromagnetism with electronic functionality brings about a fundamental shift in the design and operation of electronic components.
Exploring Multiferroics: Beyond their standalone ferromagnetic behavior, the exploration of DMS materials extends into the realm of multiferroics. The integration of magnetic ordering with other ferroic ordering parameters, such as ferroelasticity or ferroelectricity, holds great promise for the development of novel spintronics and magneto-optic devices. These multifunctional materials could pave the way for new paradigms in data storage, sensor technology, and even quantum computing [175].
Light-weight Doping for Enhanced Properties: Researchers are continually pushing the boundaries of DMS materials by exploring the impact of light-weight doping elements such as carbon (C), nitrogen (N), and lithium (Li) in metal oxide matrices. These doping strategies have been found to bolster ferromagnetic behavior, thus expanding the range of materials that can exhibit this unique property. The marriage of theoretical modeling and experimental investigations is shedding light on the intricate mechanisms that govern these enhancements, with potential implications for both fundamental physics and practical applications [176].
Innovation’s Horizon: As the technological landscape continues to evolve, DMS materials stand at the forefront of innovation. Their capacity to bridge the gap between semiconductors and ferromagnets has opened up new possibilities that were once deemed unattainable. From advancing information storage and processing to revolutionizing data communication, the journey of DMS materials promises to reshape industries and influence our daily lives in ways that were once the realm of science fiction.
The emergence of DMS materials as technological enablers exemplifies the rapid pace of scientific advancement. These materials are not only rewriting the rules of electronics but also inspiring a new wave of interdisciplinary research that blurs the boundaries between distinct fields. As DMS materials continue to reveal their potential, they will usher in a future in which the fusion of semiconductors and ferromagnetism results in transformative technologies.
DMS research has undergone significant progress over the past few decades, with key milestones and breakthroughs marking its development. The following is a concise historical framework highlighting the temporal boundaries and significant achievements in DMS research in Figure 3.
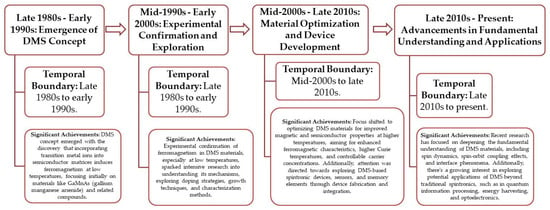
Figure 3.
A succinct historical overview delineating the temporal boundaries and notable advancements in DMS research. Data obtained from Google Scholar Citations (GSC).
By delineating these temporal boundaries and highlighting significant achievements, this historical framework provides a clearer understanding of the progressive developments in DMS research, from its conceptualization to its current state and future prospects.
3.9. Harnessing Defect Engineering for Enhanced Performance
Defect Engineering’s Impact: The strategic incorporation of defects into the matrices of transition metal-doped metal oxide semiconductors has shown itself to be a powerful method of tailoring material properties. This innovative technique, often performed through controlled ion beam irradiation, offers a transformative approach to manipulating the behavior of materials at the nanoscale. Notably, the synergy between defects and magnetic properties has gained attention, particularly in the context of enhancing the ferromagnetic behavior of materials like ZnO nanoparticles [177,178,179].
Unleashing Structural Complexity: Ion beam irradiation represents a sophisticated method for introducing controlled defects into materials. By irradiating ZnO nanoparticles with low-energy ions, researchers have managed to induce structural complexity that goes beyond conventional doping approaches. This structural manipulation serves multiple purposes—eliminating unwanted secondary impurity phases, fine-tuning lattice arrangements, and inducing localized distortions. The resulting materials exhibit enhanced ferromagnetic properties that are pivotal for diverse applications [180].
Balancing Defects and Functionalities: One of the key challenges in defect engineering is striking a delicate balance between introducing defects and preserving desired functional properties. Low-energy ion beam irradiation, particularly with inert gases, emerges as an ideal strategy in this regard. This technique enables the controlled induction of defects while simultaneously managing intrinsic structural imperfections. Additionally, it mitigates the risk of the segregation of doped transition metal clusters that could hinder desired material properties [181]. This approach aligns with the overarching goal of developing cost-effective, high-efficiency materials for multifunctional applications.
Toward Enhanced Nanocomposites: The marriage of defect engineering with DMS materials holds immense promise in the realm of advanced nanocomposites. The integration of controlled defects not only enhances ferromagnetic properties but also synergistically influences other functionalities, such as photocatalytic activity. This dual enhancement is particularly relevant in the context of materials like TiO2, where defect engineering could unlock the full potential of ferromagnetic and photocatalytic TiO2 nanocomposites [181].
Beyond Empirical Exploration: Defect engineering offers more than just empirical enhancements; it provides a pathway for rational design and optimization. Through computational simulations and theoretical modeling, researchers are gaining insight into the intricate mechanisms that govern defect-induced changes in material properties. This deeper understanding enables targeted defect engineering strategies, reducing the need for trial-and-error approaches and accelerating the development of tailored materials [180].
Future Prospects: Harnessing defect engineering to optimize material properties transcends the field of DMS materials. It underscores the versatility of this approach in enhancing a wide range of functional materials, from semiconductors to catalysts and beyond. As our ability to engineer and characterize defects advances, the potential for creating materials with unprecedented combinations of properties grows, opening up new frontiers in technology and innovation.
The art of defect engineering is revolutionizing our approach to materials design. The strategic manipulation of defects using techniques like ion beam irradiation has enabled us to craft materials with enhanced and multifunctional properties. This approach, exemplified in the context of DMS materials, promises to reshape industries and drive innovations that address some of society’s most pressing challenges. As we continue to delve into the intricacies of defect-engineered materials, we step closer to a future where materials are tailored to our needs with unprecedented precision.
3.10. A Rich Portfolio of Achievements
Past Endeavors, Ongoing Explorations: previous research reports have chronicled the photocatalytic and magnetic prowess of various TiO2-based photocatalysts, spanning metal oxide coupled TiO2 to hierarchical Sn and N co-doped TiO2 [74,75,76,182,183,184,185].
Toward Enhanced Functionalities: these studies collectively deepen the understanding of TiO2 nanocomposites, enhancing their photocatalytic and magnetic functions, and charting pathways toward diverse applications in materials science and technology.
Discerning Magnetic Realms: Figure 4A artfully captures the potential magnetic species and their distribution, hinting at intriguing interactions [186].
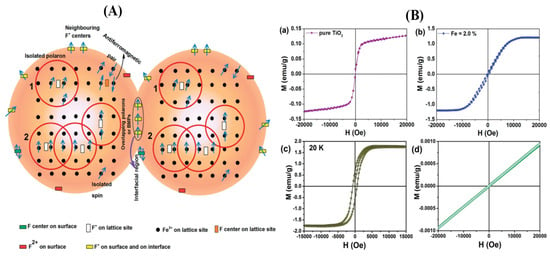
Figure 4.
(A) Various possible magnetic species, their distribution, and potential interactions [178]; (B) M–H curves of vacuum-annealed NPs for (a) pristine TiO2, (b) 2% Fe-doped TiO2 at room temperature, and (c) 2% Fe-doped TiO2 at 20 K, as well as (d) the paramagnetic M–H curve of vacuum-annealed 2% Fe-doped TiO2 after reheating in air at 450 °C [178]. Blue arrows indicate that the paramagnetic oxygen vacancies are formed by bound magnetic polarons.
Magnetism in Action: Figure 4B provides a look into the magnetic world, illustrating M–H curves of pristine and Fe-doped TiO2, evoking the magnetic transitions at play [186].
Peering into Fe-Doped TiO2: Figure 4A casts light on the magnetic landscape of Fe-doped TiO2 NPs under vacuum annealing, highlighting magnetic polarons and their alliances [186].
Mapping Magnetization: Figure 4B charts the journey of magnetization, capturing its trajectory in vacuum-annealed pristine and Fe-doped TiO2 NPs, each curve telling a magnetic tale [186].
Oxygen Vacancies’ Role: The magnetic ordering in Fe-doped TiO2 NPs toggles between paramagnetism and ferromagnetism via oxygen vacancies [186].
Defect-Induced Ferromagnetism: The interplay of defects and Fe doping emerges as a potent mechanism, triggering ferromagnetic exchange coupling [186].
3.11. Role of Ion Beam Irradiation in Defect Engineering
Defect Engineering Unveiled: ion beam irradiation stands as a masterful technique to incorporate defects and enhance ferromagnetic properties [187,188,189,190].
A Careful Approach: low-energy ion beam irradiation with inert gases strikes a balance, enhancing defects without complicating the material’s chemistry [188].
Photocatalysis at the Nexus: photocatalytic performance hinges on electrical, optical, and structural attributes, with defects playing a decisive role [185].
Charting the Trajectory: DMS, alongside defect engineering, charts the course for advancements across materials science and technology.
Magnifying Magnetic Species: Figure 4A presents a visualization of the magnetic species, their distribution, and potential interactions, elucidating the delicate dance of magnetism within Fe-doped TiO2 NPs [186].
Magnetization Under the Lens: Figure 4B visualizes magnetization’s narrative with M–H curves, showing the magnetic journey in Fe-doped TiO2 NPs [186].
In the dynamic realm of DMS, each discovery uncovers new dimensions, bolstering the quest for unparalleled functionalities. As the research continues, DMS materials stand poised to revolutionize technology, offering fascinating insight into the ever-evolving narratives of the magnetic and semiconductor domains.
4. Harnessing Visible Light for Photocatalysis: Progress and Prospects
Recent breakthroughs in the realm of visible light photocatalysis have illuminated a pathway forward in harnessing solar energy for diverse applications. Visible-light-responsive photocatalysts are key to addressing global challenges, spanning environmental remediation, energy conversion, and storage. The ability to efficiently convert sunlight into usable energy has garnered significant attention due to its potential to mitigate the environmental impact of traditional energy sources and reduce our carbon footprint. As researchers continue to uncover innovative strategies for enhancing the efficiency and selectivity of visible light photocatalysts, the prospect of realizing sustainable and ecofriendly technologies becomes increasingly attainable. These advancements not only underscore the power of interdisciplinary collaboration but also inspire a future where sunlight acts as a driving force for positive change on a global scale.
4.1. Advancements in Photocatalyst Design
Innovations in Material Choices: Researchers have spearheaded advances in visible light photocatalysts, crafting novel solutions from metal oxides, carbon-based materials, and hybrid nanocomposites [191,192]. These materials exhibit heightened light absorption and improved charge separation efficiency, unlocking the potential to capture a broader solar spectrum.
Defects and Dopants: Harnessing the power of defects and dopants, studies showcase enhanced photocatalytic activity for organic pollutant degradation and clean fuel generation, such as hydrogen. The incorporation of various dopants and defects amplifies the photocatalytic prowess of these materials.
Unraveling Mechanistic Insights: Researchers delve into the mechanics that underpin visible light photocatalysis, delving into the intricacies of bandgap engineering, energy level alignment, and charge carrier dynamics [193,194,195]. These insights illuminate the avenues for optimizing the performance of photocatalytic materials.
Beyond Conventional Approaches: Innovative strategies, like plasmonic nanoparticle integration, cocatalyst deposition, and heterostructure formation, are poised to elevate visible light photocatalytic efficiency. These innovative pathways hold promise for crafting efficient and stable photocatalysts for large-scale environmental and energy applications.
4.2. Wide-Ranging and Challenging Applications
Expanding Horizons: The potential of visible light photocatalysts is not confined to a single domain. These advancements extend to water splitting, CO2 reduction, and pollutant remediation, making headway in addressing global energy and environmental challenges in a sustainable manner. Water splitting, driven by visible light-responsive photocatalysts, offers a pathway to produce clean hydrogen fuel from water, presenting a promising alternative to conventional fossil fuels. Moreover, CO2 reduction using these materials provides a tantalizing solution to counteracting greenhouse gas emissions by converting CO2 into valuable fuels and feedstocks. In the realm of pollutant remediation, visible light photocatalysts are poised to revolutionize water and air purification technologies, paving the way for cleaner and healthier environments [196].
Pioneering Challenges: While significant strides have been made in the realm of visible light photocatalysis, challenges persist on the path to widespread implementation. The quest to enhance quantum efficiency, maximize photocatalyst stability, and mitigate the detrimental effects of photo-corrosion remains a focal point of research efforts. The intricate interplay between material properties, such as band structure, surface morphology, and defect concentration, and their ultimate impact on photocatalytic performance requires further elucidation. Addressing these challenges demands a multidisciplinary approach that combines materials science, chemistry, engineering, and theoretical modeling [197].
Tackling Efficiency: One of the central challenges in visible light photocatalysis is improving the quantum efficiency of the process ensuring that a higher percentage of absorbed light is effectively utilized for the desired photocatalytic reactions. Strategies such as bandgap engineering, surface modification and cocatalyst incorporation are being explored to enhance light absorption, charge separation, and reaction kinetics, thereby optimizing the overall efficiency of the process.
Stability and Durability: The long-term stability and durability of visible light photocatalysts are critical factors for their practical implementation. Photocatalyst degradation due to photo-corrosion, surface fouling, and other degradation mechanisms can hinder their performance over time. Researchers are delving into the development of novel materials and protective coatings that can mitigate these degradation pathways and extend the operational lifespan of photocatalysts.
Uncovering Mechanistic Insights: Understanding the intricate mechanisms that govern the interactions among photons, charge carriers, and reactants on the photocatalyst surface is paramount for designing more efficient materials [198]. This involves unraveling complex surface reaction pathways, quantifying charge transfer processes, and deciphering the role of defects in catalytic performance. Advanced characterization techniques and theoretical simulations play a pivotal role in providing insights into these fundamental processes.
A Future of Possibilities: Despite these challenges, the future of visible light photocatalysis is brimming with possibilities. As researchers continue to uncover the fundamental principles governing photocatalytic processes and explore innovative materials and strategies, the potential for scalable, sustainable, and economically viable solutions becomes increasingly evident. The convergence of scientific understanding, technological innovation, and global demand for clean energy and environmental solutions paves the way for a future where visible light photocatalysts play a pivotal role in shaping a more sustainable world.
