Catalytic Steam Reforming of Biomass-Derived Oxygenates for H2 Production: A Review on Ni-Based Catalysts
Abstract
:1. Introduction
2. Methanol Steam Reforming
2.1. Introduction
2.2. Nickel-Based Catalysts
2.2.1. Catalytic Activity and H2 Selectivity
2.2.2. Deactivation
2.2.3. Effect of the Preparation Method
2.3. Summary
3. Ethanol Steam Reforming
3.1. Introduction
3.2. Catalysts
3.2.1. Catalytic Activity and H2 Selectivity
3.2.2. Deactivation
3.2.3. Effect of the Preparation Method
3.3. Summary
4. Other Oxygenates Steam Reforming
4.1. Introduction
4.2. Nickel-Based Catalysts
4.2.1. Catalytic Activity and H2 Selectivity
4.2.2. Deactivation
4.2.3. Effect of the Preparation Method
4.3. Summary
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Notation and Glossary
| ABC | Activated Biochar |
| ATTP | Attapulgite |
| DFM | Dual Function Materials |
| ESR | Ethanol Steam Reforming |
| ISO | International Organization for Standardization |
| LDH | Layered Double Hydroxides |
| MSR | Methanol Steam Reforming |
| MMT | Montmorillonite |
| OMW | Olive Mill Wastewater |
| OSE | Oxygenates Steam Reforming |
| PF | Palygorskite |
| POME | Palm Oil Mill Effluent |
| TEM | Transmission Electron Microscopy |
| TPD | Temperature-Programmed Desorption |
| TPO | Temperature-Programmed Oxidation |
| TPR | Temperature-Programmed Reduction |
| WEFR | Water to Ethanol Feed Ratio |
| WGS | Water-Gas Shift |
| WHSV | Weight Hourly Space Velocity |
| XRD | X-Ray Diffraction |
References
- Dou, B.; Song, Y.; Wang, C.; Chen, H.; Xu, Y. Hydrogen production from catalytic steam reforming of biodiesel byproduct glycerol: Issues and challenges. Renew. Sustain. Energy Rev. 2014, 30, 950–960. [Google Scholar] [CrossRef]
- Ayalur Chattanathan, S.; Adhikari, S.; Abdoulmoumine, N. A review on current status of hydrogen production from bio-oil. Renew. Sustain. Energy Rev. 2012, 16, 2366–2372. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrog. Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yu, Y.; Liu, H.; Gong, C.; Wen, S.; Wang, X.; Tu, Z. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Processing Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Ewan, B.C.R.; Allen, R.W.K. A figure of merit assessment of the routes to hydrogen. Int. J. Hydrog. Energy 2005, 30, 809–819. [Google Scholar] [CrossRef]
- Baharudin, L.; Watson, M.J. Hydrogen applications and research activities in its production routes through catalytic hydrocarbon conversion. Rev. Chem. Eng. 2018, 34, 43–72. [Google Scholar] [CrossRef]
- Uddin, M.N.; Nageshkar, V.V.; Asmatulu, R. Improving water-splitting efficiency of water electrolysis process via highly conductive nanomaterials at lower voltages. Energy Ecol. Environ. 2020, 5, 108–117. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Xuan, J.; Leung, M.K.H.; Leung, D.Y.C.; Ni, M. A review of biomass-derived fuel processors for fuel cell systems. Renew. Sustain. Energy Rev. 2009, 13, 1301–1313. [Google Scholar] [CrossRef]
- Baneshi, J.; Haghighi, M.; Jodeiri, N.; Abdollahifar, M.; Ajamein, H. Urea–nitrate combustion synthesis of ZrO2 and CeO2 doped CuO/Al2O3 nanocatalyst used in steam reforming of biomethanol for hydrogen production. Ceram. Int. 2014, 40, 14177–14184. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S. Bio-ethanol, a suitable fuel to produce hydrogen for a molten carbonate fuel cell. J. Power Sources 2007, 173, 200–209. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrog. Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Nabgan, W.; Tuan Abdullah, T.A.; Mat, R.; Nabgan, B.; Gambo, Y.; Ibrahim, M.; Ahmad, A.; Jalil, A.A.; Triwahyono, S.; Saeh, I. Renewable hydrogen production from bio-oil derivative via catalytic steam reforming: An overview. Renew. Sustain. Energy Rev. 2017, 79, 347–357. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrog. Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Galdámez, J.R.; García, L.; Bilbao, R. Hydrogen Production by Steam Reforming of Bio-Oil Using Coprecipitated Ni−Al Catalysts. Acetic Acid as a Model Compound. Energy Fuels 2005, 19, 1133–1142. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for Lignocellulosic Biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [Green Version]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Development of Ni-Based Catalysts for Steam Reforming of Tar Derived from Biomass Pyrolysis. Chin. J. Catal. 2012, 33, 583–594. [Google Scholar] [CrossRef]
- Baddour, F.G.; Snowden-Swan, L.; Super, J.D.; Van Allsburg, K.M. Estimating Precommercial Heterogeneous Catalyst Price: A Simple Step-Based Method. Org. Process Res. Dev. 2018, 22, 1599–1605. [Google Scholar] [CrossRef]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-art catalysts for CH4 steam reforming at low temperature. Int. J. Hydrog. Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Llorca, J.; Corberán, V.C.; Divins, N.J.; Fraile, R.O.; Taboada, E. Chapter 7-Hydrogen from Bioethanol. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–169. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, F. Chapter Two-Catalytic Conversion of Biogas to Syngas via Dry Reforming Process. In Advances in Bioenergy; Li, Y., Ge, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 43–76. [Google Scholar]
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Challenges and strategies for optimization of glycerol steam reforming process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- International Organization for Standardization. Hydrogen Fuel-Product Specification-Part 2: Proton Exchange Membrane Fuel Cell Applications for Road Vehicles; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Kurtz, M.; Wilmer, H.; Genger, T.; Hinrichsen, O.; Muhler, M. Deactivation of Supported Copper Catalysts for Methanol Synthesis. Catal. Lett. 2003, 86, 77–80. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Owen, S.L.; Abrokwah, R.Y.; Kuila, D. Mesoporous nanocrystalline TiO2 supported metal (Cu, Co, Ni, Pd, Zn, and Sn) catalysts: Effect of metal-support interactions on steam reforming of methanol. J. Mol. Catal. A Chem. 2015, 408, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Abrokwah, R.Y.; Deshmane, V.G.; Kuila, D. Comparative performance of M-MCM-41 (M: Cu, Co, Ni, Pd, Zn and Sn) catalysts for steam reforming of methanol. J. Mol. Catal. A Chem. 2016, 425, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yao, S.; Johnston-Peck, A.; Xu, W.; Rodriguez, J.A.; Senanayake, S.D. Methanol steam reforming over Ni-CeO2 model and powder catalysts: Pathways to high stability and selectivity for H2/CO2 production. Catal. Today 2018, 311, 74–80. [Google Scholar] [CrossRef]
- Shetty, K.; Zhao, S.; Cao, W.; Siriwardane, U.; Seetala, N.V.; Kuila, D. Synthesis and characterization of non-noble nanocatalysts for hydrogen production in microreactors. J. Power Sources 2007, 163, 630–636. [Google Scholar] [CrossRef]
- Luo, X.; Hong, Y.; Wang, F.; Hao, S.; Pang, C.; Lester, E.; Wu, T. Development of nano NixMgyO solid solutions with outstanding anti-carbon deposition capability for the steam reforming of methanol. Appl. Catal. B Environ. 2016, 194, 84–97. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Palma, S.; Ivanova, S.; Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Steam reforming of methanol over supported Ni and Ni–Sn nanoparticles. Int. J. Hydrog. Energy 2013, 38, 6646–6656. [Google Scholar] [CrossRef]
