Photocatalytic Degradation of Tartrazine and Naphthol Blue Black Binary Mixture with the TiO2 Nanosphere under Visible Light: Box-Behnken Experimental Design Optimization and Salt Effect
Abstract
1. Introduction
2. Material and Method
2.1. Reagents
2.2. Catalyst Synthesis
2.3. Characterization
2.4. Photocatalytic Treatment
3. Results and Discussions
3.1. Characterization
3.1.1. X-ray Diffraction, Raman Spectroscopy, Fourier Transformer Infrared (FTIR) and X-ray Fluorescence of TiO2-NS
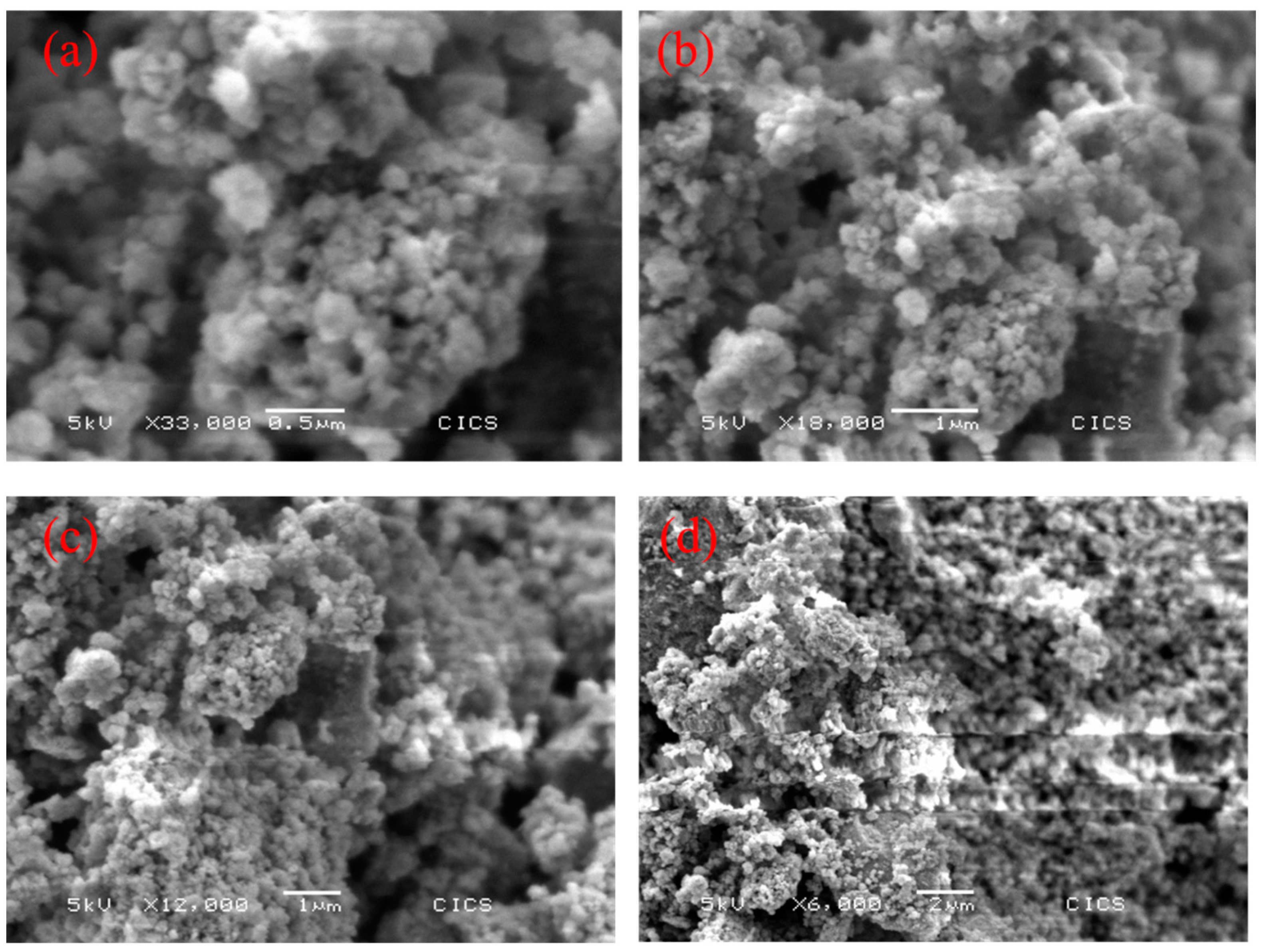
3.1.2. SEM Analysis and Specific Surface Area of TiO2-NS
3.1.3. UV-Vis Diffuse Reflectance Spectroscopy (DRS)
3.2. Photocatalytic Activity of TiO2-NS under UV and Visible Light
3.3. Catalyst Dose Effect
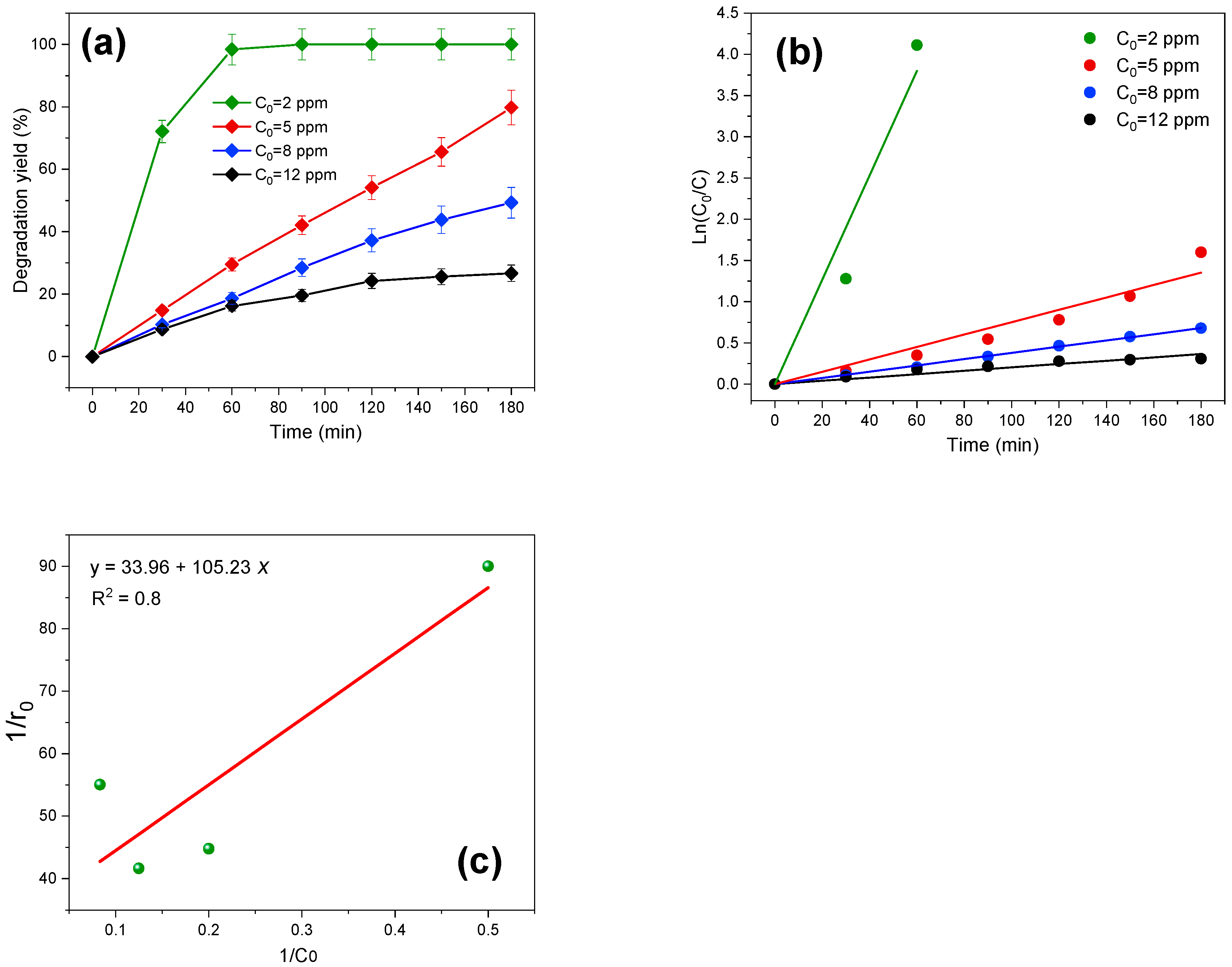
3.3.1. Initial TZZ Concentration Effect and Kinetics of Degradation
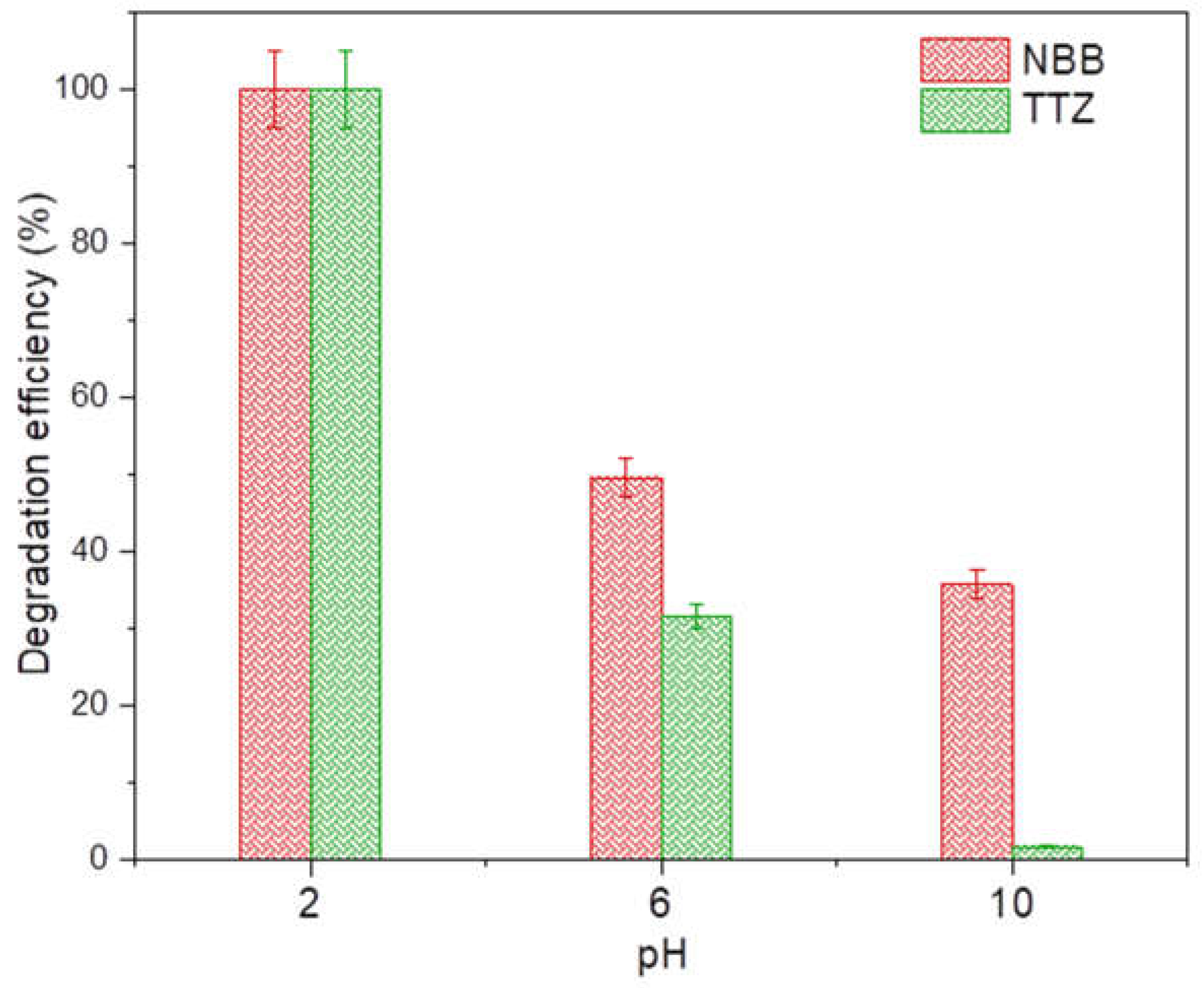
3.3.2. Effect of pH
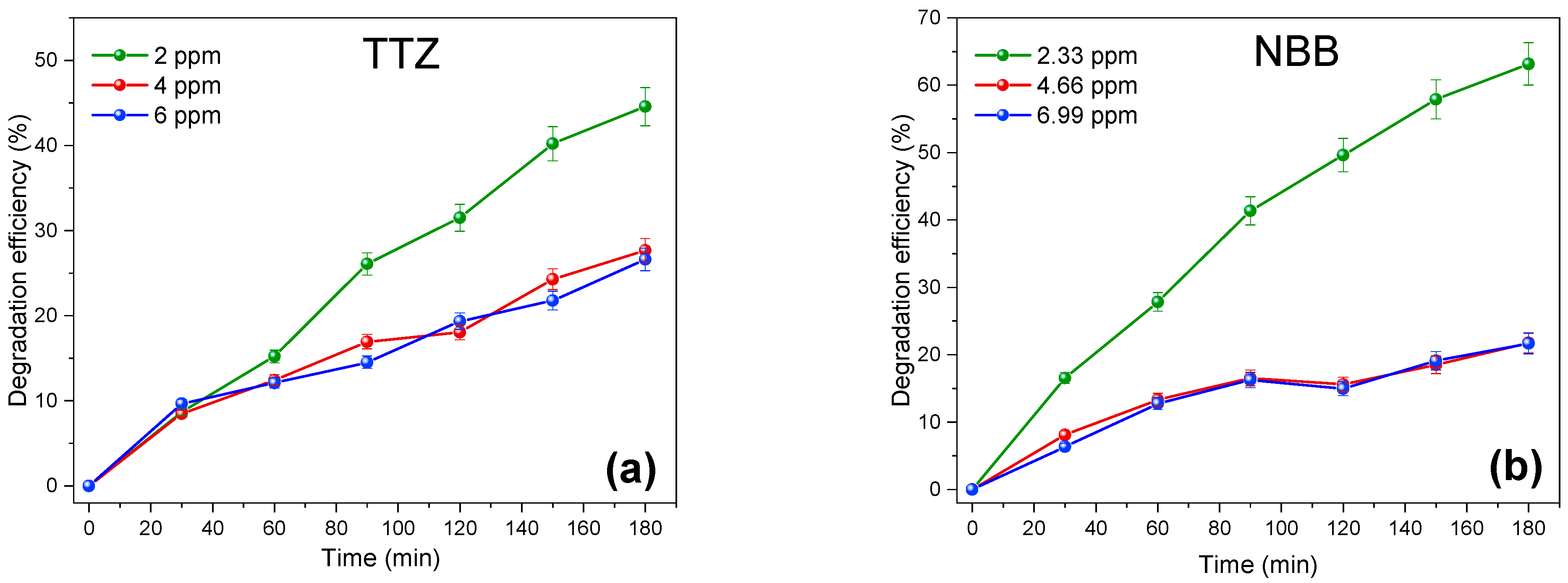
3.4. Binary System Study
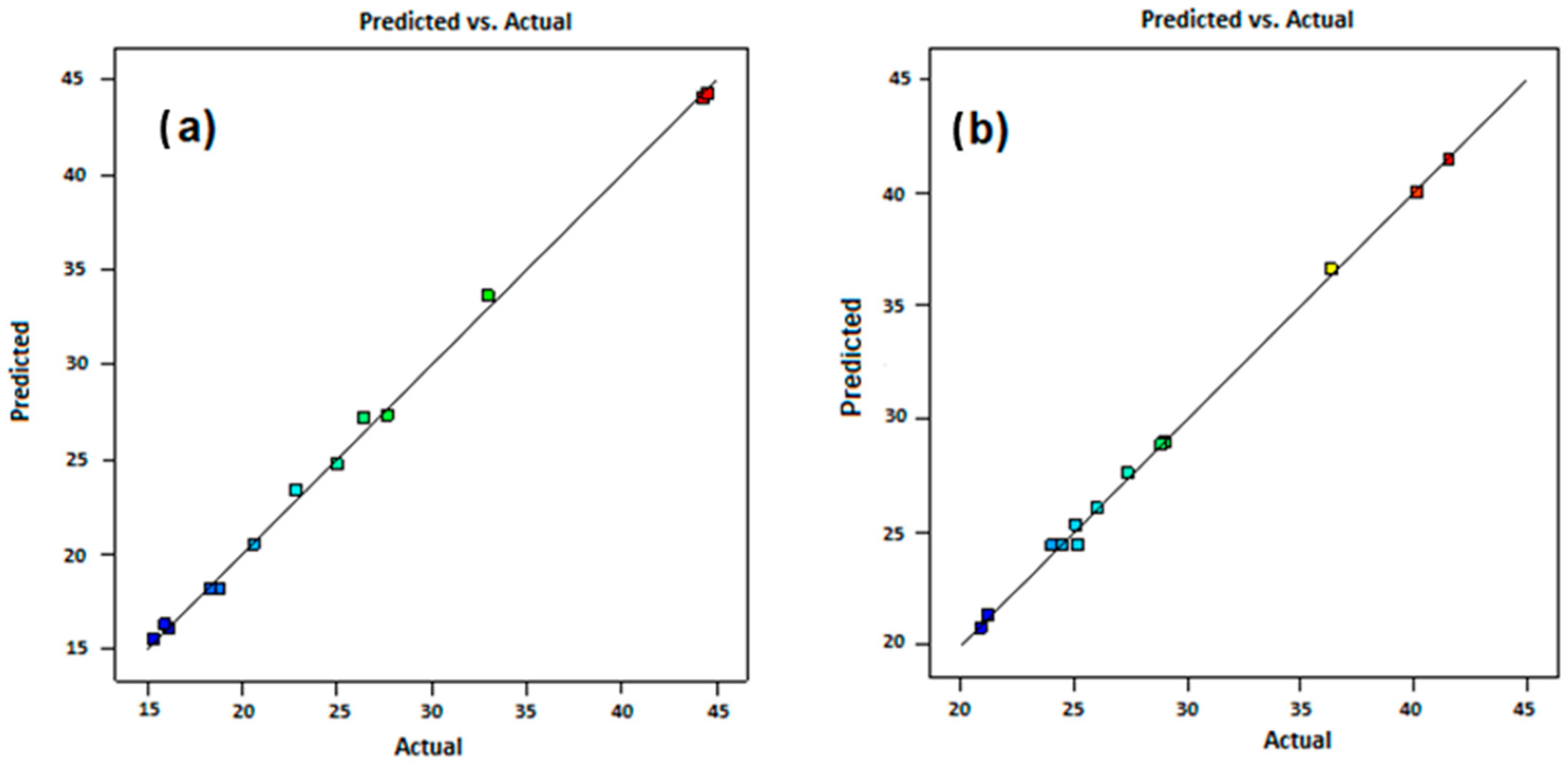
3.5. Experimental Design Results
3.5.1. Optimization of Parameters Using Response Surface Methodology (RSM)
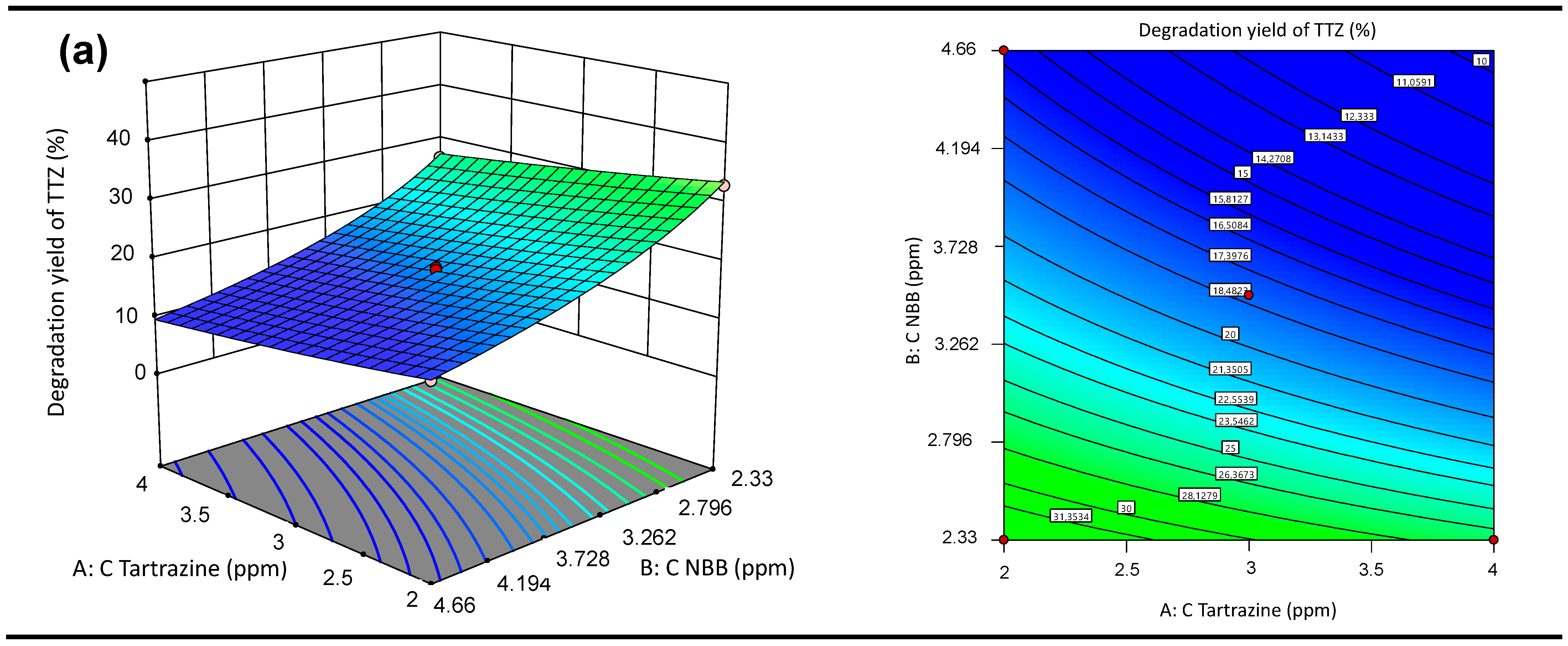
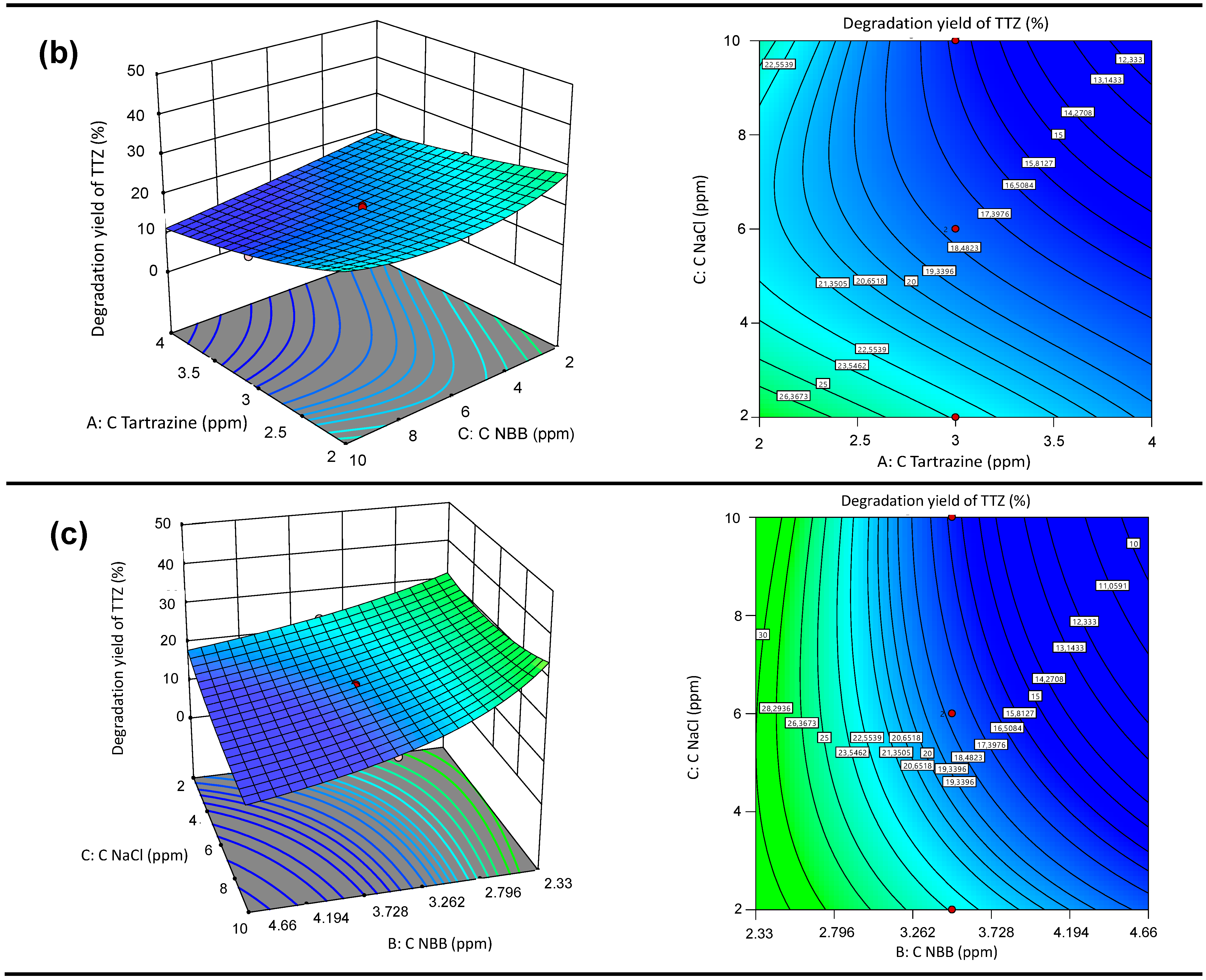
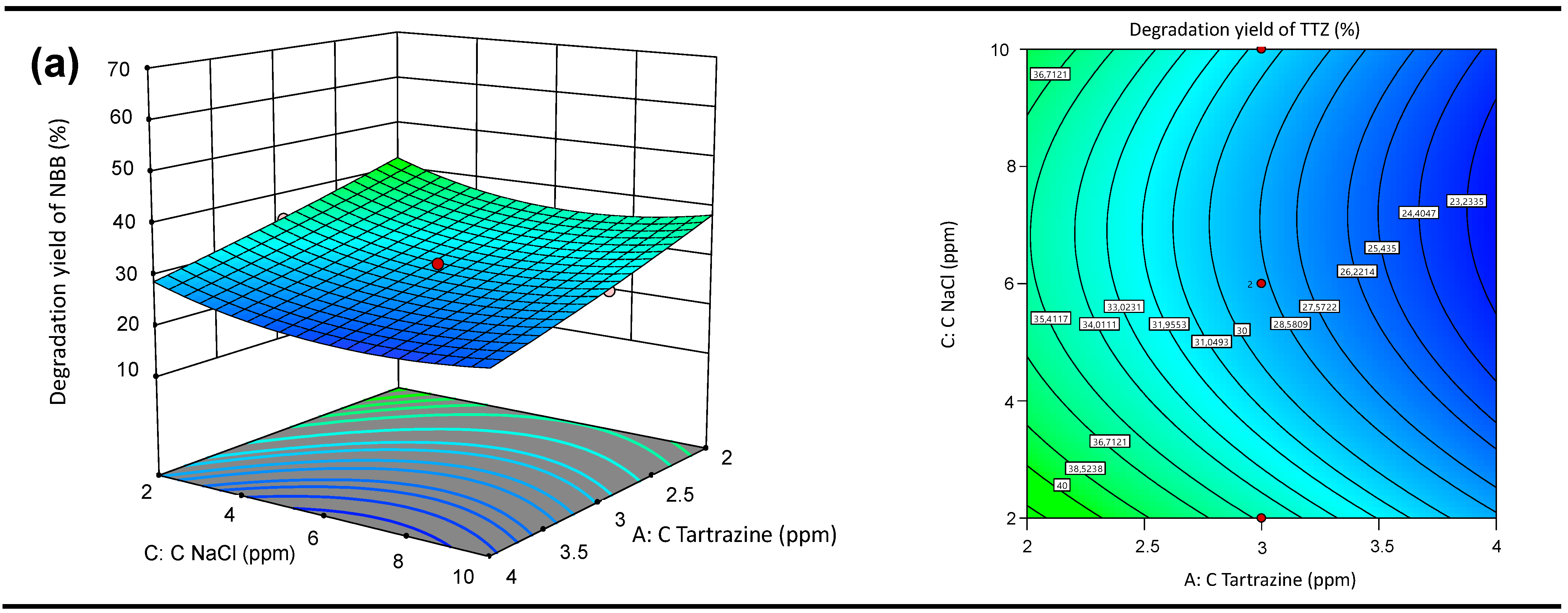
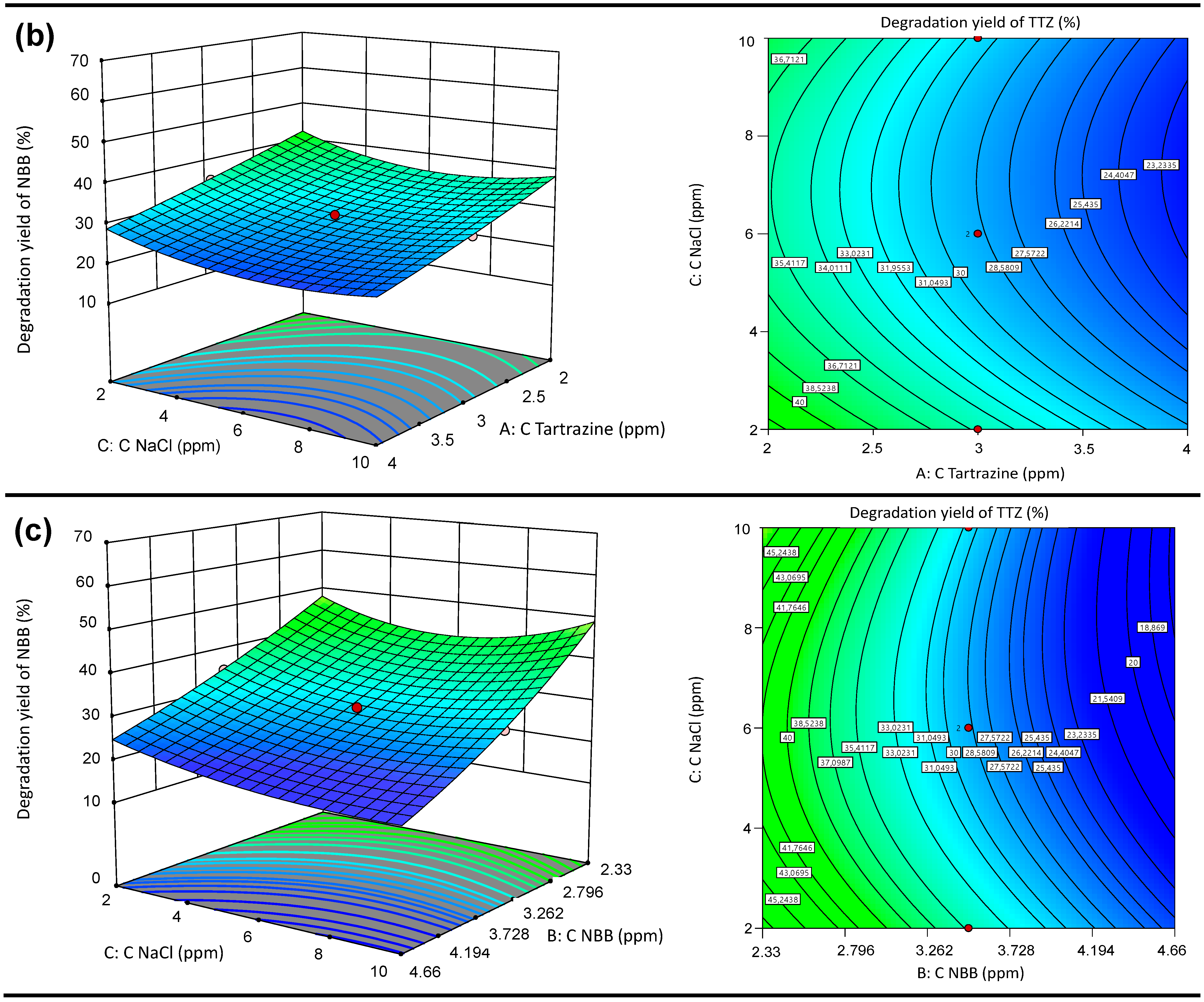
3.5.2. Response Surface 3D Graph and Contour Plots of the Interactive Effects
3.6. Mineralization
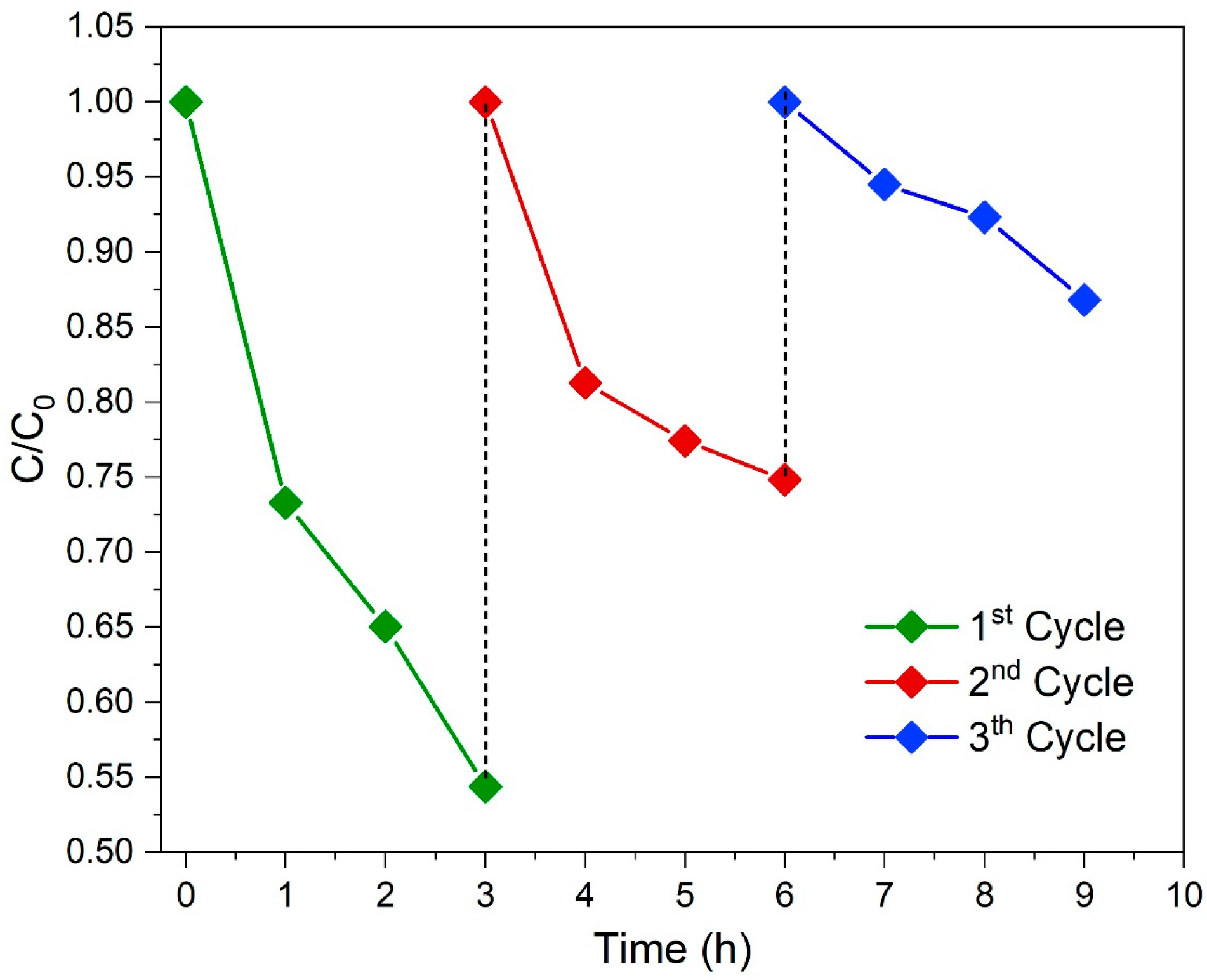
3.7. Reuse Test of Photocatalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, N.S.; Chetan, N.L.; Nataraja, M.; Suthar, S. Climate change scenarios and its effect on groundwater level in the Hiranyakeshi watershed, Groundw. Sustain. Dev. 2020, 10, 100323. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhang, K.; Wang, X.; Guo, Q.; Wu, Y.; Du, Y.; Zhang, L.; Xia, J.; Tang, H.; Zhang, X.; et al. 0D/2D Schottky junction synergies with 2D/2D S-scheme heterojunction strategy to achieve uniform separation of carriers in 0D/2D/2D quasi CNQDs/TCN/ZnIn2S4 towards photocatalytic remediating petroleum hydrocarbons polluted marine. Appl. Catal. B Environ. 2023, 325, 122387. [Google Scholar] [CrossRef]
- Zubair, M.; Aziz, H.A.; Ihsanullah, I.; Ahmad, M.A.; Al-Harthi, M.A. Biochar supported CuFe layered double hydroxide composite as a sustainable adsorbent for efficient removal of anionic azo dye from water. Environ. Technol. Innov. 2021, 23, 101614. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Xia, L.; Sun, Z.; Wu, Y.; Yu, X.-F.; Cheng, J.; Zhang, K.; Sarina, S.; Zhu, H.-Y.; Weerathunga, H.; Zhang, L.; et al. Leveraging doping and defect engineering to modulate exciton dissociation in graphitic carbon nitride for photocatalytic elimination of marine oil spill. Chem. Eng. J. 2022, 439, 135668. [Google Scholar] [CrossRef]
