Esterification of Levulinic Acid to Methyl Levulinate over Zr-MOFs Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of MOF UiO-66 and UiO-66-NH2
2.3. Characterization of MOF UiO-66 and UiO-66-NH2
2.4. Catalytic Esterification Reaction
3. Results
3.1. Physicochemical Characterization
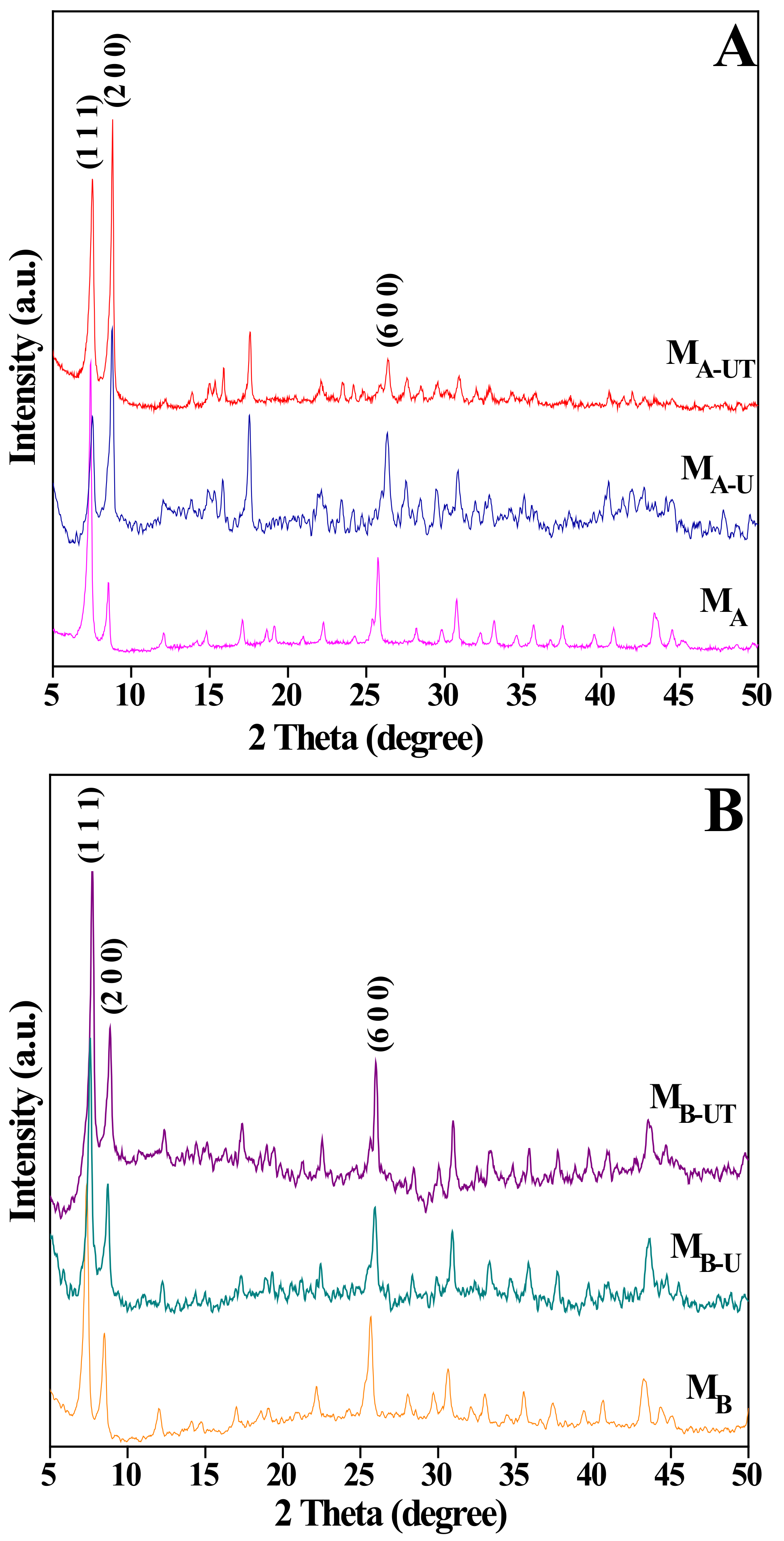
3.1.1. X-ray Diffraction
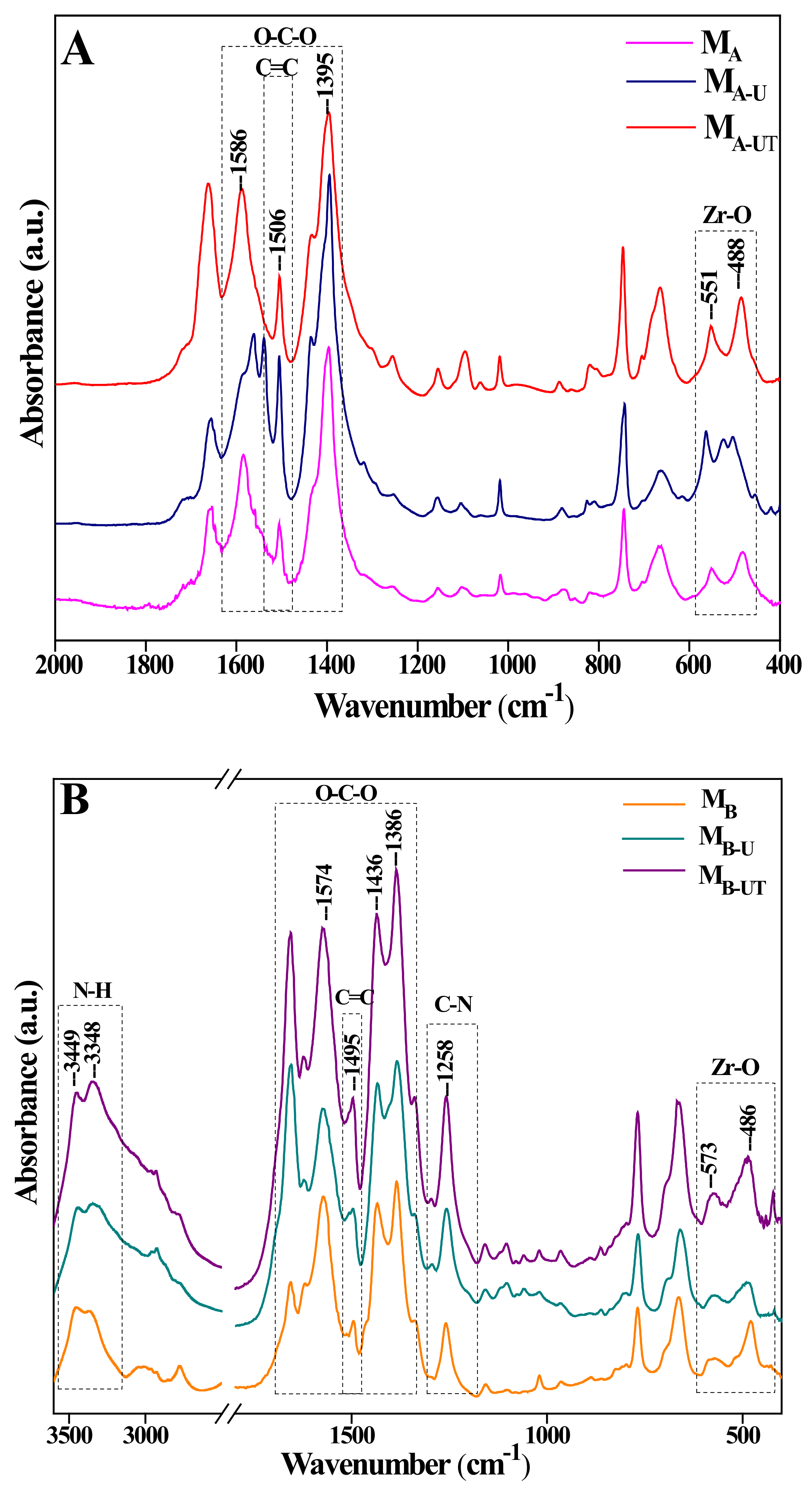
3.1.2. FTIR Spectroscopy
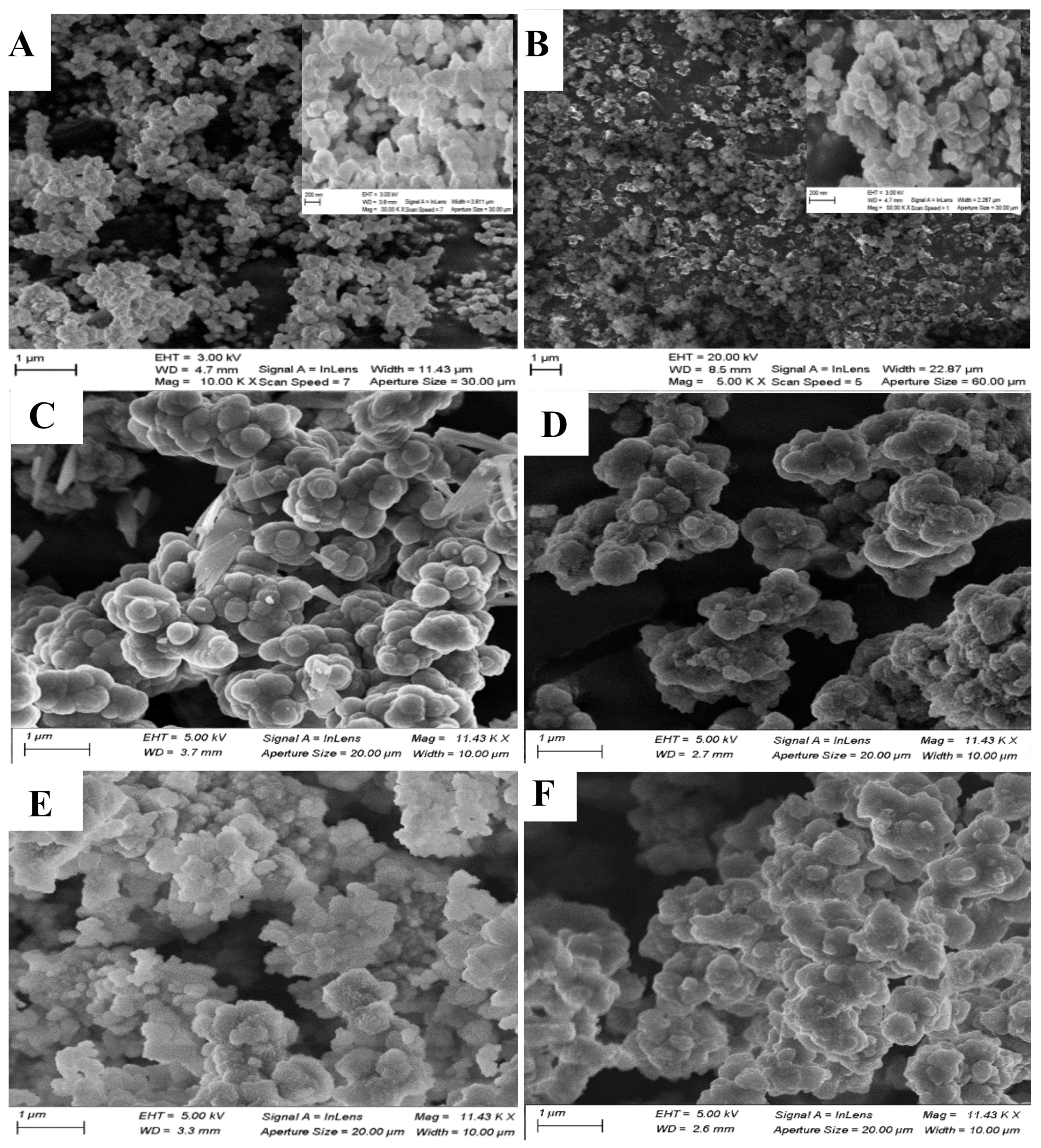
3.1.3. SEM-EDS Analysis
3.1.4. Microwave Plasma Atomic Emission Spectrometry (MP-AES) and the Surface Area by BET
3.1.5. XPS-Spectroscopy
3.1.6. Acid Analysis by FTIR-CO
3.2. Catalytic Activity: Esterification for Methyl Levulinate Production
3.2.1. Effect of Reaction Parameters on the LA Esterification with Methanol Catalyzed by MB-UT
Effect of Catalyst Loading
Effect of Temperature
Effect of Molar Ratio
3.2.2. Kinetic Model and Estimation of Kinetic Parameters
3.3. Esterification of Levulinic Acid to Methyl Levulinate in Pressure System, Study of the Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Guo, Y.; Liu, X.; Xia, Q.; Wang, Y. Catalytic conversion of lignocellulosic biomass into hydrocarbons: A mini review. Catal. Today 2019, 319, 2–13. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, G.P.; Ruiz, D.M.; Pasquale, G.A. Química de la Biomasa y los Biocombustibles; Editorial de la Universidad Nacional de La Plata (EDULP): Buenos Aires, Argentina, 2020. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Louis, H.; Akakuru, O.U.; Joseph, I.; Enudi, O.C.; Michael, D.P. A Review on the conversion of levulinic acid and its esters to various useful chemicals. AIMS Energy 2019, 7, 165–185. [Google Scholar] [CrossRef]
- Corma Canos, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Xiong, J.; Ji, N. Transformation of levulinic acid to valeric biofuels: A review on heterogeneous bifunctional catalytic systems. ChemSusChem 2019, 12, 3915–3930. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Pineda, A.; Balu, A.M.; Luque, R.; Campelo, J.M.; Romero, A.A.; Ramos-Fernández, J.M. Catalytic transformations of biomass-derived acids into advanced biofuels. Catal. Today 2012, 195, 162–168. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Recent advances in the value addition of biomass-derived levulinic acid: A review focusing on its chemical reactivity patterns. ChemCatChem 2021, 13, 3202–3222. [Google Scholar] [CrossRef]