- Xu, C.; Koel, B.E. Influence of alloyed Sn atoms on the chemisorption properties of Ni(111) as probed by RAIRS and TPD studies of CO adsorption. Surf. Sci. 1995, 327, 38–46. [Google Scholar] [CrossRef]
- Khzouz, M.; Gkanas, E.I.; Du, S.; Wood, J. Catalytic performance of Ni-Cu/Al2O
3 for effective syngas production by methanol steam reforming. Fuel 2018, 232, 672–683. [Google Scholar] [CrossRef] - Qing, S.; Hou, X.; Liu, Y.; Wang, L.; Li, L.; Gao, Z. Catalytic performance of Cu-Ni-Al spinel for methanol steam reforming to hydrogen. J. Fuel Chem. Technol. 2018, 46, 1210–1217. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Belenov, S.V.; Guterman, V.E.; Efimov, M.N.; Yaroslavtsev, A.B. Bimetallic carbon nanocatalysts for methanol steam reforming in conventional and membrane reactors. Catal. Today 2016, 268, 60–67. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wang, S.-F.; Tsai, A.-P.; Kameoka, S. Catalysts prepared from copper–nickel ferrites for the steam reforming of methanol. J. Power Sources 2015, 281, 138–145. [Google Scholar] [CrossRef]
- Khzouz, M.; Wood, J.; Pollet, B.; Bujalski, W. Characterization and activity test of commercial Ni/Al2O
3, Cu/ZnO/Al2O
3 and prepared Ni–Cu/Al2O
3 catalysts for hydrogen production from methane and methanol fuels. Int. J. Hydrog. Energy 2013, 38, 1664–1675. [Google Scholar] [CrossRef] [Green Version] - Pérez-Hernández, R.; Gutiérrez-Martínez, A.; Espinosa-Pesqueira, M.E.; Estanislao, M.L.; Palacios, J. Effect of the bimetallic Ni/Cu loading on the ZrO2 support for H2 production in the autothermal steam reforming of methanol. Catal. Today 2015, 250, 166–172. [Google Scholar] [CrossRef]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N. Highly porous monolith/TiO2 supported Cu, Cu-Ni, Ru, and Pt catalysts in methanol steam reforming process for H2 generation. Appl. Catal. A Gen. 2018, 554, 44–53. [Google Scholar] [CrossRef]
- Yang, R.-X.; Chuang, K.-H.; Wey, M.-Y. Hydrogen production through methanol steam reforming: Effect of synthesis parameters on Ni–Cu/CaO–SiO2 catalysts activity. Int. J. Hydrog. Energy 2014, 39, 19494–19501. [Google Scholar] [CrossRef]
- Suetsuna, T.; Suenaga, S.; Fukasawa, T. Monolithic Cu–Ni-based catalyst for reforming hydrocarbon fuel sources. Appl. Catal. A Gen. 2004, 276, 275–279. [Google Scholar] [CrossRef]
- Lorenzut, B.; Montini, T.; De Rogatis, L.; Canton, P.; Benedetti, A.; Fornasiero, P. Hydrogen production through alcohol steam reforming on Cu/ZnO-based catalysts. Appl. Catal. B Environ. 2011, 101, 397–408. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Zhilyaeva, N.A.; Ermilova, M.M.; Orekhova, N.V.; Yaroslavtsev, A.B. Influence of the support structure and composition of Ni–Cu-based catalysts on hydrogen production by methanol steam reforming. Int. J. Hydrog. Energy 2015, 40, 9677–9684. [Google Scholar] [CrossRef]
- Duprez, D.; Pereira, P.; Miloudi, A.; Maurel, R. Steam dealkylation of aromatic hydrocarbons: II. Role of the support and kinetic pathway of oxygenated species in toluene steam dealkylation over group VIII metal catalysts. J. Catal. 1982, 75, 151–163. [Google Scholar] [CrossRef]
- Duprez, D. Selective steam reforming of aromatic compounds on metal catalysts. Appl. Catal. A Gen. 1992, 82, 111–157. [Google Scholar] [CrossRef]
- Takezawa, N.; Iwasa, N. Steam reforming and dehydrogenation of methanol: Difference in the catalytic functions of copper and group VIII metals. Catal. Today 1997, 36, 45–56. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. The influence of the support composition and structure (MXZr1-XO2-δ) of bimetallic catalysts on the activity in methanol steam reforming. Int. J. Hydrog. Energy 2018, 43, 198–207. [Google Scholar] [CrossRef]
- Huang, H.-K.; Chih, Y.-K.; Chen, W.-H.; Hsu, C.-Y.; Lin, K.-J.; Lin, H.-P.; Hsu, C.-H. Synthesis and regeneration of mesoporous Ni–Cu/Al2O
4 catalyst in sub-kilogram-scale for methanol steam reforming reaction. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef] - Pérez-Hernández, R. Reactivity of Pt/Ni supported on CeO2-nanorods on methanol steam reforming for H2 production: Steady state and DRIFTS studies. Int. J. Hydrog. Energy 2021, 46, 25954–25964. [Google Scholar] [CrossRef]
- Penkova, A.; Bobadilla, L.; Ivanova, S.; Domínguez, M.I.; Romero-Sarria, F.; Roger, A.C.; Centeno, M.A.; Odriozola, J.A. Hydrogen production by methanol steam reforming on NiSn/MgO–Al2O
3 catalysts: The role of MgO addition. Appl. Catal. A Gen. 2011, 392, 184–191. [Google Scholar] [CrossRef] - Lu, J.; Li, X.; He, S.; Han, C.; Wan, G.; Lei, Y.; Chen, R.; Liu, P.; Chen, K.; Zhang, L.; et al. Hydrogen production via methanol steam reforming over Ni-based catalysts: Influences of Lanthanum (La) addition and supports. Int. J. Hydrog. Energy 2017, 42, 3647–3657. [Google Scholar] [CrossRef]
- Kim, W.; Mohaideen, K.K.; Seo, D.J.; Yoon, W.L. Methanol-steam reforming reaction over Cu-Al-based catalysts derived from layered double hydroxides. Int. J. Hydrog. Energy 2017, 42, 2081–2087. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. Product composition as a function of temperature over NiAl-layered double hydroxide derived catalysts in steam reforming of methanol. Appl. Catal. A Gen. 2006, 302, 237–243. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. Hydrogen production by methanol reforming on NiAl layered double hydroxide derived catalyst: Effect of the pretreatment of the catalyst. Int. J. Hydrog. Energy 2007, 32, 5098–5102. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. K (Na)-promoted Ni, Al layered double hydroxide catalysts for the steam reforming of methanol. J. Power Sources 2007, 171, 842–849. [Google Scholar] [CrossRef]
- Frank, B.; Jentoft, F.C.; Soerijanto, H.; Kröhnert, J.; Schlögl, R.; Schomäcker, R. Steam reforming of methanol over copper-containing catalysts: Influence of support material on microkinetics. J. Catal. 2007, 246, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Bepari, S.; Kuila, D. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts-A review. Int. J. Hydrog. Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Mosińska, M.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. Catalysts for Hydrogen Generation via Oxy–Steam Reforming of Methanol Process. Materials 2020, 13, 5601. [Google Scholar] [CrossRef]
- Boskovic, G.; Baerns, M. Catalyst Deactivation. In Basic Principles in Applied Catalysis; Baerns, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 477–503. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Ramôa Ribeiro, F. Catálise Heterogénea, 2nd ed.; Gulbenkian, F.C., Ed.; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2007. [Google Scholar]
- Liu, Y.; Kang, H.; Hou, X.; Zhang, L.; Qing, S.; Gao, Z.; Xiang, H. Cu-Ni-Al spinel catalyzed methanol steam reforming for hydrogen production: Effect of Al content. J. Fuel Chem. Technol. 2020, 48, 1112–1121. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Mendoza-Anaya, D.; Martínez, A.G.; Gómez-Cortés, A. Catalytic steam reforming of methanol to produce hydrogen on supported metal catalysts. In Hydrogen Energy-Challenges and Perspectives; Minic, D., Ed.; InTech: London, UK, 2012; pp. 149–174. [Google Scholar]
- López, P.; Mondragón-Galicia, G.; Espinosa-Pesqueira, M.E.; Mendoza-Anaya, D.; Fernández, M.E.; Gómez-Cortés, A.; Bonifacio, J.; Martínez-Barrera, G.; Pérez-Hernández, R. Hydrogen production from oxidative steam reforming of methanol: Effect of the Cu and Ni impregnation on ZrO2 and their molecular simulation studies. Int. J. Hydrog. Energy 2012, 37, 9018–9027. [Google Scholar] [CrossRef]