4.3. A Vision for a Transformed Landscape
Transformative Potential: The progress in harnessing the power of visible light photocatalysts resonates with the intriguing advancements made. These strides not only hold the potential to tap into solar energy but also to revolutionize how we address pressing environmental and energy-related concerns. As we stand at the intersection of scientific innovation and real-world applications, the landscape of visible light photocatalysis holds the promise of reshaping industries and redefining the way we harness and utilize energy.
A Landscape of Promise: The journey of visible light photocatalysts is characterized by relentless exploration, innovation, and collaboration across diverse scientific disciplines. With ongoing research and development, the trajectory of visible light photocatalysts is poised to reshape the renewable energy and sustainable technology arena, propelling us toward a cleaner and more efficient future [199]. From powering remote communities with solar-derived hydrogen to mitigating air and water pollution on a global scale, the potential of visible light photocatalysis is far-reaching and transformative.
Sustainable Synergy: The transformative potential of visible light photocatalysts extends beyond individual applications. The synergy between these materials and other emerging technologies, such as energy storage systems and smart grids, presents the opportunity for holistic and sustainable energy solutions. This interconnected approach has the power to usher in an era where our energy sources are not only clean but also intelligently integrated, ensuring stability and reliability in our energy infrastructure.
A Collaborative Journey: The vision of a transformed landscape driven by visible light photocatalysis is a collective endeavor. Researchers, engineers, policymakers, and industrial leaders collaborate to bridge the gap between fundamental scientific breakthroughs and practical applications. This journey is underscored by the recognition that tackling global challenges requires a multidimensional approach—one that seamlessly integrates scientific excellence with technological innovation and societal engagement.
Fostering a Resilient Future: As we envisage a landscape transformed by visible light photocatalysis, we glimpse a future marked by energy independence, environmental responsibility, and sustainable prosperity. The ability to tap into the abundant and renewable energy of the sun, combined with the creativity of the scientific community, empowers us to build a more resilient and equitable world for future generations. With each new advancement, we move closer to realizing this vision and embracing the potential of a transformed tomorrow.
4.4. Advancing Energy Conversion with TiO2-Based Materials
In the pursuit of efficient energy conversion, TiO2-based materials have emerged as pivotal players, propelling the realm of solar power-based energy conversion and wastewater treatment into new frontiers [200,201,202]. These materials stand at the forefront of harnessing solar-based light energy to drive chemical reactions and generate vital electrical power. Advancing energy conversion through the utilization of TiO2-based materials stands at the forefront of innovative research in sustainable technology. TiO2, a versatile metal oxide semiconductor, has emerged as a cornerstone for efficient energy conversion because of its exceptional photocatalytic properties. These materials possess the remarkable ability to harness sunlight and initiate catalytic reactions, such as water splitting and pollutant degradation, with remarkable efficiency [203]. This capability not only contributes to clean energy generation and environmental remediation but also holds promise for advancing the realms of hydrogen production and solar fuel synthesis [204]. Additionally, TiO2-based materials have found application in dye-sensitized solar cells, where they efficiently convert solar energy into electricity [205]. As researchers delve deeper into the design and engineering of TiO2-based materials at the nano- and microscale, novel strategies are being developed to enhance light absorption, charge separation, and overall conversion efficiency. Through synergistic efforts in materials science, chemistry, and engineering, the integration of TiO2-based materials into energy conversion technologies is poised to revolutionize our approach to sustainable energy solutions, forging a cleaner and more resourceful energy landscape.
The production of magnetic TiO2 nanocomposites employs diverse techniques to integrate magnetic components into the TiO2 matrix. Common methods include coprecipitation, where precursor solutions of titanium and magnetic metal ions are mixed, resulting in the formation of dispersed magnetic nanoparticles [206,207]. Sol-gel synthesis involves hydrolysis and condensation of metal alkoxides to form a gel, offering precise control over the composition and distribution of magnetic nanoparticles [208,209]. Hydrothermal synthesis utilizes high temperature and pressure to promote the formation of TiO2 nanoparticles decorated with magnetic nanoparticles [210]. Chemical vapor deposition (CVD) and physical vapor deposition (PVD) methods enable the growth of TiO2 thin films with embedded magnetic nanoparticles through deposition processes [211]. These techniques provide tailored control over the nanoparticle size, composition, and morphology within the TiO2 matrix, catering to diverse application requirements.
4.5. Pursuit of Efficiency: Noble Metal Doping
The pursuit of efficiency in materials science has led researchers to explore the strategy of noble metal doping in metal oxides, uncovering a pathway toward the enhancement of the catalytic and electronic properties [212]. Noble metals, known for their exceptional catalytic activity and stability, are introduced as dopants into metal oxide matrices to create hybrid materials with synergistic functionalities. By strategically incorporating elements like gold, platinum, or palladium into metal oxide structures, catalytic processes such as oxygen reduction reactions in fuel cells or CO2 conversion are accelerated, owing to the unique electronic and surface properties of these metals [213,214]. Furthermore, noble metal doping can modulate the electronic band structure of metal oxides, resulting in improved charge carrier mobility and enhanced photocatalytic efficiency [215]. This approach not only tackles the challenge of limited intrinsic catalytic activity in metal oxides but also opens doors to tailor-made materials for various applications in energy conversion, environmental remediation, and beyond [37]. The pursuit of efficiency through noble metal doping underscores the innovative nature of materials design, as researchers endeavor to unlock new avenues for sustainable technologies with higher performance and versatility. To amplify the efficiency of TiO2-based photocatalysis, noble metal doping, and modification strategies have garnered significant interest. This approach seeks to unlock enhanced photocatalytic performance through tailored modifications. The incorporation of silver (Ag) into TiO2, particularly in various forms, such as Ag cluster-incorporated AgBr NPs and Ag/AgCl in TiO2 photocatalysts, highlights a promising method of enhancing the efficiency [202,216].
4.6. Leveraging Hierarchical Assembly for Superior Performance
Strategic Nanomaterial Assembly: the concept of hierarchical heterostructures, formed through the strategic assembly of nanoscale building blocks, holds the promise of elevating photocatalytic performance by harnessing tunable dimensionality and structural complexity [217].
Multifunctional Materials: Hierarchical heterostructures offer not only enhanced performance but also a versatile platform with applications spanning various domains, making strides toward meeting multifaceted energy and environmental challenges [218]. The journey of energy conversion through TiO2-based materials stands as a testament to human innovation and the limitless potential of harnessing the sun’s energy for a sustainable and greener future.
Efficient Energy Conversion: contemporary research endeavors shine a light on solar power-based energy conversion and wastewater treatment, stirring considerable interest [183,184,185].
Photon-Powered Chemical Reactions: photocatalytic and photovoltaic solar cells, the vanguards of solar-based light energy conversion, fuel chemical reactions and generate electrical power.
TiO2’s Crucial Role: TiO2’s versatile properties find applications in diverse environmental and energy realms, including photocatalysis, photovoltaics, artificial photosynthesis, and spintronics [219].
Nanocrystals to Unleash Potential: to enhance TiO2’s photocatalytic activity with visible light, noble metal (Pt, Pd, Rh, and Au)-doped and -modified TiO2 photocatalysts have garnered attention for ability to enhance efficiency [220].
Ag-Loaded TiO2 Marvel: the integration of Ag into TiO2, such as Ag cluster-incorporated AgBr NPs, Ag NPs, CuO nanoclusters, and Ag/AgCl in TiO2 photocatalysts, has emerged as a promising approach [196,197].
Harnessing Heterojunctions: interfacial heterojunctions between TiO2 and SnO2 create a synergy that elevates photoactivity [221].
Hierarchical Heterostructures: the strategic assembly of nanoscale building blocks offers an avenue to enhance photocatalytic performance by tuning dimensionality and structural complexity [222].
Multifunctionality Unleashed: hierarchical heterostructures unlock ultrahigh specific surface areas and interconnected networks, facilitating improved performance across applications [223].
4.7. Exploring Fe-Doped TiO2 Mechanics
Exploring the mechanics of Fe-doped TiO2 reveals a fascinating realm at the intersection of materials science and energy conversion. Fe-doped TiO2 a distinguished member of the TiO2-based materials family has emerged as a compelling candidate for advancing photocatalytic and energy-related applications. By introducing Fe(III) ions into the TiO2 lattice, researchers have successfully bridged the gap between visible light absorption and efficient charge carrier transfer [224]. This strategic doping not only enhances the material’s photocatalytic activity but also brings forth its potential as a cocatalyst for multielectron reduction reactions. The synergy between surface-grafted and bulk-doped Fe(III) ions holds the key to effective charge carrier transportation, enabling the material to excel in decomposing organic compounds. Through a blend of material engineering and an in-depth understanding of charge carrier dynamics, Fe-doped TiO2 demonstrates the intricate interplay between material properties and photocatalytic performance [225]. This exploration propels us closer to unlocking the untapped potential of Fe-doped TiO2, offering a deeper understanding of the mechanisms that underlie its remarkable capabilities in advancing energy conversion and environmental remediation.
Reimagining TiO2 with Fe(III): the intricacies of the Fe(III)-FexTi1−xO2 system illuminate the potential for surface grafting and bulk doping in visible-light absorption, as shown in Figure 5 [50,52].
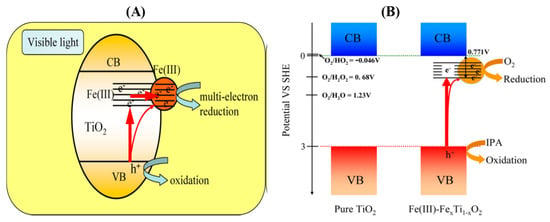
Figure 5.
Illustration of the proposed photocatalysis process (A) and the change in bandgap and photo-activity due to Fe doping (B) [52].
Balancing Efficiency: the creation of an interface junction between surface-grafted and bulk-doped Fe(III) ions is essential for efficient charge carrier transfer.
Unlocking Photocatalytic Potential: effective charge carrier transfer to the surface of Fe(III)-doped TiO2 leads to efficient cocatalyst functionality for multi-electron reduction reactions [50,52].
Efficient Decomposition: the unique property of Fe(III)-doped TiO2 allows for deep-level valence band holes to decompose organic compounds, resulting in high levels of photocatalytic activity.
4.8. Charting the Future: AgCl-Loaded Sn-Doped TiO2
The alliance between AgCl NPs and Sn-doped TiO2 microspheres stands as a compelling approach to amplify visible-light activity, leaving a prominent mark on photocatalytic and photovoltaic applications [226,227]. The journey of visible light photocatalysis is both fascinating and transformative, as researchers forge pathways to tap into solar energy’s abundance, inspiring innovations that promise to reshape the energy and environmental landscape. Charting the future, the integration of AgCl-loaded Sn-doped TiO2 emerges as a promising frontier in advanced materials research. This innovative hybrid material combines the exceptional properties of tin-doped TiO2 with the unique attributes of AgCl, paving the way for multifunctional applications. Sn-doped TiO2, already known for its enhanced charge carrier mobility and extended visible-light absorption, synergistically merges with AgCl’s exceptional visible-light photocatalytic capabilities and antibacterial properties [228]. This novel composite holds immense potential in fields ranging from sustainable energy production and water purification to medical devices. Its ability to harness sunlight efficiently for catalytic reactions while concurrently acting as an antimicrobial agent positions it at the crossroads of various technological advancements. By charting this unexplored territory, researchers are striving to shape a future in which tailored materials exhibit unprecedented multifaceted functionalities, leading us toward sustainable solutions and novel scientific horizons [229].
5. Recent Advances in Magnetic TiO2: Expanding Horizons for Ferromagnetic Photocatalysis
This review delves into the intricate interplay between magnetism and photocatalytic activity within TiO2, unveiling a realm of opportunities across diverse applications such as environmental remediation, solar energy conversion, and advanced catalysis. By strategically incorporating magnetic elements like Fe, Mn, or Co into TiO2 structures, researchers have forged a path toward magnetic TiO2 materials that exhibit both ferromagnetic behavior and exceptional photocatalytic efficiency. The article meticulously examines the spectrum of applications that magnetic TiO2 offers, including its pivotal role in purifying the environment, treating wastewater, and degrading organic pollutants under both visible and UV light [230]. As this dynamic field progresses, the review article underscores the challenges in precisely controlling magnetic properties, comprehending the underlying mechanisms governing this dual behavior, and scaling up production for practical applications. Ultimately, the review accentuates the immense potential of magnetic TiO2 as a driver for sustainable technologies and clean energy solutions, pointing toward a future shaped by innovative TiO2-based materials with remarkable multi-functionality [231,232,233].
5.1. Combining Magnetism and Photocatalysis: Unleashing TiO2’s Potential
The convergence of magnetic and photocatalytic properties within TiO2 offers a range of possibilities across environmental remediation, solar energy conversion, and advanced catalysis [34]. Although traditional TiO2 boasts exceptional photocatalytic traits, the introduction of magnetic elements like Fe, Mn, or Co into TiO2 paves the way for magnetic TiO2. This novel breed exhibits both ferromagnetic behavior and photocatalytic capabilities, yielding exciting applications across various domains.
Exploring Applications and Opportunities: Magnetic TiO2’s impact spans far and wide, from cleaning up the environment to treating wastewater and purifying air. It proficiently dismantles organic pollutants under both UV and visible light, making it pivotal for sustainable solutions. Notably, it finds its footing in solar energy conversion systems and advanced catalytic processes. Amid ongoing research, the journey is focused on enhancing both photocatalytic efficiency and magnetic properties, which is crucial for scalability and practical implementation [234].