- Omoruyi, U.; Page, S.; Hallett, J.; Miller, P.W. Homogeneous catalyzed reactions of levulinic acid: To Γ-valerolactone and beyond. ChemSusChem 2016, 9, 2037–2047. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.Y.; Ryu, G.H.; Choi, J.H.; Kim, J.H.; Choi, W.S.; Lee, S.M.; Choi, J.W.; Choi, I.G. Catalytic conversion of hemicellulosic sugars derived from biomass to levulinic acid. Catal. Commun. 2018, 117, 19–25. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Thermo-chemical energy assessment for production of energy-rich fuel additive compounds by using levulinic acid and immobilized lipase. Fuel Process. Technol. 2015, 138, 139–146. [Google Scholar] [CrossRef]
- Bhat, N.S.; Mal, S.S.; Dutta, S. Recent advances in the preparation of levulinic esters from biomass-derived furanic and levulinic chemical platforms using heteropoly acid (HPA) catalysts. Mol. Catal. 2021, 505, 111484. [Google Scholar] [CrossRef]
- Ogino, I.; Suzuki, Y.; Mukai, S.R. Esterification of levulinic acid with ethanol catalyzed by sulfonated carbon catalysts: Promotional effects of additional functional groups. Catal. Today 2018, 314, 62–69. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and applications of alkyl levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Bart, H.J.; Reidetschläger, J.; Schatka, K.; Lehmann, A. Kinetics of esterification of succinic anhydride with methanol by homogeneous catalysis. Int. J. Chem. Kinet. 1994, 26, 1013–1021. [Google Scholar] [CrossRef]
- Gong, W.; Liu, Y.; Li, H.; Cui, Y. Metal-organic frameworks as solid Brønsted acid catalysts for advanced organic transformations. Coord. Chem. Rev. 2020, 420, 213400. [Google Scholar] [CrossRef]
- Qu, H.; Liu, B.; Gao, G.; Ma, Y.; Zhou, Y.; Zhou, H.; Li, L.; Li, Y.; Liu, S. Metal-organic framework containing BrØnsted acidity and Lewis acidity for efficient conversion glucose to levulinic acid. Fuel Process. Technol. 2019, 193, 1–6. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.C. Recent progress in the synthesis of metal-organic frameworks. Sci. Technol. Adv. Mater. 2015, 16, 54202. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Lillerud, K.P.; Cavka, J.H.; Lamberti, C.; Guillou, N.; Bordiga, S.; Jakobsen, S.; Olsbye, U. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Rahmawati, I.D.; Ediati, R.; Prasetyoko, D. Synthesis of UiO-66 using solvothermal method at high temperature. IPTEK J. Proc. Ser. 2014, 1, 42–46. [Google Scholar] [CrossRef]