- De Lima Barizão, A.C.; Silva, M.F.; Andrade, M.; Brito, F.C.; Gomes, R.G.; Bergamasco, R. Green synthesis of iron oxide nanoparticles for tartrazine and bordeaux red dye removal. J. Environ. Chem. Eng. 2020, 8, 103618. [Google Scholar] [CrossRef]
- Dalhatou, S.; Pétrier, C.; Laminsi, S.; Baup, S. Sonochemical removal of naphthol blue black azo dye: Influence of parameters and effect of mineral ions. Int. J. Environ. Sci. Technol. 2015, 12, 35–44. [Google Scholar] [CrossRef]
- Dalhatou, S.; Laminsi, S.; Pétrier, C.; Baup, S. Competition in sonochemical degradation of Naphthol Blue Black: Presence of an organic (nonylphenol) and a mineral (bicarbonate ions) matrix. J. Environ. Chem. Eng. 2019, 7, 102819. [Google Scholar] [CrossRef]
- Perveen, S.; Nadeem, R.; Nosheen, F.; Asjad, M.I.; Awrejcewicz, J.; Anwar, T. Biochar-Mediated Zirconium Ferrite Nanocomposites for Tartrazine Dye Removal from Textile Wastewater. Nanomaterials 2022, 12, 2828. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef] [PubMed]
- Ichipi, E.O.; Tichapondwa, S.M.; Chirwa, E.M.N. Plasmonic effect and bandgap tailoring of Ag/Ag2S doped on ZnO nanocomposites for enhanced visible-light photocatalysis. Adv. Powder Technol. 2022, 33, 103596. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Guaraldo, T.T.; Wenk, J.; Mattia, D. Photocatalytic ZnO Foams for Micropollutant Degradation. Adv. Sustain. Syst. 2021, 5, 2000208. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review, Environ. Nanotechnology. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Sharma, K.; Raizada, P.; Hasija, V.; Singh, P.; Bajpai, A.; Nguyen, V.H.; Rangabhashiyam, S.; Kumar, P.; Nadda, A.K.; Kim, S.Y.; et al. ZnS-based quantum dots as photocatalysts for water purification. J. Water Process Eng. 2021, 43, 102217. [Google Scholar] [CrossRef]
- Sajid, M.M.; Shad, N.A.; Javed, Y.; Khan, S.B.; Zhang, Z.; Amin, N.; Zhai, H. Preparation and characterization of Vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Surf. Interfaces 2020, 19, 100502. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadur, I. TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. J. Mol. Liq. 2021, 331, 115458. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Naeem, Y.A.; Mizher, R.M.; Karim, M.M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano titanium oxide (nano-TiO2): A review of synthesis methods, properties, and applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al-Hamdani, A.A.S.; Sunghun, B.; Ko, Y.G. A review on TiO2-based composites for superior photocatalytic activity. Rev. Inorg. Chem. 2021, 41, 213–222. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Karthigadevi, G.; Manikandan, S.; Karmegam, N.; Subbaiya, R.; Chozhavendhan, S.; Ravindran, B.; Chang, S.W.; Awasthi, M.K. Chemico-nanotreatment methods for the removal of persistent organic pollutants and xenobiotics in water—A review. Bioresour. Technol. 