1. Introduction
Economic and social evolution relies extremely on energy. The global energy crisis and rising awareness of the vitality of environment preservation, are the originators behind the development and exploration of renewable energy to act as a replacement for non-renewable energy which is facing rapid depletion. Currently, noteworthy attention has been directed towards biofuels as a renewable energy resource. Biodiesel, which mostly comprises fatty acid methyl esters, possesses beneficial qualities such as low sulfur content, low toxicity, and low carbon dioxide and carbon monoxide emissions along with being biodegradable and renewable [
1,
2].
The production process of biodiesel could be converted to being mostly green through the employment of wastes. As a demonstration, wastes could be utilized in two different forms in the process which are as follows; at first, the utilization of waste cooking oil which refers to second-hand vegetable oil as a feedstock. It was proposed that waste cooking oil would act as an efficient, cost-effective, available feedstock along with offering an environmental advantage along with an economical one owing to the 60–70% reduction in feedstock costs which represents 70–95% of total production cost [
3,
4,
5,
6].
Several studies were conducted to confirm the viability of such a theory. A study was conducted by Sahar et al. where a waste cooking oil was utilized in a trans-esterification reaction in the presence KOH as an alkali catalyst to produce biodiesel. The yield of Fatty acid methyl ester attained was about 94% in the presence of a 1% catalyst at a temperature 50 °C. The characterization results confirmed the possibility of employing waste cooking oils in biodiesel production based on ASTM standards [
7]. Moreover, another study was performed by da Silva et al. which affirmed and used the waste cooking oil as a raw material to produce high-quality biodiesel and to provide a feasibility condition to use the residual glycerol [
8].
At present, the production of biodiesel is undergone in the presence of homogeneous catalysts such as potassium and sodium hydroxide due to their availability and feasibility. However, the total process cost had suffered a significant increase due to the major limitations it possesses which can only be minimized through the utilization of a heterogeneous catalyst. Heterogeneous catalysts are known to be non-corrosive, ecological, with superior selectivity, and activity, separated with ease from liquid products and minimal problems in the disposal. Furthermore, there are numerous types of heterogeneous catalysts such as acids, bases, and enzymes [
9,
10,
11].
Many researchers focused their efforts on the employment of industrial and municipal wastes as heterogeneous catalysts for biodiesel. The employment of waste-derived catalyst which could be industrial, or municipal is designated as being highly advantageous as it introduces the conversion of wastes that are readily available and requires disposal to a significant asset for biodiesel production and thus, achieving solid waste management and economic efficiency due to its low cost along with being environmentally friendly. These materials are readily available and constitute some active metal oxides such as CaO and MgO making them an appealing option [
12].
There were various research and studies concerning the employment of waste-derived catalysts. At first, the electric arc furnace dust solid waste which is significantly hazardous was analyzed in a study constructed by Khodary et.al. This study examined and confirmed the economical production of biodiesel from sunflower oil in the presence of the aforementioned solid waste as a heterogeneous catalyst with bearing mind that this catalyst is composed mainly of oxides specifically ZnO, CaO, Fe
2O
3 and SiO
2, and the optimum biodiesel yield was 96% at conditions of 20:1 methanol: oil molar ratio, 1 h as a reaction time, 57 °C as a reaction temperature and 5% catalyst loading [
1]. Another solid waste was employed as a heterogeneous catalyst for biodiesel production which was waste iron filling. Ajala et al. analyzed the utilization of solid waste in the production process of biodiesel from waste cooking oil. The waste iron filling was utilized in synthesizing α-Fe
2O
3 through co-precipitation into acidic solid catalysts and achieved a yield of biodiesel 87, 90, and 92% respectively at conditions of 12:1 methanol: oil molar ratio, 3 h as a reaction time, 80 °C as a reaction temperature and 6% catalyst loading [
13]. Furthermore, Rasouli & Esmaeili established a study that examined biodiesel production by transesterification of goat fat in the presence of a magnesium oxide (MgO) nano-catalyst at a temperature of 70 °C, a methanol/oil molar ratio of 12:1, a catalyst content of 1 wt. percent and a reaction period of 3 h, the maximum biodiesel yield of 93.12 percent was attained [
14].