- Abid, H.R.; Shang, J.; Ang, H.M.; Wang, S. Amino-functionalized Zr-MOF nanoparticles for adsorption of CO2 and CH4. Int. J. Smart Nano Mater. 2013, 4, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Cirujano, F.G.; Corma, A.; Llabrés I Xamena, F.X. Zirconium-containing metal organic frameworks as solid acid catalysts for the esterification of free fatty acids: Synthesis of biodiesel and other compounds of interest. Catal. Today 2015, 257, 213–220. [Google Scholar] [CrossRef]
- Wei, R.; Gaggioli, C.A.; Li, G.; Islamoglu, T.; Zhang, Z.; Yu, P.; Farha, O.K.; Cramer, C.J.; Gagliardi, L.; Yang, D.; et al. Tuning the properties of Zr6O8 nodes in the metal organic framework UiO-66 by selection of node-bound ligands and linkers. Chem. Mater. 2019, 31, 1655–1663. [Google Scholar] [CrossRef]
- Li, H.; Chu, H.; Ma, X.; Wang, G.; Liu, F.; Guo, M.; Lu, W.; Zhou, S.; Yu, M. Efficient heterogeneous acid synthesis and stability enhancement of UiO-66 impregnated with ammonium sulfate for biodiesel production. Chem. Eng. J. 2021, 408, 127277. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef] [Green Version]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Llabrés i Xamena, F.X.; Van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Cirujano, F.G.; Corma, A.; Llabrés i Xamena, F.X. Conversion of levulinic acid into chemicals: Synthesis of biomass derived levulinate esters over Zr-containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef] [Green Version]
- Lozano, L.A.; Iglesias, C.M.; Faroldi, B.M.C.; Ulla, M.A.; Zamaro, J.M. Efficient solvothermal synthesis of highly porous UiO-66 nanocrystals in dimethylformamide-free media. J. Mater. Sci. 2018, 53, 1862–1873. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Zaharudin, N.H.; Amin, N.A.S. Esterification of renewable levulinic acid to levulinate esters using amberlyst-15 as a solid acid catalyst. J. Teknol. 2017, 79, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Fu, Y.; Chang, J. Sustainable production of methyl levulinate from biomass in ionic liquid-methanol system with biomass-based catalyst. Fuel 2020, 259, 116246. [Google Scholar] [CrossRef]