2021, 324, 124678. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. In Titanium Dioxide-Material for a Sustainable Environment; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Samriti; Cho, J.; Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, F.; Lin, C.; Wang, Z.L. Direct growth of TiO2 nanosheet arrays on carbon fibers for highly efficient photocatalytic degradation of methyl orange. Adv. Mater. 2012, 24, 4761–4764. [Google Scholar] [CrossRef]
- Konni, M.; Dwarapureddi, B.K.; Dash, S.; Raj, A.; Karnena, M.K. Titanium dioxide electrospun nanofibers for dye removal-A review. J. Appl. Nat. Sci. 2022, 14, 450–458. [Google Scholar] [CrossRef]
- Shehab, M.A.; Sharma, N.; Valsesia, A.; Karacs, G.; Kristály, F.; Koós, T.; Leskó, A.K.; Nánai, L.; Hernadi, K.; Németh, Z. Preparation and photocatalytic performance of TiO2 nanowire-based self-supported hybrid membranes. Molecules 2022, 27, 2951. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.A.; Karoui, S.; Trabelsi, K.; Hajjaji, A.; Elfalleh, W.; Ghorbal, A.; Maghzaoui, M.; Assadi, A.A. Performance for Wastewater Treatment under Visible Light. Materials 2022, 15, 1463. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, H.; Bian, J.; Li, T.; Huang, B.; Niu, Q. High Specific Surface Area TiO2 Nanospheres for Hydrogen Production and Photocatalytic Activity. J. Nanosci. Nanotechnol. 2019, 20, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; White, T. Degradation of Methylene Blue by Three-Dimensionally Ordered Macroporous Titania. Environ. Sci. Technol. 2007, 41, 4405–4409. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Caruso, R.A. Enhancing photocatalytic activity of titania materials by using porous structures and the addition of gold nanoparticles. J. Mater. Chem. 2011, 21, 20–28. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Murugesan, S.; Valdés, H.; Selvaraj, M.; Sathishkumar, P.; Smirniotis, P.G.; Anandan, S.; Mangalaraja, R.V. Photocatalytic oxidation of ceftiofur sodium under UV–visible irradiation using plasmonic porous Ag-TiO2 nanospheres. J. Ind. Eng. Chem. 2022, 105, 384–392. [Google Scholar] [CrossRef]
- Haider, A.J.; Al-Anbari, R.H.; Kadhim, G.R.; Salame, C.T. Exploring potential Environmental applications of TiO2 Nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Askari, M.B.; Banizi, Z.T.; Seifi, M.; Dehaghi, S.B.; Veisi, P. Synthesis of TiO2 nanoparticles and decorated multi-wall carbon nanotube (MWCNT) with anatase TiO2 nanoparticles and study of optical properties and structural characterization of TiO2/MWCNT nanocomposite. Optik 2017, 149, 447–454. [Google Scholar] [CrossRef]