- Yang, W.; Parr, R.G. Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. USA 1985, 82, 6723–6726. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.-H.; He, X.-R. Synthesis of nickel ferrite nanoparticles by sol-gel method. Mater. Res. Bull. 2001, 36, 1369–1377. [Google Scholar] [CrossRef]
- Bhosale, R.; Shende, R.; Puszynski, J. H2 Generation From Thermochemical Water-Splitting Using Sol-Gel Synthesized Zn/Sn/Mn-doped Ni-Ferrite. I.RE.CH.E. 2010, 2, 852–862. [Google Scholar]
- Wu, C.; Williams, P.T. A Novel Nano-Ni/SiO2 Catalyst for Hydrogen Production from Steam Reforming of Ethanol. Environ. Sci. Technol. 2010, 44, 5993–5998. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.B.; Norton, M.G. (Eds.) Sols, Gels, and Organic Chemistry. In Ceramic Materials: Science and Engineering; Springer New York: New York, NY, USA, 2007; pp. 400–411. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Takeguchi, T.; Yamanaka, T.; Muhamad, E.N.; Mastuda, M.; Ueda, W. Effect of preparation atmosphere of Pt–SnOx/C catalysts on the catalytic activity for H2/CO electro-oxidation. Appl. Catal. B Environ. 2010, 98, 86–93. [Google Scholar] [CrossRef]
- Silva, J.M.; Trujillano, R.; Rives, V.; Soria, M.A.; Madeira, L.M. High temperature CO2 sorption over modified hydrotalcites. Chem. Eng. J. 2017, 325, 25–34. [Google Scholar] [CrossRef]
- Silva, J.M.; Trujillano, R.; Rives, V.; Soria, M.A.; Madeira, L.M. Dynamic behaviour of a K-doped Ga substituted and microwave aged hydrotalcite-derived mixed oxide during CO2 sorption experiments. J. Ind. Eng. Chem. 2019, 72, 491–503. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Effect of interlayer anion on the CO2 capture capacity of hydrotalcite-based sorbents. Sep. Purif. Technol. 2019, 219, 290–302. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Wu, R.-C.; Tang, C.-W.; Huang, H.-H.; Wang, C.-C.; Chang, M.-B.; Wang, C.-B. Effect of boron doping and preparation method of Ni/Ce0.5Zr0.5O2 catalysts on the performance for steam reforming of ethanol. Int. J. Hydrog. Energy 2019, 44, 14279–14289. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Tasnadi-Asztalos, Z.; Imre-Lucaci, A.; Katona, G.; Lazar, M.D. Hydrogen production by ethanol steam reforming on nickel catalysts: Effect of support modification by CeO2 and La2O3. Fuel 2015, 147, 260–268. [Google Scholar] [CrossRef]
- Bussi, J.; Musso, M.; Veiga, S.; Bespalko, N.; Faccio, R.; Roger, A.-C. Ethanol steam reforming over NiLaZr and NiCuLaZr mixed metal oxide catalysts. Catal. Today 2013, 213, 42–49. [Google Scholar] [CrossRef]
- Biswas, P.; Kunzru, D. Steam reforming of ethanol for production of hydrogen over Ni/CeO2–ZrO2 catalyst: Effect of support and metal loading. Int. J. Hydrog. Energy 2007, 32, 969–980. [Google Scholar] [CrossRef]
- Trane-Restrup, R.; Dahl, S.; Jensen, A.D. Steam reforming of ethanol: Effects of support and additives on Ni-based catalysts. Int. J. Hydrog. Energy 2013, 38, 15105–15118. [Google Scholar] [CrossRef]
- Dancini-Pontes, I.; DeSouza, M.; Silva, F.A.; Scaliante, M.H.N.O.; Alonso, C.G.; Bianchi, G.S.; Medina Neto, A.; Pereira, G.M.; Fernandes-Machado, N.R.C. Influence of the CeO2 and Nb2O5 supports and the inert gas in ethanol steam reforming for H2 production. Chem. Eng. J. 2015, 273, 66–74. [Google Scholar] [CrossRef]
- Song, J.H.; Yoo, S.; Yoo, J.; Park, S.; Gim, M.Y.; Kim, T.H.; Song, I.K. Hydrogen production by steam reforming of ethanol over Ni/Al2O
3-La2O3 xerogel catalysts. Mol. Catal. 2017, 434, 123–133. [Google Scholar] [CrossRef] - Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Rioche, C.; Kulkarni, S.; Meunier, F.C.; Breen, J.P.; Burch, R. Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts. Appl. Catal. B Environ. 2005, 61, 130–139. [Google Scholar] [CrossRef]
- Takanabe, K.; Aika, K.; Seshan, K.; Lefferts, L. Sustainable hydrogen from bio-oil—Steam reforming of acetic acid as a model oxygenate. J. Catal. 2004, 227, 101–108. [Google Scholar] [CrossRef]
- Matas Güell, B.; Babich, I.; Nichols, K.P.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Design of a stable steam reforming catalyst—A promising route to sustainable hydrogen from biomass oxygenates. Appl. Catal. B Environ. 2009, 90, 38–44. [Google Scholar] [CrossRef]
- Prasongthum, N.; Xiao, R.; Zhang, H.; Tsubaki, N.; Natewong, P.; Reubroycharoen, P. Highly active and stable Ni supported on CNTs-SiO2 fiber catalysts for steam reforming of ethanol. Fuel Processing Technol. 2017, 160, 185–195. [Google Scholar] [CrossRef]
- Anjaneyulu, C.; Costa, L.O.O.d.; Ribeiro, M.C.; Rabelo-Neto, R.C.; Mattos, L.V.; Venugopal, A.; Noronha, F.B. Effect of Zn addition on the performance of Ni/Al2O
3 catalyst for steam reforming of ethanol. Appl. Catal. A Gen. 2016, 519, 85–98. [Google Scholar] [CrossRef] - Mulewa, W.; Tahir, M.; Amin, N.A.S. MMT-supported Ni/TiO2 nanocomposite for low temperature ethanol steam reforming toward hydrogen production. Chem. Eng. J. 2017, 326, 956–969. [Google Scholar] [CrossRef]
- Yin, X.-m.; Xie, X.-m.; Wu, X.; An, X. Catalytic performance of nickel immobilized on organically modified montmorillonite in the steam reforming of ethanol for hydrogen production. J. Fuel Chem. Technol. 2016, 44, 689–697. [Google Scholar] [CrossRef]
- Musso, M.; Cardozo, A.; Romero, M.; Faccio, R.; Segobia, D.; Apesteguía, C.; Bussi, J. High performance Ni-catalysts supported on rare-earth zirconates (La and Y) for hydrogen production through ethanol steam reforming. Characterization and assay. Catal. Today 2021. [Google Scholar] [CrossRef]
- Niazi, Z.; Irankhah, A.; Wang, Y.; Arandiyan, H. Cu, Mg and Co effect on nickel-ceria supported catalysts for ethanol steam reforming reaction. Int. J. Hydrog. Energy 2020, 45, 21512–21522. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Tian, R.; Sun, C.; Dai, R.; Li, X.; Wu, X.; An, X.; Xie, X. Ni-hierarchical Beta zeolite catalysts were applied to ethanol steam reforming: Effect of sol gel method on loading Ni and the role of hierarchical structure. Mol. Catal. 2018, 453, 64–73. [Google Scholar] [CrossRef]
- Gac, W.; Greluk, M.; Słowik, G.; Millot, Y.; Valentin, L.; Dzwigaj, S. Effects of dealumination on the performance of Ni-containing BEA catalysts in bioethanol steam reforming. Appl. Catal. B Environ. 2018, 237, 94–109. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Yang, Z.; Liang, T.; Liu, S.; Zhou, Z.; Li, X. Effect of Mg-modified mesoporous Ni/Attapulgite catalysts on catalytic performance and resistance to carbon deposition for ethanol steam reforming. Fuel 2018, 220, 32–46. [Google Scholar] [CrossRef]
- Song, J.H.; Han, S.J.; Yoo, J.; Park, S.; Kim, D.H.; Song, I.K. Hydrogen production by steam reforming of ethanol over Ni–X/Al2O
3–ZrO2 (X=Mg, Ca, Sr, and Ba) xerogel catalysts: Effect of alkaline earth metal addition. J. Mol. Catal. A Chem. 2016, 415, 151–159. [Google Scholar] [CrossRef] - Song, J.H.; Han, S.J.; Yoo, J.; Park, S.; Kim, D.H.; Song, I.K. Effect of Sr content on hydrogen production by steam reforming of ethanol over Ni-Sr/Al2O
3-ZrO2 xerogel catalysts. J. Mol. Catal. A Chem. 2016, 418–419, 68–77. [Google Scholar] [CrossRef] - Li, L.; Tang, D.; Song, Y.; Jiang, B.; Zhang, Q. Hydrogen production from ethanol steam reforming on Ni-Ce/MMT catalysts. Energy 2018, 149, 937–943. [Google Scholar] [CrossRef]