5.2. Delving into Sn-Doped TiO2: Amplifying Performance and Potential
Tailoring Sn-Doped TiO2: Through an exploration of diverse Sn doping concentrations, our previous studies have shed light on augmenting the structural, electronic, magnetic, and photocatalytic attributes of TiO2 NPs [84,184,191,223]. A notable highlight emerges in the form of Sn-doped TiO2 NPs’ room-temperature photocatalytic and ferromagnetic prowess, particularly in the realm of environmental remediation. By integrating variable SnCl4 concentrations into Ti(NO3)4 aqueous solutions, we harnessed a facile hydrothermal technique to synthesize TiO2 NPs featuring anatase, anatase–rutile mix, and rutile phases with embedded Sn atoms.
Probing Photocatalytic Behavior: To assess the prowess of synthesized Sn-TiO2 NPs, we turned to methyl orange (MO) and RPhOH (where PhOH represents a phenol group and R is 3-NH2, H, or 4-Cl) as model pollutants, both under visible and UV light. Illumination unearthed a compelling connection between RPhOH’s Hammett substitution constant (σ) and the degradation efficiency of Sn-TiO2 NPs. The concentration of Sn doping played a pivotal role, wielding influence over structural, electronic, magnetic, and photocatalytic traits of the TiO2 NPs. Amidst extensive research, the enigma of combined ferromagnetism and photocatalytic behavior prevails, underpinned by factors like oxygen vacancies, phase transitions, and doping levels. Our current pursuit aims to unravel the role of Sn4+ ions in shaping these properties of TiO2 NPs.
5.3. Hierarchical SNT Microspheres: Pioneering Enhanced Photocatalysis and Ferromagnetism
Co-Doping for Enhanced Performance: Our exploration continued in the synthesis arena, wherein we employed a hydrothermal route followed by nitriding treatment using flowing ammonia gas to craft hierarchical Sn and nitrogen co-doped TiO2 (SNT) microspheres [65,169]. This innovation heralded improved photocatalytic efficacy and room-temperature ferromagnetism through the simultaneous incorporation of Sn and N atoms. These co-doped microspheres emerged as a breakthrough, outshining both pristine and Sn-doped TiO2 NPs. The co-doped microspheres demonstrated remarkable visible light absorption, resulting in elevated photocatalytic activity and highlighting their potential for efficient solar-driven applications. While these microspheres exhibited resilience in the face of Rhodamine B (RhB) degradation under visible light, their magnetic behavior remained an uncharted territory, intriguing us with the possibility of room-temperature ferromagnetism stemming from trapped electrons in oxygen vacancies (VO) or structural anomalies. This multifaceted study not only enriches our understanding of visible light photocatalysis and room-temperature ferromagnetism but also sets new trajectories for TiO2-based materials, potentially shaping their role in diverse applications, including photovoltaics.
Revealing Novel Hierarchical Structure: The synthesis of hierarchical SNT microspheres with co-doping introduces a novel structural dimension that goes beyond traditional TiO2 photocatalysts. The hierarchical arrangement not only enhances light trapping and charge separation but also offers an increased surface area for catalytic interactions. The integration of vanadium oxide as a co-dopant introduces additional complexity to the system, potentially leading to new electronic and magnetic phenomena. Understanding the interplay between the hierarchical structure, co-doping, and resulting properties opens the door to tailoring materials for specific applications that demand both enhanced photocatalytic activity and magnetic behavior [235].
Ferromagnetism and Trapped Electrons: The intriguing possibility of room-temperature ferromagnetism in the SNT microspheres sparks curiosity about its origins. Trapped electrons in the VO co-dopant or structural defects could potentially contribute to this magnetic behavior. Unraveling the mechanisms behind the observed ferromagnetic properties would shed light on the potential of harnessing defect engineering to achieve multifunctionality in TiO2-based materials. Furthermore, this insight could pave the way for the development of novel magnetic photocatalysts with broader implications for energy conversion and storage.
Charting New Trajectories: This study marks a significant advancement in the field of TiO2-based materials and their applications. The co-doped SNT microspheres not only showcase enhanced photocatalytic performance but also introduce the element of ferromagnetism, adding to the growing pool of multifunctional materials. The implications span various domains, including photocatalysis, magnetics, and potentially photovoltaics. By charting these new trajectories, this research exemplifies the iterative nature of scientific progress, where each discovery opens up unforeseen opportunities for innovation and exploration.
Innovative Insights and Future Directions: The synthesis and characterization of hierarchical SNT microspheres with co-doping provide innovative insights into the intricate relationship between structure, composition, and properties. As we delve deeper into understanding the origins of ferromagnetic behavior and its synergy with photocatalytic activity, new avenues for tailored materials and applications come to light. The co-doped SNT microspheres serve as a testament to the potential of materials design and engineering, offering glimpses into a future where multifunctional materials play a pivotal role in addressing complex technological challenges.
5.4. Advancing Photocatalysis through Hierarchical AgCl in Sn-TiO2 Microspheres
A novel synthesis methodology emerged, culminating in the creation of hierarchical AgCl in Sn-TiO2 (AST) microspheres through diverse postcalcination treatments [184,236]. The central aim lay in enhancing photocatalytic potency by loading AgCl NPs onto Sn-doped TiO2. These AST microspheres outperformed Sn-TiO2, AgCl, Ag/AgCl, and commercial Degussa P25 photocatalysts, flaunting superior visible light absorption. Under visible light, these hierarchical AST microspheres showcased heightened degradation rates for model systems like RhB and 3-nitrophenol aqueous solutions. A thorough exploration of varying AgCl concentrations in the AST microspheres emerged as an essential next step. Furthermore, this study pioneers the facile synthesis route, elevated visible-light photocatalysis within hierarchical AST microspheres, and uncovers the magnetic attributes through the Sn Mössbauer method. This revelation ushers in a new class of semiconductor materials, opening exciting avenues for TiO2-based innovations.
5.5. Correlation between Magnetic and Photocatalytic Properties
The simultaneous increase in ferromagnetic character and photocatalytic efficiency for metal oxide semiconductors can be attributed to specific material properties and interactions. However, it is important to note that such a correlation is not always straightforward and depends on various factors. Here’s a general explanation for why this could occur:
Crystal Structure and Defects: Both ferromagnetism and photocatalytic efficiency are influenced by the crystal structure and defects within metal oxide semiconductors [237]. Certain crystal structures can support both ferromagnetic ordering of electron spins and efficient charge carrier generation for photocatalysis. Defects, such as oxygen vacancies or dopants, can enhance both magnetic properties and photocatalytic performance by creating additional electronic states for carriers to populate [238].
Electronic Band Structure: The electronic band structure of a material plays a crucial role in determining its magnetic and photocatalytic properties [239,240]. If the material’s band structure allows for the existence of partially filled d or f orbitals, this can lead to ferromagnetism. Simultaneously, the same band structure can enable efficient charge separation and mobility necessary for effective photocatalysis.
Synergistic Charge Carrier Behavior: Efficient photocatalysis relies on the effective separation and migration of photo-generated charge carriers (electrons and holes) [241,242]. In some cases, the same processes that lead to ferromagnetic behavior, such as exchange interactions between electron spins, can also facilitate the movement of charge carriers, enhancing photocatalytic efficiency.
Surface and Interface Effects: The surface and interface properties of metal oxide semiconductors are critical in determining their catalytic and magnetic behaviors. Surface defects and exposed facets can provide active sites for photocatalysis, while also influencing magnetic interactions in the vicinity of the surface [243,244].
Doping and Elemental Composition: Controlled doping of metal oxide semiconductors can tune both their magnetic and photocatalytic properties [245]. Certain dopants can introduce magnetic moments and enhance photocatalytic activity simultaneously, making it possible to design multifunctional materials [246].
Spintronic Effects: The coupling of spin and charge in ferromagnetic materials can lead to spin-dependent charge transport phenomena. These effects can enhance the efficiency of charge separation and transport in photocatalytic processes [247,248].
Complex Interplay: The relationship between magnetic character and photocatalytic efficiency is complex and not always linear. These properties can be enhanced in tandem because of shared underlying mechanisms, but they can also exhibit opposing behaviors depending on factors such as material composition, crystal structure, and external conditions [249,250,251].
The increase in ferromagnetic character and photocatalytic efficiency for metal oxide semiconductors can arise from shared material properties, such as electronic band structure, defects, and charge carrier behavior [252,253,254,255]. However, it is important to evaluate each material system individually, as the correlations among these properties can vary based on specific conditions and material characteristics.
6. Advances in Mössbauer Spectroscopy and Ferromagnetic Photocatalytic Studies of Sn and Fe-Doped TiO2 Nanocomposites
The rapid emergence of TiO2-based nanocomposites has generated significant interest, driven by their exceptional photocatalytic properties and potential applications spanning environmental remediation, solar energy conversion, and advanced catalysis. Delving into the realm of enhancement, the incorporation of dopants like Sn and Fe has been meticulously explored, unlocking a host of structural, electronic, and magnetic augmentations within TiO2 [256]. Anchored at the atomic level, Mossbauer spectroscopy emerges as a pivotal tool to scrutinize the intricate interplay of these doped nanocomposites’ structural and magnetic attributes [257].
Revealing the Dopant Influence: The intentional introduction of dopants like Sn and Fe into TiO2 matrices marks a paradigm shift in materials engineering. These dopants, carefully selected for their electronic and magnetic properties, engender complex alterations in the host material’s structure and behavior. Understanding the nuanced effects of dopants on both lattice structure and electronic states is crucial for tailoring materials with desired properties. Mössbauer spectroscopy, a precise and sensitive technique, plays a transformative role in unraveling the intricacies of these dopant-induced modifications at the atomic scale.
Probing Local Environments: One of the distinguishing features of Mössbauer spectroscopy is its ability to probe the local atomic environment with unparalleled precision [258].
By harnessing the Mössbauer effect—a quantum mechanical phenomenon involving gamma-ray absorption and emission—researchers gain insights into the oxidation state, coordination geometry, and magnetic interactions of specific atomic species, even in the presence of complex materials [259]. In the context of Sn- and Fe-doped TiO2 nanocomposites, Mössbauer spectroscopy reveals the local environments surrounding these dopants, shedding light on their integration into the host lattice and their role in governing the resulting properties [260].
Deciphering Magnetic Phenomena: The introduction of dopants like Fe introduces the potential for magnetic behavior within TiO2, transforming it into a ferromagnetic material. Mössbauer spectroscopy is uniquely positioned to decipher the magnetic properties of these doped nanocomposites. By characterizing hyperfine interactions and magnetic hyperfine splitting patterns, researchers can elucidate the nature of magnetic ordering, the presence of magnetic clusters, and the mechanisms that underpin room-temperature ferromagnetism [261,262].
This knowledge not only enriches our fundamental understanding of the material’s behavior but also offers critical insights for the development of magnetic photocatalysts and related applications [263].
Mapping Ferromagnetic Photocatalysis: The convergence of Mössbauer spectroscopy with ferromagnetic photocatalytic studies creates a synergistic approach that bridges structural and magnetic analyses with functional performance. This integrated methodology allows researchers to correlate magnetic phenomena, such as room-temperature ferromagnetism, with enhanced photocatalytic activity. By mapping the intricate relationship between magnetic behavior and photocatalytic efficiency, this approach guides the design and optimization of materials with multifunctional properties, propelling advancements in solar energy utilization and environmental remediation.
Future Prospects: The union of Mössbauer spectroscopy and ferromagnetic photocatalytic studies ushers in a new era of materials characterization and engineering. As nanocomposites continue to evolve and find applications in diverse fields, this combined approach holds promise for uncovering hidden correlations between structure, magnetism, and functionality [28]. By leveraging the atomic-level insights provided by Mössbauer spectroscopy, researchers are set to accelerate the development of tailored materials that redefine technological possibilities and contribute to a more sustainable future.
Further, an in-depth exploration of Sn- and Fe-doped TiO2 nanocomposites is presented through the lens of Mössbauer spectroscopy. This meticulous analysis unravels the structural and magnetic intricacies, contributing to the fundamental understanding of how dopants, structural anomalies, and photocatalytic activity intertwine. Such insights are pivotal for designing durable and efficient photocatalytic materials. Peering into the heart of nanocomposites, Mössbauer spectroscopy emerges as an invaluable tool, revealing a wealth of structural and magnetic insights. As nanocomposites revolutionize materials engineering, the complex interactions between distinct components demand meticulous characterization. Mössbauer spectroscopy, with its ability to provide detailed information about the oxidation state, local environment, and magnetic behavior of atoms, offers a unique window into these intricate systems. By probing the hyperfine interactions between atomic nuclei and their surroundings, this technique facilitates the understanding of nanoscale phase distribution, chemical bonding, and magnetic coupling within composite materials. From catalytic nanoparticles on support matrices to magnetic oxide-polymer hybrids, the application of Mössbauer spectroscopy uncovers hidden correlations, guiding the design and optimization of tailored nanocomposites with enhanced performance. Its role in illuminating the inner workings of these innovative materials reinforces its status as an indispensable analytical tool, enabling researchers to sculpt the future of advanced materials with precision and insight.
7. A Glimpse into the Future: Potential and Prospects
Offering a glimpse into the future, this review article not only presents a cutting-edge overview of the advancements in Mossbauer spectroscopy and ferromagnetic photocatalytic studies of Sn and Fe-doped TiO2 nanocomposites but also extends its horizon toward the potential and prospects that lie ahead. By weaving together these two intricate fields of study, the article not only sheds light on the present state of research but also envisions novel avenues of comprehension and application for these materials in the realms of environmental remediation and renewable energy technologies. This integrative approach, fusing the analytical precision of Mossbauer spectroscopy with magnetic and photocatalytic investigations, constructs a comprehensive roadmap to unlock the full potential of these nanocomposites. This endeavor is poised to propel the advancement of high-performance, sustainable photocatalytic materials, capable of addressing pressing environmental challenges and powering future energy solutions. Drawing inspiration from the reservoir of insights gleaned from prior studies, this review not only encapsulates the multifaceted journey of exploration, understanding, and application but also serves as a foundation for ushering in an era characterized by the emergence of innovative TiO2-based materials with far-reaching implications. As researchers traverse this dynamic landscape, they are poised to shape the trajectory of scientific discovery and technological innovation, forging a transformative path toward a cleaner and more sustainable future.