- Kader, S.; Al-Mamun, M.R.; Suhan, M.B.K.; Shuchi, S.B.; Islam, M.S. Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3 and Ag doped TiO2 photocatalysts. Environ. Technol. Innov. 2022, 27, 102476. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Setayesh, S.R.; Gholami, M.R. A credible role of copper oxide on structure of nanocrystalline mesoporous titanium dioxide. J. Iran. Chem. Soc. 2008, 5, 367–374. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.T.; et al. Liquid exfoliation of g-C3N4 nanosheets to construct 2D-2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity. Appl. Catal. B Environ. 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Talami, B.; Zeghioud, H.; Dalhatou, S.; Bonnet, P.; Caperaa, C.; Ligny, R.; Assadi, A.A.; Massai, H.; Kane, A. A new sunlight active photocatalyst based on CuO-TiO2-Clay composite for wastewater remediation: Mechanistic insights and degradation optimization. Water Air Soil Pollut. 2024, 235, 104. [Google Scholar] [CrossRef]
- Perarasan, T.; Peter, I.J.; Kumar, A.M.; Rajamanickam, N.; Ramachandran, K.; Mohan, C.R. Copper doped titanium dioxide for enhancing the photovoltaic behavior in solar cell. Mater. Today Proc. 2019, 35, 66–68. [Google Scholar] [CrossRef]
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Yu, K.; Song, K.; Wang, X.; Liu, W.; Hou, J.; Liang, C. Enhanced photocatalytic performance of Nd3+-doped TiO2 nanosphere under visible light. Chem. Phys. 2020, 528, 110538. [Google Scholar] [CrossRef]
- Ma, H.; Zheng, W.; Yan, X.; Li, S.; Zhang, K.; Liu, G.; Jiang, L. Polydopamine-induced fabrication of Ag-TiO2 hollow nanospheres and their application in visible-light photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 2–7. [Google Scholar] [CrossRef]
- Kerour, A.; Boudjadar, S.; Bourzami, R.; Allouche, B. Eco-friendly synthesis of cuprous oxide (Cu2O) nanoparticles and improvement of their solar photocatalytic activities. J. Solid State Chem. 2018, 263, 79–83. [Google Scholar] [CrossRef]
- Javanroudi, S.R.; Fattahi, N.; Sharafi, K.; Arfaeinia, H.; Moradi, M. Chalcopyrite as an oxidants activator for organic pollutant remediation: A review of mechanisms, parameters, and future perspectives. Heliyon 2023, 9, e19992. [Google Scholar] [CrossRef]
- Kiwaan, H.A.; Atwee, T.M.; Azab, E.A.; El-Bindary, A.A. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide. J. Mol. Struct. 2020, 1200, 127115. [Google Scholar] [CrossRef]
- Isai, K.A.; Shrivastava, V.S. Photocatalytic degradation of methylene blue using ZnO and 2%Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: A comparative study. SN Appl. Sci. 2019, 1, 1247. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kebir, M.; Khezami, L.; Amrane, A.; Assadi, A.A. A comparative study of ceramic nanoparticles synthesized for antibiotic removal: Catalysis characterization and photocatalytic performance modeling. Environ. Sci. Pollut. Res. 2021, 28, 13900–13912. [Google Scholar] [CrossRef]