- Di, X.; Zhang, Y.; Fu, J.; Yu, Q.; Wang, Z.; Yuan, Z. Biocatalytic upgrading of levulinic acid to methyl levulinate in green solvents. Process Biochem. 2019, 81, 33–38. [Google Scholar] [CrossRef]
- Chaffey, D.R.; Bere, T.; Davies, T.E.; Apperley, D.C.; Taylor, S.H.; Graham, A.E. Conversion of levulinic acid to levulinate ester biofuels by heterogeneous catalysts in the presence of acetals and ketals. Appl. Catal. B Environ. 2021, 293, 120219. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Batten, M.P.; Polyzos, A.; Carey, K.C.; Mardel, J.I.; Lim, K.S.; Hill, M.R. Versatile, high quality and scalable continuous flow production of metal-organic frameworks. Sci. Rep. 2014, 4, 5443. [Google Scholar] [CrossRef]
- Lu, A.X.; McEntee, M.; Browe, M.A.; Hall, M.G.; Decoste, J.B.; Peterson, G.W. MOFabric: Electrospun nanofiber mats from PVDF/UiO-66-NH2 for chemical protection and decontamination. ACS Appl. Mater. Interfaces 2017, 9, 13632–13636. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Ragon, F.; Horcajada, P.; Chevreau, H.; Hwang, Y.K.; Lee, U.H.; Miller, S.R.; Devic, T.; Chang, J.S.; Serre, C. In situ energy-dispersive X-ray diffraction for the synthesis optimization and scale-up of the porous zirconium terephthalate UiO-66. Inorg. Chem. 2014, 53, 2491–2500. [Google Scholar] [CrossRef]
- Arrozi, U.S.F.; Wijaya, H.W.; Patah, A.; Permana, Y. Efficient acetalization of benzaldehydes using UiO-66 and UiO-67: Substrates accessibility or Lewis acidity of zirconium. Appl. Catal. A Gen. 2015, 506, 77–84. [Google Scholar] [CrossRef]
- Luu, C.L.; Van Nguyen, T.T.; Nguyen, T.; Hoang, T.C. Synthesis, characterization and adsorption ability of UiO-66-NH2. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025004. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wei, F.; Dong, C.; Li, J.; Zhang, C.; Han, X. UiO-66 based electrochemical sensor for simultaneous detection of Cd(II) and Pb(II). Inorg. Chem. Commun. 2021, 131, 108785. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal-organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Hou, J.; Luan, Y.; Tang, J.; Wensley, A.M.; Yang, M.; Lu, Y. Synthesis of UiO-66-NH2 derived heterogeneous copper (II) catalyst and study of its application in the selective aerobic oxidation of alcohols. J. Mol. Catal. A Chem. 2015, 407, 53–59. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.; Qi, Z.; Cai, D.; Qiu, T. Design and synthesis of ionic liquid supported hierarchically porous zr metal-organic framework as a novel Brønsted-Lewis acidic catalyst in biodiesel synthesis. Ind. Eng. Chem. Res. 2019, 58, 1123–1132. [Google Scholar] [CrossRef]
