Acid-Modified Clays for the Catalytic Obtention of 5-Hydroxymethylfurfural from Glucose
Abstract
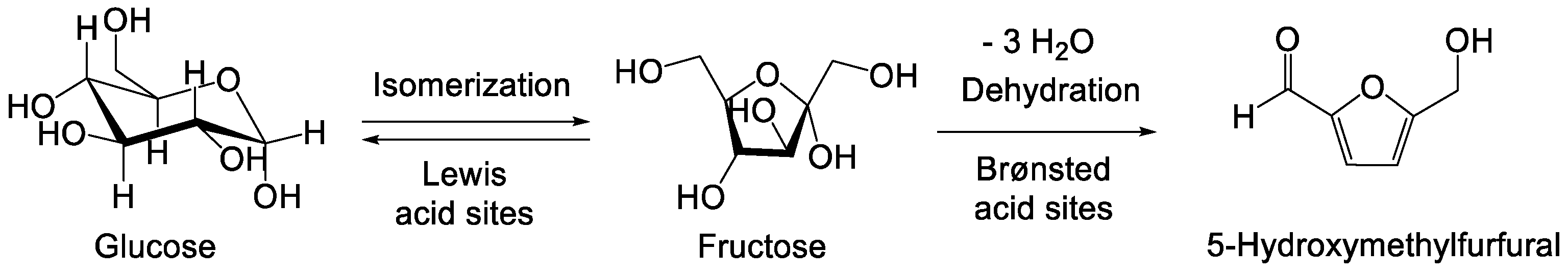
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Catalysts
2.3. Characterization of Catalysts
2.4. Catalytic Activity
3. Results and Discussion
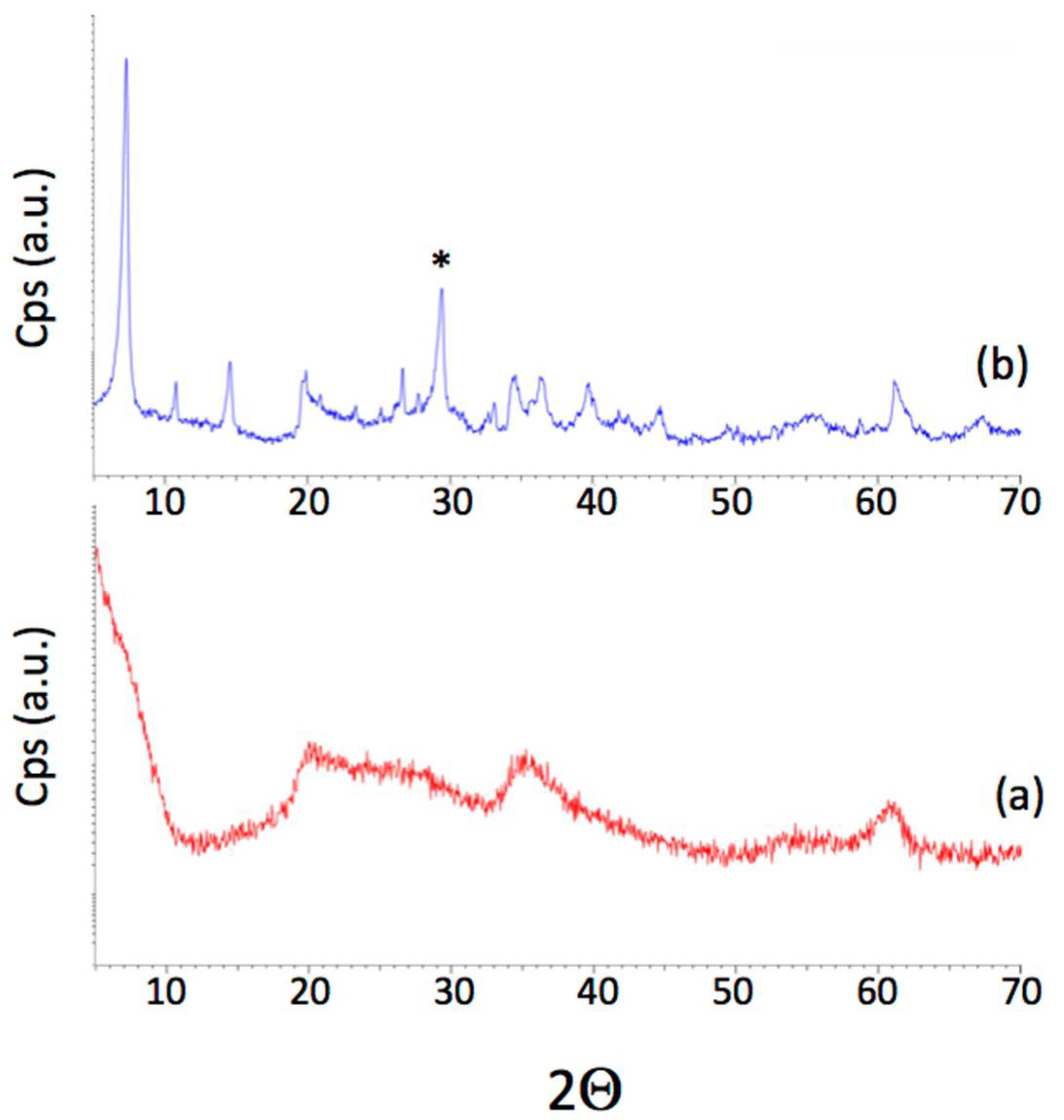
3.1. Characterization of Catalysts
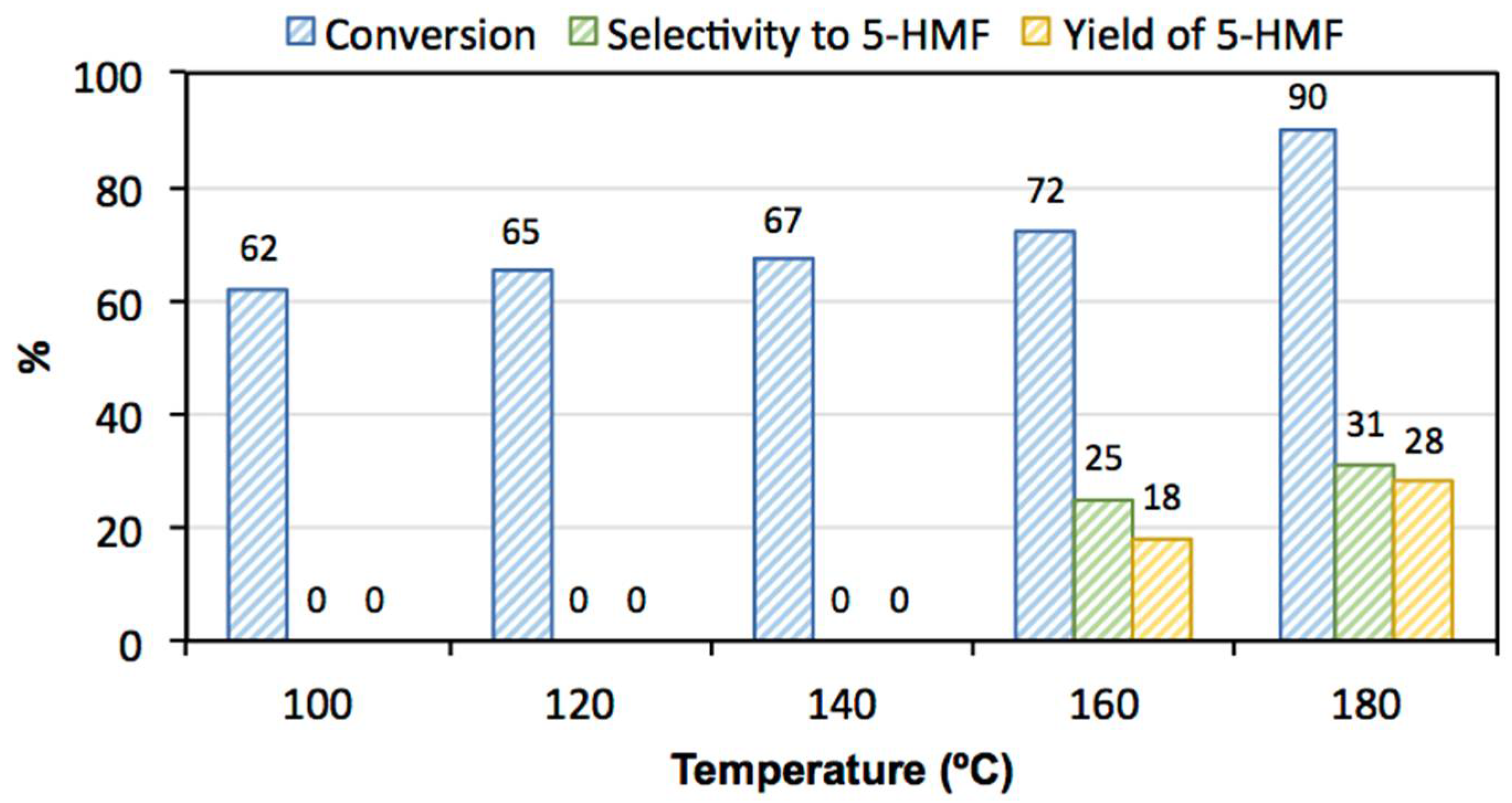
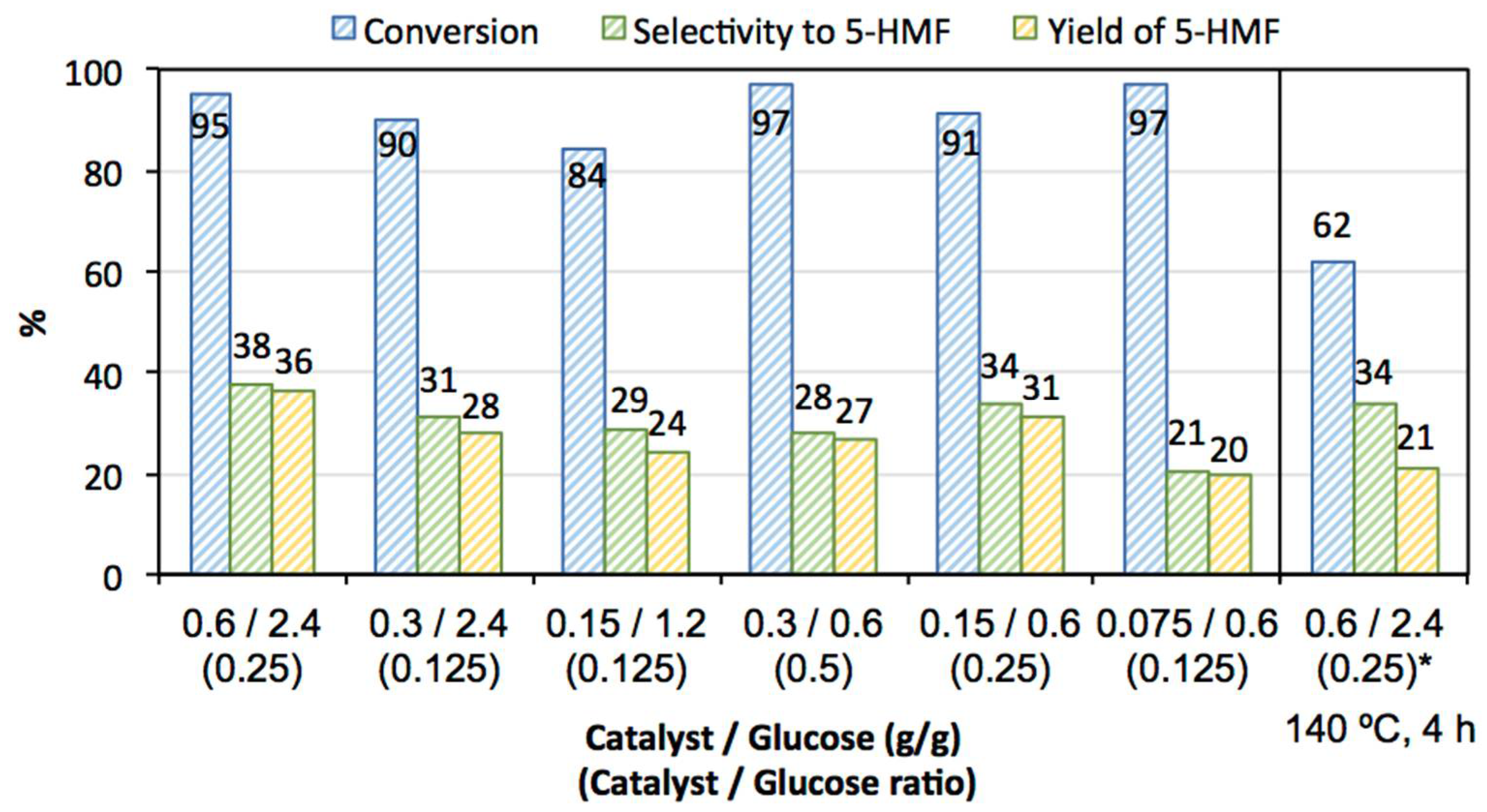
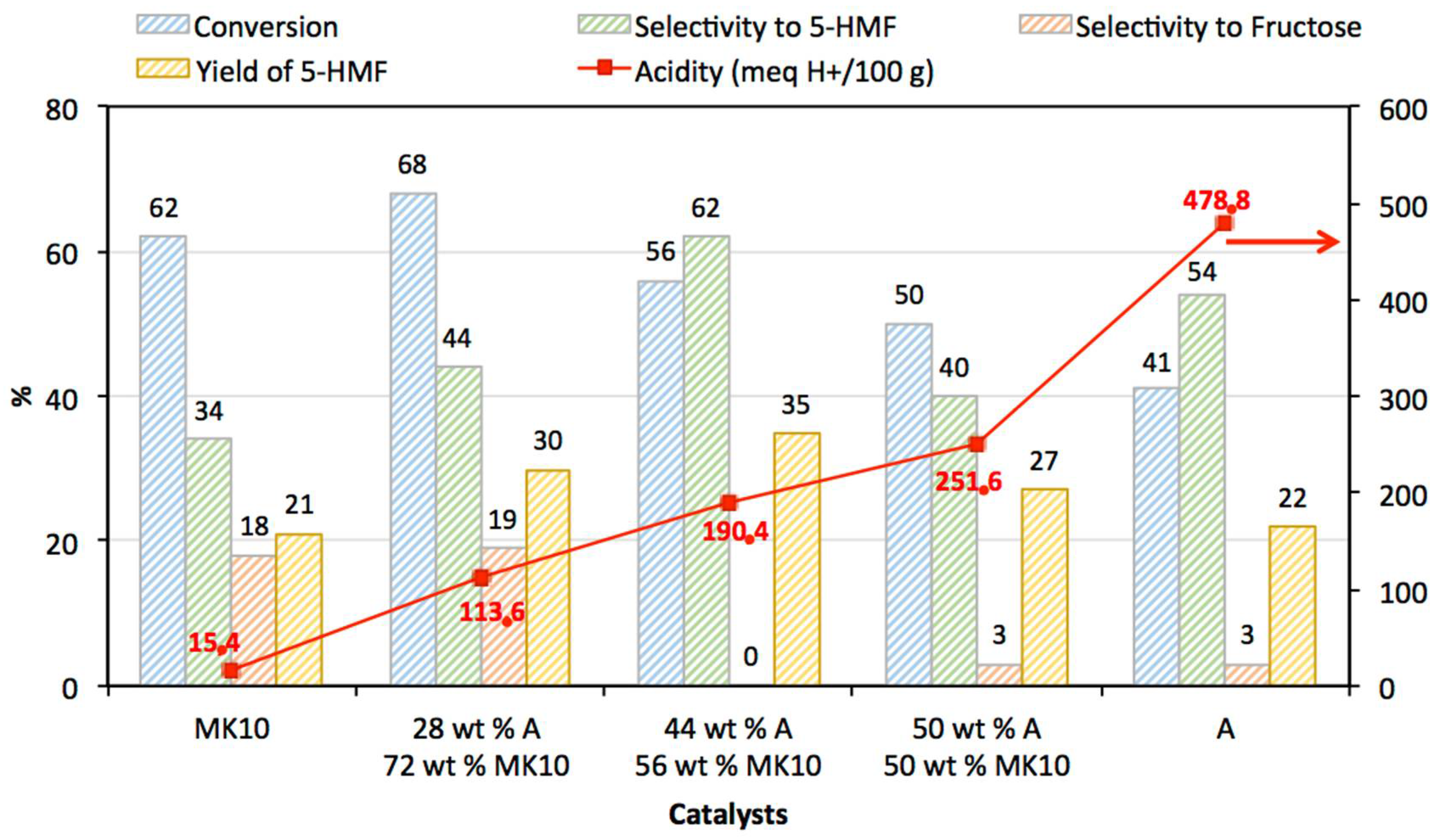
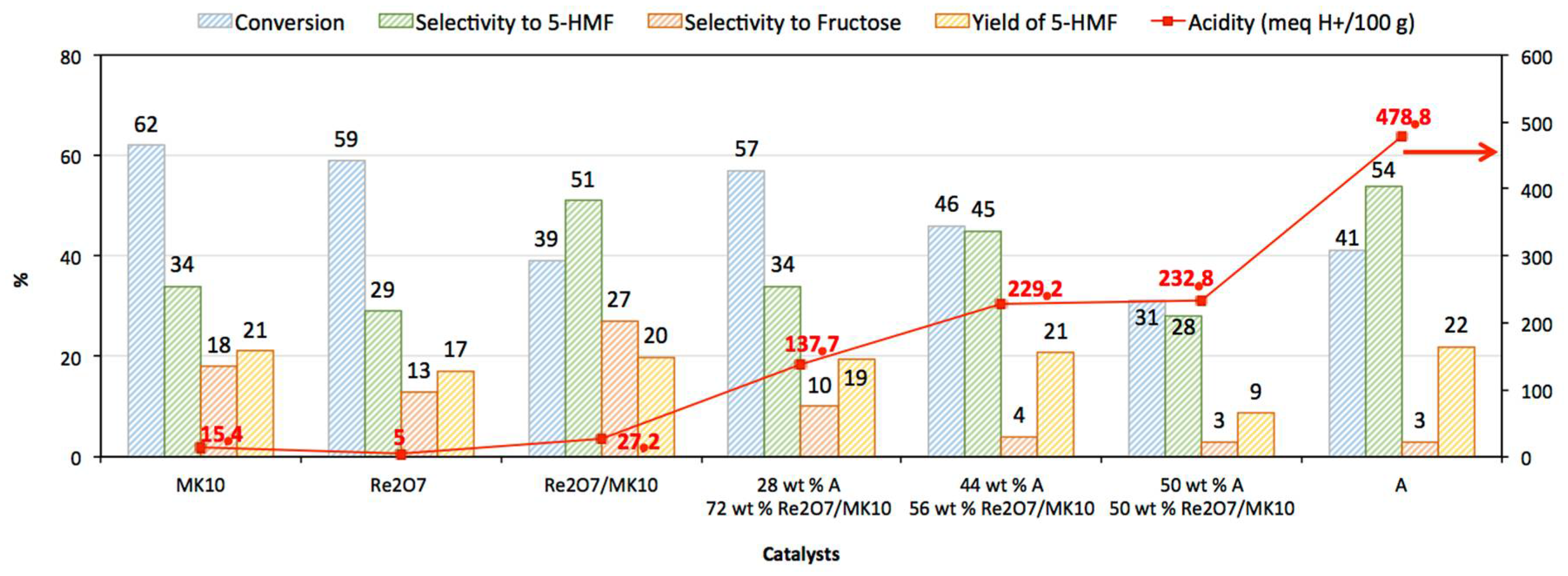
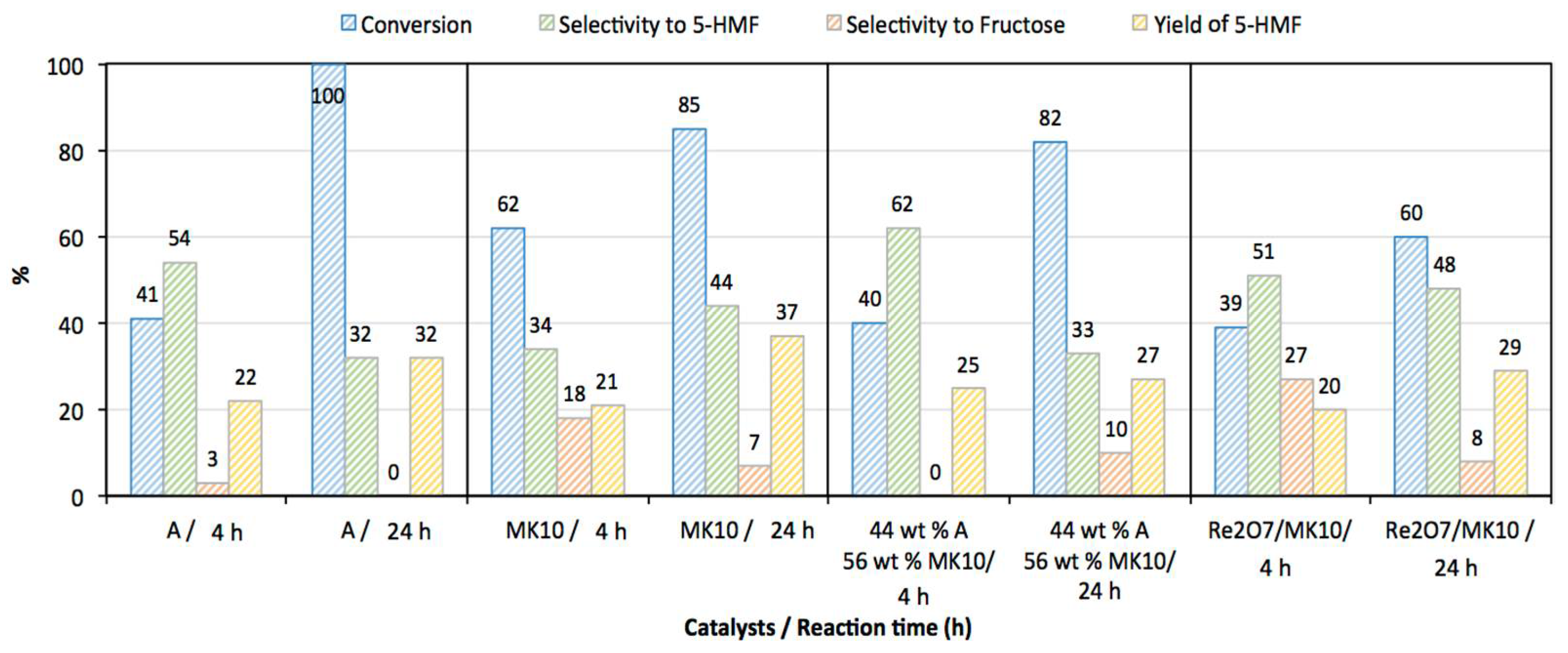
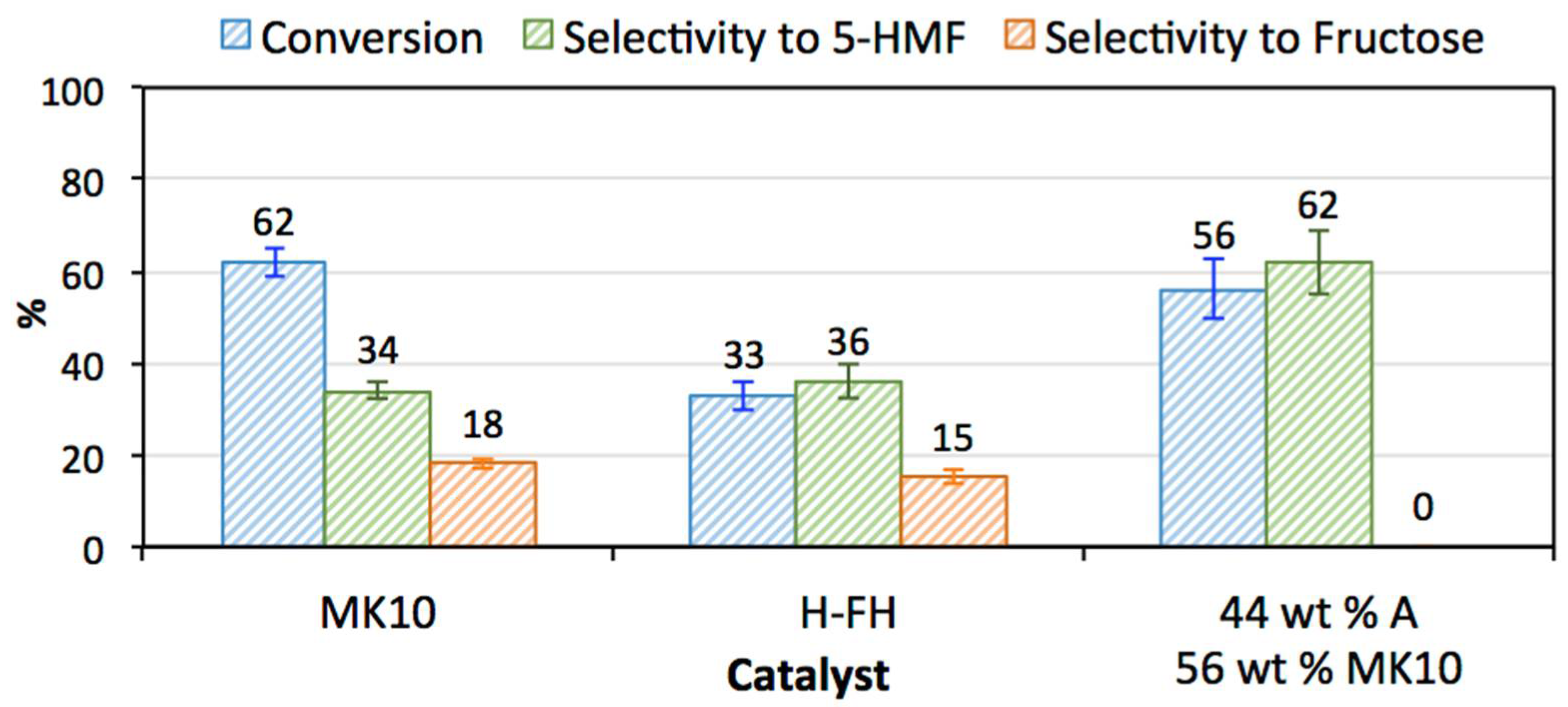
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of Biomass Platform Molecules into Fuel Additives and Liquid Hydrocarbon Fuels. Green Chem. 2014, 16, 516–547. [Google Scholar]
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar]
- Mukherjee, A.; Dumont, M.J.; Raghavan, V. Review: Sustainable Production of Hydroxymethylfurfural and Levulinic Acid: Challenges and Opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754–793. [Google Scholar]