- Aissani, T.; Yahiaoui, I.; Boudrahem, F.; Cherif, L.Y.; Fourcad, F.; Amrane, A.; Aissani-Benissad, F. Sulfamethazine degradation by heterogeneous photocatalysis with ZnO immobilized on a glass plate using the heat attachment method and its impact on the biodegradability. React. Kinet. Mech. Catal. 2020, 131, 471–487. [Google Scholar] [CrossRef]
- Zeghioud, H.; Khellaf, N.; Amrane, A.; Djelal, H.; Elfalleh, W.; Assadi, A.A.; Rtimi, S. Photocatalytic performance of TiO2 impregnated polyester for the degradation of Reactive Green 12: Implications of the surface pretreatment and the microstructure. J. Photochem. Photobiol. A Chem. 2017, 346, 493–501. [Google Scholar] [CrossRef]
- Mouhamadou, S.; Dalhatou, S.; Obada, D.O.; Fryda, L.; Mahieu, A.; Bonnet, P.; Caperaa, C.; Kane, A.; Massai, H.; Zeghioud, H. Synthesis of piliostigma reticulatum decorated TiO2 based composite and its application towards Cr(VI) adsorption and bromophenol blue degradation: Nonlinear kinetics, equilibrium modelling and optimisation photocatalytic parameters. J. Environ. Chem. Eng. 2023, 11, 109273. [Google Scholar] [CrossRef]
- Almansba, A.; Kane, A.; Nasrallah, N.; Maachi, R.; Lamaa, L.; Peruchon, L.; Brochier, C.; Béchohra, I.; Amrane, A.; Assadi, A.A. Innovative photocatalytic luminous textiles optimized towards water treatment: Performance evaluation of photoreactors. Chem. Eng. J. 2021, 416, 129195. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir-Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Almansba, A.; Kane, A.; Nasrallah, N.; Wilson, J.M.; Maachi, R.; Lamaa, L.; Peruchon, L.; Brochier, C.; Amrane, A.; Assadi, A.A. An engineering approach towards the design of an innovative compact photo-reactor for antibiotic removal in the frame of laboratory and pilot-plant scale. J. Photochem. Photobiol. A Chem. 2021, 418, 113445. [Google Scholar] [CrossRef]
- Nasr, O.; Mohamed, O.; Al-Shirbini, A.S.; Abdel-Wahab, A.M. Photocatalytic degradation of acetaminophen over Ag, Au and Pt loaded TiO2 using solar light. J. Photochem. Photobiol. A Chem. 2019, 374, 185–193. [Google Scholar] [CrossRef]
- Tamaddon, F.; Nasiri, A.; Yazdanpanah, G. Photocatalytic degradation of ciprofloxacin using CuFe2O4@methyl cellulose based magnetic nanobiocomposite. MethodsX 2020, 7, 74–81. [Google Scholar] [CrossRef]
- Singh, P.; Vishnu, M.C.; Sharma, K.K.; Borthakur, A.; Srivastava, P.; Pal, D.B.; Tiwary, D.; Mishra, P.K. Photocatalytic degradation of Acid Red dye stuff in the presence of activated carbon-TiO2 composite and its kinetic enumeration. J. Water Process Eng. 2016, 12, 20–31. [Google Scholar] [CrossRef]
- Chekir, N.; Tassalit, D.; Benhabiles, O.; Merzouk, N.K.; Ghenna, M.; Abdessemed, A.; Issaadi, R. A comparative study of tartrazine degradation using UV and solar fixed bed reactors. Int. J. Hydrogen Energy 2017, 42, 8948–8954. [Google Scholar] [CrossRef]