8. Conclusions
This review underscores the paramount importance of broadening the scope of wide-bandgap metal oxide nanoparticles (NPs) in the realm of photocatalysis. A prominent spotlight has been cast upon the advancement of room-temperature ferromagnetic TiO2, a notable photocatalyst that is adept at harnessing visible light from the solar spectrum with utmost efficiency. Nonetheless, pristine TiO2 NPs harbor limitations such as accelerated recombination of photo-generated charges and reliance on UV light for catalytic prowess. In pursuit of fully harnessing the potential of TiO2-based photocatalysts, a diverse array of strategies were meticulously explored. Within the purview of this review, two potent pathways were dissected and expounded. The first avenue involves the strategic infusion of metallic or nonmetallic dopants into TiO2, a transformation that tweaks its band structure, extends the photo-response into the visible spectrum, and, crucially, enhances the separation of charge carriers. Additionally, grafting TiO2 NPs with either anionic or cationic elements emerges as a transformative maneuver to fine tune surface properties, foster interfacial charge transfer, and enhance photocatalytic efficacy under illumination with visible light. Complementary to this trajectory, the review sheds light on another compelling development: the amalgamation of TiO2 NPs with other semiconductor counterparts. The resultant heterojunctions manifest a heightened efficiency in separating electron–hole pairs, presenting an innovative approach to the expansion of the photo-response spectrum and enhancement in the overall photocatalytic performance. Gazing into the horizon, the pathway ahead necessitates comprehensive explorations to unearth the true potential of novel ferromagnetic metal oxide-based photocatalysts, especially in the context of large-scale applications. As the continuum of photocatalysis journeys forward, the quest for efficient, enduring, and environmentally benign photocatalytic materials resonates more than ever. The imperative to combat water contamination, and air pollution, and foster renewable energy conversion spurs us to harness the expansive potential harbored by wide-bandgap metal oxide NPs, most notably room-temperature ferromagnetic TiO2. Innovative doping and coupling strategies wield the key to unlocking novel scientific vistas, emboldening sustainable and pragmatic photocatalytic technologies. This voyage calls for a harmonious alliance of researchers across diverse domains, orchestrated to usher in large-scale applications of these trailblazing ferromagnetic metal oxide-based photocatalysts, propelling us toward a cleaner, more sustainable future.
Author Contributions
Conceptualization, G.A.S.; methodology, G.A.S.; software, G.r.M.; validation, G.A.S.; formal analysis, G.r.M. and J.E.; investigation, A.S.K.K. and V.P.; resources, G.A.S. and A.S.K.K.; data curation, G.r.M., V.P. and A.S.K.K.; writing—original draft preparation, G.A.S. and G.r.M.; writing—review and editing, G.A.S., V.P., J.E. and A.S.K.K.; visualization, A.S.K.K. and J.E.; supervision, G.A.S.; funding acquisition, G.A.S. and A.S.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fischer, D.K.; de Fraga, K.R.; Choi, C.W.S. Ionic liquid/TiO2 nanoparticles doped with non-expensive metals: New active catalyst for phenol photodegradation. RSC Adv. 2022, 12, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, J.D.; Kim, S.K.R. Correlated visible-light absorption and intrinsic magnetism of SrTiO3 due to oxygen deficiency: Bulk or surface effect? Inorg. Chem. 2015, 54, 3759–3765. [Google Scholar]
- Fan, C.M.; Peng, Y.; Zhu, Q.; Lin, L.; Wang, R.X.; Xu, A.W. Synproportionation reaction for the fabrication of Sn2+ self-doped SnO2−x nanocrystals with tunable band structure and highly efficient visible light photocatalytic activity. J. Phys. Chem. C 2013, 117, 24157–24166. [Google Scholar] [CrossRef]
- Kan, D.; Terashima, T.; Kanda, R.; Masuno, A.; Tanaka, K.; Chu, S.; Kan, H.; Ishizumi, A.; Kanemitsu, Y.; Shimakawa, Y.; et al. Blue-light emission at room temperature from Ar+-irradiated SrTiO3. Nat. Mater. 2004, 4, 816–819. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P.; Xing, P. d0 ferromagnetism in undoped n and p-type In2O3 films. Appl. Phys. Lett. 2012, 101, 132417. [Google Scholar] [CrossRef]
- Lou, C.; Lei, G.; Liu, X.; Xie, J.; Li, Z.; Zheng, W.; Goel, N.; Kumar, M.; Zhang, J. Design and optimization strategies of metal oxide semiconductor nanostructures for advanced formaldehyde sensors. Coord. Chem. Rev. 2022, 452, 214280. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Tyagi, S.; Saxena, N.; Khan, Z.H.; Kumar, P. Room temperature ferromagnetism in metal oxides for spintronics: A comprehensive review. Opt. Quantum Electron. 2023, 55, 123. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, C.; Roy, V.A. Recent progress in magnetic iron oxide—Semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar]
- Xiang, Y.; Li, Y.; Zhang, X.; Zhou, A.; Jing, N.; Xu, Q. Hybrid CuxO–TiO2 porous hollow nanospheres: Preparation, characterization and photocatalytic properties. RSC Adv. 2017, 7, 31619–31627. [Google Scholar] [CrossRef]
- Gao, D.; Wu, X.; Wang, P.; Xu, Y.; Yu, H.; Yu, J. Simultaneous realization of direct photoinduced deposition and improved H2-Evolution performance of Sn-Nano particle modified TiO2 Photocatalyst. ACS Sustain. Chem. Eng. 2019, 7, 10084–10094. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Khan, R.; He, T. Visible-Light Photoreduction of CO2 to CH4 over Zn Te-Modifited TiO2 Coral-Like Nanostructures. ChemPhysChem 2017, 18, 3203–3210. [Google Scholar] [CrossRef]
- Han, F.; Kamabala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Zvesti. 2023, 77, 677–701. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A. Advances in Magnetically Separable Photocatalysts: Smart, Recyclable Materials for Water Pollution Mitigation. Catalysts 2016, 6, 79. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.Z.; Yang, H.G.; Cheng, H.M.; Lu, G.Q. Titania-based photocatalysts-crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-light-active doped metal oxide nanoparticles: Review of their synthesis, properties, and applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2006, 36, 681–694. [Google Scholar] [CrossRef]
- Alam, M.W.; Khalid, N.R.; Naeem, S.; Niaz, N.A.; Ahmad Mir, T.; Nahvi, I.; Souayeh, B.; Zaidi, N. Novel Nd-N/TiO2 Nanoparticles for Photocatalytic and Antioxidant Applications Using Hydrothermal Approach. Materials 2022, 15, 6658. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Mushtaq, S.; Al Qahtani, H.S.; Sedky, A.; Alam, M.W. Investigation of TiO2 Nanoparticles Synthesized by Sol-Gel Method for Effectual Photodegradation, Oxidation and Reduction Reaction. Crystals 2021, 11, 1456. [Google Scholar] [CrossRef]
- Govinda raj, M.; Ganeshraja, A.S.; Kaviyarasan, K.; Hector, V.; Pugazhenthiran, N.; Katayama, K.; Sekhara Theja Vaskuri, C.; John Bosco, A.; Neppolian, B. Enhanced Photocatalytic Efficacy and Stability in Antibiotic Pollution Mitigation Using BiVO4 Nanoballs Encased in Ultrathin Polymeric g-C3N4 Nanocomposites under Visible Light Exposure. J. Phys. Chem. C 2024, 128, 3214–3232. [Google Scholar] [CrossRef]
- Ayyakannu Sundaram, G.; Kuppusamy, M.; Vadivel, G.; Karthikeyan, V.; Emsaeng, K.; Anbalagan, K. Unveiling room temperature ferromagnetism in Zinc(II)-picoline complex modified TiO2 for spintronic applications. J. Solid State Chem. 2023, 327, 124278. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, J.B.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Hariram, N.P.; Mekha, K.B.; Suganthan, V.; Sudhakar, K. Sustainalism: An Integrated Socio-Economic-Environmental Model to Address Sustainable Development and Sustainability. Sustainability 2023, 15, 10682. [Google Scholar] [CrossRef]
- Hossain, N.; Mobarak, M.-H.; Mimona, M.K.; Islam, M.A.; Hossain, A.; Zohura, F.T.; Chowdhury, M.A. Advances and significances of nanoparticles in semiconductor applications—A review. Results Eng. 2023, 19, 101347. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-based nanocomposites for sustainable applications: A review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Paudel, H.P.; Syamlal, N.; Crawford, S.; Lee, Y.-L.; Shugayev, R.; Lu, P.; Ohodnicki, P.R.; Mollot, D.; Duan, Y. Quantum computing and simulations for energy applications: Review and perspective. ACS Eng. Au 2022, 2, 151–196. [Google Scholar] [CrossRef]
- Bergmann, M.; Collard, F.; Fabres, J.; Gabrielsen, G.W.; Provencher, J.F.; Rochman, C.M.; van Sebille, E.; Tekman, M.B. Plastic pollution in the Arctic. Nat. Rev. Earth Environ. 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 13, 95–112. [Google Scholar] [CrossRef]
- O’Neill, S.; Robertson, J.M.C.; Héquet, V.; Chazarenc, F.; Pang, X.; Ralphs, K.; Skillen, N.; Robertson, P.K. Comparison of Titanium Dioxide and Zinc Oxide Photocatalysts for the Inactivation of Escherichia coli in Water Using Slurry and Rotating-Disk Photocatalytic Reactors. Ind. Eng. Chem. Res. 2023, 62, 18952–18959. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Khatoon, A.; Rha, E.S.; Jamil, M. Synergistic Effects of Zinc Oxide Nanoparticles and Bacteria Reduce Heavy Metals Toxicity in Rice (Oryza sativa L.). Plant. Toxics 2021, 9, 113. [Google Scholar] [CrossRef]
- Wang, S.; Alenius, H.; El-Nezami, H.; Karisola, P. A New Look at the Effects of Engineered ZnO and TiO2 Nanoparticles: Evidence from Transcriptomics Studies. Nanomaterials 2022, 12, 1247. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- El-sheikh, S.M.; Zhang, G.; El-hosainy, H.M.; Ismail, A.A.; Shea, K.E.O.; Falaras, P.; Kontos, A.G.; Dionysiou, D.D. High performance sulfur, nitrogen and carbon doped mesoporous anatase—Brookite TiO2 photocatalyst for the removal of microcystin-LR under visible light irradiation. J. Hazard. Mater. 2014, 280, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, L.; Bouzerar, G.; Srivastava, S.K. d0 Ferromagnetism in Ag-doped monoclinic ZrO2 compounds. Vacuum 2020, 182, 109716. [Google Scholar] [CrossRef]
- Tsurkan, V.; Nidda, H.K.; Deisenhofer, J.; Lunkenheimer, P.; Loidl, A. On the complexity of spinels: Magnetic, electronic, and polar ground states. Phys. Rep. 2021, 926, 1–86. [Google Scholar] [CrossRef]
- Fu, L.; You, S.; Li, G.; Li, X.; Fan, Z. Application of semiconductor metal oxide in chemiresistive methane gas sensor: Recent developments and future perspectives. Molecules 2023, 28, 6710. [Google Scholar] [CrossRef]
- Anbalagan, K. UV-Sensitized generation of phase pure cobalt-doped anatase: CoxTi1-xO2-δ nanocrystals with ferromagnetic behavior using nano-TiO2/cis-[CoIII(en)2(MeNH2)Cl]2+. J. Phys. Chem. C 2011, 115, 3821–3832. [Google Scholar] [CrossRef]
- Spitaler, J.; Estreicher, S.K. Perspectives on the theory of defects. Front. Mater. 2018, 5, 70. [Google Scholar] [CrossRef]
- Girish Kumar, S.; Koteswara Rao, K.S.R. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Sowmya, B.; John, A.; Panda, P.K. A review on metal-oxide based p-n and n-n hetero-structured nano-materials for gas sensing applications. Sens. Int. 2021, 2, 100085. [Google Scholar]
- Samriti; Rajput, V.; Raju Kumar, G.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2022, 10, 73. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered double hydroxides-based photocatalysts and visible-light-driven photodegradation of organic pollutants: A review. Chem. Eng. J. 2020, 392, 123684. [Google Scholar] [CrossRef]
- Queraltó, A.; Pérez del Pino, A.; Logofatu, C.; Datcu, A.; Amade, R.; Bertran-Serra, E.; György, E. Reduced graphene oxide/iron oxide nanohybrid flexible electrodes grown by laser-based technique for energy storage applications. Ceram. Int. 2018, 44, 20409–20416. [Google Scholar] [CrossRef]