- Assary, R.S.; Curtiss, L.A. Comparison of Sugar Molecule Decomposition through Glucose and Fructose: A High-Level Quantum Chemical Study. Energy Fuels 2012, 26, 1344–1352. [Google Scholar]
- Yu, S.; Kim, E.; Park, S.; Song, I.K.; Jung, J.C. Isomerization of Glucose into Fructose over Mg-Al Hydrotalcite Catalysts. Catal. Commun. 2012, 29, 63–67. [Google Scholar]
- Pidko, E.A.; Degirmenci, V.; Hensen, E.J.M. On the Mechanism of Lewis Acid Catalyzed Glucose Transformations in Ionic Liquids. ChemCatChem 2012, 4, 1263–1271. [Google Scholar]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)Furfural from Carbohydrates Using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar]
- Moliner, M.; Roman-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar]
- Song, X.; Yue, J.; Zhu, Y.; Wen, C.; Chen, L.; Liu, Q.; Ma, L.; Wang, C. Efficient Conversion of Glucose to 5-Hydroxymethylfurfural over a Sn-Modified SAPO-34 Zeolite Catalyst. Ind. Eng. Chem. Res. 2021, 60, 5838–5851. [Google Scholar]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Nb2O5·nH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar]
- Fan, C.; Guan, H.; Zhang, H.; Wang, J.; Wang, S.; Wang, X. Conversion of Fructose and Glucose into 5-Hydroxymethylfurfural Catalyzed by a Solid Heteropolyacid Salt. Biomass Bioenergy 2011, 35, 2659–2665. [Google Scholar]
- Wang, J.; Xi, J.; Xia, Q.; Liu, X.; Wang, Y. Recent Advances in Heterogeneous Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural via Green Routes. Sci. China Chem. 2017, 60, 870–886. [Google Scholar]
- Zou, B.; Chen, X.; Zhou, C.; Yu, X.; Ma, H.; Zhao, J.; Bao, X. Highly-efficient and Low-cost Synthesis of 5-hydroxymethylfurfural from Monosaccharides. Can. J. Chem. Eng. 2018, 96, 1337. [Google Scholar]
- Nahavandi, M.; Kasanneni, T.; Yuan, Z.S.; Xu, C.C.; Rohani, S. Efficient Conversion of Glucose into 5-Hydroxymethylfurfural Using a Sulfonated Carbon-Based Solid Acid Catalyst: An Experimental and Numerical Study. ACS Sustain. Chem. Eng. 2019, 7, 11970–11984. [Google Scholar]
