Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review
Abstract
:1. Introduction
Occurrence of Dyes in the Aquatic Environment
2. Dye-Removal Methods
2.1. Physical
2.2. Biological
2.3. Chemical
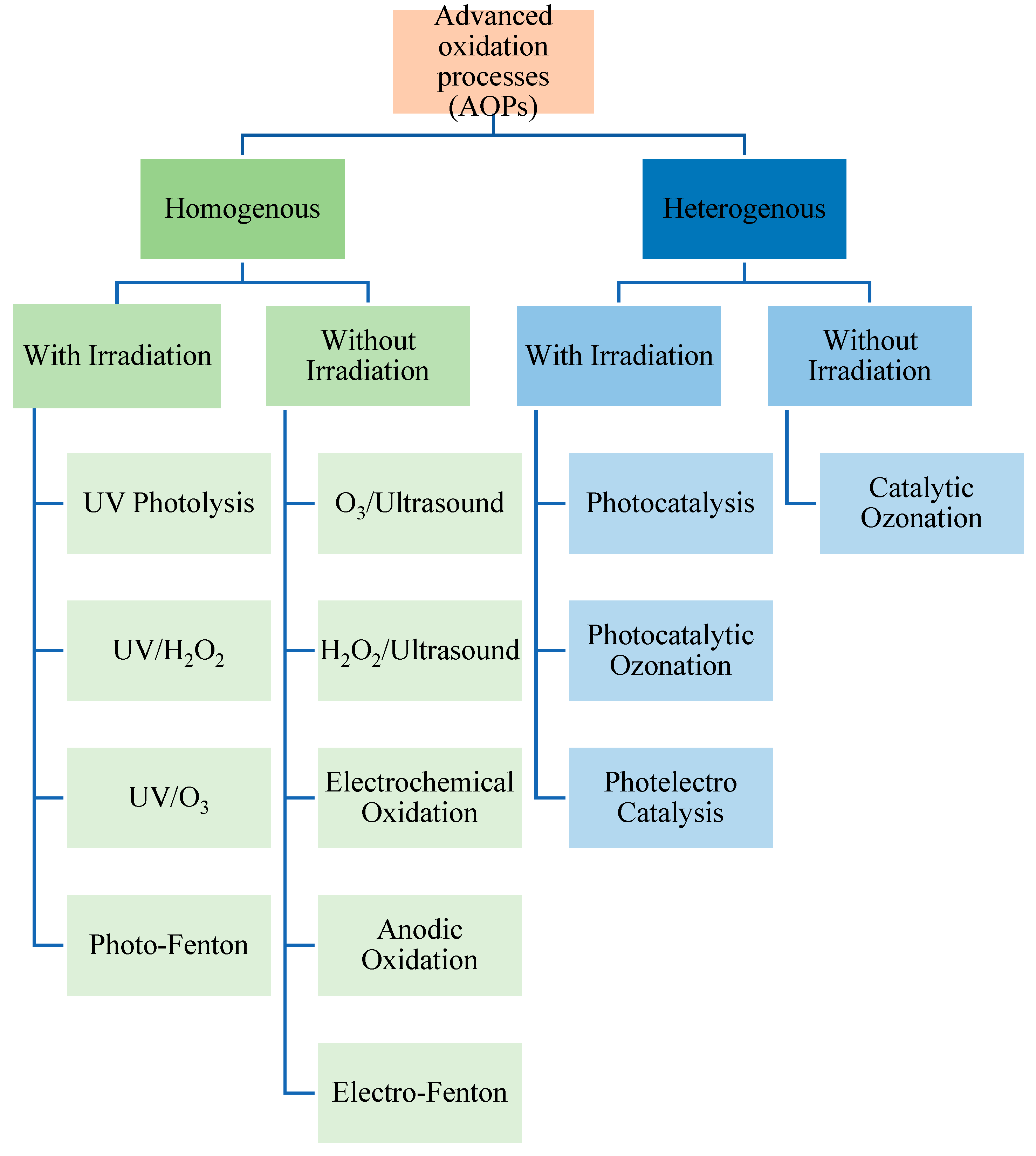
3. Advanced Oxidation Processes (AOPs)
4. Photochemical Treatment of Dyes
History
5. Current Developments in UV Radiation Sources
5.1. Pulsed UV Lamps
5.2. Ultraviolet Light Emitting Diodes (LEDs)
5.3. Microwaved Electrodeless Discharge Lamp
6. Photolysis
6.1. Direct Photolysis
6.2. Photolysis Method Based on H2O2 (UV/H2O2)
6.3. Photolysis Method Based on O3 (UV/O3)
6.4. Photo-Fenton
7. Factors Affecting the Degradation Rate of Photolysis Methods
7.1. Contact Time
7.2. Radiation Source
7.3. pH of the Medium
7.4. Initial Concentration of Dyes
8. Photocatalysis
8.1. Photocatalysts
8.1.1. Titanium Dioxide, TiO2, and Metal Oxide Semiconductors
8.1.2. Modifications to Enhance Photocatalyst Activity
Metal Doping
Non-Metal Doping
8.2. Mechanism of Photocatalysis
8.3. Factors Affecting Photocatalysis Process on Degradation of Dyes
8.3.1. Effect of pH
8.3.2. Effect of Photocatalyst Loading
9. Energy Consumption and Cost–Benefit Analysis
10. Reaction Kinetics Model
11. Conclusions
- AOPs are confirmed as a highly competitive technology in water treatment for removing organic pollutants, especially dyes.
- Different types of AOPs, such as photocatalysis, photolysis, UV/H2O2, photolysis, UV/O3, photo-Fenton, electrochemical oxidation, ozonation, sonolysis, etc., can be effectively used for the treatment of dye-containing wastewater.
- This paper thoroughly investigated the current utilization of photolysis and photocatalytic treatment processes, which are among effective AOPs, for the degradation of dye-containing wastewater.
- The study confirmed that photocatalysis could be used for the complete mineralization of various dyes present in water using light and a photocatalyst by the simultaneous occurrence of oxidation and reduction reactions.
- pH, the initial concentration of the dye, catalyst loading, etc., were identified to have an influence on the photocatalytic degradation of the dye.
- A recent development in the photocatalytic process using TiO2 is the photocatalyst modification by metal and non-metal doping, which results in improved photocatalytic activity in the presence of visible radiation.
- Utilizing a cost-effective and sustainable energy source for the photocatalytic degradation of pollutants is very effective.