- Gharahshiran, V.S.; Yousefpour, M. Synthesis and characterization of Zr-promoted Ni-Co bimetallic catalyst supported OMC and investigation of its catalytic performance in steam reforming of ethanol. Int. J. Hydrog. Energy 2018, 43, 7020–7037. [Google Scholar] [CrossRef]
- Nejat, T.; Jalalinezhad, P.; Hormozi, F.; Bahrami, Z. Hydrogen production from steam reforming of ethanol over Ni-Co bimetallic catalysts and MCM-41 as support. J. Taiwan Inst. Chem. Eng. 2019, 97, 216–226. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, G. Modulating and controlling active species dispersion over Ni–Co bimetallic catalysts for enhancement of hydrogen production of ethanol steam reforming. Int. J. Hydrog. Energy 2016, 41, 3349–3362. [Google Scholar] [CrossRef]
- Llorca, J.; Homs, N.; Ramirez de la Piscina, P. In situ DRIFT-mass spectrometry study of the ethanol steam-reforming reaction over carbonyl-derived Co/ZnO catalysts. J. Catal. 2004, 227, 556–560. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Processing Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Campos, C.H.; Pecchi, G.; Fierro, J.L.G.; Osorio-Vargas, P. Enhanced bimetallic Rh-Ni supported catalysts on alumina doped with mixed lanthanum-cerium oxides for ethanol steam reforming. Mol. Catal. 2019, 469, 87–97. [Google Scholar] [CrossRef]
- Mondal, T.; Pant, K.K.; Dalai, A.K. Oxidative and non-oxidative steam reforming of crude bio-ethanol for hydrogen production over Rh promoted Ni/CeO2-ZrO2 catalyst. Appl. Catal. A Gen. 2015, 499, 19–31. [Google Scholar] [CrossRef]
- Le Valant, A.; Can, F.; Bion, N.; Duprez, D.; Epron, F. Hydrogen production from raw bioethanol steam reforming: Optimization of catalyst composition with improved stability against various impurities. Int. J. Hydrog. Energy 2010, 35, 5015–5020. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Lytkina, A.A.; Ermilova, M.M.; Efimov, M.N.; Zemtsov, L.M.; Orekhova, N.V.; Karpacheva, G.P.; Bondarenko, G.N.; Muraviev, D.N.; Yaroslavtsev, A.B. Ethanol and methanol steam reforming on transition metal catalysts supported on detonation synthesis nanodiamonds for hydrogen production. Int. J. Hydrog. Energy 2015, 40, 3557–3565. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Ermilova, M.M.; Orekhova, N.V.; Muraviev, D.N.; Yaroslavtsev, A.B. Production of high purity hydrogen by ethanol steam reforming in membrane reactor. Catal. Today 2014, 236, 64–69. [Google Scholar] [CrossRef]
- He, S.; He, S.; Zhang, L.; Li, X.; Wang, J.; He, D.; Lu, J.; Luo, Y. Hydrogen production by ethanol steam reforming over Ni/SBA-15 mesoporous catalysts: Effect of Au addition. Catal. Today 2015, 258, 162–168. [Google Scholar] [CrossRef]
- Kim, D.; Kwak, B.S.; Min, B.-K.; Kang, M. Characterization of Ni and W co-loaded SBA-15 catalyst and its hydrogen production catalytic ability on ethanol steam reforming reaction. Appl. Surf. Sci. 2015, 332, 736–746. [Google Scholar] [CrossRef]
- Romero, A.; Jobbágy, M.; Laborde, M.; Baronetti, G.; Amadeo, N. Ni(II)–Mg(II)–Al(III) catalysts for hydrogen production from ethanol steam reforming: Influence of the Mg content. Appl. Catal. A Gen. 2014, 470, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Bepari, S.; Basu, S.; Pradhan, N.C.; Dalai, A.K. Steam reforming of ethanol over cerium-promoted Ni-Mg-Al hydrotalcite catalysts. Catal. Today 2017, 291, 47–57. [Google Scholar] [CrossRef]
- Shejale, A.D.; Yadav, G.D. Cu promoted Ni-Co/hydrotalcite catalyst for improved hydrogen production in comparison with several modified Ni-based catalysts via steam reforming of ethanol. Int. J. Hydrog. Energy 2017, 42, 11321–11332. [Google Scholar] [CrossRef]
- Passos, A.R.; Pulcinelli, S.H.; Santilli, C.V.; Briois, V. Operando monitoring of metal sites and coke evolution during non-oxidative and oxidative ethanol steam reforming over Ni and NiCu ex-hydrotalcite catalysts. Catal. Today 2019, 336, 122–130. [Google Scholar] [CrossRef]
- Wang, F. Hydrogen Production from Steam Reforming of Ethanol Over an Ir/Ceria-Based Catalyst: Catalyst Ageing Analysis and Performance Improvement upon Ceria Doping; Université Claude Bernard-Lyon I: Villeurbanne, France, 2012. [Google Scholar]
- Mhadmhan, S.; Natewong, P.; Prasongthum, N.; Samart, C.; Reubroycharoen, P. Investigation of Ni/SiO2 Fiber Catalysts Prepared by Different Methods on Hydrogen production from Ethanol Steam Reforming. Catalysts 2018, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Stach, E.A.; Rodriguez, J.A. Steam Reforming of Ethanol on Ni/CeO2: Reaction Pathway and Interaction between Ni and the CeO2 Support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Santander, J.A.; Tonetto, G.M.; Pedernera, M.N.; López, E. Ni/CeO2–MgO catalysts supported on stainless steel plates for ethanol steam reforming. Int. J. Hydrog. Energy 2017, 42, 9482–9492. [Google Scholar] [CrossRef]
- Zhuang, Q.; Qin, Y.; Chang, L. Promoting effect of cerium oxide in supported nickel catalyst for hydrocarbon steam-reforming. Appl. Catal. 1991, 70, 1–8. [Google Scholar] [CrossRef]
- Tahir, M.; Mulewa, W.; Amin, N.A.S.; Zakaria, Z.Y. Thermodynamic and experimental analysis on ethanol steam reforming for hydrogen production over Ni-modified TiO2/MMT nanoclay catalyst. Energy Convers. Manag. 2017, 154, 25–37. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Niu, G.; Yang, Z.; Bian, M.; He, A. High temperature thermal stabilization of alumina modified by lanthanum species. Appl. Catal. A Gen. 2001, 205, 159–172. [Google Scholar] [CrossRef]
- Carrera Cerritos, R.; Fuentes Ramírez, R.; Aguilera Alvarado, A.F.; Martínez Rosales, J.M.; Viveros García, T.; Galindo Esquivel, I.R. Steam Reforming of Ethanol over Ni/Al2O
3−La2O3 Catalysts Synthesized by Sol−Gel. Ind. Eng. Chem. Res. 2011, 50, 2576–2584. [Google Scholar] [CrossRef] - Lee, S. Concepts in Syngas Manufacture. By Jens Rostrup-Nielsen and Lars J. Christiansen. Energy Technol. 2013, 1, 419–420. [Google Scholar] [CrossRef]
- Melo, F.; Morlanés, N. Synthesis, characterization and catalytic behaviour of NiMgAl mixed oxides as catalysts for hydrogen production by naphtha steam reforming. Catal. Today 2008, 133–135, 383–393. [Google Scholar] [CrossRef]
- Melo, F.; Morlanés, N. Naphtha steam reforming for hydrogen production. Catal. Today 2005, 107-108, 458–466. [Google Scholar] [CrossRef]
- Conner, W.C.; Falconer, J.L. Spillover in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- He, S.; Mei, Z.; Liu, N.; Zhang, L.; Lu, J.; Li, X.; Wang, J.; He, D.; Luo, Y. Ni/SBA-15 catalysts for hydrogen production by ethanol steam reforming: Effect of nickel precursor. Int. J. Hydrog. Energy 2017, 42, 14429–14438. [Google Scholar] [CrossRef]
- Villagrán-Olivares, A.C.; Gomez, M.F.; Barroso, M.N.; Abello, M.C. Hydrogen production from ethanol: Synthesis of Ni catalysts assisted by chelating agents. Mol. Catal. 2020, 481, 110164. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Barroso, N.; Galetti, A.E.; Arrúa, L.A.; Cornaglia, L.; Abello, M.C. XPS study of the surface properties and Ni particle size determination of Ni-supported catalysts. Surf. Interface Anal. 2014, 46, 521–529. [Google Scholar] [CrossRef]