- Zyoud, A.H.; Zubi, A.; Hejjawi, S.; Zyoud, S.H.; Helal, M.H.; Zyoud, S.H.; Qamhieh, N.; Hajamohideen, A.R.; Hilal, H.S. Removal of acetaminophen from water by simulated solar light photodegradation with ZnO and TiO2 nanoparticles: Catalytic efficiency assessment for future prospects. J. Environ. Chem. Eng. 2020, 8, 104038. [Google Scholar] [CrossRef]
- Surya, L.; Sheilatin; Praja, P.V.; Sepia, N.S. Preparation and characterization of titania/bentonite composite application on the degradation of naphthol blue black dye. Res. J. Chem. Environ. 2018, 22, 48–53. [Google Scholar]
- Essandoh, M.; Garcia, R.A. Efficient removal of dyes from aqueous solutions using a novel hemoglobin/iron oxide composite. Chemosphere 2018, 206, 502–512. [Google Scholar] [CrossRef]
- Ahmedi, A.; Abouseoud, M.; Couvert, A.; Amrane, A. Enzymatic degradation of Congo Red by turnip (Brassica rapa) peroxidase. Z. Naturforsch. Sect. C J. Biosci. 2012, 67, 429–436. [Google Scholar] [CrossRef]
- Christy, E.J.S.; Amalraj, A.; Rajeswari, A.; Pius, A. Enhanced photocatalytic performance of Zr(IV) doped ZnO nanocomposite for the degradation efficiency of different azo dyes. Environ. Chem. Ecotoxicol. 2021, 3, 31–41. [Google Scholar] [CrossRef]
- Dhatshanamurthi, P.; Shanthi, M. Enhanced photocatalytic degradation of azo dye in aqueous solutions using Ba@Ag@ZnO nanocomposite for self-sensitized under sunshine irradiation. Int. J. Hydrogen Energy 2017, 42, 5523–5536. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.S.D.; Ali, H.; Banu, T.; Farzana, M.H.; Min, C. Chemosphere Hydrothermal synthesis of hydroxyapatite-reduced graphene oxide (1D e 2D) hybrids with enhanced selective adsorption properties for methyl orange and hexavalent chromium from aqueous solutions. Chemosphere 2021, 276, 130200. [Google Scholar] [CrossRef]
- Hassan, F.; Bonnet, P.; Marie, J.; Dikdim, D.; Bandjoun, N.G.; Caperaa, C.; Dalhatou, S.; Kane, A.; Zeghioud, H. Synthesis and Investigation of TiO2/g-C3N4 Performance for Photocatalytic Degradation of Bromophenol Blue and Eriochrome Black T: Experimental Design Optimization and Reactive Oxygen Species Contribution. Water 2022, 14, 3331. [Google Scholar] [CrossRef]
- Shahnaz, T.; Sharma, V.; Subbiah, S.; Narayanasamy, S. Multivariate optimisation of Cr (VI), Co (III) and Cu (II) adsorption onto nanobentonite incorporated nanocellulose/chitosan aerogel using response surface methodology. J. Water Process Eng. 2020, 36, 101283. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, D.; Xu, Y.; Liu, C. Optimization of parameters on photocatalytic degradation of chloramphenicol using TiO2 as photocatalyist by response surface methodology. J. Environ. Sci. 2010, 22, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Çatlıoğlu, F.; Akay, S.; Turunç, E.; Gözmen, B.; Anastopoulos, I.; Kayan, B.; Kalderis, D. Preparation and application of Fe-modified banana peel in the adsorption of methylene blue: Process optimization using response surface methodology. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100517. [Google Scholar] [CrossRef]