- Hu, Z.; Peng, Y.; Kang, Z.; Qian, Y.; Zhao, D. A modulated hydrothermal (MHT) approach for the facile synthesis of UiO-66-type MOFs. Inorg. Chem. 2015, 10, 4862–4868. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Z.; Chen, H.; Goetjen, T.A.; Li, P.; Wang, F.X.; Alayoglu, S.; Ma, K.; Chen, Y.; Wang, T.; et al. Interplay of Lewis and brønsted acid sites in Zr-based metal-organic frameworks for efficient esterification of biomass-derived levulinic acid. ACS Appl. Mater. Interfaces 2019, 11, 32090–32096. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Z.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction. Appl. Catal. B Environ. 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp., Eden Prairie: Chichester, UK, 1992; ISBN 9780470014226. [Google Scholar]
- Wiersum, A.D.; Soubeyrand-Lenoir, E.; Yang, Q.; Moulin, B.; Guillerm, V.; Yahia, M.B.; Bourrelly, S.; Vimont, A.; Miller, S.; Vagner, C.; et al. An evaluation of UiO-66 for gas-based applications. Chem.-Asian J. 2011, 6, 3270–3280. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Jun, J.W.; Hasan, Z.; Matrosova, M.M.; Jhung, S.H. Effects of linker substitution on catalytic properties of porous zirconium terephthalate UiO-66 in acetalization of benzaldehyde with methanol. Appl. Catal. A Gen. 2014, 471, 91–97. [Google Scholar] [CrossRef]
- Strauss, I.; Chakarova, K.; Mundstock, A.; Mihaylov, M.; Hadjiivanov, K.; Guschanski, N.; Caro, J. UiO-66 and UiO-66-NH2 based sensors: Dielectric and FTIR investigations on the effect of CO2 adsorption. Microporous Mesoporous Mater. 2020, 302, 110227. [Google Scholar] [CrossRef]
- Caretto, A.; Perosa, A. Upgrading of levulinic acid with dimethylcarbonate as solvent/reagent. ACS Sustain. Chem. Eng. 2013, 1, 989–994. [Google Scholar] [CrossRef]
- Langlois, D.P.; Wolff, H. Pseudo esters of levulinic acid. J. Am. Chem. Soc. 1948, 70, 2624–2626. [Google Scholar] [CrossRef]