- Kumar, N.; Chamoli, P.; Misra, M.; Manoj, M.K.; Sharma, A. Advanced metal and carbon nanostructures for medical, drug delivery and bio-imaging applications. Nanoscale 2022, 14, 3987. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, M.; Jing, X.; Zhang, P.; Zhu, Y.; Zhang, Z. Electrospun Semiconductor-based nano-heterostructures for photocatalytic energy conversion and environmental remediation: Opportunities and challenges. Energy Environ. Mater. 2023, 6, e12338. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Energy-Level Matching of Fe(III) Ions Grafted at Surface and Doped in Bulk for Efficient Visible-Light Photocatalysts. J. Am. Chem. Soc. 2013, 135, 10064–10072. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.; Kumar, R.; Kumar, S.; Srivastava Joshi, M. Visible light induced photodegradation of chlorinated organic pollutants using highly efficient magnetic Fe3O4/TiO2 nanocomposite. Optik 2021, 243, 167309. [Google Scholar] [CrossRef]
- Abdel-Messih, M.F.; Ahmed, M.A.; El-sayed, A.S. Photocatalytic decolorization of Rhodamine B dye using novel mesoporous SnO2–TiO2 nano mixed oxides prepared by sol–gel method. J. Photochem. Photobiol. A Chem. 2013, 260, 1–8. [Google Scholar] [CrossRef]
- Mourão, H.A.J.L.; Avansi, W.J.; Ribeiro, C. Hydrothermal synthesis of Ti oxide nanostructures and TiO2: SnO2 heterostructures applied to the photodegradation of Rhodamine B. Mater. Chem. Phys. 2012, 135, 524–532. [Google Scholar] [CrossRef]
- Cao, Y.; He, T.; Zhao, L.; Wang, E.; Yang, W.; Cao, Y. Structure and Phase Transition Behavior of Sn4+-Doped TiO2 Nanoparticles. J. Phys. Chem. C 2009, 113, 18121–18124. [Google Scholar] [CrossRef]
- Boppana, V.B.R.; Lobo, R.F. Photocatalytic degradation of organic molecules on mesoporous visible-light-active Sn (II)-doped titania. J. Catal. 2011, 281, 156–168. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Liu, X.; Yu, C.; Yan, D.; Sun, Z.; Pan, L. Sn doped TiO2 nanotube with oxygen vacancy for highly efficient visible light photocatalysis. J. Alloys Compd. 2016, 679, 454–462. [Google Scholar] [CrossRef]
- Lübke, M.; Johnson, I.; Makwana, N.M.; Brett, D.; Shearing, P.; Liu, Z.; Darr, J.A. High power TiO2 and high capacity Sn-doped TiO2 nanomaterial anodes for lithium-ion batteries. J. Power Source 2015, 294, 94–102. [Google Scholar] [CrossRef]
- Dhanapandian, S.; Arunachalam, A.; Manoharan, C. Highly oriented and physical properties of sprayed anatase Sn-doped TiO2 thin films with an enhanced antibacterial activity. Appl. Nanosci. 2016, 6, 387–397. [Google Scholar] [CrossRef]
- Duan, Y.; Fu, N.; Liu, Q.; Fang, Y.; Zhou, X.; Zhang, J.; Lin, Y. Sn-doped TiO2 photoanode for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 8888–8893. [Google Scholar] [CrossRef]
- Abbas, H.; Nadeem, K.; Hassan, A.; Rahman, S.; Krenn, H. Enhanced Photocatalytic activity of ferromagnetic Fe-doped NiO Nanoparticles. Optik 2020, 20, 163637. [Google Scholar] [CrossRef]
- Joseph, A.; Ayyappan, A.; Subair, T.; Pandibayal, M.; Nair, S.; Ramany, R.; Varma, M.R.; Thomas, S. Pure and Sm doped CeO2 nanoparticles: An insight into the room temperature ferromagnetism and photocatalytic dye degradation. ChemistrySelect 2023, 8, e202301020. [Google Scholar] [CrossRef]
- Hezam, F.A.; Rajesh, A.; Nur, O.; Mustafa, M.A. Synthesis and physical properties of spinel ferrites/MWCNTs hybrids nanocomposites for energy storage and photocatalytic applications. Phys. B Phys. Condens. Matter. 2020, 596, 412389. [Google Scholar] [CrossRef]
- Shah Saqib, A.N.; Thu Huong, N.T.; Kim, S.-W.; Jung, M.-H.; Lee, Y.H. Structural and magnetic properties of highly Fe-doped ZnO nanoparticles synthesized by one-step solution plasma process. J. Alloys Compd. 2021, 853, 157153. [Google Scholar] [CrossRef]
- Sanchis-Gual, R.; Coronado-Puchau, M.; Mallah, T.; Coronado, E. Hybrid nanostructures based on gold nanoparticles and functional coordination polymers: Chemistry, physics and applications in biomedicine, catalysis and magnetism. Coord. Chem. Rev. 2023, 480, 215025. [Google Scholar] [CrossRef]
- Barad, H.-N.; Kwon, H.; Alarcón-Correa, M.; Fischer, P. Large Area Patterning of Nanoparticles and Nanostructures: Current Status and Future Prospects. ACS Nano 2021, 15, 5861–5875. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Bihari Yadav, A.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: A review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Deac, I.G.; Petean, I.; Borodi, G.; Cadar, O. Sol-Gel Synthesis, Structure, Morphology and Magnetic Properties of Ni0.6Mn0.4Fe2O4 Nanoparticles Embedded in SiO2 Matrix. Nanomaterials 2021, 11, 3455. [Google Scholar] [CrossRef]
- Murzin, S.P.; Kazanskiy, N.L. Creation of One- and Two-Dimensional Copper and Zinc Oxides Semiconductor Structures. Appl. Sci. 2023, 13, 11459. [Google Scholar] [CrossRef]
- Huseien, G.F. Potential Applications of Core-Shell Nanoparticles in Construction Industry Revisited. Appl. Nano 2023, 4, 75–114. [Google Scholar] [CrossRef]
- Yadav, A.; Follink, B.; Funston, A.M. Anion-Directed Synthesis of Core–Shell and Janus Hybrid Nanostructures. Chem. Mater. 2022, 34, 8987–8998. [Google Scholar] [CrossRef]
- Xu, M.; Da, P.; Wu, H.; Zhao, D.; Zheng, G. Controlled Sn-doping in TiO2 nanowire photoanodes with enhanced photoelectrochemical conversion. Nano Lett. 2012, 12, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Asefa, B.A.A.; Pan, C.J.; Su, W.N.; Chen, H.M.; Rick, J.; Hwang, B.J. Facile one-pot controlled synthesis of Sn and C co-doped single crystal TiO2 nanowire arrays for highly efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2015, 163, 478–486. [Google Scholar]
- Chang, S.; Chen, S.; Huang, Y. Synthesis, structural correlations, and photocatalytic properties of TiO2 nanotube/SnO2–Pd nanoparticle heterostructures. J. Phys. Chem. C 2011, 115, 1600–1607. [Google Scholar] [CrossRef]
- Dalapati, G.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Sai Krishna, A.M.; Mukhopadhyay, S.; Dey, A.; et al. Tin oxide for optoelectronic, photovoltaic and energy storage devices: A review. J. Mater. Chem. A 2021, 9, 16621. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.W.; Kong, K.J.; Sun Kim, E.; Lee, K.H.; Sung Lee, J. Anion-doped mixed metal oxide nanostructures derived from layered double hydroxide as visible light photocatalysts. Adv. Funct. Mater. 2013, 23, 2348–2356. [Google Scholar] [CrossRef]
- Martha, S.; Chandra Sahoo, P.; Parida, K.M. An overview on visible light responsive metal oxide based photocatalysts for hydrogen energy production. RSC Adv. 2015, 5, 61535. [Google Scholar] [CrossRef]
- Feng, X.; Chen, H.; Jiang, F.; Wang, X. Enhanced visible-light photocatalytic nitrogen fixation over semi crystalline graphitic carbon nitride: Oxygen and sulfur co-doping for crystal and electronic structure modulation. J. Colloid Interface Sci. 2018, 509, 298–306. [Google Scholar] [CrossRef]
- Nair, R.V.; Siva Gummaluri, V.; Vadakke Matham, M.; Vijayan, C. A review on optical bandgap engineering in TiO2 nanostructures via doping and intrinsic vacancy modulation towards visible light applications. J. Phys. D Appl. Phys. 2022, 55, 313003. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S. Self-cleaning applications of TiO2 by photoinduced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef]
- Ayyakannu Sundaram, G.; Samy, A.; Rajkumar, K.; Wang, Y.; Wang, Y.; Wang, J.; Anbalagan, K. Simple hydrothermal synthesis of metal oxides coupled nanocomposites: Structural, optical, magnetic and photocatalytic studies. Appl. Surf. Sci. 2015, 353, 553–563. [Google Scholar]
- Ganeshraja, A.S.; Thirumurugan, S.; Rajkumar, K.; Zhu, K.; Wang, Y.; Anbalagan, K.; Wang, J. Effects of structural, optical and ferromagnetic states on the photocatalytic activities of Sn–TiO2 nanocrystals. RSC Adv. 2016, 6, 409–421. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yu, F.; Jin, C.; Liu, X.; Ma, J.; Wang, Y.; Huang, Y.; Wang, J. Correlation investigation on the visible-light-driven photocatalytic activity and coordination structure of rutile Sn-Fe-TiO2 nanocrystallites for methylene blue degradation. Catal. Today 2015, 258, 112–119. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Mao, Y.; Xing, M.; Zhang, J. Preparation of homogeneous nitrogen-doped mesoporous TiO2 spheres with enhanced visible-light photocatalysis. Appl. Catal. B Environ. 2015, 164, 352–359. [Google Scholar] [CrossRef]
- Pu, X.; Hu, Y.; Cui, S.; Cheng, L.; Jiao, Z. Preparation of N-doped and oxygen-deficient TiO2 microspheres via a novel electron beam-assisted method. Solid State Sci. 2017, 70, 66–73. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Lim, J.; Murugan, P.; Lakshminarasimhan, N.; Kim, J.Y.; Lee, J.S.; Lee, S.H.; Choi, W. Synergic photocatalytic effects of nitrogen and niobium co-doping in TiO2 for the redox conversion of aquatic pollutants under visible light. J. Catal. 2014, 310, 91–99. [Google Scholar] [CrossRef]
- Pal, A.; Zhang, S.; Chavan, T.; Agashiwala, K.; Yeh, C.-H.; Cao, W.; Banerjee, K. Quantum-engineered devices based on 2D materials for next-generation information processing and storage. Adv. Mater. 2023, 35, 2109894. [Google Scholar] [CrossRef]
- Zhao, G.; Deng, Z.; Jin, C. Advances in new generation diluted magnetic semiconductors with independent spin and charge doping. J. Semicond. 2019, 40, 081505. [Google Scholar] [CrossRef]
- Zou, B.; Tian, Y.; Shi, L.; Liu, R.; Zhang, Y.; Zhong, H. Excitonic magnetic polarons in II-VI diluted magnetic semiconductor nanostructures. J. Lumin. 2022, 252, 119334. [Google Scholar] [CrossRef]
- Dieny, B.; Prejbeanu, I.L.; Garello, K.; Gambardella, P.; Freitas, P.; Lehndorff, R.; Raberg, W.; Ebels, U.; Demokritov, S.O.; Akerman, J.; et al. Opportunities and challenges for spintronics in the microelectronics industry. Nat. Electron. 2020, 3, 446–459. [Google Scholar] [CrossRef]
- Goel, S.; Duy Khang, N.H.; Osada, Y.; Anh, L.D.; Hai, P.N.; Tanaka, M. Room-temperature spin injection from a ferromagnetic semiconductor. Sci. Rep. 2023, 13, 2181. [Google Scholar] [CrossRef]
- Dusanowski, Ł.; Nawrath, C.; Portalupi, S.L.; Jetter, M.; Huber, T.; Klembt, S.; Michler, P.; Höfling, S. Optical charge injection and coherent control of a quantum-dot spin-qubit emitting at telecom wavelengths. Nat. Commun. 2022, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mizukami, S.; Kubota, T.; Ma, Q.; Oogane, M.; Naganuma, H.; Ando, Y.; Miyazaki, T. Observation of a large spin-dependent transport length in organic spin valves at room temperature. Nat. Commun. 2013, 4, 1392. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-C.; Wu, W.-H.; Wu, M.-T.; Chuang, C.; Pai, C.-F.; Hsieh, Y.-P.; Hofmann, M. Realizing High-Quality Interfaces in Two-Dimensional Material Spin Valves. ACS Mater. Lett. 2024, 6, 94–99. [Google Scholar] [CrossRef]
- Malavolti, L.; McMurtrie, G.; Rolf-Pissarczyk, S.; Yan, S.; Burgess, J.A.J.; Loth, S. Minimally invasive spin sensing with scanning tunneling microscopy. Nanoscale 2020, 12, 11619–11626. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Shigidi, I.; Otaibi, S.A.; Althubeiti, K.; Abdullaev, S.S.; Rahman, N.; Sohail, M.; Khan, A.; Iqbal, S.; Rosso, T.D.; et al. Room temperature dilute magnetic semiconductor response in (Gd, Co) co-doped ZnO for efficient spintronics applications. RSC Adv. 2022, 12, 36126–36137. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Zhang, R.; Kumar, P.; Kumar, V.; Kumar, A. Nano-Structured Dilute Magnetic Semiconductors for Efficient Spintronics at Room Temperature. Magnetochemistry 2020, 6, 15. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. Synthesis of Magnetic Ferrite Nanoparticles with High Hyperthermia Performance via a Controlled Co-Precipitation Method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef]