- van Dillen, A.J.; Terörde, R.J.A.M.; Lensveld, D.J.; Geus, J.W.; de Jong, K.P. Synthesis of supported catalysts by impregnation and drying using aqueous chelated metal complexes. J. Catal. 2003, 216, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Dupont, V.; Nahil, M.A.; Dou, B.; Chen, H.; Williams, P.T. Investigation of Ni/SiO2 catalysts prepared at different conditions for hydrogen production from ethanol steam reforming. J. Energy Inst. 2017, 90, 276–284. [Google Scholar] [CrossRef]
- Nichele, V.; Signoretto, M.; Menegazzo, F.; Rossetti, I.; Cruciani, G. Hydrogen production by ethanol steam reforming: Effect of the synthesis parameters on the activity of Ni/TiO2 catalysts. Int. J. Hydrog. Energy 2014, 39, 4252–4258. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Bridgwater, T. Biomass for energy. J. Sci. Food Agric. 2006, 86, 1755–1768. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Recent developments on the catalytic technologies for the transformation of biomass into biofuels: A patent survey. Renew. Sustain. Energy Rev. 2015, 51, 273–287. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Martinez-Hernandez, E.; Murphy, R.J.; Ng, D.K.S.; Hassim, M.H.; Siew Ng, K.; Yoke Kin, W.; Jaye, I.F.M.; Leung Pah Hang, M.Y.; Andiappan, V. Role of bioenergy, biorefinery and bioeconomy in sustainable development: Strategic pathways for Malaysia. Renew. Sustain. Energy Rev. 2018, 81, 1966–1987. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Mondal, P.; Dang, G.S.; Garg, M.O. Syngas production through gasification and cleanup for downstream applications—Recent developments. Fuel Processing Technol. 2011, 92, 1395–1410. [Google Scholar] [CrossRef]
- Raffelt, K.; Henrich, E.; Koegel, A.; Stahl, R.; Steinhardt, J.; Weirich, F. The BTL2 process of biomass utilization entrained-flow gasification of pyrolyzed biomass slurries. Appl. Biochem. Biotechnol. 2006, 129, 153–164. [Google Scholar] [CrossRef]
- Soria, M.A.; Barros, D.; Madeira, L.M. Hydrogen production through steam reforming of bio-oils derived from biomass pyrolysis: Thermodynamic analysis including in situ CO2 and/or H2 separation. Fuel 2019, 244, 184–195. [Google Scholar] [CrossRef]
- Basagiannis, A.C.; Verykios, X.E. Reforming reactions of acetic acid on nickel catalysts over a wide temperature range. Appl. Catal. A Gen. 2006, 308, 182–193. [Google Scholar] [CrossRef]
- Marquevich, M.; Czernik, S.; Chornet, E.; Montané, D. Hydrogen from Biomass: Steam Reforming of Model Compounds of Fast-Pyrolysis Oil. Energy Fuels 1999, 13, 1160–1166. [Google Scholar] [CrossRef]
- Radlein, D.; Piskorz, J.; Scott, D.S. Fast pyrolysis of natural polysaccharides as a potential industrial process. J. Anal. Appl. Pyrolysis 1991, 19, 41–63. [Google Scholar] [CrossRef]
- Paredes, M.J.; Moreno, E.; Ramos-Cormenzana, A.; Martinez, J. Characteristics of soil after pollution with wastewaters from olive oil extraction plants. Chemosphere 1987, 16, 1557–1564. [Google Scholar] [CrossRef]
- DellaGreca, M.; Monaco, P.; Pinto, G.; Pollio, A.; Previtera, L.; Temussi, F. Phytotoxicity of low-molecular-weight phenols from olive mill wastewaters. Bull. Environ. Contam. Toxicol. 2001, 67, 352–359. [Google Scholar] [CrossRef]
- Rana, G.; Rinaldi, M.; Introna, M. Volatilisation of substances alter spreading olive oil waste water on the soil in a Mediterranean environment. Agric. Ecosyst. Environ. 2003, 96, 49–58. [Google Scholar] [CrossRef]
- Montero, C.; Oar-Arteta, L.; Remiro, A.; Arandia, A.; Bilbao, J.; Gayubo, A.G. Thermodynamic comparison between bio-oil and ethanol steam reforming. Int. J. Hydrog. Energy 2015, 40, 15963–15971. [Google Scholar] [CrossRef]
- Casanovas, A.; Galvis, A.; Llorca, J. Catalytic steam reforming of olive mill wastewater for hydrogen production. Int. J. Hydrog. Energy 2015, 40, 7539–7545. [Google Scholar] [CrossRef] [Green Version]
- Tosti, S.; Fabbricino, M.; Pontoni, L.; Palma, V.; Ruocco, C. Catalytic reforming of olive mill wastewater and methane in a Pd-membrane reactor. Int. J. Hydrog. Energy 2016, 41, 5465–5474. [Google Scholar] [CrossRef]
- Tosti, S.; Accetta, C.; Fabbricino, M.; Sansovini, M.; Pontoni, L. Reforming of olive mill wastewater through a Pd-membrane reactor. Int. J. Hydrog. Energy 2013, 38, 10252–10259. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Mazzei, R.; Giorno, L. Trends and current practices of olive mill wastewater treatment: Application of integrated membrane process and its future perspective. Sep. Purif. Technol. 2016, 162, 45–60. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef]
- Daâssi, D.; Lozano-Sánchez, J.; Borrás-Linares, I.; Belbahri, L.; Woodward, S.; Zouari-Mechichi, H.; Mechichi, T.; Nasri, M.; Segura-Carretero, A. Olive oil mill wastewaters: Phenolic content characterization during degradation by Coriolopsis gallica. Chemosphere 2014, 113, 62–70. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Kyriacou, A.; Lasaridi, K.E.; Kotsou, M.; Balis, C.; Pilidis, G. Combined bioremediation and advanced oxidation of green table olive processing wastewater. Process Biochem. 2005, 40, 1401–1408. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Kaleh, Z.; Geißen, S.U. Selective isolation of valuable biophenols from olive mill wastewater. J. Environ. Chem. Eng. 2016, 4, 373–384. [Google Scholar] [CrossRef]
- Caputo, A.C.; Scacchia, F.; Pelagagge, P.M. Disposal of by-products in olive oil industry: Waste-to-energy solutions. Appl. Therm. Eng. 2003, 23, 197–214. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Loizides, M.; Karlis, P.K. Integrated strategic approach for reusing olive oil extraction by-products. J. Clean. Prod. 2004, 12, 603–611. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Bernal, M.P. Characterization of olive mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Feki, M.; Allouche, N.; Bouaziz, M.; Gargoubi, A.; Sayadi, S. Effect of storage of olive mill wastewaters on hydroxytyrosol concentration. Eur. J. Lipid Sci. Technol. 2006, 108, 1021–1027. [Google Scholar] [CrossRef]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem.-Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef]
- O-Thong, S.; Suksong, W.; Promnuan, K.; Thipmunee, M.; Mamimin, C.; Prasertsan, P. Two-stage thermophilic fermentation and mesophilic methanogenic process for biohythane production from palm oil mill effluent with methanogenic effluent recirculation for pH control. Int. J. Hydrog. Energy 2016, 41, 21702–21712. [Google Scholar] [CrossRef]
- Bizkarra, K.; Bermudez, J.M.; Arcelus-Arrillaga, P.; Barrio, V.L.; Cambra, J.F.; Millan, M. Nickel based monometallic and bimetallic catalysts for synthetic and real bio-oil steam reforming. Int. J. Hydrog. Energy 2018, 43, 11706–11718. [Google Scholar] [CrossRef]
- Kechagiopoulos, P.N.; Voutetakis, S.S.; Lemonidou, A.A.; Vasalos, I.A. Hydrogen Production via Steam Reforming of the Aqueous Phase of Bio-Oil in a Fixed Bed Reactor. Energy Fuels 2006, 20, 2155–2163. [Google Scholar] [CrossRef]
- Remiro, A.; Arandia, A.; Oar-Arteta, L.; Bilbao, J.; Gayubo, A.G. Regeneration of NiAl2O
4 spinel type catalysts used in the reforming of raw bio-oil. Appl. Catal. B Environ. 2018, 237, 353–365. [Google Scholar] [CrossRef] - Remiro, A.; Valle, B.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Operating conditions for attenuating Ni/La2O3–αAl2O
3 catalyst deactivation in the steam reforming of bio-oil aqueous fraction. Fuel Processing Technol. 2013, 115, 222–232. [Google Scholar] [CrossRef] - Valle, B.; Aramburu, B.; Olazar, M.; Bilbao, J.; Gayubo, A.G. Steam reforming of raw bio-oil over Ni/La2O3-αAl2O