The ductile cast iron industry is a prosperous industry where the manufacturing had seen rapid growth due to ductility, elevated strength, and impact toughness in comparison to other steel grades, corrosion, and wear resistance because of graphite morphology modification which involves pure or an alloy of magnesium addition converting lamellar to a globular shape when crystallized. Initially, the core wire technique is defined as a graphite morphology modification method of simple mannerism where the core wire is injected into molten cast iron. The difficulty arises in magnesium’s reaction with molten iron due to Mg’s lower boiling point leading to spontaneous MgO fumes release and low yield in adsorption. The aversion to air pollution through a collection of dust formed by filtration leads to solid waste creation which requires handling to avert risks of land contamination and respiratory diseases [
15].
This paper examines the utilization of ductile cast iron solid waste as a heterogeneous catalyst in a trans-esterification reaction to produce biodiesel using optimum, low energy, and economic process. This research examines biodiesel production using waste cooking oil, and ductile cast iron solid wastes which are considered dangerous materials to the environment so this research will have environmental benefit in addition to the economic benefit because using waste materials as a replacement for raw materials.
2. Methodology for Research
2.1. Raw Materials
The materials used in this research are described as follows:
- (a)
Ductile cast iron supplied from Cairo Great Foundries, Cairo, Egypt.
- (b)
Methanol 99% was supplied by Morgan Chemical company Ltd., Cairo, Egypt.
- (c)
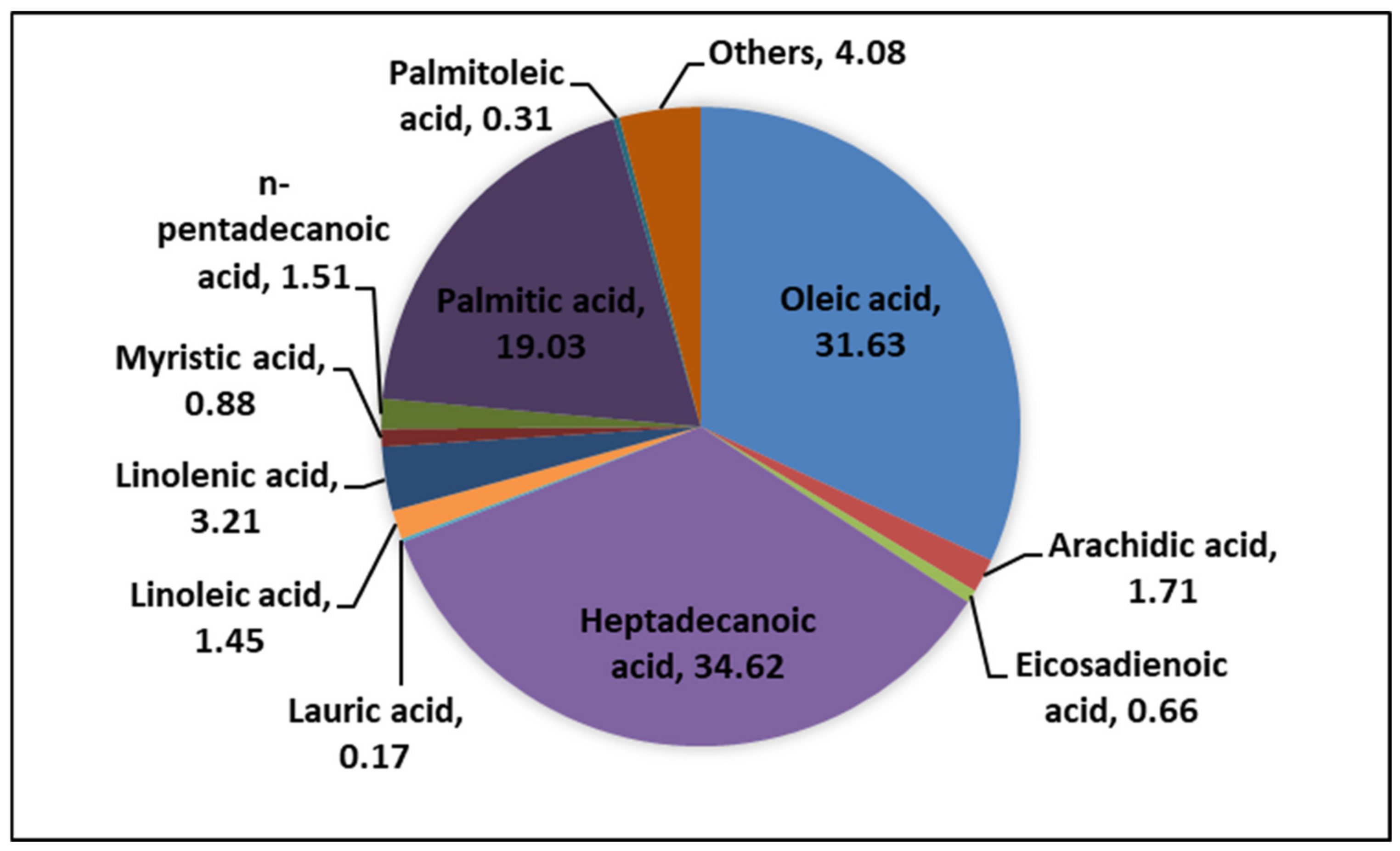
Sunflower waste cooking oil provided (SFWCO) by Egyptian restaurants and cafes which is characterized by chemical and physicochemical properties as shown in
Figure 1 and
Table 1. The method used for determining the Physicochemical properties of oil was mentioned in Roushdy [
16].
2.2. Solid Waste Preparation
The solid molds were collected from the ductile cast iron factory from the dust accumulated around the furnace that was used to produce the ductile cast iron using the core wire technique.