- Kanwal, S.; Tahir Khan, M.; Tirth, V.; Algahtani, A.; Al-Mughanam, T.; Zaman, A. Room-Temperature Ferromagnetism in Mn-Doped ZnO Nanoparticles Synthesized by the Sol–Gel Method. ACS Omega 2023, 8, 28749–28757. [Google Scholar] [CrossRef]
- Ayyakannu Sundaram, G.; Kanniah, R.; Anbalagan, K.; Kulandaivelu, K.; Valdés, H. Impact of Copper(II)-Imidazole Complex Modification on Polycrystalline TiO2: Insights into Formation, Characterization, and Photocatalytic Performance. Catalysts 2024, 14, 169. [Google Scholar] [CrossRef]
- Ahmad, N.; Khan, S.; Nizam Ansari, M.M. Optical, dielectric and magnetic properties of Mn doped SnO2 diluted magnetic semiconductors. Ceram. Int. 2018, 44, 15972–15980. [Google Scholar] [CrossRef]
- Yao, C.; Ismail, M.; Hao, A.; Thatikonda, S.K.; Huang, W.; Qin, N.; Bao, D. Annealing atmosphere effect on the resistive switching and magnetic properties of spinel Co3O4 thin films prepared by a sol-gel technique. RSC Adv. 2019, 9, 12615–12625. [Google Scholar] [CrossRef]
- Tadic, M.; Kralj, S.; Jagodic, M.; Hanzel, D.; Makovec, D. Magnetic properties of novel superparamagnetic iron oxide nanoclusters and their peculiarity under annealing treatment. Appl. Surf. Sci. 2014, 322, 255–264. [Google Scholar] [CrossRef]
- Aleinawi, M.H.; Uddin Ammar, A.; Buldu-Akturk, M.; Selin Turhan, N.; Nadupalli, S.; Erdem, E. Spectroscopic Probing of Mn-Doped ZnO Nanowires Synthesized via a Microwave-Assisted Route. J. Phys. Chem. C 2022, 126, 4229–4240. [Google Scholar] [CrossRef]
- Talebian, N.; Jafarinezhad, F. Morphology-controlled synthesis of SnO2 nanostructures using hydrothermal method and their photocatalytic applications. Ceram. Int. 2013, 39, 8311–8317. [Google Scholar] [CrossRef]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Dai, J.Y.; Ong, H.C. Hydrothermal Synthesis and Properties of Diluted Magnetic Semiconductor Zn1-xMnxO Nanowires. Open J. Phys. Chem. 2011, 1, 6–10. [Google Scholar] [CrossRef]
- Di, M.; Fu, L.; Zhou, Y.; Pan, H.; Xu, Y.; Du, Y.; Tang, N. Comprehensive mechanism of ferromagnetism enhancement in nitrogen-doped graphene. New J. Phys. 2021, 23, 103003. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Ying, M.; Li, M.; Wang, X.; Nan, C.-W. Room-temperature ferromagnetic and ferroelectric behavior in polycrystalline ZnO-based thin films. Appl. Phys. Lett. 2007, 90, 222110. [Google Scholar] [CrossRef]
- Vargas-Ortiz, J.R.; Gonzalez, C.; Esquivel, K. Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges. Processes 2022, 10, 2282. [Google Scholar] [CrossRef]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A comprehensive review on various techniques used for synthesizing nanoparticle. J. Mater. Res. Technol. 2023, 27, 1739–1763. [Google Scholar] [CrossRef]
- Dong, Y.X.; Wang, X.L.; Jin, E.M.; Jeong, S.M.; Jin, B.; Lee, S.H. One-step hydrothermal synthesis of Ag decorated TiO2 nanoparticles for dye-sensitized solar cell application. Renew. Energy 2019, 135, 1207–1212. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Trpkov, D.; Panjan, M.; Kopanja, L.; Tadić, M. Hydrothermal synthesis, morphology, magnetic properties and self-assembly of hierarchical α-Fe2O3 (hematite) mushroom-, cube- and sphere-like superstructures. Appl. Surf. Sci. 2018, 457, 427–438. [Google Scholar] [CrossRef]
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; Molnár, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A spin-based electronics vision for the future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, L.; Wang, C.; Wang, J.; Guo, J.; Tang, R.; Zhu, J.; Zou, G. Seed engineering toward layer-regulated growth of magnetic semiconductor VS2. Adv. Funct. Mater. 2023, 33, 2213295. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mondal, P.; Makkar, M.; Moretti, L.; Cerullo, G.; Viswanatha, R. Transition metal doping in CdS quantum dots: Diffusion, magnetism, and ultrafast charge carrier dynamics. Chem. Mater. 2023, 35, 2146–2154. [Google Scholar] [CrossRef]
- Pawar, S.; Duadi, H.; Fixler, D. Recent advances in the spintronic application of carbon-based nanomaterials. Nanomaterials 2023, 13, 598. [Google Scholar] [CrossRef]
- Haider, T. A Review of Magneto-Optic Effects and Its Application. Int. J. Electromagn. Appl. 2017, 7, 17–24. [Google Scholar]
- Ando, K. Magneto-Optics of Diluted Magnetic Semiconductors: New Materials and Applications. In Magneto-Optics; Sugano, S., Kojima, N., Eds.; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 2000; Volume 128. [Google Scholar] [CrossRef]
- Rudno-Rudziński, W.; Burakowski, M.; Reithmaier, J.P.; Musiał, A.; Benyoucef, M. Magneto-Optical Characterization of Trions in Symmetric InP-Based Quantum Dots for Quantum Communication Applications. Materials 2021, 14, 942. [Google Scholar] [CrossRef]
- Telegin, A.; Sukhorukov, Y. Magnetic Semiconductors as Materials for Spintronics. Magnetochemistry 2022, 8, 173. [Google Scholar] [CrossRef]
- Tatzenko, O.M.; Markevsev, I.M.; Pavlovskii, A.I.; Platonov, V.V.; Sosnin, P.V.; Druzhinin, V.V.; Lugutin, A.S.; Nikitin, P.I.; Savchuk, A.I. The Faraday Effect in Dilute Magnetic Semiconductors in Ultrahigh Magnetic Field. In Proceedings of the 1993 IEEE International Magnetics Conference (INTERMAG), Stockhom, Sweden, 13–16 April 1993. [Google Scholar] [CrossRef]
- Portugall, O.; Krämer, S.; Skourski, Y. Magnetic Fields and Measurements. In Handbook of Magnetism and Magnetic Materials; Coey, M., Parkin, S., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.O.; Cao, G.; Xiao, M.; Guo, G.C.; Guo, G.P. Semiconductor quantum computation. Natl. Sci. Rev. 2019, 6, 32–54. [Google Scholar] [CrossRef] [PubMed]
- Young, D.K.; Gupta, J.A.; Johnston-Halperin, E.; Epstein, Y.; Awschalom, K.D.D. Optical, electrical and magnetic manipulation of spins in semiconductors. Semicond. Sci. Technol. 2002, 17, 275. [Google Scholar] [CrossRef]
- Eills, J.; Budker, D.; Cavagnero, S.; Chekmenev, E.Y.; Elliott, S.J.; Jannin, S.; Lesage, A.; Matysik, J.; Meersmann, T.; Prisner, T.; et al. Spin Hyperpolarization in Modern Magnetic Resonance. Chem. Rev. 2023, 123, 1417–1551. [Google Scholar] [CrossRef] [PubMed]
- Da, H.; Song, Q.; Dai, H.; Dong, P.; Bao, Q.; Ye, H.; An, Y.; Chen, J.; Guo, J.; Wang, X.; et al. Electrically controllable magneto-optic effects in a two-dimensional hexagonal organometallic lattice. Phys. Rev. B 2020, 101, 035423. [Google Scholar] [CrossRef]
- Pham, Y.T.; Liu, M.; Jimenez, V.O.; Yu, Z.; Kalappattil, V.; Zhang, F.; Wang, K.; Williams, T.; Terrones, M.; Phan, M.-H. Tunable ferromagnetism and thermally induced spin flip in vanadium-doped tungsten diselenide monolayers at room temperature. Adv. Mater. 2020, 32, 2003607. [Google Scholar] [CrossRef]
- Anbuselvan, D.; Nilavazhagan, S.; Santhanam, A.; Chidhambaram, N.; Gunavathy, K.V.; Ahamad, T.; Alshehri, S.M. Room temperature ferromagnetic behavior of nickel-doped zinc oxide dilute magnetic semiconductor for spintronics applications. Phys. E Low-Dimens. 2021, 129, 114665. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, C.; Fu, L.; Gu, Y.; Zhi, G.; Dong, J.; Zhao, X.; Xie, L.; Zhang, H.; Cao, C.; et al. Manipulation of the ferromagnetic ordering in magnetic semiconductor (La,Ca)(Zn,Mn)AsO by chemical pressure. J. Magn. Magn. Mater. 2022, 554, 169276. [Google Scholar] [CrossRef]
- Feng, S.; Duan, H.; Tan, H.; Hu, F.; Liu, C.; Wang, Y.; Li, Z.; Cai, L.; Cao, Y.; Wang, C.; et al. Intrinsic room-temperature ferromagnetism in a two-dimensional semiconducting metal-organic framework. Nat. Commun. 2023, 14, 7063. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Sathish, C.I.; Geng, X.; Guan, X.; Liu, Y.; Wang, L.; Qiao, L.; Vinu, A.; Yi, J. Manipulation of ferromagnetism in intrinsic two-dimensional magnetic and nonmagnetic materials. Matter 2022, 5, 4212–4273. [Google Scholar] [CrossRef]
- Wang, H.; Lu, H.; Guo, Z.; Li, A.; Wu, P.; Li, J.; Xie, W.; Sun, Z.; Li, P.; Damas, H.; et al. Interfacial engineering of ferromagnetism in wafer-scale van der Waals Fe4GeTe2 far above room temperature. Nat. Commun. 2023, 29, 2483. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.H.; Kumar Yadav, V.; Singh, J.K. Recent Advances in the Carrier Mobility of Two-Dimensional Materials: A Theoretical Perspective. ACS Omega 2020, 5, 14203–14211. [Google Scholar] [CrossRef] [PubMed]
- Tuček, J.; Holá, K.; Bourlinos, A.; Błoński, P.; Bakandritsos, A.; Ugolotti, J.; Dubecký, M.; Karlický, F.; Ranc, V.; Čépe, K.; et al. Room temperature organic magnets derived from sp3 functionalized graphene. Nat. Commun. 2017, 8, 14525. [Google Scholar] [CrossRef]
- Hanson, R.; Awschalom, D. Coherent manipulation of single spins in semiconductors. Nature 2008, 453, 1043–1049. [Google Scholar] [CrossRef]
- Fursina, A.A.; Sinitskii, A. Toward Molecular Spin Qubit Devices: Integration of Magnetic Molecules into Solid-State Devices. ACS Appl. Electron. Mater. 2023, 5, 3531–3545. [Google Scholar] [CrossRef]
- Wu, J.; Guo, R.; Wu, D.; Li, X.; Wu, X. Turning Nonmagnetic Two-Dimensional Molybdenum Disulfides into Room-Temperature Ferromagnets by the Synergistic Effect of Lattice Stretching and Charge Injection. J. Phys. Chem. Lett. 2024, 15, 2293–2300. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Menelaou, M.; Sarkar, K.J.; Sofer, Z.; Polavarapu, L.; Mourdikoudis, S. Ferromagnetic Elements in Two-Dimensional Materials: 2DMagnets and Beyond. Adv. Funct. Mater. 2024, 34, 2309046. [Google Scholar] [CrossRef]
- Dung, D.D.; Lam, N.H.; Nguyen, A.D.; Trung, N.N.; Van Duc, N.; Hung, N.T.; Kim, Y.S.; Odkhuu, D. Experimental and theoretical studies on induced ferromagnetism of new (1 − x)Na0.5Bi0.5TiO3 + xBaFeO3−δ solid solution. Sci. Rep. 2021, 11, 8908. [Google Scholar] [CrossRef]
- He, W.; Kong, L.; Zhao, W.; Yu, P. Atomically Thin 2D van der Waals Magnetic Materials: Fabrications, Structure, Magnetic Properties and Applications. Coatings 2022, 12, 122. [Google Scholar] [CrossRef]
- Scott, J. Room-temperature multiferroic magnetoelectrics. NPG Asia Mater. 2013, 5, e72. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Sun, H.; Wang, N.; He, J.; Wang, N.; Long, Y.; Huang, C.; Li, Y. Induced Ferromagnetic Order of Graphdiyne Semiconductors by Introducing a heteroatom. ACS Cent. Sci. 2020, 6, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Dhamodaran, M.; Yadav, R.K.; Karuppannan, R.; Ramaswamy, M.; Boukhvalov, D.W.; Yadav, A.K.; Gupta, R. Dopant-activated magnetism and local structure properties of cubic shape Co, Mn:In2O3. Mater. Sci. Semicond. Process. 2023, 168, 107818. [Google Scholar] [CrossRef]
- Seema, K.; Kumar, R. Effect of Dopant Concentration on Electronic and Magnetic Properties of Transition Metal-Doped ZrO2. J. Supercond. Nov. Magn. 2015, 28, 2735–2742. [Google Scholar] [CrossRef]
- Nan, T.; Quintela, C.X.; Irwin, J.G.; Gurung, D.F.; Shao, J.; Gibbons, N.; Campbell, K.; Song, S.-Y.; Choi, L.; Guo, R.D.; et al. Controlling spin current polarization through non-collinear antiferromagnetism. Nat. Commun. 2020, 11, 4671. [Google Scholar] [CrossRef]
- Gu, X.; Guo, L.; Qin, Y.; Yang, T.; Meng, K.; Hu, S.; Sun, X. Challenges and Prospects of Molecular Spintronics. Precis. Chem. 2024, 2, 1–13. [Google Scholar] [CrossRef]
- Ahn, E.C. 2D materials for spintronic devices. npj 2d Mater. Appl. 2020, 4, 17. [Google Scholar] [CrossRef]
- Salinas, R.I.; Chen, P.-C.; Yang, C.-Y.; Lai, C.-H. Spintronic materials and devices towards an artificial neural network: Accomplishments and the last mile. Mater. Res. Lett. 2023, 11, 305–326. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Cao, Y.; Kamra, A.; Duine, R.; Yan, P. Quantum magnonics: When magnon spintronics meets quantum information science. Phys. Rep. 2022, 965, 1–74. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, X.; Zhang, T.; Yu, X.; Ma, Y.; Santos, E.C.; White, J.; Liu, H.; Sang, Y. Construction of diluted magnetic semiconductor to endow nonmagnetic semiconductor with spin-regulated photocatalytic performance. Nano Energy 2023, 110, 108381. [Google Scholar] [CrossRef]