- Sohrabi, S.; Akhlaghian, F. Modeling and optimization of phenol degradation over copper-doped titanium dioxide photocatalyst using response surface methodology. Process Saf. Environ. Prot. 2016, 99, 120–128. [Google Scholar] [CrossRef]
- Evgenidou, E.; Chatzisalata, Z.; Tsevis, A.; Bourikas, K.; Torounidou, P.; Sergelidis, D.; Koltsakidou, A.; Lambropoulou, D.A. Photocatalytic degradation of a mixture of eight antibiotics using Cu-modified TiO2 photocatalysts: Kinetics, mineralization, antimicrobial activity elimination and disinfection. J. Environ. Chem. Eng. 2021, 9, 105295. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef]





| Dye | Molecular Formula | Structure | λmax (nm) |
|---|---|---|---|
| Tartrazine | C16H9N4Na3O9S2 | 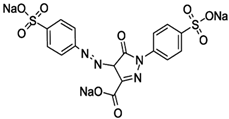 | 426 |
| Naphthol Blue Black | C22H14N6Na2O9S2 |  | 618 |
| Materials | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) | Nanoparticle Size (Å) |
|---|---|---|---|---|
| TiO2-NS | 33.3 | 0.2 | 201.0 | 470.9 |
| Pollutant | Photocatalyst | Irradiation Source | k (mg∙min−1.L−1) | K (L∙mg−1) | Ref. |
|---|---|---|---|---|---|
| Acetaminophen | TiO2 | Simulated solar light | 0.385 | 0.0970 | [60] |
| Ciprofloxacin | CuFe2O4 | UV-C light irradiation | 0.141 | 0.202 | [61] |
| Acid Red dye | Activated carbon-TiO2 composite | UV light irradiation | 1.78 | 0.06 | [62] |
| Reactive green 12 | TiO2 loading on polyester | UV light irradiation | 0.035 | 0.796 | [55] |
| Tartrazine | Synthesized TiO2-NS | Visible light irradiation | 0.029 | 0.32 | This work |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | |
|---|---|---|---|---|---|
| Run | A: CTTZ | B: CNBB | C: CNaCl | Degradation Yield TZ | Degradation Yield NBB |
| (ppm) | (ppm) | (ppm) | (%) | (%) | |
| 1 | 2 | 2.33 | 10 | 44.55 | 63.14 |
| 2 | 2 | 2.33 | 6 | 32.97 | 52.71 |
| 3 | 3 | 3.495 | 10 | 15.94 | 30.2 |
| 4 | 2 | 2.33 | 2 | 44.32 | 60.31 |
| 5 | 4 | 2.33 | 2 | 25 | 38.06 |
| 6 | 2 | 4.66 | 6 | 15.38 | 22.33 |
| 7 | 4 | 2.33 | 6 | 26.44 | 32 |
| 8 | 4 | 2.33 | 10 | 27.68 | 37.74 |
| 9 | 3 | 3.495 | 6 | 18.37 | 30.33 |
| 10 | 3 | 3.495 | 2 | 22.82 | 34.74 |
| 11 | 2 | 4.66 | 10 | 16.15 | 21.75 |
| 12 | 2 | 4.66 | 2 | 20.61 | 28.99 |
| 13 | 3 | 3.495 | 6 | 18.79 | 28.09 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2.001 × 10−6 | 9 | 2.224 × 10−7 | 110.00 | 0.0013 | Significant |
| A-CTTZ | 9.303 × 10−8 | 1 | 9.303 × 10−8 | 46.02 | 0.0065 | |
| B-CNBB | 8.724 × 10−7 | 1 | 8.724 × 10−7 | 431.59 | 0.0002 | |