- Sunlight as energy for the photocatalytic degradation of various dyes and other organic pollutants will be more efficient in terms of energy utilization.
- The combination of various AOPs such as photocatalysis combined with sonolysis, ozonation, electrolysis, Fenton, etc., will be major aspects for the complete mineralization of various organic pollutants. It will be effective, as there is a synergistic effect, by combining one or more AOPs, which will eliminate the drawbacks of individual processes.
- Scaling up an energy-efficient, cost-effective, and sustainable technique for the complete mineralization of various types of organic pollutants from water by using a reusable energy source, sunlight, and individual AOPs or hybrid AOPs is the challenging future aspect.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and pigments: Their structure and properties. In Dyes and Pigments; Springer: Berlin/Heidelberg, Germany, 2016; pp. 13–29. [Google Scholar]
- Morsy, S.A.G.Z.; Tajudin, A.A.; Ali, M.S.M.; Shariff, F.M. Current development in decolorization of synthetic dyes by immobilized laccases. Front. Microbiol. 2020, 11, 572309. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.M.; Rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; anf Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Navarro, P.; Gabaldón, J.A.; Gómez-López, V.M. Degradation of an azo dye by a fast and innovative pulsed light/H2O2 advanced oxidation process. Dye. Pigment. 2017, 136, 887–892. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [Green Version]
- Carmen, Z.; Daniela, S. Textile organic dyes—Characteristics, polluting effects and separation/elimination procedures from industrial effluents-A critical overview. In Organic Pollutants Ten Years after the Stockholm Convention—Environmental and Analytical Update; IntechOpen: Rijeka, Croatia, 2012; pp. 55–86. [Google Scholar]
- Ghaly, A.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V. Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process Technol. 2013, 5, 182. [Google Scholar]
- Naveed, S.; Bhatti, I. Membrane technology and its suitability for treatment of textile wastewater in Pakistan. J. Res. 2006, 17, 155–164. [Google Scholar]
- Mohajerani, M.; Mehrvar, M.; Ein-Mozaffari, F. An overview of the integration of advanced oxidation technologies and other processes for water and wastewater treatment. Int. J. Eng. IJE 2009, 3, 131. [Google Scholar]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Yeap, K.L.; Teng, T.T.; Poh, B.T.; Morad, N.; Lee, K.E. Preparation and characterization of coagulation/flocculation behavior of a novel inorganic–organic hybrid polymer for reactive and disperse dyes removal. Chem. Eng. J. 2014, 243, 305–314. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 2. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.P.S.; Jafari, S.; Sillanpää, M.; Lee, G.-J.; Wu, J.J.; Swaminathan, M. Recent developments in homogeneous advanced oxidation processes for water and wastewater treatment. Int. J. Photoenergy 2014, 2014, 821674. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Peter, A.; Mihaly-Cozmuta, A.; Nicula, C.; Mihaly-Cozmuta, L.; Jastrzębska, A.; Olszyna, A.; Baia, L. UV light-assisted degradation of methyl orange, methylene blue, phenol, salicylic acid, and rhodamine B: Photolysis versus photocatalyis. Water Air Soil Pollut. 2017, 228, 1. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.H.; Yadav, V.K. Advanced Oxidation Processes for Wastewater Remediation: An Overview. In Removal of Emerging Contaminants Through Microbial Processes; Springer: Singapore, 2021. [Google Scholar]
- Britannica, T. (Ed.) Ultraviolet radiation. In Encyclopedia Britannica; 2021; Available online: https://www.britannica.com/science/ultraviolet-radiation (accessed on 15 June 2022).
- Britannica, T. (Ed.) Heterogeneous reaction. In Encyclopedia Britannica; 1998; Available online: https://www.britannica.com/science/heterogeneous-reaction (accessed on 15 June 2022).
- Britannica, T. (Ed.) Homogeneous reaction. In Encyclopedia Britannica; 2016; Available online: https://www.britannica.com/science/homogeneous-reaction (accessed on 15 June 2022).
- Hossain, S.; Chun, D.-M. ZnO decorated polydimethylsiloxane sponges as photocatalysts for effective removal of methylene blue dye. Mater. Chem. Phys. 2020, 255, 123589. [Google Scholar] [CrossRef]
- Mullapudi, V.B.K.; Salveru, A.; Kora, A.J. An in-house UV-photolysis setup for the rapid degradation of both cationic and anionic dyes in dynamic mode through UV/H2O2-based advanced oxidation process. Int. J. Environ. Anal. Chem. 2020, 100, 1–17. [Google Scholar] [CrossRef]
- Lovato, M.E.; Fiasconaro, M.L.; Martín, C.A. Degradation and toxicity depletion of RB19 anthraquinone dye in water by ozone-based technologies. Water Sci. Technol. 2017, 75, 813–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentealba, D.; Venegas, C.; Morales, M.; Waissbluth, O. Comparing photo-Fenton degradation of malachite green using FeII and FeIII salts under UVA light irradiation. J. Braz. Chem. Soc. 2016, 27, 341–348. [Google Scholar]