- Lima, C.G.S.; Monteiro, J.L.; de Melo Lima, T.; Weber Paixão, M.; Corrêa, A.G. Angelica lactones: From biomass-derived platform chemicals to value-added products. ChemSusChem 2018, 11, 25–47. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Ciptonugroho, W.; Holzhäuser, F.J.; Mensah, J.B.; Hausoul, P.J.C.; Palkovits, R. Catalytic upgrading of α-angelica lactone to levulinic acid esters under mild conditions over heterogeneous catalysts. Catal. Sci. Technol. 2015, 5, 5168–5173. [Google Scholar] [CrossRef] [Green Version]
- Ramli, N.A.S.; Sivasubramaniam, D.; Amin, N.A.S. Esterification of levulinic acid using ZrO2-supported phosphotungstic acid catalyst for ethyl levulinate production. Bioenergy Res. 2017, 10, 1105–1116. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Sonar, S.K.; Niphadkar, P.S.; Joshi, P.N.; Deshpande, S.S.; Patil, V.S.; Bokade, V.V. Catalytic upgrading of renewable levulinic acid to ethyl levulinate biodiesel using dodecatungstophosphoric acid supported on desilicated H-ZSM-5 as catalyst. Appl. Catal. A Gen. 2013, 460–461, 90–98, ISBN 9120259026. [Google Scholar] [CrossRef]
- Zubir, M.I.; Chin, S.Y. Kinetics of modified Zirconia-catalyzed heterogeneous esterification reaction for biodiesel production. J. Appl. Sci. 2010, 10, 2584–2589. [Google Scholar] [CrossRef] [Green Version]
- Jrad, A.; Abu Tarboush, B.J.; Hmadeh, M.; Ahmad, M. Tuning acidity in zirconium-based metal organic frameworks catalysts for enhanced production of butyl butyrate. Appl. Catal. A Gen. 2019, 570, 31–41. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Altuntepe, E.; Held, C.; Pimerzin, A.A.; Verevkin, S.P. Renewable platform chemicals: Thermochemical study of levulinic acid esters. Thermochim. Acta 2018, 659, 213–221. [Google Scholar] [CrossRef]
- Feng, J.; Li, M.; Zhong, Y.; Xu, Y.; Meng, X.; Zhao, Z.; Feng, C. Hydrogenation of levulinic acid to γ-valerolactone over Pd@UiO-66-NH2 with high metal dispersion and excellent reusability. Microporous Mesoporous Mater. 2020, 294, 109858. [Google Scholar] [CrossRef]
- Cao, W.; Lin, L.; Qi, H.; He, Q.; Wu, Z.; Wang, A.; Luo, W.; Zhang, T. In-situ synthesis of single-atom Ir by utilizing metal-organic frameworks: An acid-resistant catalyst for hydrogenation of levulinic acid to Γ-valerolactone. J. Catal. 2019, 373, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Sosa, L.F.; da Silva, V.T.; de Souza, P.M. Hydrogenation of levulinic acid to γ-valerolactone using carbon nanotubes supported nickel catalysts. Catal. Today 2020, 381, 86–95. [Google Scholar] [CrossRef]