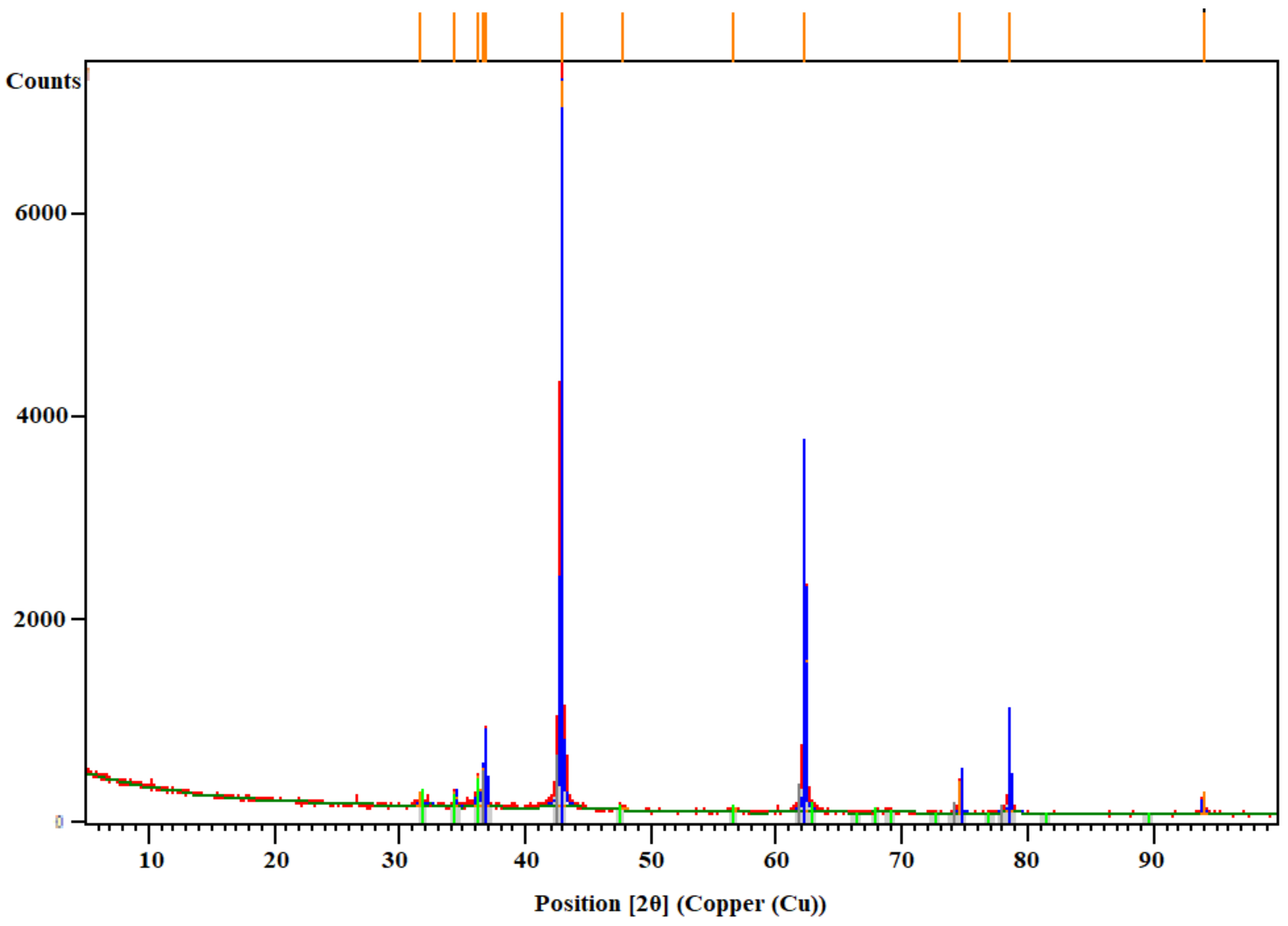
2.3. Assessment of Solid Waste
The used characterization methods for the solid waste are described in the
Table 2.
2.4. Collection and Preparation of Waste Sunflower Cooking Oil
Sunflower waste cooking oil (SFWCO) was a discarded item in many households. A centrifuge and filter were used to remove any suspended particulates, fried food particles, and other pollutants, and it was then dried at 105 °C for two hours to eliminate the water.
2.5. Experimental Work Done to Produce Biodiesel
The experimental step that was used for biodiesel production as shown in
Figure 2 can be described as follow:
Round bottom flask was used as a batch biodiesel reactor
Magnetic stirrer on which the biodiesel reactor is put. This stirrer is used for providing a good reaction mixing.
Heater that is provided with the stirrer to provide the required reaction temperature for the transesterification reaction.
Thermometer is used to measure the reaction temperature
A reflux fitted with the batch reactor to prevent methanol escape by condensation.