- Chanikya, P.; Nidheesh, P.V.; Babu, D.S.; Gopinath, A.; Kumar, M.S. Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep. Purif. Technol. 2021, 254, 117570. [Google Scholar] [CrossRef]
- Dias, N.C.; Bassin, J.P.; Sant’Anna, G.L.; Dezotti, M. Ozonation of the dye reactive red 239 and biodegradation of ozonation products in a moving-bed biofilm reactor: Revealing reaction products and degradation pathways. Int. Biodeterior. Biodegrad. 2019, 144, 104742. [Google Scholar] [CrossRef]
- Eren, Z.; Ince, N.H. Sonolytic and sonocatalytic degradation of azo dyes by low and high frequency ultrasound. J. Hazard. Mater. 2010, 177, 1019–1024. [Google Scholar] [CrossRef]
- Vilhunen, S.; Sillanpää, M. Recent developments in photochemical and chemical AOPs in water treatment: A mini-review. Rev. Environ. Sci. Bio/Technol. 2010, 9, 323–330. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–112. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Gallagher, R.P.; Lee, T.K.; Bajdik, C.D.; Borugian, M. Ultraviolet radiation. Chronic Dis. Inj. Can. 2010, 29, 51–68. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2019, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.A.; Khaleefa, S.A.; Basheer, I.M. Photolysis of methylene blue dye using an advanced oxidation process (ultraviolet light and hydrogen peroxide). J. Eng. Sustain. Dev. 2021, 25, 59–67. [Google Scholar] [CrossRef]
- Asano, T.; Burton, F.; Leverenz, H. Water reuse. In Issues, Technologies and Applications, 1st ed.; Inc. Metcalf and Eddy, McGraw-Hill Companies Inc.: New York, NY, USA, 2006. [Google Scholar]
- Navarro, P.; Zapata, J.P.; Gotor, G.; Gonzalez-Olmos, R.; Gómez-López, V.M. Degradation of malachite green by a pulsed light/H2O2 process. Water Sci. Technol. 2019, 79, 260–269. [Google Scholar] [CrossRef]
- Joseph, C.; Taufiq-Yap, Y.H.; Elilarasi, L.; Krishnan, V. Remediation of cationic dye simulated wastewater using photolysis: Parametric and kinetic studies. Malays. J. Chem. 2017, 19, 82–98. [Google Scholar]
- Jamal, M.; Muneer, M.; Iqbal, M. Photo-degradation of monoazo dye blue 13 using advanced oxidation process. Chem. Int. 2015, 1, 12–16. [Google Scholar]
- De Lima, L.B.; Pereira, L.O.; de Moura, S.G.; Magalhães, F. Degradation of organic contaminants in effluents—Synthetic and from the textile industry-by Fenton, photocatalysis, and H2O2 photolysis. Environ. Sci. Pollut. Res. 2016, 24, 6299–6306. [Google Scholar] [CrossRef]
- Grčić, I.; Papić, S.; Mesec, D.; Koprivanac, N.; Vujević, D. The kinetics and efficiency of UV assisted advanced oxidation of various types of commercial organic dyes in water. J. Photochem. Photobiol. A Chem. 2014, 273, 49–58. [Google Scholar] [CrossRef]
- Nikravesh, B.; Shomalnasab, A.; Nayyer, A.; Aghababaei, N.; Zarebi, R.; Ghanbari, F. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater. J. Environ. Chem. Eng. 2020, 8, 104244. [Google Scholar] [CrossRef]
- Wang, S.; Luo, C.; Tan, F.; Cheng, X.; Ma, Q.; Wu, D.; Li, P.; Zhang, F.; Ma, J. Degradation of Congo red by UV photolysis of nitrate: Kinetics and degradation mechanism. Sep. Purif. Technol. 2021, 262, 118276. [Google Scholar] [CrossRef]
- Wen, D.; Li, W.; Lv, J.; Qiang, Z.; Li, M. Methylene blue degradation by the VUV/UV/persulfate process: Effect of pH on the roles of photolysis and oxidation. J. Hazard. Mater. 2020, 391, 121855. [Google Scholar] [CrossRef]
- Bendjama, H.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. UV-photolysis of Chlorazol Black in aqueous media: Process intensification using acetone and evidence of methyl radical implication in the degradation process. J. Photochem. Photobiol. A Chem. 2019, 368, 268–275. [Google Scholar] [CrossRef]
- Ghodbane, H.; Hamdaoui, O. Decolorization of antraquinonic dye, C.I. Acid Blue 25, in aqueous solution by direct UV irradiation, UV/ H2O2 and UV/Fe(II) processes. Chem. Eng. J. 2010, 160, 226–231. [Google Scholar] [CrossRef]
- Egerton, T.A.; Purnama, H. Does hydrogen peroxide really accelerate TiO2 UV-C photocatalyzed decolouration of azo-dyes such as reactive orange 16? Dye. Pigment. 2014, 101, 280–285. [Google Scholar] [CrossRef]
- Mitrovic, J.; Radovic, M.; Bojic, D.; Andjelkovic, T.; Purenovic, M.; Bojic, A. Decolorization of textile azo dye reactive orange 16 with UV/ H2O2 process. J. Serb. Chem. Soc. 2012, 77, 465–481. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Guedes Maniero, M.; de Araújo, R.N. A comparative study on the degradation of RB-19 dye in an aqueous medium by advanced oxidation processes. J. Environ. Manag. 2012, 110, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-M.; Hong, G.-B.; Chen, H.-W.; Hang, N.-T.; Shen, Y.-S. Photooxidation contribution study on the decomposition of azo dyes in aqueous solutions by VUV-based AOPs. Int. J. Photoenergy 2011, 2011, 156456. [Google Scholar] [CrossRef]
- Dhruv, B.; Abhipsa, M. UV/Fe+3 Photolysis process optimization using response surface methodology for decolorization of reactive red 120 dye simulated wastewater. In Recent Trends in Civil Engineering; Springer: Singapore, 2021. [Google Scholar]