3: Influence of temperature on product yields and catalyst deactivation. Fuel 2018, 216, 463–474. [Google Scholar] [CrossRef] - Xie, H.; Yu, Q.; Wei, M.; Duan, W.; Yao, X.; Qin, Q.; Zuo, Z. Hydrogen production from steam reforming of simulated bio-oil over Ce–Ni/Co catalyst with in continuous CO2 capture. Int. J. Hydrog. Energy 2015, 40, 1420–1428. [Google Scholar] [CrossRef]
- Silva, J.M.; Ribeiro, L.S.; Órfão, J.J.M.; Soria, M.A.; Madeira, L.M. Low temperature glycerol steam reforming over a Rh-based catalyst combined with oxidative regeneration. Int. J. Hydrog. Energy 2019, 44, 2461–2473. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Olive mill wastewater valorization through steam reforming using hybrid multifunctional reactors for high-purity H2 production. Chem. Eng. J. 2022, 430, 132651. [Google Scholar] [CrossRef]
- Ng, K.H.; Cheng, Y.W.; Lee, Z.S.; Cheng, C.K. A study into syngas production from catalytic steam reforming of palm oil mill effluent (POME): A new treatment approach. Int. J. Hydrog. Energy 2019, 44, 20900–20913. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Ng, K.H.; Lam, S.S.; Lim, J.W.; Wongsakulphasatch, S.; Witoon, T.; Cheng, C.K. Syngas from catalytic steam reforming of palm oil mill effluent: An optimization study. Int. J. Hydrog. Energy 2019, 44, 9220–9236. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. Factor effects and interactions in steam reforming of biomass bio-oil. Chem. Pap. 2020, 74, 1459–1470. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Steam reforming of olive oil mill wastewater with in situ hydrogen and carbon dioxide separation–Thermodynamic analysis. Fuel 2017, 207, 449–460. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Thermodynamic analysis of olive oil mill wastewater steam reforming. J. Energy Inst. 2019, 92, 1599–1609. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.A.; Madeira, L.M. Glycerol steam reforming for hydrogen production: Traditional versus membrane reactor. Int. J. Hydrog. Energy 2019, 44, 24719–24732. [Google Scholar] [CrossRef]
- Leal, A.L.; Soria, M.A.; Madeira, L.M. Autothermal reforming of impure glycerol for H2 production: Thermodynamic study including in situ CO2 and/or H2 separation. Int. J. Hydrog. Energy 2016, 41, 2607–2620. [Google Scholar] [CrossRef]
- Medrano, J.A.; Oliva, M.; Ruiz, J.; Garcia, L.; Arauzo, J. Catalytic steam reforming of acetic acid in a fluidized bed reactor with oxygen addition. Int. J. Hydrog. Energy 2008, 33, 4387–4396. [Google Scholar] [CrossRef]
- Matas Güell, B.; Silva, I.M.T.d.; Seshan, K.; Lefferts, L. Sustainable route to hydrogen–Design of stable catalysts for the steam gasification of biomass related oxygenates. Appl. Catal. B Environ. 2009, 88, 59–65. [Google Scholar] [CrossRef]
- Hoang, T.M.C.; Geerdink, B.; Sturm, J.M.; Lefferts, L.; Seshan, K. Steam reforming of acetic acid–A major component in the volatiles formed during gasification of humin. Appl. Catal. B Environ. 2015, 163, 74–82. [Google Scholar] [CrossRef]
- Chen, G.; Tao, J.; Liu, C.; Yan, B.; Li, W.; Li, X. Steam reforming of acetic acid using Ni/Al2O
3 catalyst: Influence of crystalline phase of Al2O
3 support. Int. J. Hydrog. Energy 2017, 42, 20729–20738. [Google Scholar] [CrossRef] - Savuto, E.; Navarro, R.M.; Mota, N.; Di Carlo, A.; Bocci, E.; Carlini, M.; Fierro, J.L.G. Steam reforming of tar model compounds over Ni/Mayenite catalysts: Effect of Ce addition. Fuel 2018, 224, 676–686. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Li, J.; Gao, G.; Dong, D.; Westerhof, R.; Hu, S.; Xiang, J.; Wang, Y. Steam reforming of acetic acid over Ni/Al2O
3 catalysts: Correlation of nickel loading with properties and catalytic behaviors of the catalysts. Fuel 2018, 217, 389–403. [Google Scholar] [CrossRef] - Borges, R.P.; Ferreira, R.A.R.; Rabelo-Neto, R.C.; Noronha, F.B.; Hori, C.E. Hydrogen production by steam reforming of acetic acid using hydrotalcite type precursors. Int. J. Hydrog. Energy 2018, 43, 7881–7892. [Google Scholar] [CrossRef]
- He, L.; Hu, S.; Jiang, L.; Liao, G.; Chen, X.; Han, H.; Xiao, L.; Ren, Q.; Wang, Y.; Su, S.; et al. Carbon nanotubes formation and its influence on steam reforming of toluene over Ni/Al2O
3 catalysts: Roles of catalyst supports. Fuel Processing Technol. 2018, 176, 7–14. [Google Scholar] [CrossRef] - Pu, J.; Nishikado, K.; Wang, N.; Nguyen, T.T.; Maki, T.; Qian, E.W. Core-shell nickel catalysts for the steam reforming of acetic acid. Appl. Catal. B Environ. 2018, 224, 69–79. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Yu, Z.; Zhang, Z.; Chen, G.; Li, C.; Liu, Q.; Xiang, J.; Wang, Y.; Hu, S. Steam reforming of acetic acid for hydrogen production over attapulgite and alumina supported Ni catalysts: Impacts of properties of supports on catalytic behaviors. Int. J. Hydrog. Energy 2019, 44, 5230–5244. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Wang, S.; Li, X. Hydrogen production via steam reforming of acetic acid over biochar-supported nickel catalysts. Int. J. Hydrog. Energy 2018, 43, 18160–18168. [Google Scholar] [CrossRef]
- Kechagiopoulos, P.N.; Voutetakis, S.S.; Lemonidou, A.A.; Vasalos, I.A. Hydrogen Production via Reforming of the Aqueous Phase of Bio-Oil over Ni/Olivine Catalysts in a Spouted Bed Reactor. Ind. Eng. Chem. Res. 2009, 48, 1400–1408. [Google Scholar] [CrossRef]
- Liu, S.; Chen, M.; Chu, L.; Yang, Z.; Zhu, C.; Wang, J.; Chen, M. Catalytic steam reforming of bio-oil aqueous fraction for hydrogen production over Ni–Mo supported on modified sepiolite catalysts. Int. J. Hydrog. Energy 2013, 38, 3948–3955. [Google Scholar] [CrossRef]
- Garcia, L.a.; French, R.; Czernik, S.; Chornet, E. Catalytic steam reforming of bio-oils for the production of hydrogen: Effects of catalyst composition. Appl. Catal. A Gen. 2000, 201, 225–239. [Google Scholar] [CrossRef]
- Bangala, D.N.; Abatzoglou, N.; Chornet, E. Steam reforming of naphthalene on Ni–Cr/Al2O
3 catalysts doped with MgO, TiO2, and La2O3. AIChE J. 1998, 44, 927–936. [Google Scholar] [CrossRef] - Zhang, Z.; Hu, X.; Gao, G.; Wei, T.; Dong, D.; Wang, Y.; Hu, S.; Xiang, J.; Liu, Q.; Geng, D. Steam reforming of acetic acid over NiKOH/Al2O
3 catalyst with low nickel loading: The remarkable promotional effects of KOH on activity. Int. J. Hydrog. Energy 2019, 44, 729–747. [Google Scholar] [CrossRef] - Choi, I.-H.; Hwang, K.-R.; Lee, K.-Y.; Lee, I.-G. Catalytic steam reforming of biomass-derived acetic acid over modified Ni/γ-Al2O
3 for sustainable hydrogen production. Int. J. Hydrog. Energy 2019, 44, 180–190. [Google Scholar] [CrossRef] - Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3–ZrO2 catalyst for hydrogen production through the glycerol steam reforming reaction. Int. J. Hydrog. Energy 2020, 45, 10442–10460. [Google Scholar] [CrossRef]
- Souza, I.C.A.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen production from steam reforming of acetic acid over Pt–Ni bimetallic catalysts supported on ZrO2. Biomass Bioenergy 2022, 156, 106317. [Google Scholar] [CrossRef]
- Baamran, K.S.; Tahir, M.; Mohamed, M.; Hussain Khoja, A. Effect of support size for stimulating hydrogen production in phenol steam reforming using Ni-embedded TiO2 nanocatalyst. J. Environ. Chem. Eng. 2020, 8, 103604. [Google Scholar] [CrossRef]
- Baamran, K.S.; Tahir, M. Ni-embedded TiO2-ZnTiO3 reducible perovskite composite with synergistic effect of metal/support towards enhanced H2 production via phenol steam reforming. Energy Convers. Manag. 2019, 200, 112064. [Google Scholar] [CrossRef]