- Tulchinsky, M.L.; Briggs, J.R. One-pot synthesis of alkyl 4-alkoxypentanoates by esterification and reductive etherification of levulinic acid in alcoholic solutions. ACS Sustain. Chem. Eng. 2016, 4, 4089–4093. [Google Scholar] [CrossRef]
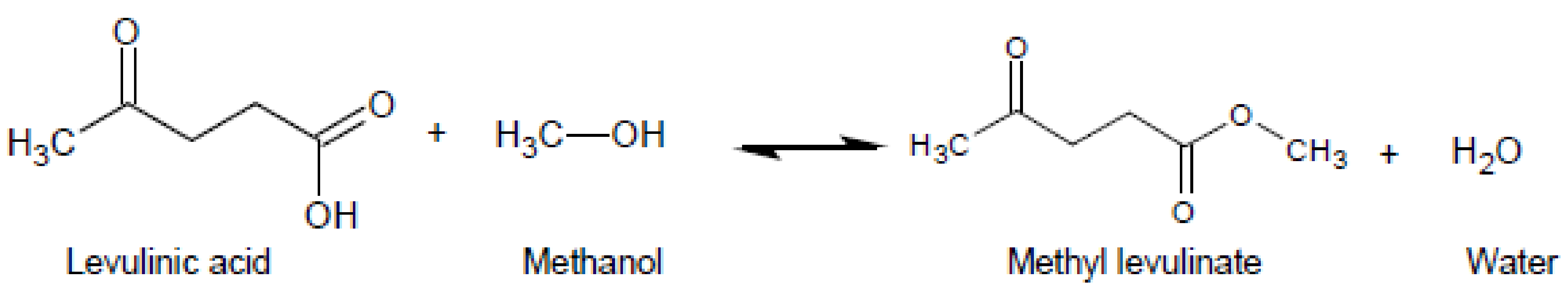
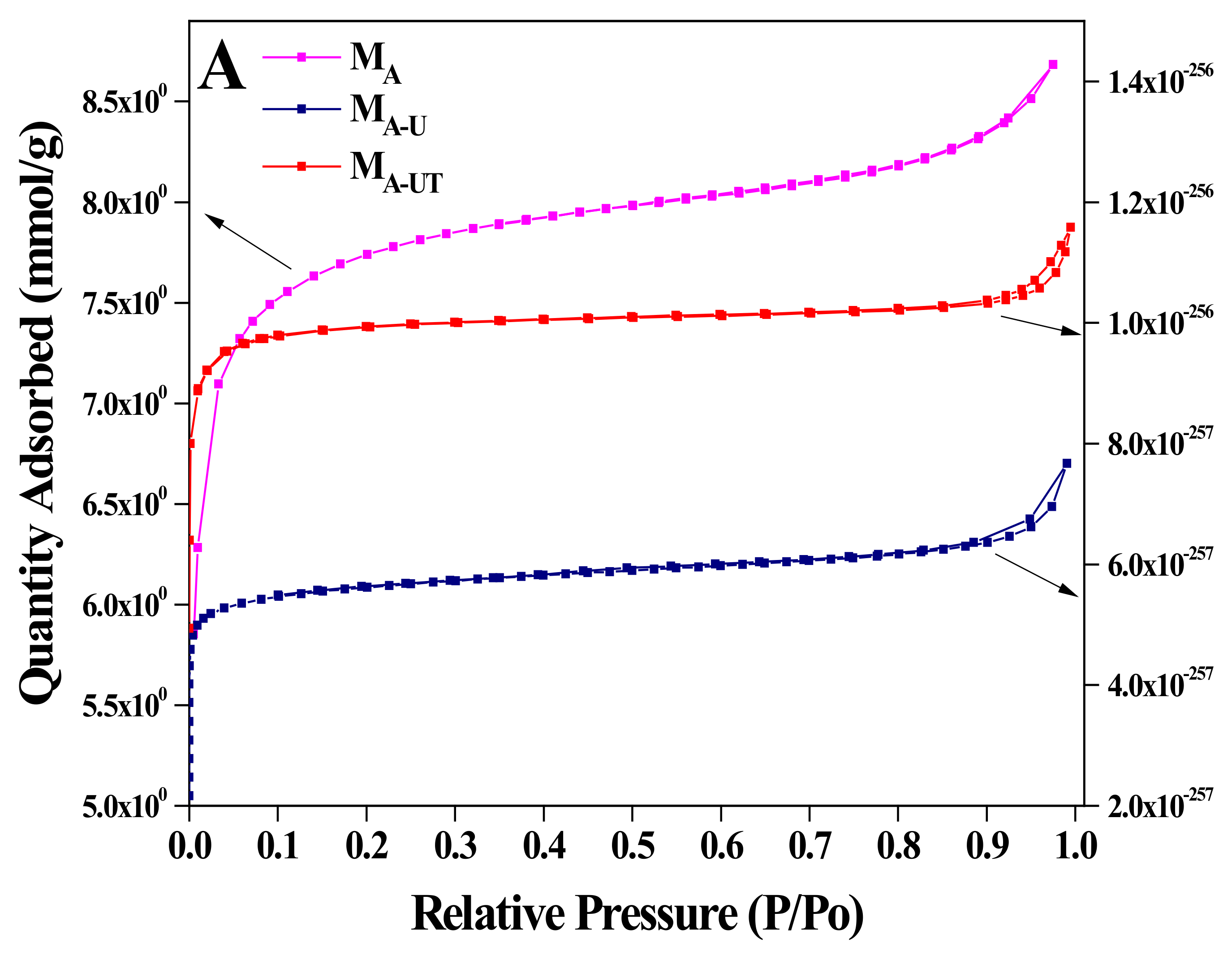
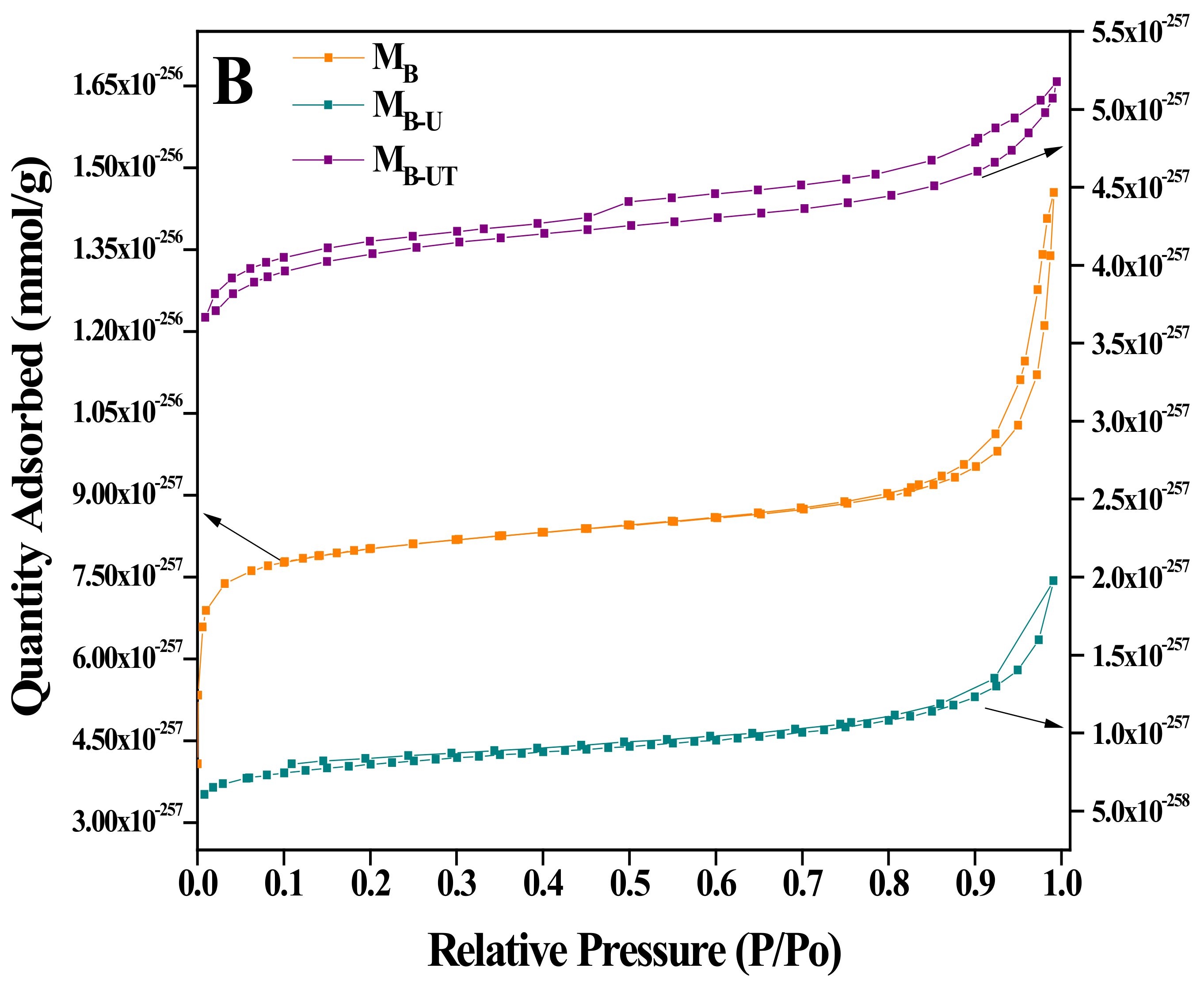


| Catalyst | Zr (w %) Experimental | BET (m2 g−1) | Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|---|
| MA | 28.4 ± 0.6 | 683 | 2.64 | 0.248 |
| MA-U | 16.5 ± 0.3 | 640 | 2.46 | 0.163 |
| MA-UT | 17.2 ± 0.3 | 658 | 2.30 | 0.153 |
| MB | 20.5 ± 0.4 | 400 | 2.36 | 0.164 |
| MB-U | 14.8 ± 0.3 | 300 | 1.21 | 0.099 |
| MB-UT | 16.2 ± 0.3 | 312 | 1.07 | 0.095 |
| Sample | C% | Zr% | O% | N% |
|---|---|---|---|---|
| MA | 62.59 | 5.59 | 31.51 | 0.31 |
| MA-U | 57.94 | 7.02 | 34.72 | 0.33 |
| MA-UT | 56.03 | 7.76 | 35.29 | 0.92 |
| MB | 60.54 | 4.97 | 29.87 | 4.62 |
| MB-U | 57.95 | 5.63 | 31.60 | 4.83 |
| MB-UT | 59.52 | 5.42 | 29.96 | 5.09 |
| Sample | C 1s | Zr 3d5/2 | O 1s | N 1s |
|---|---|---|---|---|
| MA | 284.8 (86) | 182.8 | 530.4 (14) | 400.6 |
| 288.8 (14) | 531.8 (86) | |||
| MA-U | 284.8 (82) | 182.8 | 530.1 (18) | 401.4 |
| 288.8 (18) | 531.8 (82) | |||
| MA-UT | 284.8 (83) | 182.7 | 530.2 (23) | 400.7 |
| 288.8 (17) | 531.8 (77) | |||
| MB | 284.8 (64) | 182.9 | 530.3 (12) | 399.8 |
| 286.1 (18) | 532.0 (88) | |||
| 288.7 (18) | ||||
| MB-U | 284.8 (65) | 182.9 | 530.5 (12) | 399.5 |
| 286.1 (17) | 531.9 (88) | |||
| 288.8 (18) | ||||
| MB-UT | 284.8 (65) | 182.8 | 530.6 (17) | 399.4 |
| 286.0 (17) | 531.9 (83) | |||
| 288.8 (18) |
| Sample | % Area (50 °C) Weak Sites | % Area (100 °C) Medium Sites | % Area Total Sites | ||||
|---|---|---|---|---|---|---|---|
| Lewis | Brønsted | Lewis | Brønsted | Lewis | Brønsted | L/B | |
| MA | 45.87 | 54.13 | 53.40 | 46.60 | 49.64 | 50.36 | 0.99 |
| MA-U | 57.38 | 42.62 | 52.68 | 47.32 | 55.03 | 44.97 | 1.22 |
| MA-UT | 60.43 | 39.57 | 54.87 | 45.13 | 57.65 | 42.35 | 1.36 |
| MB | 42.97 | 57.03 | 42.74 | 57.26 | 42.85 | 57.15 | 0.75 |
| MB-U | 45.40 | 54.60 | 46.74 | 53.26 | 46.07 | 53.93 | 0.85 |
| MB-UT | 48.25 | 51.75 | 45.75 | 54.25 | 47.00 | 53.00 | 0.89 |
| Catalyst | Conversion % | Selectivity ML % | Yield % |
|---|---|---|---|