- Zuorro, A.; Lavecchia, R. Evaluation of UV/ H2O2 advanced oxidation process (AOP) for the degradation of diazo dye reactive green 19 in aqueous solution. Desalination Water Treat. 2014, 52, 1571–1577. [Google Scholar] [CrossRef]
- Marhoon, A.A.; Saeed, S.I.; Ahmed, L.M. Application of some effects on the degradation of the aqueous solution of fuchsine dye by photolysis. J. Glob. Pharma Technol. 2019, 11, 76–81. [Google Scholar]
- Gole, V.L.; Gogate, P.R. Degradation of brilliant green dye using combined treatment strategies based on different irradiations. Sep. Purif. Technol. 2014, 133, 212–220. [Google Scholar] [CrossRef]
- Sartaj, S.; Ali, N.; Khan, A.; Malik, S.; Bilal, M.; Khan, M.; Ali, N.; Hussain, S.; Khan, H.; Khan, S. Performance evaluation of photolytic and electrochemical oxidation processes for enhanced degradation of food dyes laden wastewater. Water Sci. Technol. 2020, 81, 971–984. [Google Scholar] [CrossRef] [Green Version]
- Kalsoom, U.; Ashraf, S.S.; Meetani, M.A.; Rauf, M.A.; Bhatti, H.N. Degradation and kinetics of H2O2 assisted photochemical oxidation of Remazol Turquoise Blue. Chem. Eng. J. 2012, 200, 373–379. [Google Scholar] [CrossRef]
- Rauf, M.A.; Marzouki, N.; Körbahti, B.K. Photolytic decolorization of Rose Bengal by UV/H2O2 and data optimization using response surface method. J. Hazard. Mater. 2008, 159, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Aliouche, S.; Djebbar, K.; Sehili, T. Removal of an azo dye (Alizarin yellow) in homogeneous medium using direct photolysis, acetone/UV, H2O2/UV, /UV, H2O2//UV, and /heat. Desalination Water Treat. 2016, 57, 18182–18193. [Google Scholar] [CrossRef]
- Körbahti, B.K.; Rauf, M.A. Determination of optimum operating conditions of carmine decoloration by UV/H2O2 using response surface methodology. J. Hazard. Mater. 2009, 161, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Elmorsi, T.M.; Riyad, Y.M.; Mohamed, Z.H.; Abd El Bary, H.M.H. Decolorization of mordant red 73 azo dye in water using H2O2/UV and photo-Fenton treatment. J. Hazard. Mater. 2010, 174, 352–358. [Google Scholar] [CrossRef]
- Bousnoubra, I.; Djebbar, K.; Abdessemed, A.; Sehili, T. Decolorization of methyl green and bromocresol purple in mono and binary systems by photochemical processes: Direct UV photolysis, Acetone/UV and H2O2/UV. A comparative study. Desalination Water Treat. 2016, 57, 27710–27725. [Google Scholar] [CrossRef]
- Chenini, H.; Djebbar, K.; Zendaoui, S.M.; Sehili, T.; Zouchoune, B. Removal of an azo dye (Orange G) by various methods in homogeneous phase: Comparative study. Jordan J. Chem. 2011, 6, 307–319. [Google Scholar]
- Ding, X.; Gutierrez, L.; Croue, J.-P.; Li, M.; Wang, L.; Wang, Y. Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/ H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison. Chemosphere 2020, 253, 126655. [Google Scholar] [CrossRef]
- Maleki, A.; Mahvi, A.H.; Ebrahimi, R.; Zandsalimi, Y. Study of photochemical and sonochemical processes efficiency for degradation of dyes in aqueous solution. Korean J. Chem. Eng. 2010, 27, 1805–1810. [Google Scholar] [CrossRef]
- Kasiri, M.B.; Khataee, A.R. Photooxidative decolorization of two organic dyes with different chemical structures by UV/ H2O2 process: Experimental design. Desalination 2011, 270, 151–159. [Google Scholar] [CrossRef]
- Haji, S.; Benstaali, B.; Al-Bastaki, N. Degradation of methyl orange by UV/ H2O2 advanced oxidation process. Chem. Eng. J. 2011, 168, 134–139. [Google Scholar] [CrossRef]
- Jian-xiao, L.V.; Ying, C.; Guo-hong, X.; Ling-yun, Z.; Su-fen, W. Decoloration of methylene blue simulated wastewater using a UV- H2O2 combined system. J. Water Reuse Desalination 2011, 1, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Chekir, N.; Tassalit, D.; Benhabiles, O.; Kasbadji Merzouk, N.; Ghenna, M.; Abdessemed, A.; Issaadi, R. A comparative study of tartrazine degradation using UV and solar fixed bed reactors. Int. J. Hydrogen Energy 2017, 42, 8948–8954. [Google Scholar] [CrossRef]
- Zukawa, T.; Sasaki, Y.; Kurosawa, T.; Kamiko, N. Photolysis of Indigo Carmine solution by planar vacuum-ultraviolet (147 nm) light source. Chemosphere 2019, 214, 123–129. [Google Scholar] [CrossRef]
- Safni, M.; Putri, R.; Wellia, D.; Septiani, U. Photodegradation of orange F3R dyes: Effect of light sources and the addition of CN—Codoped TiO2 catalyst. Pharma Chem. 2017, 9, 1–5. [Google Scholar]
- Algubili, A.M.; Alrobayi, E.M.; Alkaim, A.F. Photocatalytic degradation of remazol brilliant blue dye by ZnO/UV process. Int. J. Chem. Sci. 2015, 13, 911–921. [Google Scholar]
- Daneshvar, N.; Behnajady, M.A.; Mohammadi, M.K.A.; Dorraji, M.S.S. UV/ H2O2 treatment of rhodamine b in aqueous solution: Influence of operational parameters and kinetic modeling. Desalination 2008, 230, 16–26. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Mishra, N.; Reddy, R.; Kuila, A.; Rani, A.; Nawaz, A.; Pichiah, S. A review on advanced oxidation processes for effective water treatment. Curr. World Environ. 2017, 12, 469–489. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J. Performance of an integrated membrane photocatalytic reactor for the removal of Reactive Black 5. Sep. Purif. Technol. 2010, 71, 44–49. [Google Scholar] [CrossRef]