The oil, methanol, and the solid catalyst were added to the batch biodiesel reactor taking into consideration the required catalyst percentage and methanol to oil ratio than the reaction temperature was adjusted, and the reaction timer started and adjust for a certain time. When the reaction ended the solid catalyst was removed by the filter media and then glycerol was separated from the resulted biodiesel using a separating funnel finally the excess methanol was removed using 80 °C, 30 min drying. The biodiesel conversion was calculated by the weight ratio between the resulted biodiesel and the used SFWCO.
2.6. Experimental Design
The surface methodological technique (RSM) was utilized to design the experimental work, and a detailed analysis of the process was generated using Design-Expert version 13 [
20]. The process response is the conversion of biodiesel and glycerol while the reaction variables are shown in
Table 3.
Based on reaction parameters used by Rasouli & Esmaeili in their paper, the processing parameters and ranges were chosen [
14]. Thirty experimental runs were generated by the design expert program [
22] using the central composite design technique (CCD) as shown in
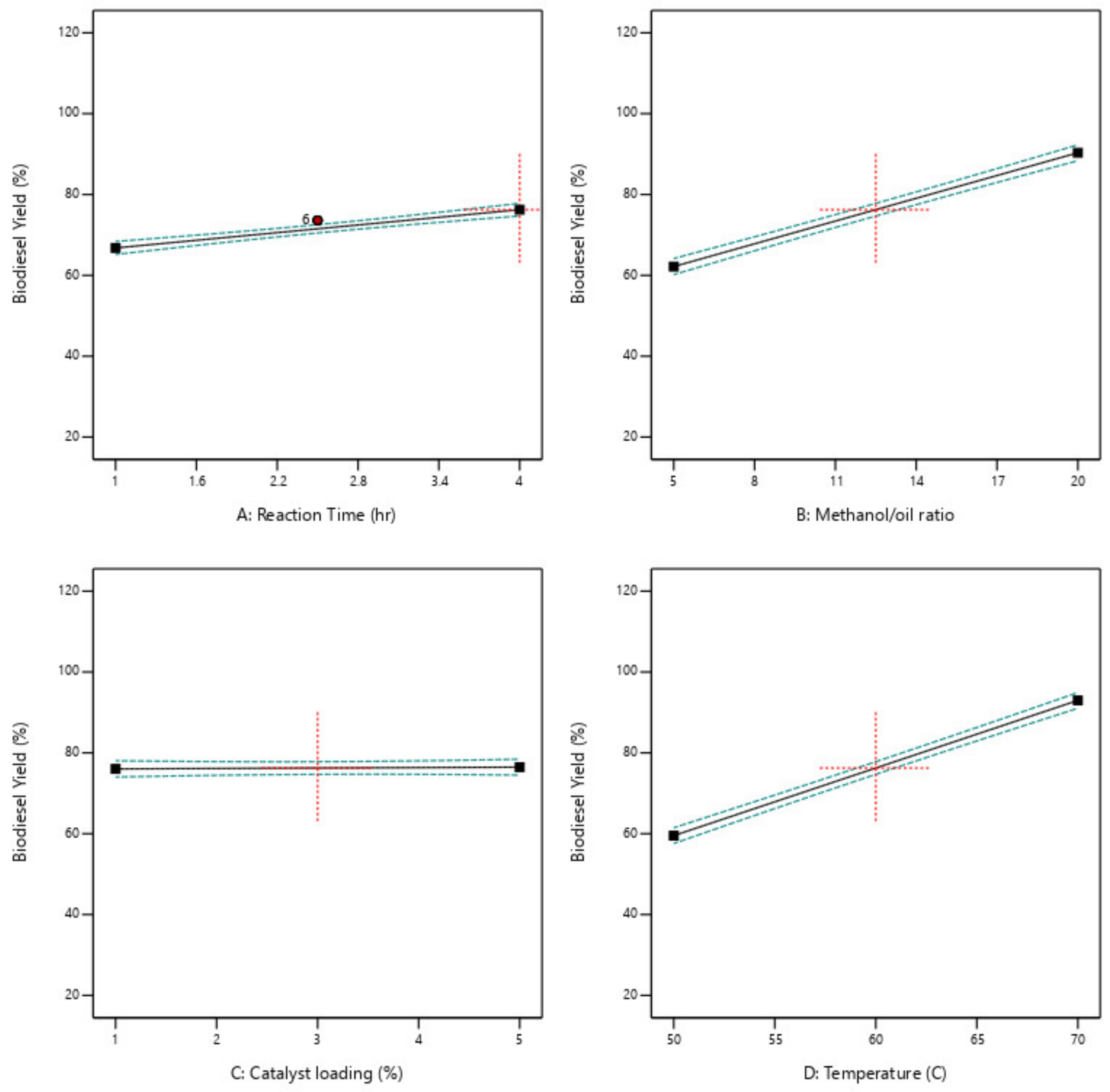
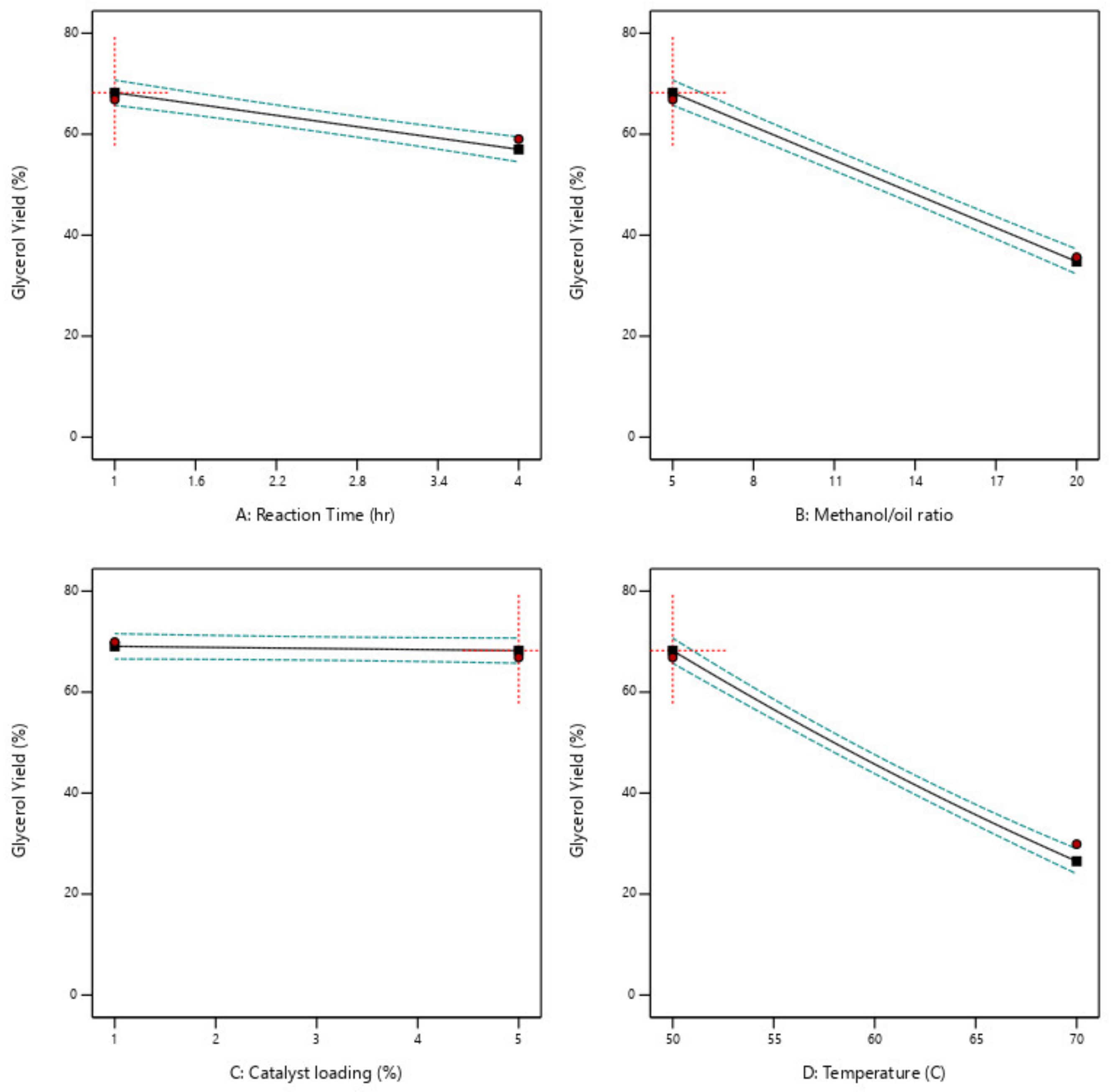
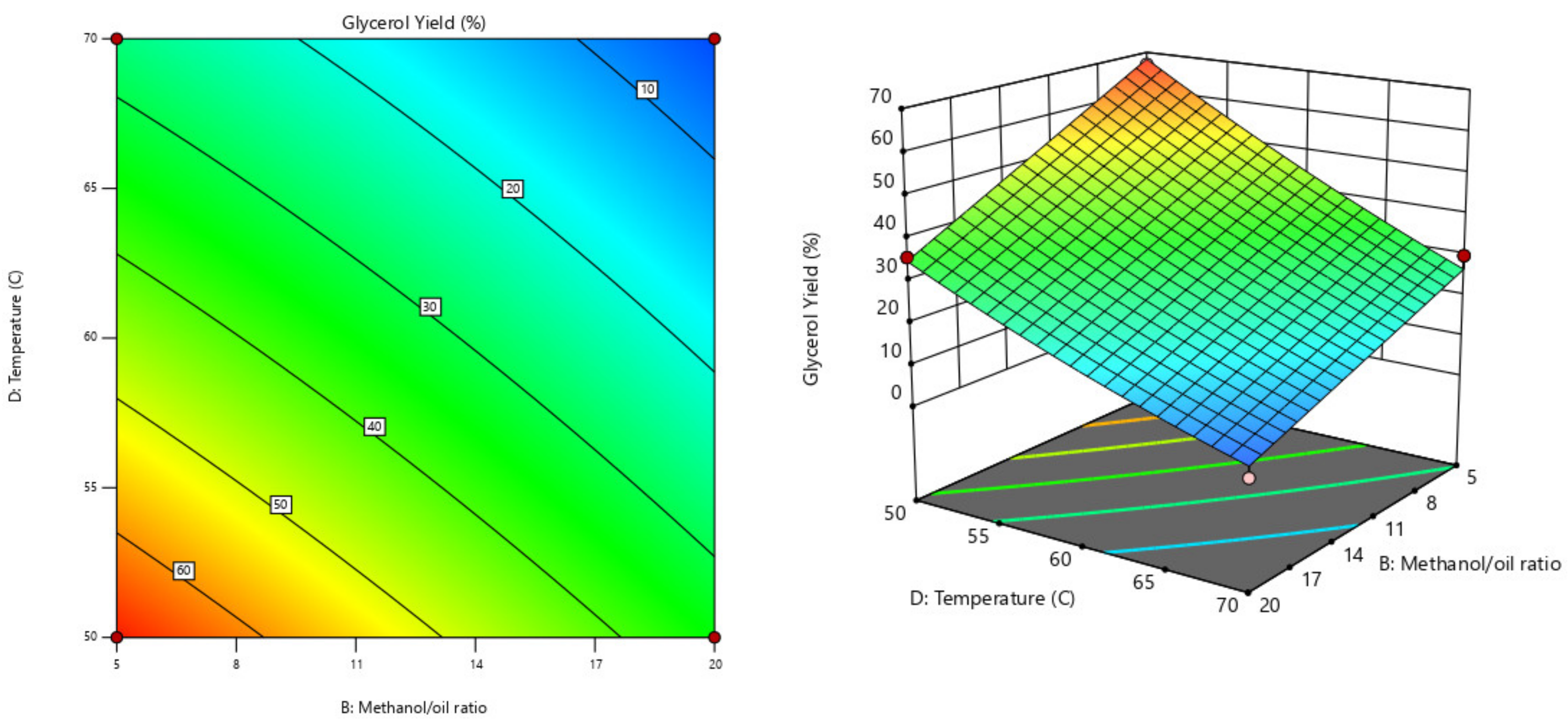
Table 4. The conditions in experimental runs 25 to 30 represent the design center point. The optimization process was done based on the economic purpose to maximize biodiesel production while minimizing production cost. This target was reached by minimizing both reaction time and temperature, maximizing the biodiesel production rate, and minimizing the glycerol production rate.
2.7. Optimum Biodiesel Sample Analysis
Two important tests must be done to make sure that the resulted product is biodiesel and complied with the standards required. The first test is gas chromatography (GC) which determines the amount of total fatty acid methyl ester (FAME), glycerol, and triglycerides in the biodiesel sample and compared it with the standards EN 14103 [
23] and EN 14105 [
24]. The second test is physicochemical determination and compares their results with the standards ASTM D6751 [