- Goyal, R.; Abraham, B.M.; Singh, O.; Sameer, S.; Bal, R.; Mondal, P. One-pot transformation of glucose into hydroxymethyl furfural in water over Pd decorated acidic ZrO2. Renew. Energy 2022, 183, 791–801. [Google Scholar]
- Li, X.; Peng, K.; Liu, X.; Xia, Q.; Wang, Y. Comprehensive Understanding of the Role of Brønsted and Lewis Acid Sites in Glucose Conversion into 5-Hydromethylfurfural. ChemCatChem 2017, 9, 2739–2746. [Google Scholar]
- Megías-Sayago, C.; Navarro-Jaén, S.; Drault, F.; Ivanova, S. Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics. Catalysts 2021, 11, 1395. [Google Scholar]
- Sánchez, T.; Salagre, P.; Cesteros, Y.; Bueno-López, A. Use of delaminated hectorites as supports of copper catalysts for the hydrogenolysis of glycerol to 1,2-propanediol. Chem. Eng. J. 2012, 179, 302–311. [Google Scholar]
- Sánchez, V.; Dafinov, A.; Salagre, P.; Llorca, J.; Cesteros, Y. Microwave-Assisted Furfural Production Using Hectorites and Fluorohectorites as Catalysts. Catalysts 2019, 9, 706. [Google