- Bansal, P.; Sud, D. Photodegradation of commercial dye, Procion Blue HERD from real textile wastewater using nanocatalysts. Desalination 2011, 267, 244–249. [Google Scholar] [CrossRef]
- Soni, H.; Kumar, N.J.I. UV light induced photocatalytic degradation of malachite green on TiO2 nanoparticles. Int. J. Recent Res. Rev. 2014, 3, 10–15. [Google Scholar]
- Putri, R.A.; Safni, S.; Jamarun, N.; Septiani, U.; Kim, M.-K.; Zoh, K.-D. Degradation and mineralization of violet-3B dye using C-N-codoped TiO2 photocatalyst. Environ. Eng. Res. 2020, 25, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shrivastava, M. Removal of the hazardous dye—Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Hadjltaief, B.H.; Ben Zina, M.; Galvez, M.E.; Da Costa, P. Photocatalytic degradation of methyl green dye in aqueous solution over natural clay-supported ZnO–TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2016, 315, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.-W.; Ong, S.-T.; Hung, Y.-T.; Lee, S.-L. Photodegradation of malachite green by immobilization of titanium dioxide on glass plates. Asian J. Chem. 2013, 25, 755–758. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Willington, T.D.; Hatshan, M.R.; Chen, S.-M.; Chen, T.-W. Environmental photochemistry with Sn/F simultaneously doped TiO2 nanoparticles: UV and visible light induced degradation of thiazine dye. Environ. Res. 2022, 207, 112108. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bhunia, H.; Bajpai, P.K. Photocatalytic decolorization kinetics and mineralization of Reactive Black 5 aqueous solution by UV/TiO2 nanoparticles. CLEAN—Soil Air Water 2012, 40, 1290–1296. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Ishak, M.A.M.; Ismail, K.; Nawawi, W.I. Kinetics of photocatalytic decolourization of cationic dye using porous TiO2 film. J. Taibah Univ. Sci. 2018, 10, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Joseph, C.G.; Taufiq-Yap, Y.H.; Puma, G.L.; Sanmugam, K.; Quek, K.S. Photocatalytic degradation of cationic dye simulated wastewater using four radiation sources, UVA, UVB, UVC and solar lamp of identical power output. Desalination Water Treat. 2016, 57, 7976–7987. [Google Scholar] [CrossRef]
- Laohaprapanon, S.; Matahum, J.; Tayo, L.; You, S.-J. Photodegradation of Reactive Black 5 in a ZnO/UV slurry membrane reactor. J. Taiwan Inst. Chem. Eng. 2015, 49, 136–141. [Google Scholar] [CrossRef]
- Kansal, S.K.; Kaur, N.; Singh, S. Photocatalytic degradation of two commercial reactive dyes in aqueous phase using nanophotocatalysts. Nanoscale Res. Lett. 2009, 4, 709–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.-Z.; Li, A.-D.; Li, X.-Y.; Zhai, H.-F.; Zhang, W.-Q.; Gong, Y.-P.; Li, H.; Wu, D. Photo-degradation of methylene blue using Ta-doped ZnO nanoparticle. J. Solid State Chem. 2010, 183, 1359–1364. [Google Scholar] [CrossRef]
- Juang, R.-S.; Lin, S.-H.; Hsueh, P.-Y. Removal of binary azo dyes from water by UV-irradiated degradation in TiO2 suspensions. J. Hazard. Mater. 2010, 182, 820–826. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl. Catal. B Environ. 2015, 170, 153–161. [Google Scholar] [CrossRef]
- Shinde, D.R.; Tambade, P.S.; Chaskar, M.G.; Gadave, K.M. Photocatalytic degradation of dyes in water by analytical reagent grades ZnO, TiO2 and SnO2: A comparative study. Drink. Water Eng. Sci. 2017, 10, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Tolia, J.; Chakraborty, M.; Murthy, Z. Photocatalytic degradation of malachite green dye using doped and undoped ZnS nanoparticles. Pol. J. Chem. Technol. 2012, 14, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Azami, M.S.; Nawawi, W.I.; Jawad, A.H.; Ishak, M.A.M.; Ismail, K. N-doped TiO2 synthesised via microwave induced photocatalytic on RR4 dye removal under LED light irradiation. Sains Malays. 2017, 46, 1309–1316. [Google Scholar] [CrossRef]
- Tammina, S.K.; Mandal, B.K.; Kadiyala, N.K. Photocatalytic degradation of methylene blue dye by nonconventional synthesized SnO2 nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 10, 339–350. [Google Scholar] [CrossRef]
- Khatri, A.; Rana, P.S. Visible light assisted photocatalysis of Methylene Blue and Rose Bengal dyes by iron doped NiO nanoparticles prepared via chemical co-precipitation. Phys. B Condens. Matter 2020, 579, 411905. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Ravindranadh, K.; Reddy, K.R.; Kim, D.; Shim, J. Ni-dopant concentration effect of ZrO2 photocatalyst on photoelectrochemical water splitting and efficient removal of toxic organic pollutants. Sep. Purif. Technol. 2020, 252, 117352. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118, 102900. [Google Scholar] [CrossRef]