- Tang, J.; Wang, C.-Y.; Chang, L.; Fan, Y.; Nie, T.; Chan, M.; Jiang, W.; Chen, Y.; Yang, H.; Tuan, H.; et al. Electrical Spin Injection and Detection in Mn5Ge3/Ge/Mn5Ge3 nanowire transistors. Nano Lett. 2013, 13, 4036–4043. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Cui, X.; Yao, W.; Duan, T. One-step hydrothermal synthesis of iron and nitrogen co-doped TiO2 nanotubes with enhanced visible-light photocatalytic activity. CrystEngComm 2015, 17, 8368–8376. [Google Scholar] [CrossRef]
- Irie, H.; Washizuka, S.; Yoshino, N.; Hashimoto, K.Y. Visible-light induced hydrophilicity on nitrogen-substituted titanium dioxide films. Chemcomm 2003, 11, 1298–1299. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–272. [Google Scholar] [CrossRef]
- Wang, W.; Tadé, M.O.; Shao, Z. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, Y.; Chu, Z.; Long, J.; An, X.; Zhang, H.; Lin, H.; Zhang, Z.; Wang, X. Synergy of metal and nonmetal dopants for visible-light photocatalysis: A case-study of Sn and N co-doped TiO2. Phys. Chem. Chem. Phys. 2016, 18, 9636–9644. [Google Scholar] [CrossRef] [PubMed]
- Phokha, S.; Pinitsoontorn, S.; Maensiri, S. Structure and Magnetic Properties of Monodisperse Fe3+ -doped CeO2 Nanospheres. Nano-Micro Lett. 2013, 3, 223–233. [Google Scholar] [CrossRef]
- Dakhel, A.A. Microstructural, optical and magnetic properties of TiO2:Fe:M (M = Ga, Zn) dilute magnetic semiconductor nanoparticles: A comparative study. Appl. Phys. A 2021, 127, 440. [Google Scholar] [CrossRef]
- Lu, A.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, L.; Song, J.; Mi, W.; Zou, J.; Wang, L.; Zhang, X. Titanium-defected undoped anatase TiO2 with p-Type conductivity, room-temperature ferromagnetism, and remarkable photocatalytic performance. J. Am. Chem. Soc. 2015, 137, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Charles Cao, Y. Impurities enhance semiconductor nanocrystal performance. Science 2011, 332, 48–49. [Google Scholar]
- Chetri, P.; Basyach, P.; Choudhury, A. Exploring the structural and magnetic properties of TiO2/SnO2 core/shell nanocomposite: An experimental and density functional study. J. Solid. State Chem. 2014, 220, 124–131. [Google Scholar] [CrossRef]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.; Song, H.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef]
- Charanpahari, A.; Ghugal, S.G.; Umare, S.S.; Sasikala, R. Mineralization of malachite green dye over visible light responsive bismuth doped TiO2-ZrO2 ferromagnetic nanocomposites. New J. Chem. 2015, 39, 3629–3638. [Google Scholar] [CrossRef]
- Khang, N.C.; Khanh, N.; Anh, N.H.; Nga, D.; Minh, N. The origin of visible light photocatalytic activity of N-doped and weak ferromagnetism of Fe-doped TiO2 anatase. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 015008. [Google Scholar] [CrossRef]
- Na, C.; Park, S.; Kim, S.J.; Woo, H.; Kim, H.J.; Chung, J.; Lee, J. Chemical synthesis of CoO–ZnO:Co hetero-nanostructures and their ferromagnetism at room temperature. CrystEngComm 2012, 14, 5390–5393. [Google Scholar] [CrossRef]
- Alivov, Y.; Singh, V.; Ding, Y.; Cerkovnik, L.J.; Nagpal, P. Doping of wide-bandgap titanium-dioxide nanotubes: Optical, electronic and magnetic properties. Nanoscale 2014, 6, 10839–10849. [Google Scholar] [CrossRef]
- Thakare, V.P.; Game, O.S.; Ogale, S.B. Ferromagnetism in metal oxide systems: Interfaces, dopants, and defects. J. Mater. Chem. C 2013, 1, 1545–1557. [Google Scholar] [CrossRef]
- Rahman, G. Nitrogen-induced ferromagnetism in BaO. RSC Adv. 2015, 5, 33674–33680. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Choudhury, B.; Verma, R.; Choudhury, A. Oxygen defect assisted paramagnetic to ferromagnetic conversion in Fe doped TiO2 nanoparticles. RSC Adv. 2014, 4, 29314–29323. [Google Scholar] [CrossRef]
- Neogi, S.K.; Midya, N.; Pramanik, P.; Banerjee, A.; Bhattacharyya, A.; Taki, G.S.; Krishna, J.B.M.; Bandyopadhyay, S. Correlation between defect and magnetism of low energy Ar+9 implanted and un-implanted Zn0.95Mn0.05O thin films suitable for electronic application. J. Magn. Magn. Mater. 2016, 408, 217–227. [Google Scholar] [CrossRef]
- Kumar, S.; Asokan, K.; Singh, R.; Chatterjee, S.; Kanjilal, D.; Ghosh, A.K. Investigations on structural and optical properties of ZnO and ZnO:Co nanoparticles under dense electronic excitations. RSC Adv. 2014, 4, 62123–62131. [Google Scholar] [CrossRef]
- Borges, R.; Silva, R.; Magalhaes, S.; Cruz, M.; Godinho, M. Magnetism in Ar-implanted ZnO. J. Phys. Condens. Matter 2007, 19, 476207. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Ganeshraja, A.S.; Rajkumar, K.; Zhu, K.; Li, X.; Thirumurugan, S.; Xu, W.; Zhang, J.; Yang, M.; Anbalagan, K.; Wang, J. Facile synthesis of iron oxide coupled and doped titania nanocomposites: Tuning of physicochemical and photocatalytic properties. RSC Adv. 2016, 6, 72791–72802. [Google Scholar] [CrossRef]
- Ganeshraja, A.S.; Thirumurugan, S.; Rajkumar, K.; Wang, J.; Anbalagan, K. Ferromagnetic nickel(II) imidazole-anatase framework: An enhanced photocatalytic performance. J. Alloys Compd. 2017, 706, 485–494. [Google Scholar] [CrossRef]
- Ganeshraja, A.S.; Yang, M.; Nomura, K.; Maniarasu, S.; Veerappan, G.; Liu, T.; Wang, J. 119Sn Mössbauer and ferromagnetic studies on hierarchical Tin- and nitrogen-codoped TiO2 microspheres with efficient photocatalytic performance. J. Phys. Chem. C 2017, 121, 6662–6673. [Google Scholar]
- Ganeshraja, A.S.; Zhu, K.; Nomura, K.; Wang, J. Hierarchical assembly of AgCl@Sn-TiO2 microspheres with enhanced visible light photocatalytic performance. Appl. Surf. Sci. 2018, 441, 678–687. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Liu, Y.; Chen, S.; Zheng, X.; Gao, C.; He, C.; Chen, N.; Qi, Z.; Song, L.; et al. Isolation of Cu atoms in Pd lattice: Forming highly selective sites. J. Am. Chem. Soc. 2017, 139, 4486–4492. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Lv, L.; Zhao, Y.; Qu, L. Vertically aligned graphene sheets membrane for highly efficient solar thermal generation of clean water. ACS Nano 2017, 11, 5087–5093. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmuki, P. Photocatalysis with TiO2 Nanotubes: “Colorful” reactivity and designing site-specific photocatalytic centers into TiO2 nanotubes. ACS Catal. 2017, 7, 3210–3235. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Xu, S.; Yaowen Ruan, Y. Carbon quantum dot-sensitized hollow TiO2 spheres for high-performance visible light photocatalysis. New J. Chem. 2021, 45, 8693–8700. [Google Scholar] [CrossRef]
- Araújo, E.S.; Pereira, M.F.G.; da Silva, G.M.G.; Tavares, G.F.; Oliveira, C.Y.B.; Faia, P.M. The role of environmental contamination in the spread of COVID-19; A Review. Toxics 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Jamjoum, H.A.A.; Umar, K.; Adnan, R.; Razali, M.R.; Mohamad Ibrahim, M.N. Synthesis, characterization, and photocatalytic activities of graphene oxide/metal oxides nanocomposites: A Review. Front. Chem. 2021, 9, 752276. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E. State-of-the-art developments in carbon-based metal nanocomposites as a catalyst: Photocatalysis. Nanoscale Adv. 2021, 3, 1887–1900. [Google Scholar] [CrossRef]
- Rani, M.; Murtaza, M.; Amjad, A.; Zahra, M.; Waseem, A.; Alhodaib, A. NiSe2/Ag3PO4 nanocomposites for enhanced Visible light photocatalysts for environmental remediation applications. Catalysts 2023, 13, 929. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Xu, T.; Ji, W.; Zong, X. Recent advances on small band gap semiconductor materials (≤2.1 eV) for solar water splitting. Catalysts 2023, 13, 728. [Google Scholar] [CrossRef]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, G.; Bonapasta, A.A.; Bovi, D.; Giannozzi, P. Photocatalytic and photovoltaic properties of TiO2 nanoparticles investigated by Ab Initio simulations. J. Phys. Chem. C 2014, 118, 29928–29942. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Shen, S.; Zhu, J.; Liu, Y.; Mao, X. Understanding photo (electro)catalysts for energy conversion via operando functional imaging. Chem. Biomed. Imaging 2023, 1, 522–536. [Google Scholar] [CrossRef]
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent progress on semiconductor heterogeneous photocatalysts in clean energy production and environmental remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Ganeshraja, A.S.; Yang, M.; Xu, W.; Anbalagan, K.; Wang, J. Photoinduced interfacial electron transfer in 2,2′-bipyridyl iron(III) complex-TiO2 nanoparticles in aqueous medium. ChemistrySelect 2017, 2, 10648–10653. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Y.; Lawes, D.J.; Ball, G.E.; Zhou, C.; Liu, Z.; Amal, R. Analysis of the promoted activity and molecular mechanism of hydrogen production over Fine Au–Pt Alloyed TiO2 photocatalysts. ACS Catal. 2015, 5, 3924–3931. [Google Scholar] [CrossRef]
- Seh, Z.W.; Liu, S.; Low, M.; Zhang, S.; Liu, Z.; Milayah, A.; Han, M. Janus Au-TiO2 photocatalysts with strong localization of plasmonic near-fields for efficient visible-light hydrogen generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, P.; Bansal, N.; Paul, M.; Dixit, H.; Porwal, S.; Mishra, S.; Singh, T. Recent progress in perovskite transition metal oxide-based photocatalyst and photoelectrode materials for solar-driven water splitting. J. Environ. Chem. Eng. 2022, 10, 108429. [Google Scholar] [CrossRef]
- Lei, W.; Zhou, T.; Pang, X.; Xue, S.; Xu, Q. Low-dimensional MXenes as noble metal-free co-catalyst for solar-to-fuel production: Progress and prospects. J. Mater. Sci. Technol. 2022, 114, 143–164. [Google Scholar] [CrossRef]
- Hendi, A.; Alanazi, M.; Alharbi, W.; Ali, T.; Awad, M.; Ortashi, K.; Aldosari, H.; Alfaifi, F.; Qindeel, R.; Naz, G.; et al. Significance of ternary hybrid nanoparticles on the dynamics of nanofluids over a stretched surface subject to gravity modulation. J. King Saud. Univ. Sci. 2023, 35, 102555. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.A.; Martínez-Montemayor, S.; Leyva-Porras, C.C.; Longoria-Rodríguez, F.E.; Martínez-Guerra, E.; Sánchez-Domínguez, M. CoFe2O4-TiO2 Hybrid Nanomaterials: Synthesis Approaches Based on the Oil-in-Water Microemulsion Reaction Method. J. Nanomater. 2017, 2017, 2367856. [Google Scholar] [CrossRef]
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic titanium dioxide based nanomaterials: Synthesis, characteristics, and photocatalytic application in pollutant degradation. J. Mater. Chem. A 2015, 3, 17511. [Google Scholar] [CrossRef]
- Cheng, C.; Saeed, R.; Lei, G.; Zitao, L.; Junfeng, D.; Asfandyar, S. Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process. Nanotechnol. Rev. 2023, 12, 20230150. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 21, 5102014. [Google Scholar] [CrossRef]
- Hameed, H.G.; Abdulrahman, N.A. Synthesis of TiO2 Nanoparticles by Hydrothermal Method and Characterization of their Antibacterial Activity: Investigation of the Impact of Magnetism on the Photocatalytic Properties of the Nanoparticles. Phys. Chem. Res. 2023, 11, 771–782. [Google Scholar]
- Byun, D.; Jin, Y.; Kim, B.; Kee Lee, J.; Park, D. Photocatalytic TiO2 deposition by chemical vapor deposition. J. Hazard. Mater. 2000, 73, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Shi, M.; Liu, J.; Han, X.; Lan, Z.; Gu, H.; Wang, X.; Sun, H.; Zhang, Q.; Li, H.; et al. A novel approach to enhance the photocatalytic activity of g-C3N4 by constructing a Z-scheme heterojunction with Bi2WO6. J. Hazard. Mater. 2020, 394, 122540. [Google Scholar] [CrossRef]