25] and European Biodiesel Standard, EN 14214 [
26].
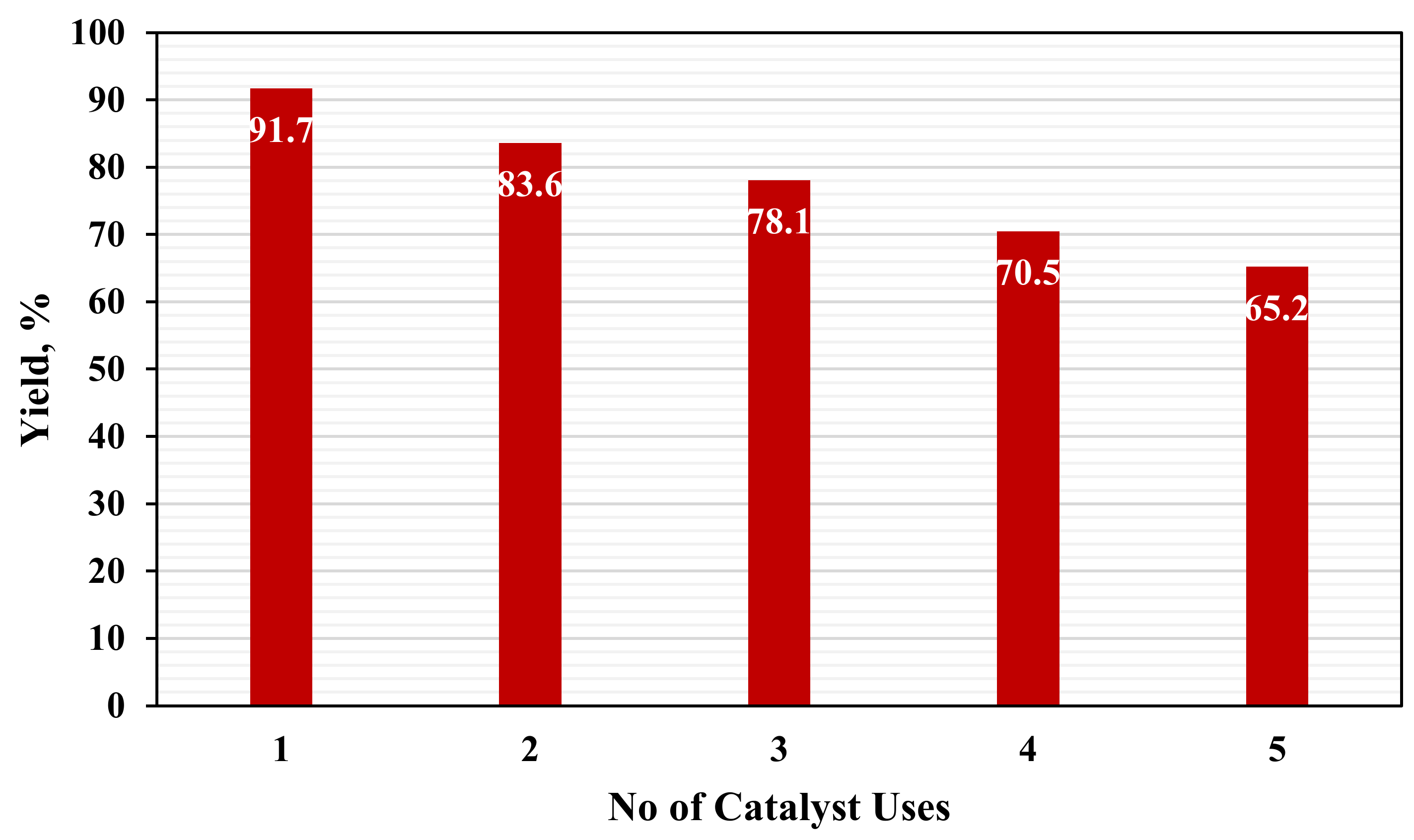
2.8. Reusability Test of Biodiesel Catalyst
A reusable test was done using two methods under the resulted optimum conditions. Once the reaction ended the reaction product was filtered to remove the heterogeneous catalyst. The used method can be summarized in
Table 5. The reaction conversion was calculated at the point of reuse to determine the catalyst efficiency and strength.
5. Conclusions
This paper examined the utilization of ductile cast iron solid waste as a heterogeneous catalyst in a trans-esterification reaction to produce biodiesel using optimum, low energy, and economic process. This research examined biodiesel production using waste cooking oil, and ductile cast iron solid wastes which are considered dangerous materials to the environment, so this research has environmental benefit in addition to the economic benefit because using waste materials as a replacement for raw materials. Four reaction parameters were chosen to determine their effect on the reaction responses. The reaction parameters are M:O ratio, reaction time and temperature, and catalyst loading. The reaction responses are the biodiesel and glycerol conversions. The design expert program was used in the analysis, models generation, and optimization. It generated 25 different experimental runs and determine the impact of each reaction parameter using resulted models, 2D graphs, 3D plots, and contour figures. Optimization was done with economic and environmental targets. 100 possible optimum solutions which include lowering the cost of biodiesel production, increasing the volume of biodiesel produced, and decreasing the amount of resulting glycerol. The optimum reactions are 20:1 M:O molar ratio, 65 °C reaction temperature, 5 wt% catalyst loading, 2 h reaction time, and a stirring rate of 750 rpm. The biodiesel conversion resulting at this optimum reaction conditions is 91.7 percent with agreed with all biodiesel standards. The catalyst usability test was done it was found the catalyst can be used up to 4 times after that a fresh catalyst is required to be used.