- Kazeminezhad, I.; Sadollahkhani, A. Influence of pH on the photocatalytic activity of ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 4206–4215. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Assadi, A.A.; Jellali, S.; Bouzaza, A.; Wolbert, D.; Rtimi, S. Discoloration of simulated textile effluent in continuous photoreactor using immobilized titanium dioxide: Effect of zinc and sodium chloride. J. Photochem. Photobiol. A Chem. 2018, 358, 111–120. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Sacco, O.; Stoller, M.; Vaiano, V.; Ciambelli, P.; Chianese, A.; Sannino, D. Photocatalytic degradation of organic dyes under visible light on N-doped TiO2 Photocatalysts. Int. J. Photoenergy 2012, 2012, 626759. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.T.; Bari, G.A.K.M.R.; Kang, H.-J.; Lee, T.-G.; Park, J.-W.; Hwang, H.; Hossain, S.; Mun, J.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-doped TiO2 for efficient photocatalytic degradation of atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, M.; Zhang, J. Gel-hydrothermal synthesis of carbon and boron co-doped TiO2 and evaluating its photocatalytic activity. J. Hazard. Mater. 2011, 192, 368–373. [Google Scholar] [CrossRef]
- Eskandarian, M.R.; Ganjkhanloo, M.; Rasoulifard, M.H.; Hosseini, S.A. Energy-efficient removal of acid red 14 by UV-LED/persulfate advanced oxidation process: Pulsed irradiation, duty cycle, reaction kinetics, and energy consumption. J. Taiwan Inst. Chem. Eng. 2021, 127, 129–139. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Raju, A.; Vishnuganth, M.; Asha, R.; Remya, N.; Selvaraju, N.; Kumar, M. Livestock wastewater treatment in batch and continuous photocatalytic systems: Performance and economic analyses. Water Air Soil Pollut. 2015, 226, 132. [Google Scholar]
- Basturk, E.; Karatas, M. Decolorization of antraquinone dye Reactive Blue 181 solution by UV/H2O2 process. J. Photochem. Photobiol. A Chem. 2015, 299, 67–72. [Google Scholar] [CrossRef]
- Rabahi, A.; Assadi, A.A.; Nasrallah, N. Photocatalytic treatment of petroleum industry wastewater using recirculating annular reactor: Comparison of experimental and modeling. Environ. Sci. Pollut. Res. 2019, 26, 19035–19046. [Google Scholar] [CrossRef]

| Industrial Application Class | Applications | Chemical Type/Chromophore Structure | Example |
|---|---|---|---|
| Disperse dye (non-ionic) | Polyester, nylon, cellulose, cellulose acetate, acrylic fibers, polyamide, plastics | Azo, nitro, styryl, anthraquinone, benzodifuranone, | Disperse violet 26 (DV26), Disperse blue 27 (DB27) |
| Direct dye (anionic) | Paper, cellulose fibers, nylon, rayon, cotton, viscose, leather | Azo, oxazine, thiazole, stibene, phthalocyanine | Direct red 28 (DR28), Direct orange 26 (DR26) |
| Reactive dye (anionic) | Cellulose fibers, silk, cotton, wool fibers, nylon | Anthraquinone, formazan, oxazine, phthalocyanine, azo, triphenylmethane | Reactive blue 19 (RB19), Reactive blue 5 (RB5) |
| Vat dye (non-ionic) | Cellulose fibers, cotton, viscose, wool | Anthraquinone, Indigoid | Vat blue 1 (VB1), Vat blue 4 (VB4) |
| Basic dye (cationic) | Acrylic, ink, paper, silk, wool, cotton, treated nylon, modified polyester, polyacrylonitrile | Triarylmethane, azo, xanthene, Triphenylmethane, hemicyanine, cyanine, acridine, diazahemicyanine, anthraquinone, oxazine, thiazine | Basic blue 6 (BB6), MB, MG |
| Acid dye (anionic) | Nylon, wool, leather, food, silk, cotton, cosmetics, ink-jet printing, paper, modified acrylics | Anthraquinone, xanthene, azo, nitrodiphenylamine, triphenylmethane, nitroso, azine, nitro, indigoid | AO7, Acid yellow 36 (AY36) |
| Process | Quality of Textile Effluent |
|---|---|
| Desizing | High TSS, high BOD, neutral pH |
| Scouring | High TTS, high BOD, high alkalinity, high temperature |
| Bleaching, Mercerizing | High TSS, high BOD, alkaline wastewater |
| Heat-setting | Low TSS, low BOD, alkaline wastewater |
| Dyeing, printing, and finishing | High TSS, BOD, COD, wasted dyes, neutral to alkaline wastewater |
| Dye | Type of Photolysis | Experimental Conditions | COD/TOC/Degradation Percentage/other Remarks | Reaction Kinetics | Reference |
|---|---|---|---|---|---|
| MB—cationic dye, thiazine | UV/H2O2 | UV light source (UV lamp = 6 W) |
| N/A | [39] |
| MB | UV | UV lamps (UV-A, UV-B, UV-C) |
| First-order | [42] |
| Blue 13 -monoazo dye | UV/H2O2 | UV-A (6, 12 and 18 W) |
| N/A | [43] |
| RhB | UV/H2O2 | [H2O2] = 0.10–1020 mg/L, pH 4.5, UV light (low pressure, λ = 254 nm) |
| N/A | [44] |
| RB 19—anionic, anthraquinone | UV, UV/O3 | Two monochromatic germicidal lamps (40 W, 253.7 nm), T = 20 °C, [RB19]0 = 230 ± 1.5 mg/L, pH 6.0, [O3]0 = 50 ± 2 mg/L |
| Pseudo-first-order | [28] |
| MO | UV | Medium pressure Hg lamp (150 W, 350–400 nm), pH 7.3, [MO]0 = 100 μM |
| Pseudo-first-order | [20] |
| Basic Red 1 (BR1)—cationic dye | UV/Fenton | Mercury lamp (9.5 W, 254 nm), pH 3.0, [BR1]0 = 100 mg/L |
| N/A | [45] |
| Brilliant blue FCF (BBF) -Triarylmethane dye, anionic | UV/Cl | Radiation sources: UV lamp (4W, 254 nm, Philips) as UVC source, solar irradiation as an alternative |
| Pseudo-first-order | [46] |
| Congo red (CR) -Azo dye | UV/NO3 | Low-pressure UV (254 nm) |
| Pseudo-first-order | [47] |
| MB—Cationic dye | VUV/UV/ Persulfate | [MB]0 = 10 μM, [PS]0 = 0.5 mM, T = 25 °C, Reaction time = 10 min. |
| N/A | [48] |
| Chlorazol black (CB) | UV/acetone | [CB] = 20 mg/L at 25 °C, [Acet.]0 = 50 mM, pH = 3–9 |
| First-order | [49] |
| Direct yellow 106 (DY106)—Azo dye, anionic | PL direct photolysis, PL/H2O2 | Pulsed light, [DY106]0 = 20 mg/L, [H2O2]0600 mg/L, pH 9.5 |