Scholar]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct Conversion of Carbohydrates to 5-Hydroxymethylfurfural Using Sn-Mont Catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar]
- Qiu, G.; Huang, C.; Sun, X.; Chen, B. Highly active niobium-loaded montmorillonite catalysts for the production of 5-hydroxymethylfurfural from glucose. Green Chem. 2019, 21, 3930–3939. [Google Scholar]
- Yang, F.L.; Weng, J.S.; Ding, J.J.; Zhao, Z.Y.; Qin, L.Z.; Xia, F.F. Effective conversion of saccharides into hydroxymethylfurfural catalyzed by a natural clay, attapulgite. Renew. Energy 2020, 151, 829–836. [Google Scholar]
- Sánchez, T.; Salagre, P.; Cesteros, Y. Ultrasounds and microwave-assisted synthesis of mesoporous hectorites. Microporous Mesoporous Mater. 2013, 171, 24–34. [Google Scholar]
- Plaza de los Reyes, J.; Cid, R.; Pecchi, G.; Reyes, P. Platinium-Rhenium Catalysts. I. Thermal Study of Precursor Salts. J. Chem. Res. 1983, 12, 318. [Google Scholar]
- Melero, J.A.; Stucky, G.D.; van Grieken, R.; Morales, G. Direct Syntheses of Ordered SBA-15 Mesoporous Materials Containing Arenesulfonic Acid Groups. J. Mater. Chem. 2002, 12, 1664–1670. [Google Scholar]
- Chuseang, J.; Nakwachara, R.; Kalong, M.; Ratchahat, S.; Koo-Amornpattana, W.; Klysubun, W.; Khemthong, P.; Faungnawakij, K.; Assabumrungrat, S.; Itthibenchapong, V.; et al. Selective Hydrogenolysis of Furfural into Fuel-Additive 2-Methylfuran over a Rhenium-Promoted Copper Catalyst. Sustain. Energy Fuels 2021, 5, 1379–1393. [Google Scholar]

| Starting Clay | Acid Modification | Sample |
|---|---|---|
| Na-DH | NH4+ cation exchange +calcination | H-DH |
| Li-FH | NH4+ cation exchange +calcination | H-FH |
| MK10 | Physical mixtures withAmberlyst-15 (A) | 28 wt % A: 72 wt % MK10 44 wt % A: 56 wt % MK10 50 wt % A: 50 wt % MK10 |
| MK10 | Impregnation with NH4ReO4 to obtain 5 wt % of Re + calcination | Re2O7/MK10 |
| H-DH | Impregnation with NH4ReO4 to obtain 5 wt % of Re + calcination | Re2O7/H-DH |
| Re2O7/MK10 | Physical mixtures withAmberlyst-15 (A) | 28 wt % A: 72 wt % Re2O7/ MK10 44 wt % A: 56 wt % Re2O7/ MK10 50 wt % A: 50 wt % Re2O7/ MK10 |