- Ramli, Z.; Pasupuleti, J.; Tengku Saharuddin, T.; Yusoff, Y.; Roslam Wan Isahak, W.; Baharudin, L.; Yaw, C.; Koh, S.P.; Tiong Kiong, S. Electrocatalytic activities of platinum and palladium catalysts for enhancement of direct formic acid fuel cells: An updated progress. Alex. Eng. J. 2023, 76, 701–733. [Google Scholar] [CrossRef]
- Okatenko, V.; Loiudice, A.; Newton, M.; Stoian, D.; Blokhina, A.; Chen, A.; Rossi, K.; Buonsanti, R. Alloying as a strategy to boost the stability of copper nano-catalysts during the electrochemical CO2 reduction reaction. J. Am. Chem. Soc. 2023, 145, 5370–5383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ou, L.; Mao, L.; Wu, X.; Liu, Y.; Lu, H. Advances in noble metal-decorated metal oxide nanomaterials for Chemiresistive gas sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Fontelles-carceller, O.; Muñoz-batista, M.J.; Rodríguez-castellón, E.; Conesa, J.C.; Fernández-garcía, M.; Kubacka, A. Measuring and interpreting quantum efficiency for hydrogen photo-production using Pt-titania catalysts. J. Catal. 2017, 347, 157–169. [Google Scholar] [CrossRef]
- Hayashido, Y.; Naya, S.; Tada, H. Local Electric Field-enhanced plasmonic photocatalyst: Formation of Ag cluster-incorporated AgBr nanoparticles on TiO2. J. Phys. Chem. C 2016, 120, 19663–19669. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodreguez-Lopez, J.L.; Remita, H. Surface modification of TiO2 with Ag nanoparticles and CuO nanoclusters for application in photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.; Kley, C.; Punke, P.; Patrick, C.; Jacobson, P.; Giustino, F.; Jung Jung, S.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kweon, D.; Jae Jang, B.; Jong Ju, M.; Baek, J. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced faradaic efficiency. Nat. Commun. 2020, 4, 2000197. [Google Scholar]
- Yang, L.; Wang, F.; Shu, C.; Liu, P.; Zhang, W.; Hu, S. An in-situ synthesis of Ag/AgCl/TiO2/hierarchical porous magnesian material and its photocatalytic performance. Sci. Rep. 2016, 6, 21617. [Google Scholar] [CrossRef]
- Shah, Z.H.; Wang, J.; Ge, Y.; Wang, C.; Mao, W.; Zhang, S.; Lu, R. Highly enhanced plasmonic photocatalytic activity of Ag/Agcl/TiO2 by CuO co-catalyst. J. Mater. Chem. 2015, 3, 3568–3575. [Google Scholar] [CrossRef]
- Zhu, L.; Hong, M.; Ho, G.W. Hierarchical assembly of SnO2/ZnO nanostructures for enhanced photocatalytic performance. Sci. Rep. 2015, 5, 11609. [Google Scholar] [CrossRef]
- Yalçın, Y.; Kılıç, M.; Çınar, Z. Fe+3-doped TiO2: A combined experimental and computational approach to the evaluation of visible light activity. Appl. Catal. B Environ. 2010, 99, 469–477. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Wang, W.; Wang, Y.; Gong, X.; Geng, C.; Tang, J. Self-healing nanocomposites with carbon nanotube/graphene/Fe3O4 nanoparticle tri-continuous networks for electromagnetic radiation shielding. ACS Appl. Nano Mater. 2022, 5, 16423–16439. [Google Scholar]
- Her, Y.; Yeh, B.; Huang, S. Vapor–solid growth of p Te/n-SnO2 hierarchical heterostructures and their enhanced room-temperature gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 9150–9159. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.B.; Christopher, P.; Bauer, J.L.; Linic, S. Predictive model for the design of plasmonic metal/semiconductor composite photocatalysts. ACS Catal. 2011, 1, 1441–1447. [Google Scholar] [CrossRef]
- Sirivallop, A.; Areerob, T.; Chiarakorn, S. Enhanced visible light photocatalytic activity of N and Ag doped and Co-doped TiO2 synthesized by using an in-situ solvothermal method for gas phase ammonia removal. Catalysts 2020, 10, 251. [Google Scholar] [CrossRef]
- Kallel, W.; Chaabene, S.; Bouattour, S. Novel (Ag,Y) doped TiO2 plasmonic photocatalyst with enhanced photocatalytic activity under visible light. Physicochem. Probl. Miner. Process. 2019, 55, 745–759. [Google Scholar]
- Kabir Suhan, M.B.; Al-Mamun, M.R.; Farzana, N.; Munira Aishee, S.; Islam, M.S.; Marwani, H.; Hasan, M.M.; Asiri, A.M.; Rahman, M.M.; Islam, A.; et al. Sustainable pollutant removal and wastewater remediation using TiO2-based nanocomposites: A critical review. Nano-Struct. Nano-Objects 2023, 36, 101050. [Google Scholar] [CrossRef]
- Saliba, M.; Zhang, W.; Burlakov, V.M.; Stranks, S.D.; Sun, Y.; Ball, J.M.; Johnston, M.B.; Goriely, A.; Wiesner, U.; Snaith, H.J. Plasmonic-induced photon recycling in metal halide perovskite solar cells. Adv. Funct. Mater. 2015, 25, 5038–5046. [Google Scholar] [CrossRef]
- Ganeshraja, A.S.; Kiyoshi, G.; Wang, J. 119Sn Mossbauer studies on ferromagnetic and photocatalytic Sn–TiO2 nanocrystals. Hyperfine Interact. 2016, 237, 139. [Google Scholar] [CrossRef]
- Vázquez-Robaina, O.; Cabrera, A.F.; Cruz, A.F.; Torres, C.E.R. Observation of room-temperature ferromagnetism induced by high-pressure hydrogenation of Anatase TiO2. J. Phys. Chem. C 2021, 125, 14366–14377. [Google Scholar] [CrossRef]
- Sakar, M.; Mithun Prakash, R.; Do, T.-O. Insights into the TiO2-based photocatalytic systems and their mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Hu, P.; Hu, P.; Duc Vu, T.; Li, M.; Wang, S.; Ke, Y.; Zeng, X.; Mai, L.; Long, Y. Vanadium oxide: Phase diagrams, structures, synthesis, and applications. Chem. Rev. 2023, 123, 4353–4415. [Google Scholar] [CrossRef]
- Sundaram, A.G.; Maniarsu, S.; Vijendra, R.P.; Ganapathy, V.; Karthikeyan, V.; Nomura, K.; Wang, J. Hierarchical Sn and AgCl co-doped TiO2 microspheres as electron transport layer for enhanced perovskite solar cell performance. Catal. Today 2020, 355, 333–339. [Google Scholar] [CrossRef]
- Birajdar, S.D.; Saraf, A.; Maharolkar, A.P.; Gattu, K.P.; Patil, N.G.; Chavan, R.B.; Jamkar, M.V.; Mundhe, Y.S.; Kambale, R.N.; Alange, R.C.; et al. Intrinsic defect-induced magnetism and enhanced photocatalytic activity in Zn1−xZrxO (0.0 ≤ x ≤ 0.07) nanoparticles for spintronic device and photocatalytic application. J. Alloys Compd. 2022, 929, 167272. [Google Scholar] [CrossRef]
- Pascariu, P.; Gherasim, C.; Airinei, A. Metal oxide nanostructures (MONs) as photocatalysts for ciprofloxacin degradation. Int. J. Mol. Sci. 2023, 24, 9564. [Google Scholar] [CrossRef] [PubMed]
- Lizeth Katherine, T.N.; Vendula, B.; Jaroslav, K.; Jaroslav, C. Structure and photocatalytic properties of Ni-, Co-, Cu-, and Fe-doped TiO2 Aerogels. Gels 2023, 9, 357. [Google Scholar] [CrossRef]
- Liton, M.N.H.; Roknuzzaman, M.; Helal, M.A.; Kamruzzaman, M.; Islam, A.K.M.F.U.; Ostrikov, K.; Khan, M.K.R. Electronic, mechanical, optical and photocatalytic properties of perovskite RbSr2Nb3O10 compound. J. Alloys Compd. 2021, 867, 159077. [Google Scholar] [CrossRef]
- Huang, M.; Lian, J.; Si, R.; Wang, L.; Pan, X.; Liu, P. Spatial separation of electrons and holes among ZnO polar {0001} and {1010} facets for enhanced photocatalytic performance. ACS Omega 2022, 7, 26844–26852. [Google Scholar] [CrossRef]
- Feng, H.; Du, Y.; Wang, C.; Hao, W. Efficient visible-light photocatalysts by constructing dispersive energy band with anisotropic p and s-p hybridization states. Curr. Opin. Green. Sustain. Chem. 2017, 6, 93–100. [Google Scholar] [CrossRef]
- Raizada, P.; Soni, V.; Kumar, A.; Singh, P.; Parwaz Khan, A.; Asiri, A.M.; Thakur, V.; Nguyen, V. Surface defect engineering of metal oxides photocatalyst for energy application and water treatment. J. Mater. 2021, 7, 388–418. [Google Scholar] [CrossRef]
- Sun, S.; Yu, X.; Yang, Q.; Yang, Z.; Liang, S. Mesocrystals for photocatalysis: A comprehensive review on synthesis engineering and functional modifications. Nanoscale Adv. 2019, 1, 34–63. [Google Scholar] [CrossRef]
- Siriwong, C.; Wetchakun, N.; Inceesungvorn, B.; Channei, D.; Samerjai, T.; Phanichphant, S. Doped-metal oxide nanoparticles for use as photocatalysts. Prog. Cryst. Growth Charact. Mater. 2012, 58, 145–163. [Google Scholar] [CrossRef]
- Sultana, S.; Mansingh, S.; Parida, K.M. Crystal facet and surface defect engineered low dimensional CeO2 (0D, 1D, 2D) based photocatalytic materials towards energy generation and pollution abatement. Mater. Adv. 2021, 2, 6942–6983. [Google Scholar] [CrossRef]
- Davidson, A.; Amin, V.; Aljuaid, W.; Haney, P.; Fan, X. Perspectives of electrically generated spin currents in ferromagnetic materials. Phys. Lett. A 2020, 384, 126228. [Google Scholar] [CrossRef]
- Hoffmann, A. Spin transport modified by magnetic order. J. Magn. Magn. Mater 2022, 563, 169896. [Google Scholar] [CrossRef]
- Cervera-Gabalda, L.; Zielińska-Jurek, A.; Gómez-Polo, C. Tuning the photocatalytic performance through magnetization in Co-Zn ferrite nanoparticles. J. Magn. Magn. Mater 2022, 560, 169617. [Google Scholar] [CrossRef]
- Ghozza, M.H.; Yahia, I.S.; Hussien, M.S.A. Structure, magnetic, and photocatalysis of La0.7Sr0.3MO3 (M = Mn, Co, and Fe) perovskite nanoparticles: Novel photocatalytic materials. Environ. Sci. Pollut. Res. Int. 2023, 30, 61106–61122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gong, W.; Wang, J.; Ning, X.; Wang, Z.; Zhao, X.; Ren, X.; Zhang, Z. Size-dependent magnetic, photo-absorbing, and photocatalytic properties of single-crystalline Bi2Fe4O9 semiconductor nanocrystals. J. Phys. Chem. C 2011, 115, 25241–25246. [Google Scholar] [CrossRef]
- Lv, S.; Du, Y.; Wu, F.; Cai, Y.; Zhou, T. Review on LSPR assisted photocatalysis: Effects of physical fields and opportunities in multifield decoupling. Nanoscale Adv. 2022, 28, 2608–2631. [Google Scholar] [CrossRef]
- Duan, K.; Que, T.; Koppala, S.; Balan, R.; Lokesh, B.; Pillai, R.; David, S.; Karthikeyan, P.; Ramamoorthy, S.; Lekshmi, I.C.; et al. A facile route to synthesize n-SnO2/p-CuFe2O4 to rapidly degrade toxic methylene blue dye under natural sunlight. RSC Adv. 2022, 12, 16544–16553. [Google Scholar] [CrossRef]
- Stiadi, Y.; Wendari, T.P. Tuning the structural, magnetic, and optical properties of ZnO/NiFe2O4 heterojunction photocatalyst for simultaneous photodegradation of Rhodamine B and Methylene Blue under natural sunlight. Environ. Eng. Res. 2023, 28, 220074. [Google Scholar] [CrossRef]
- Hezam, F.A.; Nur, O.; Mustafa, M.A. Synthesis, structural, optical and magnetic properties of NiFe2O4/MWCNTs/ZnO hybrid nanocomposite for solar radiation driven photocatalytic degradation and magnetic separation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124586. [Google Scholar] [CrossRef]
- Supin, K.K.; George, A.; Kumar, Y.R.; Thejas, K.K.; Mandal, G.; Chanda, A.; Vasundhara, M. Structural, optical and magnetic properties of pure and 3d metal dopant-incorporated SnO2 nanoparticles. RSC Adv. 2022, 12, 26712–26726. [Google Scholar]
- Schmidbauer, E.; Keller, M. Magnetic properties and rotational hysteresis of Fe3O4 and γ-Fe2O3 particles ∼ 250 nm in diameter. J. Magn. Magn. Mater. 2006, 297, 107–117. [Google Scholar] [CrossRef]
- Li, X.; Zhu, K.; Pang, J.; Tian, M.; Liu, J.; Rykov, A.; Zheng, M.; Wang, X.; Zhu, X.; Huang, Y.; et al. Unique role of Mössbauer spectroscopy in assessing structural features of heterogeneous catalysts. Appl. Catal. B 2018, 224, 518–532. [Google Scholar] [CrossRef]
- Gütlich, P. Fifty Years of Mössbauer spectroscopy in solid state research—Remarkable achievements, future perspectives. J. Inorg. Chem. Gen. Chem. 2012, 638, 15–43. [Google Scholar] [CrossRef]
- Moon, S.; Shim, I.; Kim, C. Crystallographic and magnetic properties of KFeO. IEEE Trans. Magn. 2006, 42, 2879–2881. [Google Scholar] [CrossRef]
- Locovei, C.; Radu, C.; Kuncser, A.; Iacob, N.; Schinteie, G.; Stanciu, A.; Iftimie, S.; Kuncser, V. Relationship between the formation of magnetic clusters and hexagonal phase of gold matrix in AuxFe1−x nanophase thin films. Nanomaterials 2022, 12, 1176. [Google Scholar] [CrossRef]
- Balamurugan, K.; Harish Kumar, N.; Arout Chelvane, J.; Santhosh, P.N. Room temperature ferromagnetism in Fe-doped BaSnO3. J. Alloys Compd. 2009, 472, 9–12. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the roadmap for photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).