- Abbas, T.; Tahir, M.; Saidina Amin, N.A. Enhanced Metal–Support Interaction in Ni/CO3O4/TiO2 Nanorods toward Stable and Dynamic Hydrogen Production from Phenol Steam Reforming. Ind. Eng. Chem. Res. 2019, 58, 517–530. [Google Scholar] [CrossRef]
- Pu, J.; Ikegami, F.; Nishikado, K.; Qian, E.W. Effect of ceria addition on NiRu/CeO2Al2O
3 catalysts in steam reforming of acetic acid. Int. J. Hydrog. Energy 2017, 42, 19733–19743. [Google Scholar] [CrossRef] - Wang, Y.; Chen, M.; Yang, J.; Liu, S.; Yang, Z.; Wang, J.; Liang, T. Hydrogen Production from Steam Reforming of Acetic Acid over Ni-Fe/Palygorskite Modified with Cerium. BioResources 2017, 12, 4830–4853. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jiang, B.; Tang, D.; Zhang, Q.; Zheng, Z. Hydrogen generation by acetic acid steam reforming over Ni-based catalysts derived from La1−xCexNiO3 perovskite. Int. J. Hydrog. Energy 2018, 43, 6795–6803. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, Y.; Lu, Z.; Huang, Y.; Guo, C.; Yan, C. Encapsulating Ni/CeO2-ZrO2 with SiO2 layer to improve it catalytic activity for steam reforming of toluene. Catal. Commun. 2017, 101, 138–141. [Google Scholar] [CrossRef]
- Liang, T.; Wang, Y.; Chen, M.; Yang, Z.; Liu, S.; Zhou, Z.; Li, X. Steam reforming of phenol-ethanol to produce hydrogen over bimetallic NiCu catalysts supported on sepiolite. Int. J. Hydrog. Energy 2017, 42, 28233–28246. [Google Scholar] [CrossRef]
- Pant, K.K.; Mohanty, P.; Agarwal, S.; Dalai, A.K. Steam reforming of acetic acid for hydrogen production over bifunctional Ni–Co catalysts. Catal. Today 2013, 207, 36–43. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Jha, A.; Jang, W.-J.; Shim, J.-O.; Jeon, K.-W.; Na, H.-S.; Kim, H.-M.; Lee, D.-W.; Yoo, S.-Y.; Jeon, B.-H.; et al. Optimization of Cobalt Loading in Co–CeO2 Catalyst for the High Temperature Water–Gas Shift Reaction. Top. Catal. 2017, 60, 721–726. [Google Scholar] [CrossRef]
- Sabnis, K.D. Structure-Activity Relationships for the Water-Gas Shift Reaction over Supported Metal Catalysts; Purdue University: West Lafayette, IN, USA, 2015. [Google Scholar]
- Mizuno, S.C.M.; Braga, A.H.; Hori, C.E.; Santos, J.B.O.; Bueno, J.M.C. Steam reforming of acetic acid over MgAl2O4-supported Co and Ni catalysts: Effect of the composition of Ni/Co and reactants on reaction pathways. Catal. Today 2017, 296, 144–153. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Screening of commercial catalysts for steam reforming of olive mill wastewater. Renew. Energy 2021, 169, 765–779. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Use of Ni-containing catalysts for synthetic olive mill wastewater steam reforming. Renew. Energy 2022, 185, 1329–1342. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.D.; To, S.D.F.; Bricka, R.M.; Steele, P.H.; Haryanto, A. Conversion of Glycerol to Hydrogen via a Steam Reforming Process over Nickel Catalysts. Energy Fuels 2008, 22, 1220–1226. [Google Scholar] [CrossRef]
- Pant, K.K.; Jain, R.; Jain, S. Renewable hydrogen production by steam reforming of glycerol over Ni/CeO2 catalyst prepared by precipitation deposition method. Korean J. Chem. Eng. 2011, 28, 1859. [Google Scholar] [CrossRef]
- Buffoni, I.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Nickel catalysts applied in steam reforming of glycerol for hydrogen production. Catal. Commun. 2009, 10, 1656–1660. [Google Scholar] [CrossRef]
- Silva, O.C.V.; Silveira, E.B.; Rabelo-Neto, R.C.; Borges, L.E.P.; Noronha, F.B. Hydrogen Production Through Steam Reforming of Toluene Over Ni Supported on MgAl Mixed Oxides Derived from Hydrotalcite-Like Compounds. Catal. Lett. 2018, 148, 1622–1633. [Google Scholar] [CrossRef]
- Manfro, R.L.; Ribeiro, N.F.P.; Souza, M.M.V.M. Production of hydrogen from steam reforming of glycerol using nickel catalysts supported on Al2O3, CeO2 and ZrO2. Catal. Sustain. Energy 2013, 1, 60–70. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaíno, A.J. Production of Renewable Hydrogen from Glycerol Steam Reforming over Bimetallic Ni-(Cu,Co,Cr) Catalysts Supported on SBA-15 Silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Dupont, V. Nickel catalyst auto-reduction during steam reforming of bio-oil model compound acetic acid. Int. J. Hydrog. Energy 2013, 38, 15160–15172. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Wang, S. Effect of La2O3 replacement on γ-Al2O3 supported nickel catalysts for acetic acid steam reforming. Int. J. Hydrog. Energy 2017, 42, 20540–20548. [Google Scholar] [CrossRef]
- Matas Güell, B.; Babich, I.V.; Lefferts, L.; Seshan, K. Steam reforming of phenol over Ni-based catalysts–A comparative study. Appl. Catal. B Environ. 2011, 106, 280–286. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Spadaro, L.; Di Blasi, O.; Bonura, G.; Cavallaro, S. Steam reforming of bio-ethanol on alkali-doped Ni/MgO catalysts: Hydrogen production for MC fuel cell. Appl. Catal. A Gen. 2004, 270, 1–7. [Google Scholar] [CrossRef]
- Ahmed, T.; Xiu, S.; Wang, L.; Shahbazi, A. Investigation of Ni/Fe/Mg zeolite-supported catalysts in steam reforming of tar using simulated-toluene as model compound. Fuel 2018, 211, 566–571. [Google Scholar] [CrossRef]
- Fally, F.; Perrichon, V.; Vidal, H.; Kaspar, J.; Blanco, G.; Pintado, J.M.; Bernal, S.; Colon, G.; Daturi, M.; Lavalley, J.C. Modification of the oxygen storage capacity of CeO2–ZrO2 mixed oxides after redox cycling aging. Catal. Today 2000, 59, 373–386. [Google Scholar] [CrossRef]
- Vidal, H.; Kašpar, J.; Pijolat, M.; Colon, G.; Bernal, S.; Cordón, A.; Perrichon, V.; Fally, F. Redox behavior of CeO2–ZrO2 mixed oxides: II. Influence of redox treatments on low surface area catalysts. Appl. Catal. B Environ. 2001, 30, 75–85. [Google Scholar] [CrossRef]
- Sánchez, E.A.; D’Angelo, M.A.; Comelli, R.A. Hydrogen production from glycerol on Ni/Al2O3 catalyst. Int. J. Hydrog. Energy 2010, 35, 5902–5907. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Ma, H.; Xu, Z.; Tian, Z. Production of hydrogen by aqueous-phase reforming of glycerol. Int. J. Hydrog. Energy 2008, 33, 6657–6666. [Google Scholar] [CrossRef]
- Cakiryilmaz, N.; Arbag, H.; Oktar, N.; Dogu, G.; Dogu, T. Effect of W incorporation on the product distribution in steam reforming of bio-oil derived acetic acid over Ni based Zr-SBA-15 catalyst. Int. J. Hydrog. Energy 2018, 43, 3629–3642. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, X.; Jia, P.; Zhang, Z.; Dong, D.; Hu, G.; Hu, S.; Wang, Y.; Xiang, J. Steam reforming of acetic acid over nickel-based catalysts: The intrinsic effects of nickel precursors on behaviors of nickel catalysts. Appl. Catal. B Environ. 2018, 237, 538–553. [Google Scholar] [CrossRef]
- Pekmezci Karaman, B.; Cakiryilmaz, N.; Arbag, H.; Oktar, N.; Dogu, G.; Dogu, T. Performance comparison of mesoporous alumina supported Cu & Ni based catalysts in acetic acid reforming. Int. J. Hydrog. Energy 2017, 42, 26257–26269. [Google Scholar] [CrossRef]
- Goicoechea, S.; Kraleva, E.; Sokolov, S.; Schneider, M.; Pohl, M.-M.; Kockmann, N.; Ehrich, H. Support effect on structure and performance of Co and Ni catalysts for steam reforming of acetic acid. Appl. Catal. A Gen. 2016, 514, 182–191. [Google Scholar] [CrossRef]
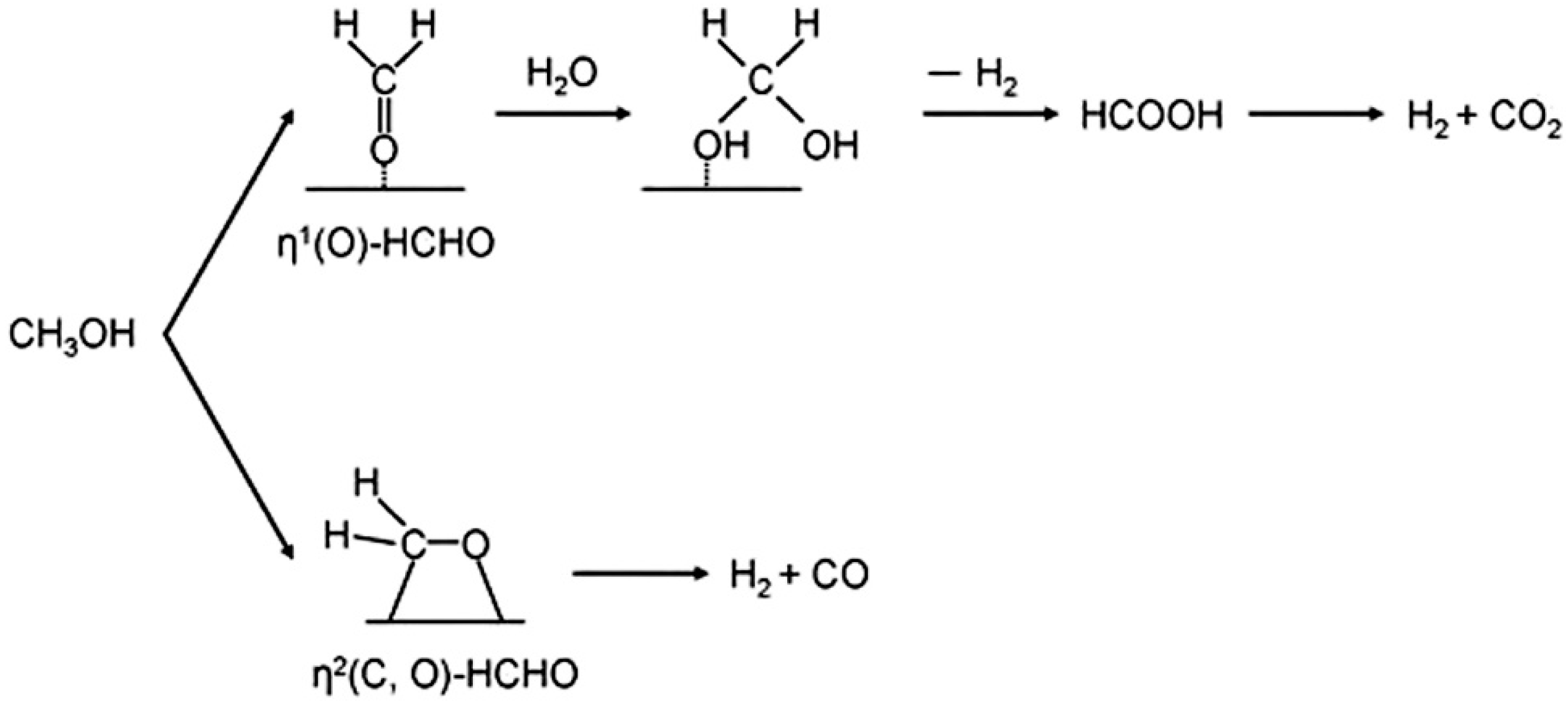
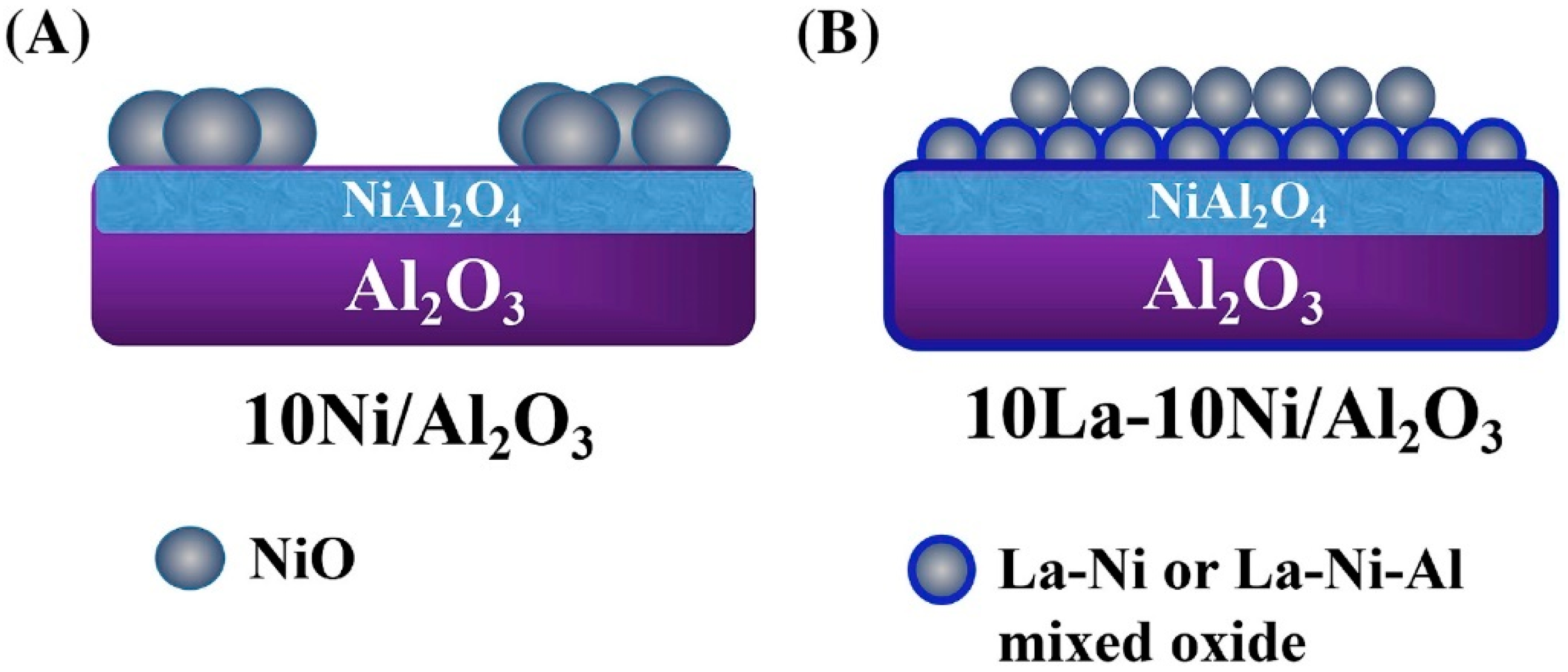