| Pseudo-first-order | [8] |
| C.I. Acid Blue 25 (AB25) Anthraquinone | Direct UV irradiation, UV/H2O2, UV/Fe(II) | Direct photolysis:
|
| Pseudo-first-order | [50] |
| Reactive orange 16 (RO16)—anionic dye | UV, UV-C/H2O2 | UV-C germicidal tubes (8 W), pH = 6.5, t = 30 min |
| Pseudo-first-order | [51] |
| RO16 -Anionic mono-azo dye | UV/H2O2 | Low-pressure mercury vapor lamp (28 W, 253.7 nm) |
| Pseudo-first-order | [52] |
| RB19 | UV, UV/H2O2 | Low pressure mercury lamp (65 W, 254 nm), [RB19]0 = 10–100 mg/L for photolysis, a fixed [RB19]0 = 100 mg/L and [H2O2] = 100,300, 500 and 800 mg/L for UV/ H2O2, pH = 3 |
| N/A | [53] |
| Acid Orange 8 (AO8), Acid blue 29 (AB29), Acid blue 113 (AB113) (Azo dyes) | VUV/H2O2 | Low-pressure mercury 185 nm vacuum UV lamp (6 W), |
| First-order | [54] |
| Reactive red 120 (RR120) | UV/Fe3 | Low pressure mercury lamp, [Fe+3]0 = 0.25–2.75 mM, [MB]0 = 100–200 mg/L, initial pH 1–11 |
| N/A | [55] |
| Reactive green 19 (RG19) | UV/ H2O2 | Low-pressure mercury lamp (6 W, 254 nm), pH 2–10, [RG19]0 = 90 mg/L |
| Pseudo-first-order | [56] |
| Acid red 27 (AR27) (anionic dyes) | UV/H2O2 | Low-pressure mercury lamp (8 W) H2O2 (0.03% (v/v), [RG19]0 = 50 μg mL−1, sample flow rate of 6 mL min−1 |
| N/A | [27] |
| Basic Fuchsine dye | UV | UV-A light, pH 6.4 |
| N/A | [57] |
| Brilliant green (BG) | UV | UV tubes (11 W, 350–450 nm), [BG] = 10–50 ppm |
| First-order | [58] |
| Allura red | UV | T = 35 °C, t = 1–6 h, pH =3–12 |
| N/A | [59] |
| Erythrosine | UV | T = 35 °C, t = 1–6 h, pH =3–12 |
| N/A | [59] |
| Remazol turquoise blue (RTB) | UV/H2O2 | UV lamp (6 W, 254 nm), [RTB]0 = 25 ppm |
| First-order | [60] |
| Acid red 94 (AR94)—xanthene dye | UV/H2O2 | UV lamp (254 nm), |
| Pseudo-first-order | [61] |
| Alizarin yellow (AY)—azo dye | UV/acetone, UV/H2O2, UV/S2O82− | Low-pressure mercury lamps (15 W, 254 nm), T = 18 °C and 20 °C, pH = 1.7, 2, 11.5 and 12 |
| N/A | [62] |
| Carmine (C.I. natural Red 4) | UV/H2O2 | UV lamp (254 nm), T = 25 °C, [dye]0 = 20–160 µM, [H2O2]0 = 0.83–6.64 mM, pH = 2–10, t = 30 min |
| N/A | [63] |
| Mordant red 73 (MR73) | UV/H2O2 | [MR73] = 0.1 mM, 0.05 mM, 0.05 mM, [H2O2]0 = 2.5 mM, pH 3 and T = 25 °C |
| Pseudo-first-order | [64] |
| Direct red 23 (DR23) AB25 Mordant Orange 1 (MO1) | UV/Fenton | Mercury lamp (9.5 W, 254 nm) |
| First-order | [45] |
| Blue 13 Monoazo dye | UV/ H2O2 | UV lamp (6,12 and 18 W, 254 nm), Comparing the UV intensity |
| N/A | [43] |
| MG, Bromocresol purple (BCP) | UV | Low-pressure mercury lamp (15 W, 254 nm), T = 18–20 °C, pH 5.8 for MG and pH 4.5 for BCP |
| Pseudo-first-order | [65] |
| Orange G Azo dye, anionic | UV, UV/acetone, UV/H2O2, UV/S2O82 | Low-pressure mercury lamp (15 W, 254 nm), T = 18–20 °C, pH 5.8 for MG and pH 4.5 for BCP |
| N/A | [66] |
| RhB—xanthene | UV, UV H2O2, UV/Persulfate | Medium-pressure mercury lamp (330 W, 365 nm) |
| Pseudo first order | [67] |
| Reactive black 5 (RB5)—anionic dye | UV, UV/H2O2 | Low-pressure mercury lamp (55 W), [RB5]0 = 10–50 mg/L, t=120 min |
| Pseudo-first-order | [68] |
| Disperse orange 25—non-ionic | UV, UV/H2O2 | Low-pressure mercury lamp (55 W), [RB5]0 = 10–50 mg/L, t = 120 min |
| Pseudo-first-order | [68] |
| Basic blue 3 (BB3), Acid green 25 (AG25) | UV/ H2O2 | Mercury lamp (30 W, 254 nm), pH 6.5, [H2O2]0 = 1.2 g/L, [BB3]0 = 10 mg/L, [AG25]0= 10 mg/L |
| Pseudo-first-order | [69] |
| MO | UV/ H2O2 | UV lamp (254 nm), [MO]0 = 7.80 × 10−5 M, [H2O2]0 = 4.58 × 10−2 M |
| Pseudo-first-order | [70] |
| MB | UV/ H2O2 | Medium pressure lamp (300 W, 365 nm), |
| First-order | [71] |
| Tartrazine | UV | Solar UV, UV lamp (24 W, 365 nm), t = 300 min, flow rate of solution = 60 mL/s, [dye]0 = 10 mg/L, pH 8.2–8.5 |
| N/A | [72] |
| Indigo carmine | VUV | VUV light from Xe-Ne plasma (147–172 nm), |
| N/A | [73] |
| Dye | Photocatalyst | Experimental Conditions | COD/TOC/Degradation Percentage/Remarks | Reaction Kinetics | Reference |
|---|---|---|---|---|---|
| MB | Ferrite Bismuth nanoparticles | Direct solar irradiation; [MB]0 = 15 mg/L; acidic medium; photocatalyst (0.5 g/L) |
| Pseudo-first-order | [77] |
| RB5 | TiO2 | Low-pressure Hg UV-C lamp (15 W, 254 nm); [TiO2]0 = 0.5 g/L; [RB5]0 = 25–125 mg/L; pH 6.4–6.9 |
| Pseudo-first-order | [79] |
| Procion Blue HERD (PBH) | TiO2 and ZnO | UV lamp (30 W); [PBH]0 = 10–100 ppm; photocatalyst loading = 0.5–2 g/L; pH 2–10 |
| First-order | [80] |
| Methyl orange (MO) | Ag-doped titania-silica | Medium-pressure Hg lamp (150 W, 350–400 nm); [MO]0 = 100 µM; pH 7.3; T = ~20 °C; 0.15 g catalyst |
| Pseudo-first-order | [20] |
| Orange F3R | C-N-codoped TiO2 | UV (10 W, 365 nm), visible (13 W), solar irradiation; [dye]0 = 30 mg/L; dosage of C-N-codoped TiO2 is 3–15 mg |
| N/A | [74] |
| MG | TiO2 | UV lamp (15 W, 365 nm); [MG]0 = 40 mg/L; 20 mg of TiO2 |
| N/A | [81] |
| Remazol Brilliant Blue (RBB) | ZnO | High-pressure Hg lamp (125 W, 365 nm), [ZnO] = 1.5 g/L, [RBB]0 = 100 mg/L |
| Pseudo-first-order | [75] |
| MB | ZnO/PDMS | Three different types of light sources such as halogen (100 W), metal-halide (150 W), and UV (4 W) light sources. | The highest degradation of MB achieved was 93% under UV/Vis irradiation after 3 h. | N/A | [26] |