| MA | 16.73 | 13.41 | 2.24 |
| MA-U | 42.03 | 58.31 | 24.51 |
| MA-UT | 53.85 | 78.02 | 42.02 |
| MB | 21.08 | 73.10 | 15.41 |
| MB-U | 58.48 | 84.88 | 49.64 |
| MB-UT | 63.57 | 92.22 | 58.62 |
| Catalyst Mass | Conversion % | Yield % | ΔY/ΔC |
|---|---|---|---|
| 0.025 g | 56.79 | 45.22 | ----- |
| 0.05 g | 63.57 | 58.62 | 536 |
| 0.1 g | 67.55 | 63.31 | 93.8 |
| 0.15 g | 70.00 | 65.72 | 48.2 |
| Temperature | Conversion % | Selectivity ML % | Yield % | ΔY/ΔT |
|---|---|---|---|---|
| 55 °C | 48.20 | 70.29 | 33.88 | ---- |
| 60 °C | 62.86 | 88.79 | 55.81 | 4.26 |
| 65 °C | 70.87 | 89.99 | 63.77 | 1.59 |
| 70 °C | 73.79 | 91.23 | 67.32 | 0.71 |
| Molar Ratio | Conversion % | Yield % | ΔY/Δmol [M] |
|---|---|---|---|
| 1:10 | 59.97 | 54.19 | --- |
| 1:15 | 70.87 | 63.77 | 1.92 |
| 1:20 | 67.28 | 64.47 | 0.14 |
| Catalyst | Conversion % | Selectivity ML % | Yield % |
|---|---|---|---|
| MA | 50.18 | 58.63 | 29.42 |
| MA-U | 50.08 | 67.64 | 33.87 |
| MA-UT | 72.05 | 87.82 | 63.27 |
| MB | 73.06 | 83.11 | 60.73 |
| MB-U | 77.85 | 100 | 77.85 |
| MB-UT | 85.89 (4) * | 100 | 85.89 |
| MB-UT R1 | 70.18 (24) * | 100 | 70.18 |
| MB-UT R2 | 50.46 (5) * | 100 | 50.46 |
| MB-UT R3 | 40.20 (12) * | 63.12 | 25.37 |
| Cl4Zr | 98.24 | 100 | 98.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo Fuchineco, D.A.; Heredia, A.C.; Mendoza, S.M.; Rodríguez-Castellón, E.; Crivello, M.E. Esterification of Levulinic Acid to Methyl Levulinate over Zr-MOFs Catalysts. ChemEngineering 2022, 6, 26. https://doi.org/10.3390/chemengineering6020026
Bravo Fuchineco DA, Heredia AC, Mendoza SM, Rodríguez-Castellón E, Crivello ME. Esterification of Levulinic Acid to Methyl Levulinate over Zr-MOFs Catalysts. ChemEngineering. 2022; 6(2):26. https://doi.org/10.3390/chemengineering6020026
Chicago/Turabian StyleBravo Fuchineco, Daiana A., Angélica C. Heredia, Sandra M. Mendoza, Enrique Rodríguez-Castellón, and Mónica E. Crivello. 2022. "Esterification of Levulinic Acid to Methyl Levulinate over Zr-MOFs Catalysts" ChemEngineering 6, no. 2: 26. https://doi.org/10.3390/chemengineering6020026
APA StyleBravo Fuchineco, D. A., Heredia, A. C., Mendoza, S. M., Rodríguez-Castellón, E., & Crivello, M. E. (2022). Esterification of Levulinic Acid to Methyl Levulinate over Zr-MOFs Catalysts. ChemEngineering, 6(2), 26. https://doi.org/10.3390/chemengineering6020026