| C-CNaCl | 1.199 × 10−7 | 1 | 1.199 × 10−7 | 59.29 | 0.0046 | |
| AB | 4.421 × 10−8 | 1 | 4.421 × 10−8 | 21.87 | 0.0185 | |
| AC | 3.440 × 10−8 | 1 | 3.440 × 10−8 | 17.02 | 0.0258 | |
| BC | 1.004 × 10−7 | 1 | 1.004 × 10−7 | 49.65 | 0.0059 | |
| C2 | 2.006 × 10−8 | 1 | 2.006 × 10−8 | 9.92 | 0.0513 | |
| ABC | 4.730 × 10−8 | 1 | 4.730 × 10−8 | 23.40 | 0.0168 | |
| AC2 | 3.452 × 10−8 | 1 | 3.452 × 10−8 | 17.07 | 0.0257 | |
| Residual | 6.064 × 10−9 | 3 | 2.021 × 10−9 | |||
| R2 | 0.9970 | |||||
| Adjusted R2 | 0.9879 | |||||
| Predicted R2 | 0.7922 | |||||
| Adeq Precision | 29.6421 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1.11 | 8 | 0.1389 | 193.93 | <0.0001 | Significant |
| A-C tartrazine | 0.0780 | 1 | 0.0780 | 108.87 | 0.0005 | |
| B-C NBB | 0.1623 | 1 | 0.1623 | 226.57 | 0.0001 | |
| C-C NaCl | 0.0208 | 1 | 0.0208 | 29.09 | 0.0057 | |
| AB | 0.0059 | 1 | 0.0059 | 8.17 | 0.0460 | |
| AC | 0.0008 | 1 | 0.0008 | 1.18 | 0.3388 | |
| BC | 0.0206 | 1 | 0.0206 | 28.77 | 0.0058 | |
| C2 | 0.0339 | 1 | 0.0339 | 47.36 | 0.0023 | |
| BC2 | 0.0022 | 1 | 0.0022 | 3.02 | 0.1573 | |
| Residual | 0.0029 | 4 | 0.0007 | |||
| R2 | 0.9974 | |||||
| Adjusted R2 | 0.9923 | |||||
| Predicted R2 | 0.9805 | |||||
| Adeq Precision | 42.6727 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, F.; Talami, B.; Almansba, A.; Bonnet, P.; Caperaa, C.; Dalhatou, S.; Kane, A.; Zeghioud, H. Photocatalytic Degradation of Tartrazine and Naphthol Blue Black Binary Mixture with the TiO2 Nanosphere under Visible Light: Box-Behnken Experimental Design Optimization and Salt Effect. ChemEngineering 2024, 8, 50. https://doi.org/10.3390/chemengineering8030050
Hassan F, Talami B, Almansba A, Bonnet P, Caperaa C, Dalhatou S, Kane A, Zeghioud H. Photocatalytic Degradation of Tartrazine and Naphthol Blue Black Binary Mixture with the TiO2 Nanosphere under Visible Light: Box-Behnken Experimental Design Optimization and Salt Effect. ChemEngineering. 2024; 8(3):50. https://doi.org/10.3390/chemengineering8030050
Chicago/Turabian StyleHassan, Fadimatou, Bouba Talami, Amira Almansba, Pierre Bonnet, Christophe Caperaa, Sadou Dalhatou, Abdoulaye Kane, and Hicham Zeghioud. 2024. "Photocatalytic Degradation of Tartrazine and Naphthol Blue Black Binary Mixture with the TiO2 Nanosphere under Visible Light: Box-Behnken Experimental Design Optimization and Salt Effect" ChemEngineering 8, no. 3: 50. https://doi.org/10.3390/chemengineering8030050
APA StyleHassan, F., Talami, B., Almansba, A., Bonnet, P., Caperaa, C., Dalhatou, S., Kane, A., & Zeghioud, H. (2024). Photocatalytic Degradation of Tartrazine and Naphthol Blue Black Binary Mixture with the TiO2 Nanosphere under Visible Light: Box-Behnken Experimental Design Optimization and Salt Effect. ChemEngineering, 8(3), 50. https://doi.org/10.3390/chemengineering8030050