| Reaction Parameter | Reaction Conditions |
|---|---|
| Temperature | 100, 120, 140, 160 and 180 °C |
| Time | 1, 4 and 24 h |
| Catalyst wt /glucose wt ( ratio) | 0.6 g/2.4 g (0.25) |
| 0.3 g/2.4 g (0.125) | |
| 0.15 g/1.2 g (0.125) | |
| 0.3 g/0.6 g (0.5) | |
| 0.15 g/0.6 g (0.25) | |
| 0.075 g/0.6 g (0.125) |
| Catalyst | Crystalline Phases (XRD) | BET Area a (m2/g) | Acidity b (meq/g) |
|---|---|---|---|
| MK10 | Montmorillonite | 233 | --- |
| Na-DH | Hectorite | 327 | 0.90 |
| H-DH | Hectorite | 334 | 0.67 |
| Li-FH | Fluorohectorite, anthophyllite | 17 | 0.23 |
| H-FH | Fluorohectorite, anthophyllite | 21 | 0.53 |
| Catalyst | Brønsted Acidity (meq H+/100g) |
|---|---|
| Re2O7 | 5.0 |
| MK10 | 15.4 |
| Re2O7/ MK10 | 27.2 |
| A | 478.8 |
| 28 wt % A: 72 wt % MK10 | 113.6 |
| 44 wt % A: 56 wt % MK10 | 190.4 |
| 50 wt % A: 50 wt % MK10 | 251.6 |
| 28 wt % A: 72 wt % Re2O7/ MK10 | 137.7 |
| 44 wt % A: 56 wt % Re2O7/ MK10 | 229.2 |
| 50 wt % A: 50 wt % Re2O7/ MK10 | 232.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, V.; González, M.D.; Salagre, P.; Cesteros, Y. Acid-Modified Clays for the Catalytic Obtention of 5-Hydroxymethylfurfural from Glucose. ChemEngineering 2022, 6, 57. https://doi.org/10.3390/chemengineering6040057
Sánchez V, González MD, Salagre P, Cesteros Y. Acid-Modified Clays for the Catalytic Obtention of 5-Hydroxymethylfurfural from Glucose. ChemEngineering. 2022; 6(4):57. https://doi.org/10.3390/chemengineering6040057
Chicago/Turabian StyleSánchez, Vladimir, María Dolores González, Pilar Salagre, and Yolanda Cesteros. 2022. "Acid-Modified Clays for the Catalytic Obtention of 5-Hydroxymethylfurfural from Glucose" ChemEngineering 6, no. 4: 57. https://doi.org/10.3390/chemengineering6040057
APA StyleSánchez, V., González, M. D., Salagre, P., & Cesteros, Y. (2022). Acid-Modified Clays for the Catalytic Obtention of 5-Hydroxymethylfurfural from Glucose. ChemEngineering, 6(4), 57. https://doi.org/10.3390/chemengineering6040057