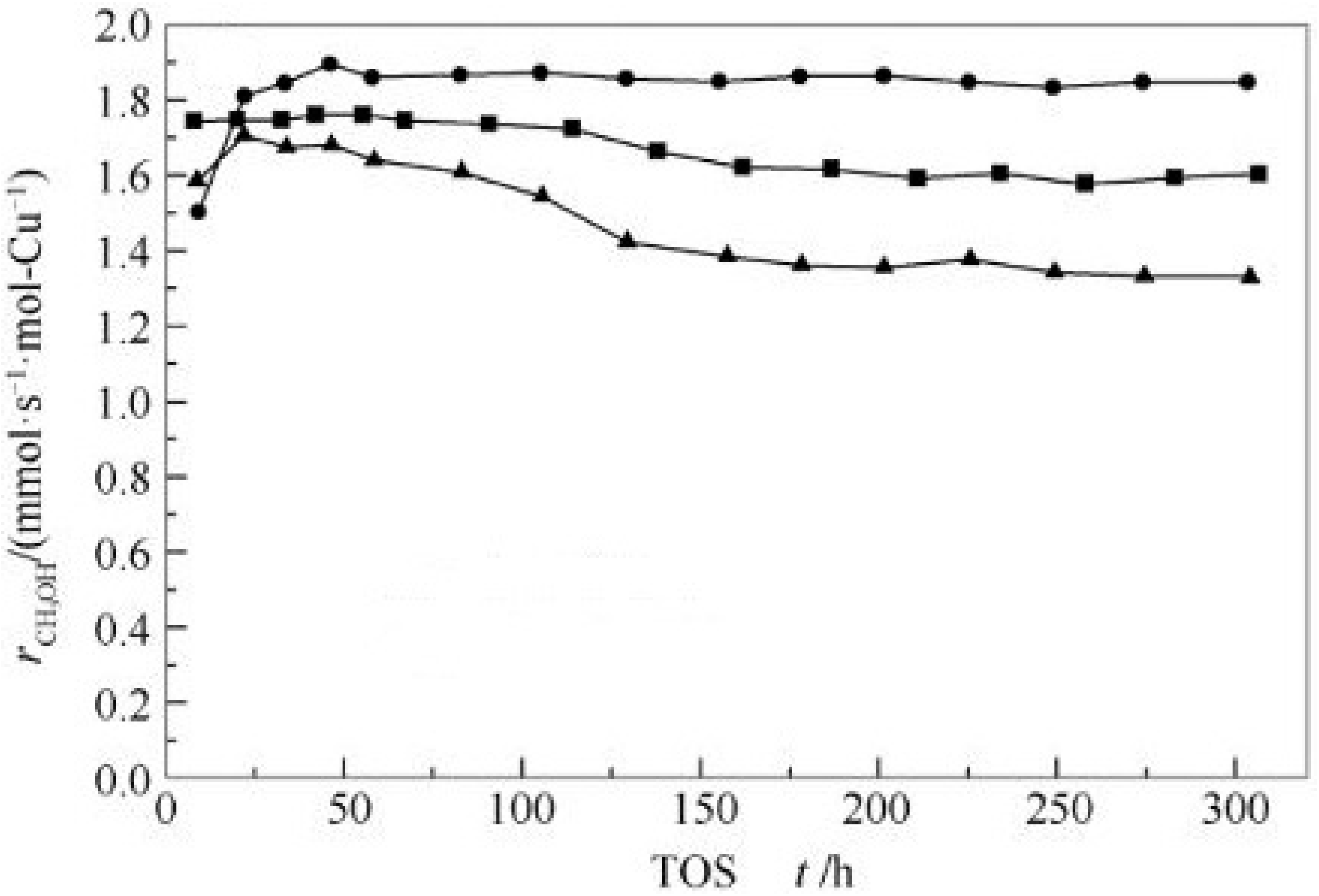

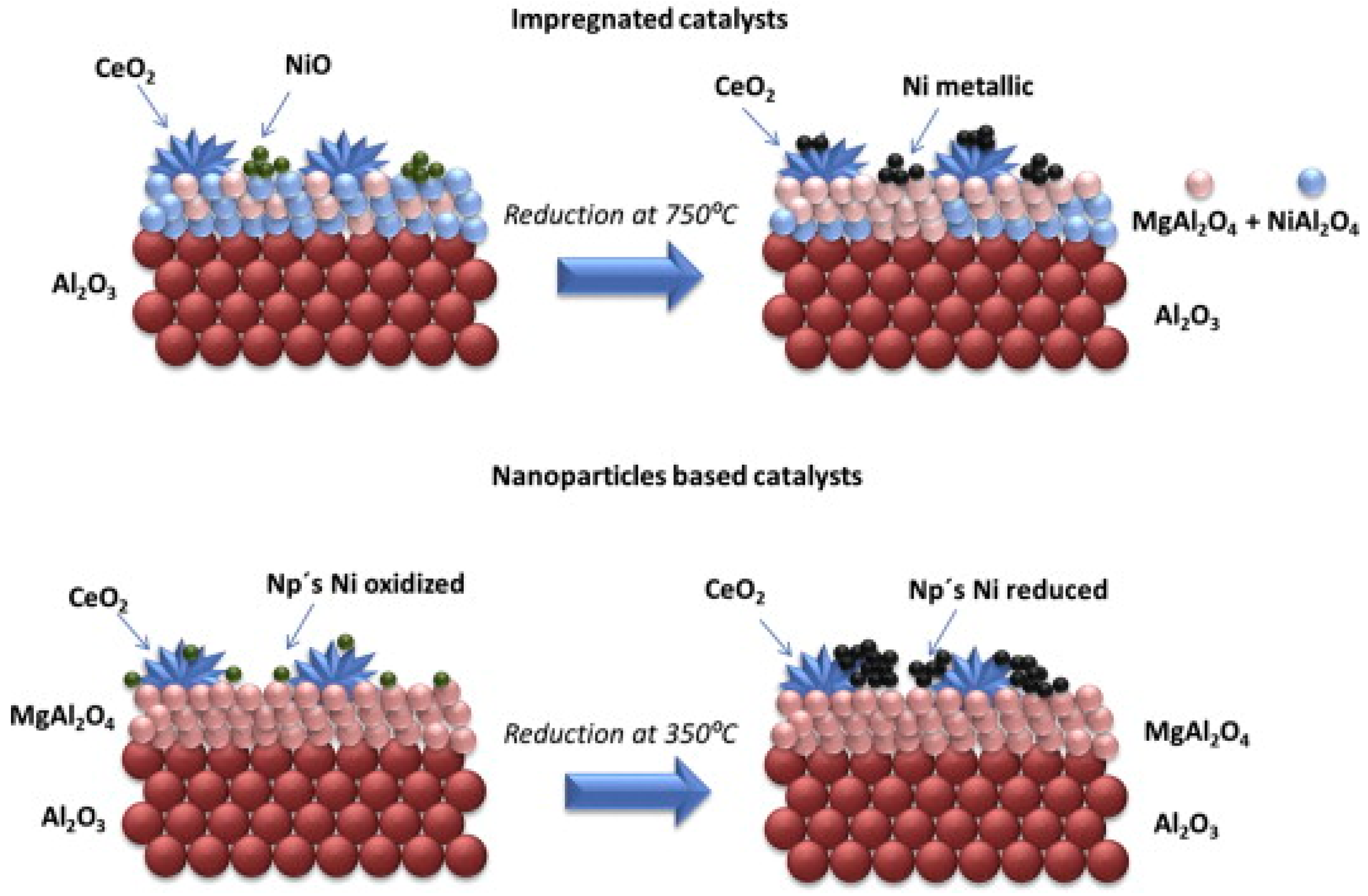

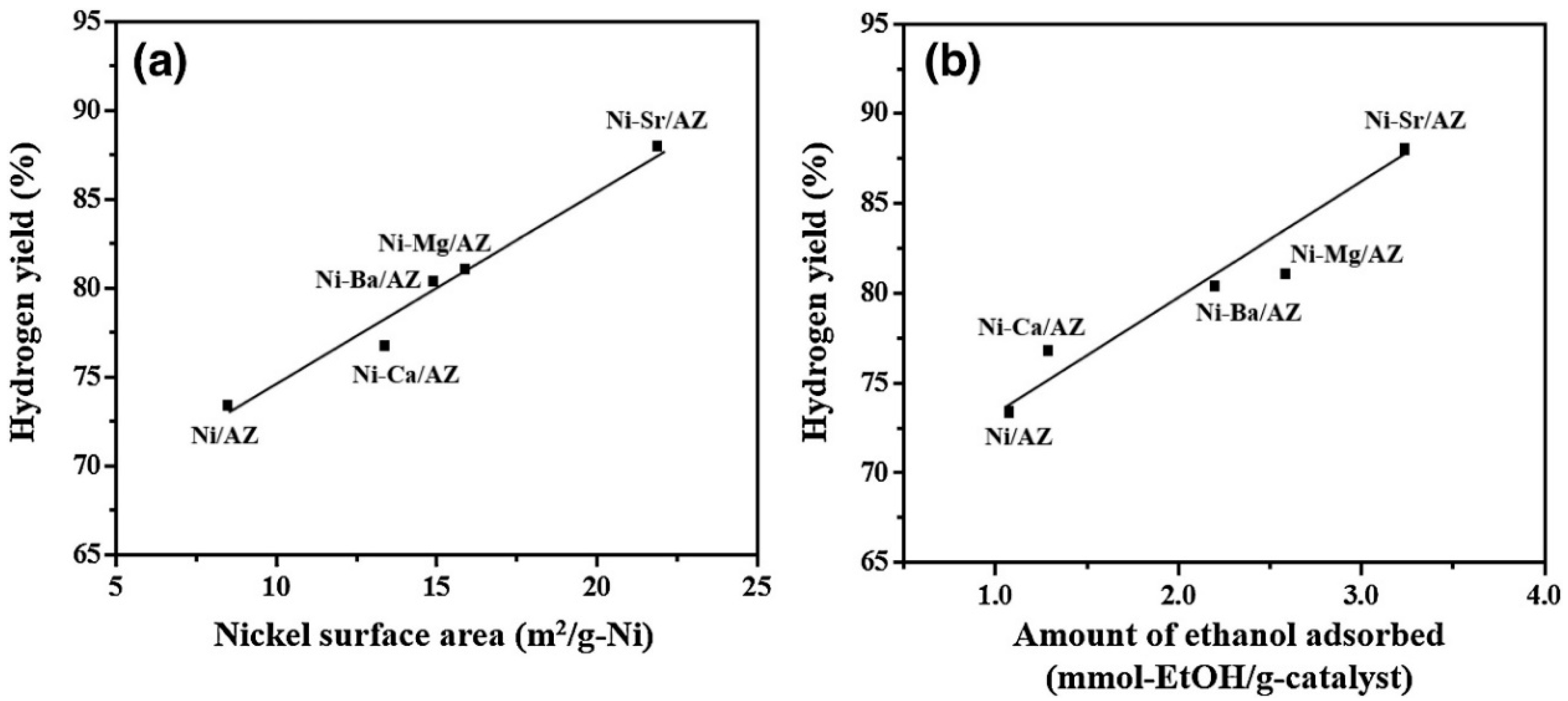
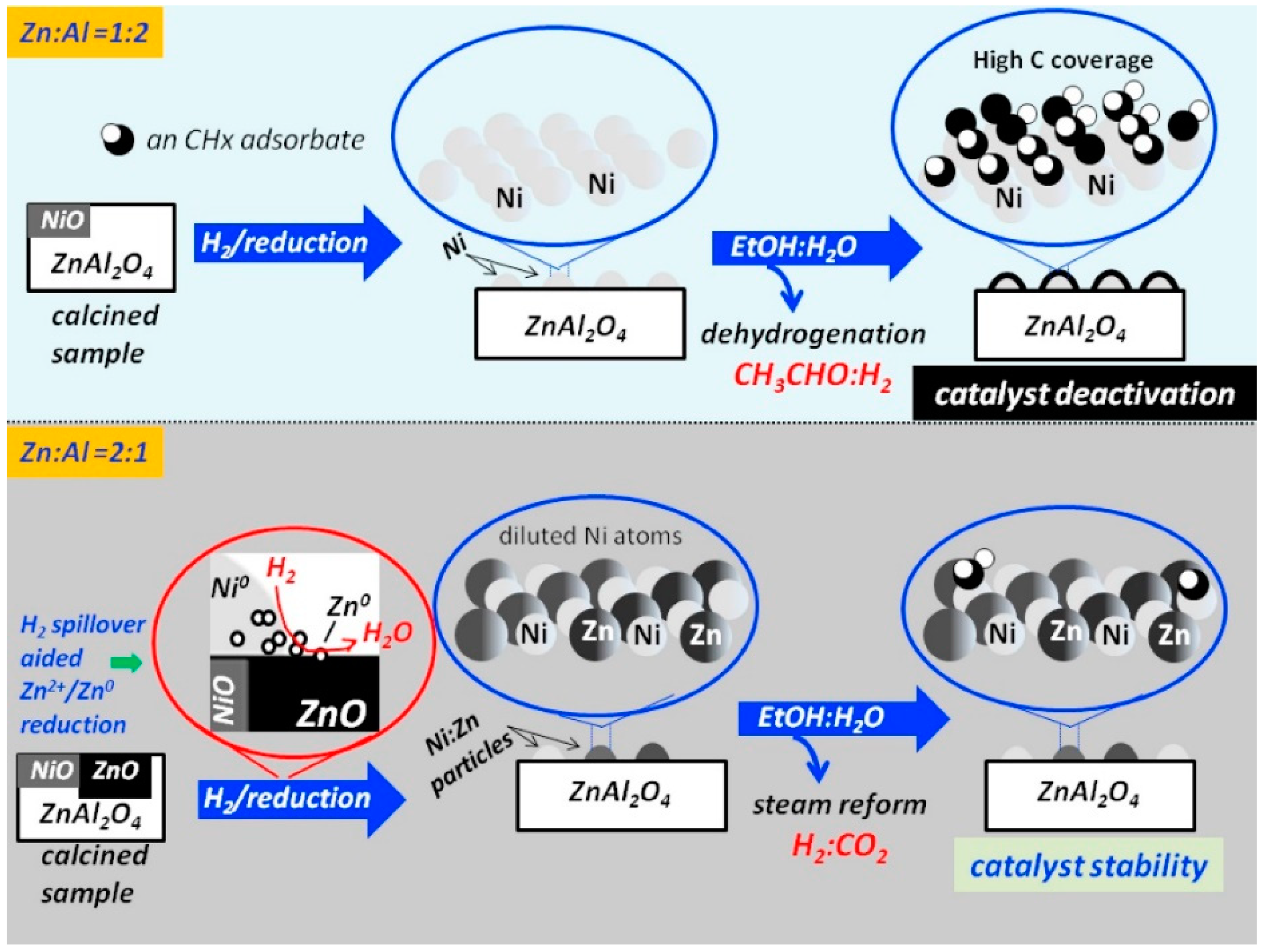
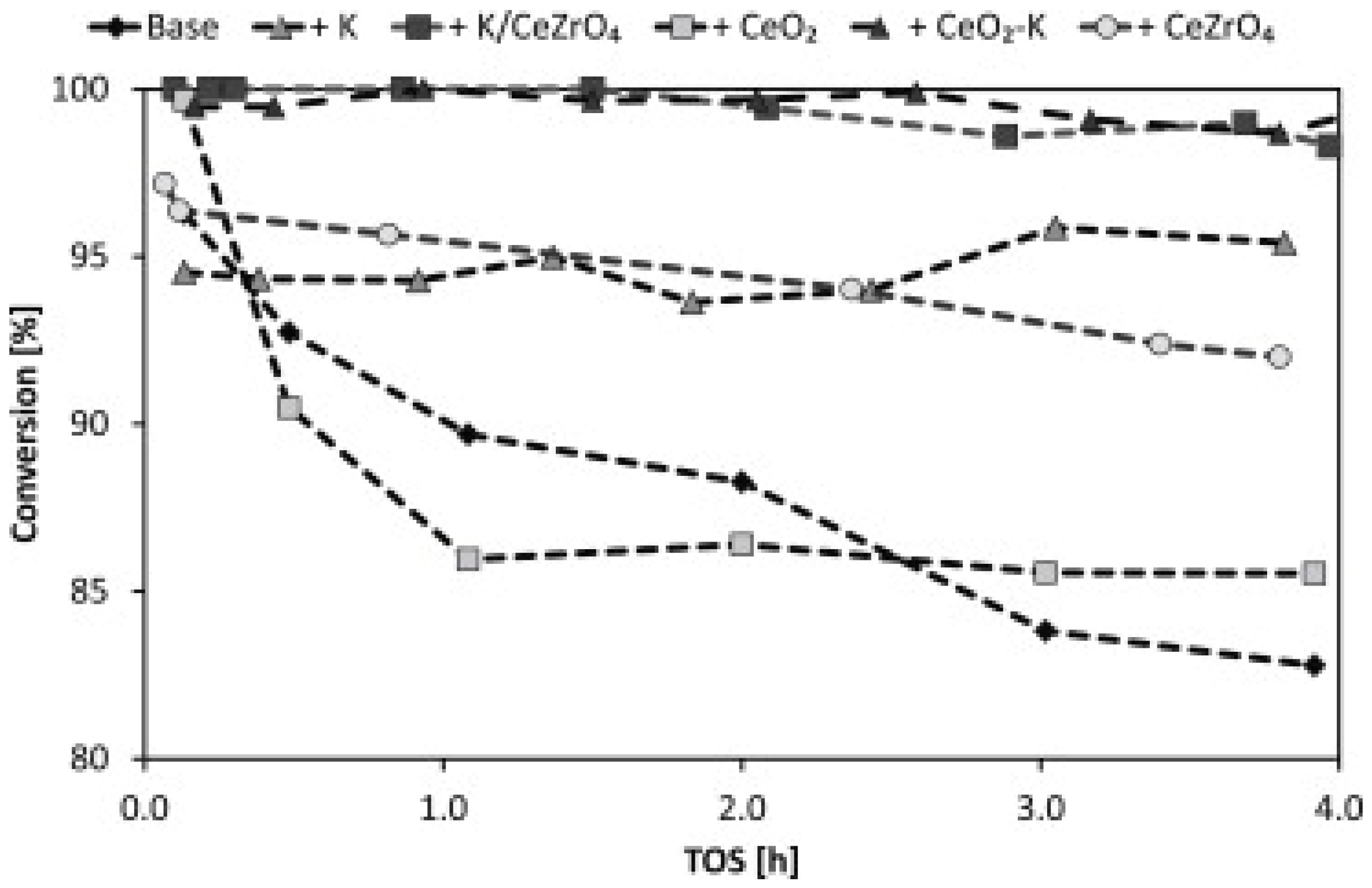
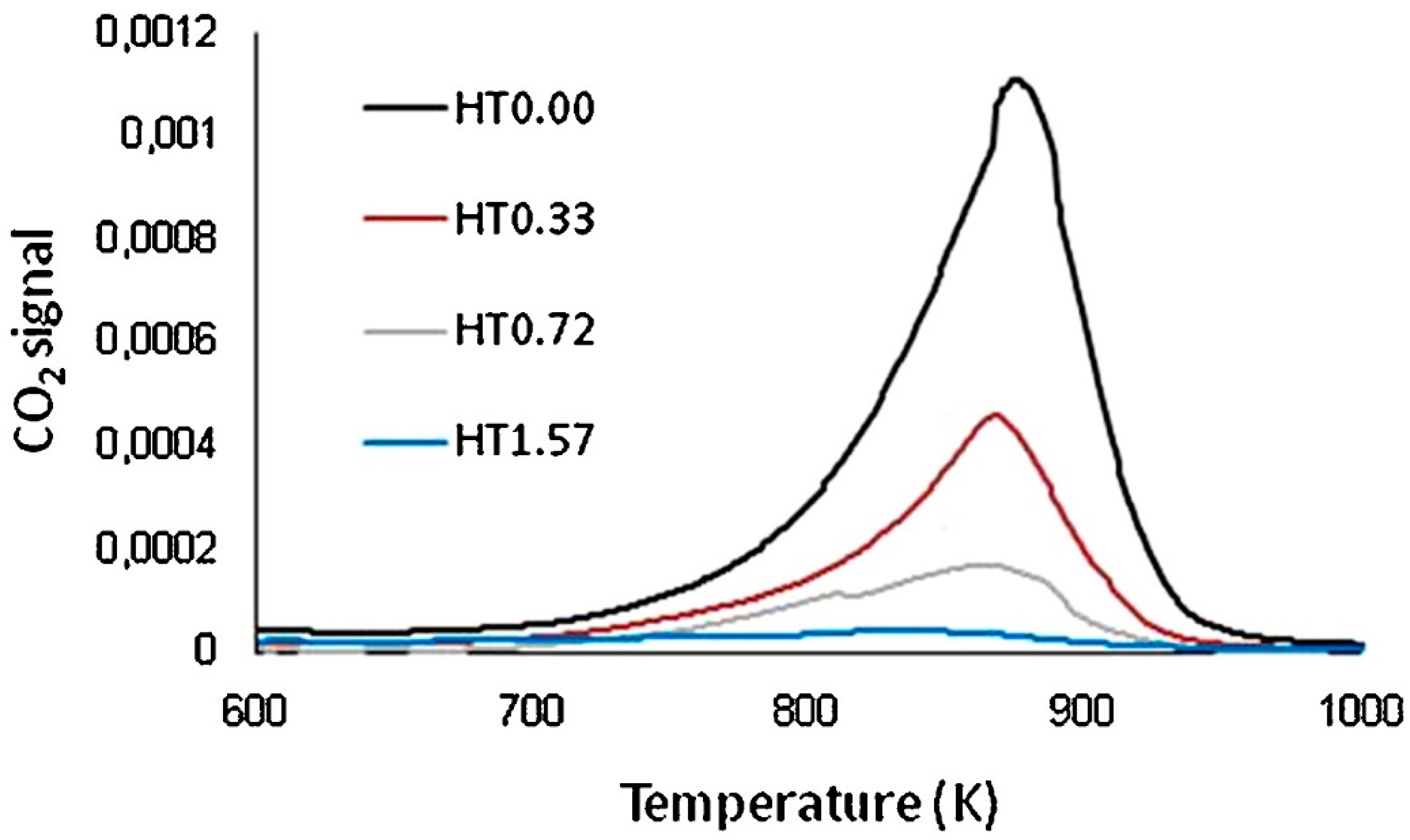


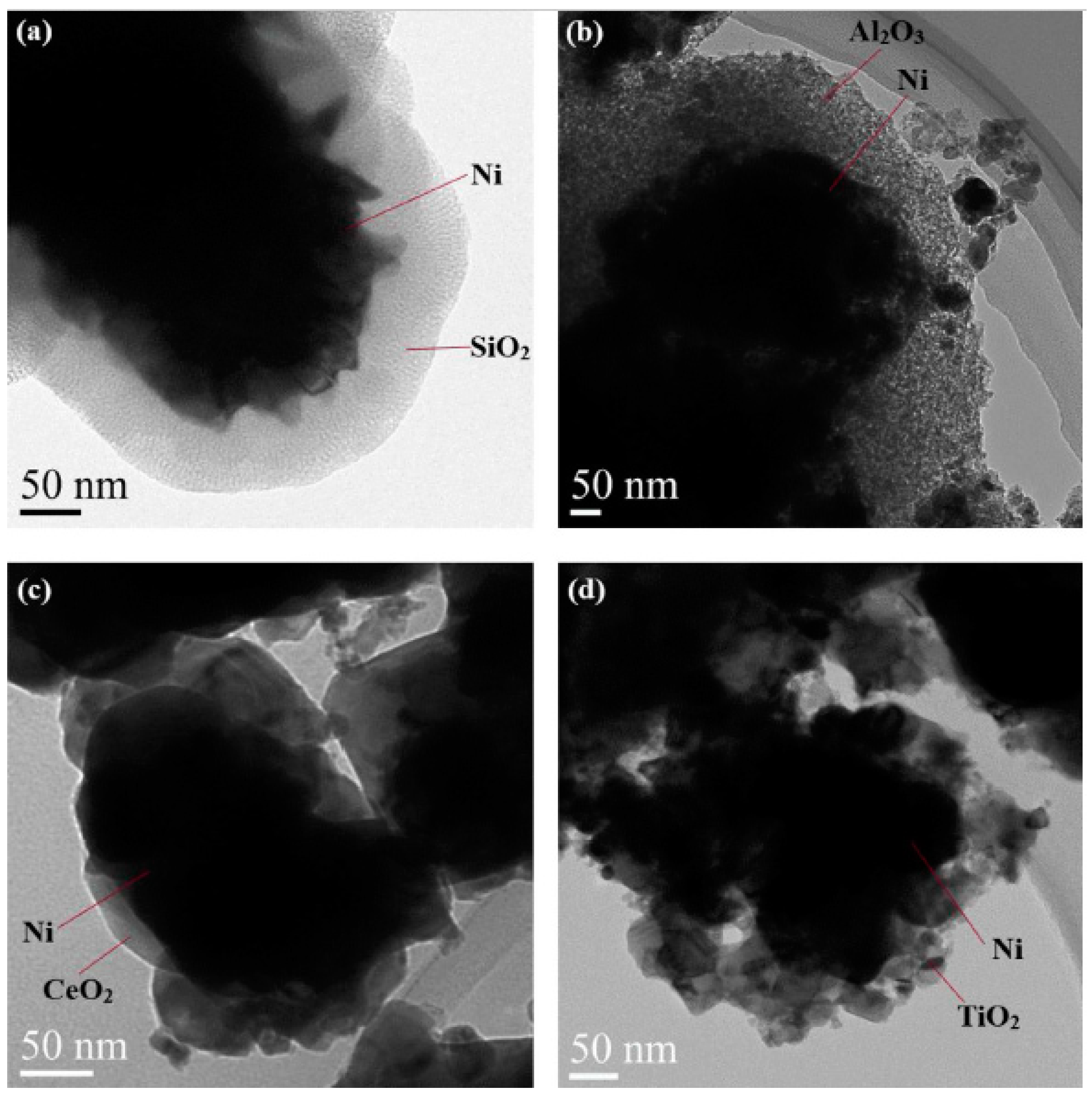
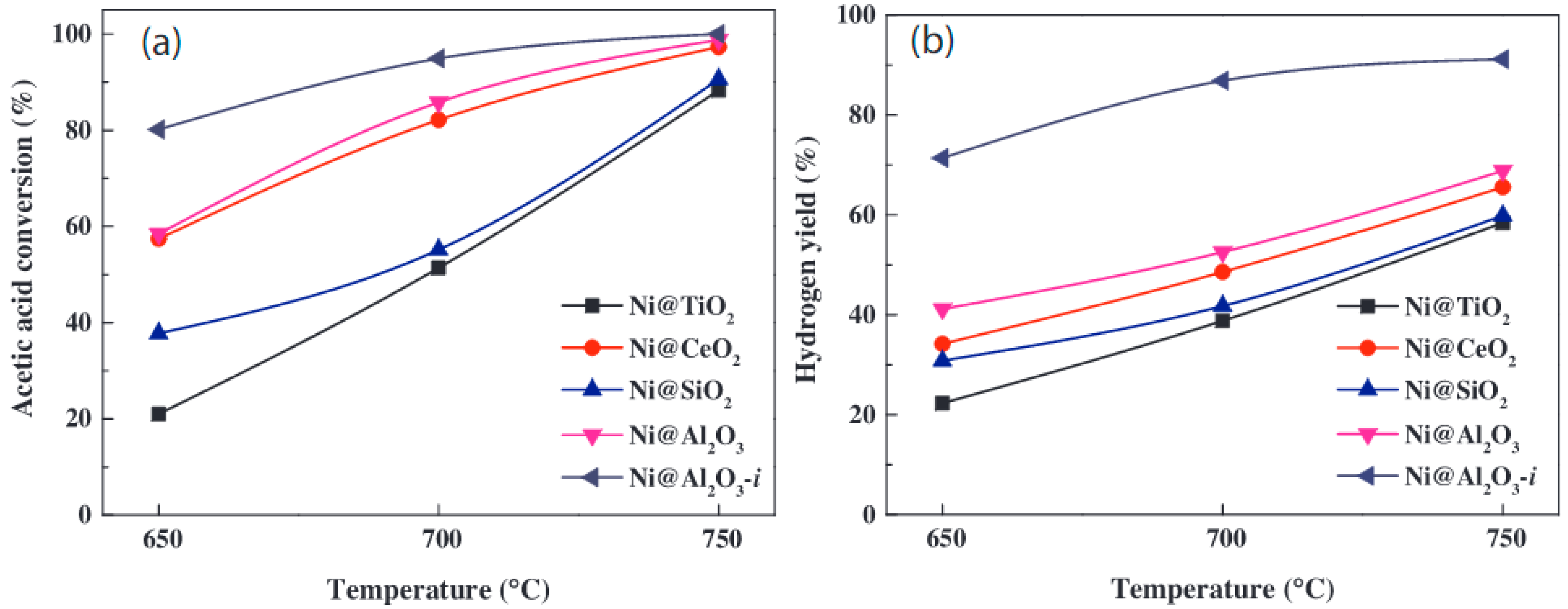

| Catalyst | Temperature | Feed Flow Rate | Mass of Catalyst | S/C a | Conversion of Methanol | H2 Yield/Selectivity | Stability | Preparation Method | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 5 wt% Ni-5 wt% Cu/Al2O3 | 325 °C | 0.06 mL·min−1 | 3 g | 1.7 | 98.5% | 2.2 b/n.d. | Result after 3 h | Impregnation | [43] |
| 10.8 wt% Ni-Cu/TiO2/Monolith | 300 °C | 1.8 h−1 c | n.d. | 2 | 92.6% | n.d./92.7% g | n.d. | Impregnation | [49] |
| Ni/Cu/ZnO/Al2O3 (22.5/22.5/45/10) d | 350 °C | 150,000 mL·g−1·h−1 | 0.031 g | 1 | 100% | ≈83.3% f/n.d. | n.d. | Coating and impregnation | [52] |
| Ni0.2-Cu0.8/ZrO2 (Metals/Carrier = 0.2/1) | 325 °C | n.d. | 0.3 g | 1 | ≈100% | ≈66.7% f/n.d. | n.d. | Sequential impregnation over support prepared via precipitation | [53] |
| Ni0.2-Cu0.8/Ce0.1Zr0.9O2 (Metals/Carrier = 0.2/1) | 350 °C | 172 h−1 e | 0.3 g | 1 | ≈86% | n.d./ ≈ 99.9% g | 90 h without deactivation | Sequential impregnation over support prepared via co-precipitation | [57] |
| 10 wt% Ni-10 wt% La/Al2O3 | 350 °C | 0.02 mL·min−1 10,920 h−1 e | 0.2 g | 3 | 100% | n.d./ ≈ 69% g | n.d. | Co-incipient wetness impregnation | [61] |
| 10 wt% Ni/TiO2 | 350 °C | 2838 h−1 e | n.d. | 3 | ≈86% | n.d./ ≈ 97% g | n.d. | Facile one-step synthesis | [36] |
| 10 wt% Ni nanoparticles/15 wt% CeO2-10 wt% MgO-Al2O3 | 350 °C | 8000 mL·g−1·h−1 | n.d. | 2 | ≈ 66% | 66.7%/n.d. | Result after 24 h (no significant deactivation) | Polyol and support via co-impregnation | [41] |
| NiAl-LDH (Ni/Al = 4.9) | 390 °C | 0.05 mL·min−1 | 0.15 g | 1 | 94.6% | 70% f/n.d. | Result after 100 h (no significant deactivation) | Co-precipitation | [64] |
| 3wt% K/Ni0.78Al0.16(OH)2(CO3)0.15·0.66H2O | 390 °C | 0.05 mL·min−1 | 0.15 g | 1.2 | 91% | 80% f/n.d. | Result after 60 h (no significant deactivation) | Co-precipitation | [65] |
| (30 wt% Ni)-Cu/Al2O4 | 300 °C | 1 mL·min−1 | 30 g | 1.5 | ≈100% | 98% f/n.d. | Result after 30 h (no significant deactivation) | Impregnation | [58] |
| Category | Biomass |
|---|---|
| Sugar sources | Sugarcane |
| Sugar beet | |
| Sweet sorghum | |
| Cane | |
| Molasses | |
| Beet molasses | |
| Grape | |
| Dates | |
| Watermelon | |
| Apple | |
| Starch sources | Corn |
| Wheat | |
| Cassava | |
| Barley | |
| Canna | |
| Sorghum grain | |
| Potato | |
| Sweet potato | |
| Yam | |
| Jerusalem artichoke | |
| Iles-iles | |
| Oat | |
| Banana | |
| Lignocellulosic biomass | Perennial grasses |
| Aquatic plants | |
| Softwood | |
| Hard wood | |
| Sawdust | |
| Pruning | |
| Bark thinning residues | |
| Cereal straws | |
| Stovers | |
| Bagasse | |
| Organic municipal solid wastes |
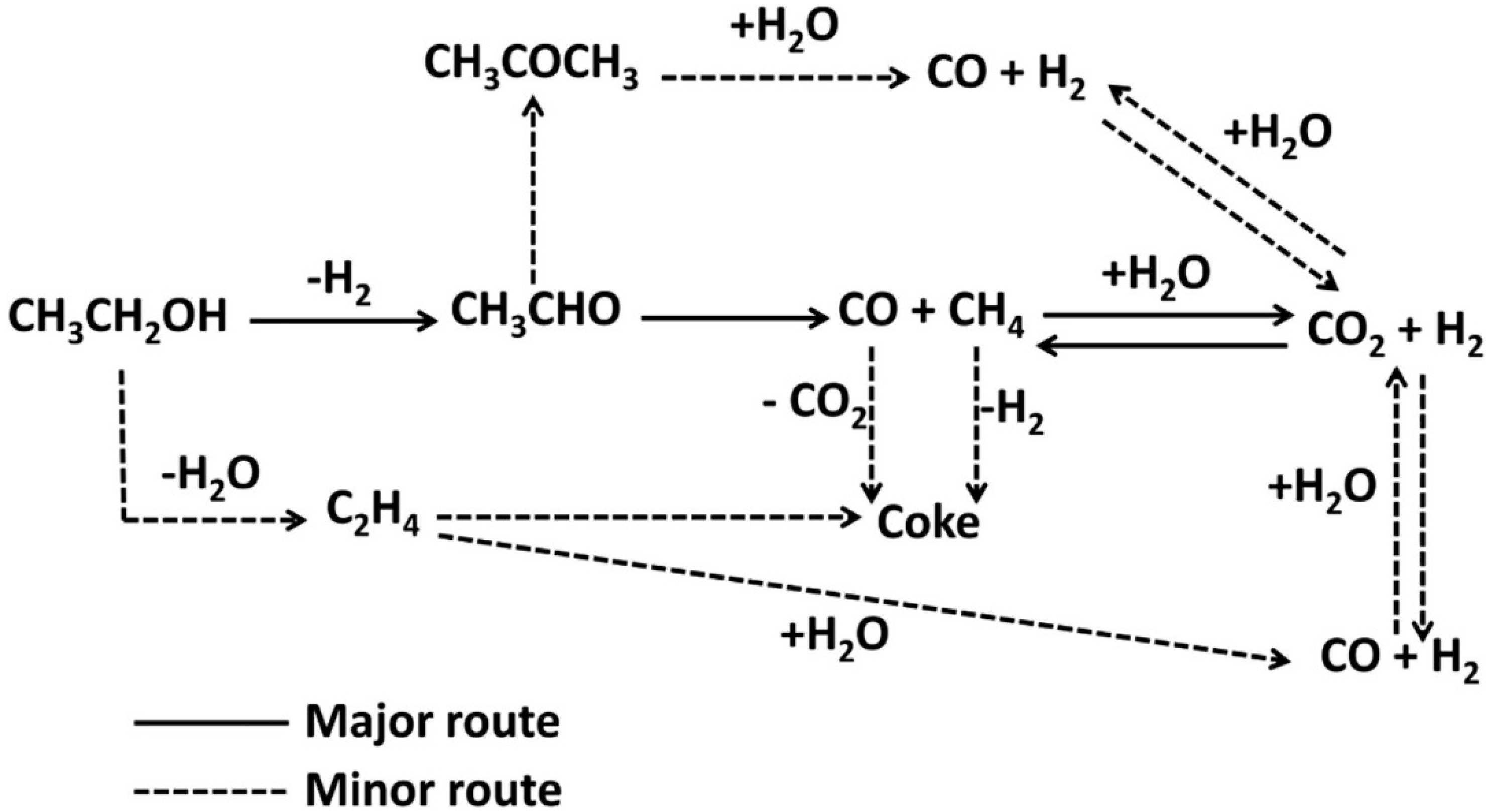
| Type | Reaction | Eq. Number | |
|---|---|---|---|
| Major | (ethanol dehydrogenation to acetaldehyde) | 68 | |
(acetaldehyde decomposition) | - | ||
(methane steam reforming; the reverse of Equation (4)) | 206 | ||
| Minor | (ethanol dehydration to ethylene) | 45 | |
(ethylene polymerization to coke) | - | ||
(acetaldehyde condensation into acetone and subsequent decarboxylation) | - | ||
(Boudouard reaction) | −172 | ||
(reduction of carbon monoxide) | −131 | ||
(methane cracking) | 75 |
| Catalyst | Temperature | Feed Flow Rate | Mass of Catalyst | S/C a | Conversion of Ethanol | H2 Yield/Selectivity | Stability | Preparation Method | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 15 wt.% Ni/zeolite | 550 °C | 0.05 mL·min−1 | 0.1 g | 6 | ≈100% | 76% b/n.d. | Result after 27 h (no significant deactivation) | Sol-gel + Impregnation | [103] |
| 10 wt.% Ni/CNTs–SF | 450 °C | 8 gcat·h·mol−1 | n.d. | 9 | ≈100% | 40% b/n.d. | Result after 22 h (no significant deactivation) | Sol-gel + Impregnation | [97] |
| Ni/Al-0.1La | 450 °C | 23,140 mL·h−1 gcat−1 c | 0.1 g | 6 | 100% | 124% b/n.d. | Result after 15 h (no significant deactivation) | Epoxide-initiated sol-gel + Impregnation | [92] |
| 15 wt.% Ni/Y-ZrO2 | 650 °C | 41,000 h−1 c | 0.1 g | 4.5 | 100% | 91% b/74% d | Result after 8 h (no significant deactivation) | Sol-gel | [101] |
| 13 wt.% Ni-4 wt.% Cu/CeO2 | 600 °C | 20,00 mL·h−1 gcat−1 c | 0.3 g | 6 | ≈100% | n.d./70 d | Result after 20 h (no significant deactivation) | Impregnation | [102] |
| Component | [wt.%] |
|---|---|
| Water | 20–23 |
| Acids | 3–22 |
| Sugars | 4–9 |
| Phenols | 3–4 |
| Lignin | 2–18 |
| PAH (a) | 8 [ppm] |
| Others (b) | 2–21 |
| Catalyst | Temperature | Feed Flow Rate | Mass of Catalyst | S/C a | Conversion | H2 Yield/Selectivity | Stability | Preparation Method | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 3.5 wt.% Ni/5 wt.% La2O3-ZrO2 1 | 700 °C | 240,000 h−1 b | 0.050 g | 5 | 100 | 87 c/64 d | Lost 7% of H2 yield in 20 h | Impregnation | [194] |
| (2.5+2.5) wt.% Ni-Cu/Al2O3 1 | 750 °C | 28 h−1 e | 0.1 g | 1.25 | 100 | 67 c/57 d | Result of 7.5 h (no deactivation) | Impregnation over support prepared by following evaporation-induced-self-assembly | [243] |
| 15 wt.% Ni/α-Al2O3 1 | 600 °C | 20 h−1 e | 1.5 g | 1 | ≈100 | 90 c/66 d | n.d. | Impregnation | [196] |
| 10 wt.% Ni/La2O3-Al2O3 1 (La2O3/Al2O3 = 1:3, weight ratio) | 700 °C | 10 h−1 f | 0.2 g | 2.5 | 100 | 73 c/59 d | Result of 30 h (no deactivation) | Co-precipitation | [233] |
| 15 wt.% Ni/Al2O3 1 | 700 °C | 7400–10,000 h−1 b | 0.2 g | 1 | 100 | 57 c/54 d | n.d. | Incipient wetness impregnation | [244] |
| 5.5 wt.% Cu–2.5 wt.% Ni/MgAl2O4 1 | 450 °C | 9 h−1 e | 0.1 mg | 2 | 100 | 83 c/63 d | n.d. | Impregnation | [223] |
| (6.6+10) wt.% Ni-Fe/(CeO2)0.4-PG0.6 1 | 600 °C | 14,427 h−1 f | n.d. | 3 | ≈93 | 85 c/63 d | Result of 20 h (no deactivation) | Co-precipitation | [216] |
| Ni(NO3)2/Al2O3 1 | 600 °C | 12.7 h−1 f | 0.5 g | 5 | ≈100 | 77 c/71 d | n.d. | Incipient wetness impregnation | [242] |
| Ni/ABC 1 | 700 °C | 10 h−1 f | 0.15 g | 2.5 | 91.2 | 71 c/61 d | n.d. | Impregnation | [203] |
| 10 wt.% Ni/ATTP 1 | 600 °C | 7.2 h−1 f | 0.5 g | 5 | ≈100 | 75 c/65 d | n.d. | Impregnation | [202] |
| (10+1) wt.% Ni-Ru/SiO2 2 | 400 °C | n.d. | 0.65 g | 694 | ≈100 | 84 c/68 d | Lost 10% of H2 yield in 20 h | Co-precipitation | [225] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; Rocha, C.; Soria, M.A.; Madeira, L.M. Catalytic Steam Reforming of Biomass-Derived Oxygenates for H2 Production: A Review on Ni-Based Catalysts. ChemEngineering 2022, 6, 39. https://doi.org/10.3390/chemengineering6030039
Silva J, Rocha C, Soria MA, Madeira LM. Catalytic Steam Reforming of Biomass-Derived Oxygenates for H2 Production: A Review on Ni-Based Catalysts. ChemEngineering. 2022; 6(3):39. https://doi.org/10.3390/chemengineering6030039
Chicago/Turabian StyleSilva, Joel, Cláudio Rocha, M. A. Soria, and Luís M. Madeira. 2022. "Catalytic Steam Reforming of Biomass-Derived Oxygenates for H2 Production: A Review on Ni-Based Catalysts" ChemEngineering 6, no. 3: 39. https://doi.org/10.3390/chemengineering6030039
APA StyleSilva, J., Rocha, C., Soria, M. A., & Madeira, L. M. (2022). Catalytic Steam Reforming of Biomass-Derived Oxygenates for H2 Production: A Review on Ni-Based Catalysts. ChemEngineering, 6(3), 39. https://doi.org/10.3390/chemengineering6030039