| Rhodamine B (RhB) | TiO2 | Low-pressure UV lamp (15 W, 254 nm), 120 mg TiO2, pH 4.5, [RhB]0 = 5 mg/L | Color removal achieved 29% after 60 min and TOC, 25% | Pseudo-first-order | [44] |
| RO16—anionic monoazo dye | UV-C/TiO2 and UV-C/H2O2/TiO2 | UV-C germicidal tubes (8 W), pH 6.5 |
| Pseudo-first-order | [51] |
| MO, RhB, MB | Ag-doped titania-silica | Medium-pressure mercury lamp (150 W, 350–400 nm), Catalyst was prepared by sol-gel, catalyst loading is 0.15 g, T = 20 °C |
| Pseudo-first-order | [20] |
| Violet-3B | C-N-codoped TiO2 | Visible-halogen lamp (500 W), [dye]0 = 5 mg/L, catalyst dosage is 0.3 g/L, pH 5.6 |
| Pseudo-first-order | [82] |
| Tartrazine | UV/TiO2, UV/H2O2/TiO2 | UV lamp (6 W, 254 nm), [dye]0 = 2 × 10−5 to 8 × 10−5 M, pH 2.2–11, 0.02–0.18 mg/L catalyst dosage, T = 30 °C |
| Pseudo-first-order | [83] |
| MG | ZnO–TiO2/clay | UV-A lamp (100 W, 365 nm), catalyst dosage = 1 g, [dye]0 = 75 mg/L, pH 5.2 |
| Pseudo-first-order | [84] |
| MG | TiO2 dip-coating | UV lamp, solar irradiation, |
| Pseudo-first-order | [85] |
| AO8, AC29, AB113 (Azo dyes) | VUV/TiO2 | Low-pressure Hg lamp (18 W, 185 nm), TiO2 dosage = 0.5 g/L, [dye]0 = 0.0523 mM, T = 25 °C, pH 3, 5, 7, 9 and 11 |
| Pseudo-first-order | [54] |
| MB | TiO2, Sn–F/TiO2 NPs | Sol-gel method, UV, and visible light irradiation |
| Pseudo-first-order | [86] |
| RB5—anionic dye | TiO2 | UV lamps (40 W, 365 nm), pH (3–11), catalyst load (0.5–3.0 g/L), and [RB5]0 = 20–100 mg/L |
| Pseudo-first-order | [87] |
| MB—cationic dye | TiO2, TiO2 ENR | Fluorescent lamp, |
| Pseudo-first-order | [88] |
| MB | TiO2 | UV-A, UV-B, UV-C and solar light, [MB]0 = 2–10 ppm, pH (4–10), t = 1 h. |
| First-order | [89] |
| RB5—azo dye | ZnO, TiO2 | Catalyst load = 0.5–1.5 g/L, [RB5]0 = 25–150 mg/L), pH = 3.0–11.0 |
| Pseudo-first-order | [90] |
| RB5 | TiO2, ZnO | UV lamp (20 W, 365 nm), Catalyst load = 1.25 g/L, [RB5]0 = 10–100 mg/L), pH = 3.0–11.0 |
| Pseudo-first-order | [91] |
| Reactive orange 4 (RO4) | TiO2, ZnO | UV lamp (20 W, 365 nm), Catalyst load = 1.0 g/L, [RO4]0 = 10–100 mg/L), pH = 3.0–11.0 |
| Pseudo-first-order | [91] |
| MB—cationic | Ta-doped ZnO | Xe arc lamp (300 W), [MB]0 = 10 mg/L, 50 mg, pH 8 | −1 mol% Ta-doped ZnO annealed at 700 °C exhibits the highest degradation rate. | Pseudo-first-order | [92] |
| AO7 | TiO2 | High-pressure mercury lamp (400 W) |
| First-order | [93] |
| Reactive red | TiO2 | High-pressure mercury lamp (400 W) |
| First-order | [93] |
| Procion yellow H-EXL | N-doped TiO2 | UV lamp (100 W) |
| N/A | [94] |
| Tartrazine—anionic azo dye | TiO2 | Solar UV, UV lamp (24 W, 365 nm), t = 300 min, flow rate of solution = 60 mL/s, [dye]0 = 10 mg/L, TiO2 dosage = 0.3 mg/cm2, pH 8.2–8.5 |
| N/A | [72] |
| CV, Methyl red (MR), Basic blue (BB) | ZnO, TiO2, SnO | Solar irradiation, [dye]0 = 10 mg/L, pH 9 |
| N/A | [95] |
| MG | ZnS, Mn-doped ZnS | Medium-pressure lamp (125 W), pH 2–5, t = 90 min, [MG]0 = 25 g/L |
| Pseudo-second-order | [96] |
| Reactive red 4 (RR4) | TiO2, N-doped TiO2 | LED light irradiation, [RR4]4 = 30 mg/L, 0.030 g catalyst |
| N/A | [97] |
| MB | SnO | Low-pressure mercury lamp (125 W, 254 nm), 0.02 g SnO2, [MB]0 = 10 mg/L, |
| First-order | [98] |
| MB, RB | Fe-doped NiO | Sunlight radiations, [RB]0 = 5 ppm, [MB]0 = 5 ppm, t = 60 min, |
| Pseudo-first-order | [99] |
| MB | Ni-doped ZrO2 | Visible light lamp (>400 nm), [dye]0 = 5 ppm, 15 mg of photocatalyst |
| Pseudo-first-order | [100] |
| MB, MO | Pd-doped TiO2 | High-pressure lamp (100 W), [MB]0 = 20 mg/L, [MO]0 = 20 mg/L, |
| Pseudo-first-order | [101] |
| MB | Co-doped TiO2 | UV-C lamp, [MB]0 = 10 ppm, 0.5 g/L catalyst |
| Pseudo-first-order | [102] |
| RB | ZnO nanoparticles | UV lamp (15 W, 256 nm), 0.05 g ZnONPs, [dye]0 = 20 mg/L, pH 4,8 and 11 | 96, 100 and 83% of RB degraded at pH 4, 8 and 11 respectively after 240 UV irradiation | First-order | [103] |
| MB | Immobilised TiO2 | [MB]0 = 75 mg/l, [Zinc] = 60 mg/L, [NaCl] = 0.250 M, flowrate of 0.7 L/min. | After 180 min of UV radiation, a 79.27% reduction in initial dye concentration was observed. | N/A | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anisuzzaman, S.M.; Joseph, C.G.; Pang, C.K.; Affandi, N.A.; Maruja, S.N.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering 2022, 6, 58. https://doi.org/10.3390/chemengineering6040058
Anisuzzaman SM, Joseph CG, Pang CK, Affandi NA, Maruja SN, Vijayan V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering. 2022; 6(4):58. https://doi.org/10.3390/chemengineering6040058
Chicago/Turabian StyleAnisuzzaman, S M, Collin G. Joseph, Chuan Kian Pang, Nur Ammarah Affandi, Sitti Nurazida Maruja, and Veena Vijayan. 2022. "Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review" ChemEngineering 6, no. 4: 58. https://doi.org/10.3390/chemengineering6040058
APA StyleAnisuzzaman, S. M., Joseph, C. G., Pang, C. K., Affandi, N. A., Maruja, S. N., & Vijayan, V. (2022). Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering, 6(4), 58. https://doi.org/10.3390/chemengineering6040058







