Sensitivity Control of Hydroquinone and Catechol at Poly(Brilliant Cresyl Blue)-Modified GCE by Varying Activation Conditions of the GCE: An Experimental and Computational Study
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Preparation of Modified Electrodes
2.3. Instrumentation
3. Results and Discussion
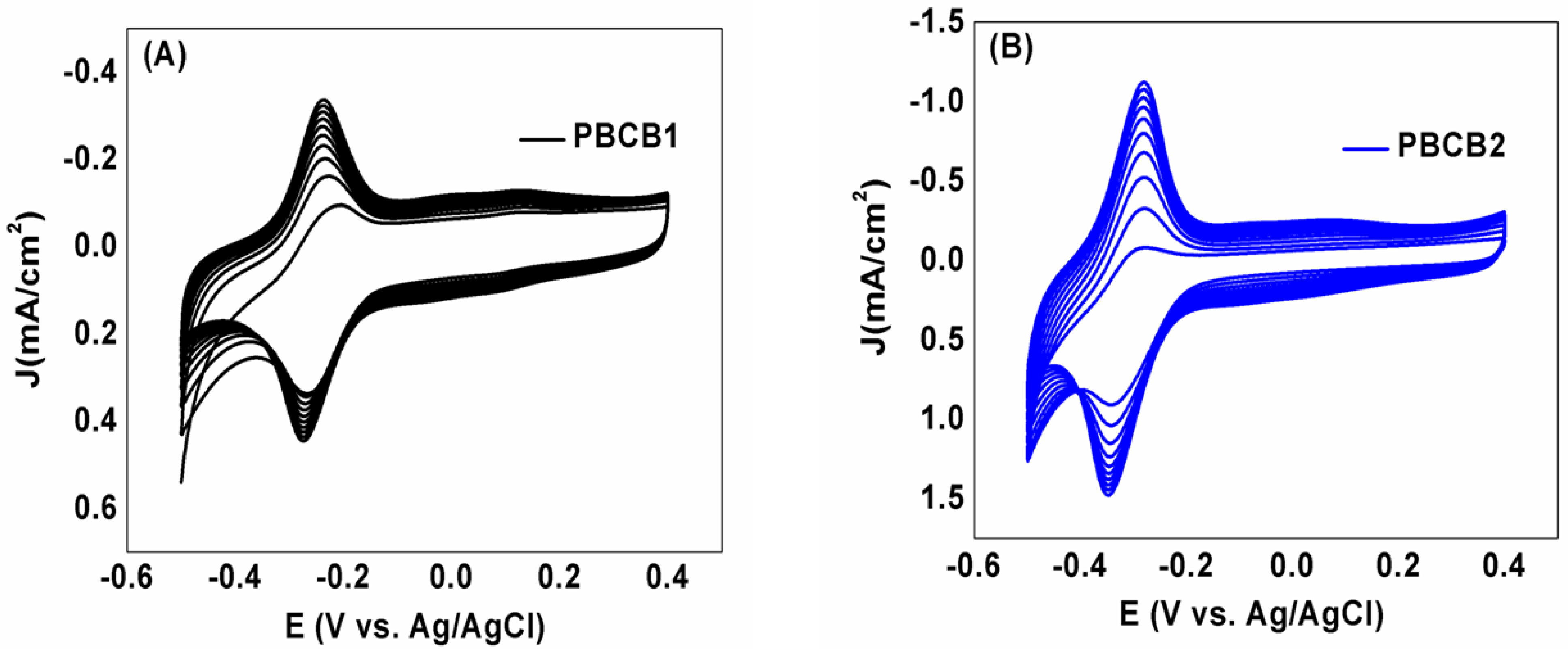
3.1. Electropolymerization of BCB on AGCE
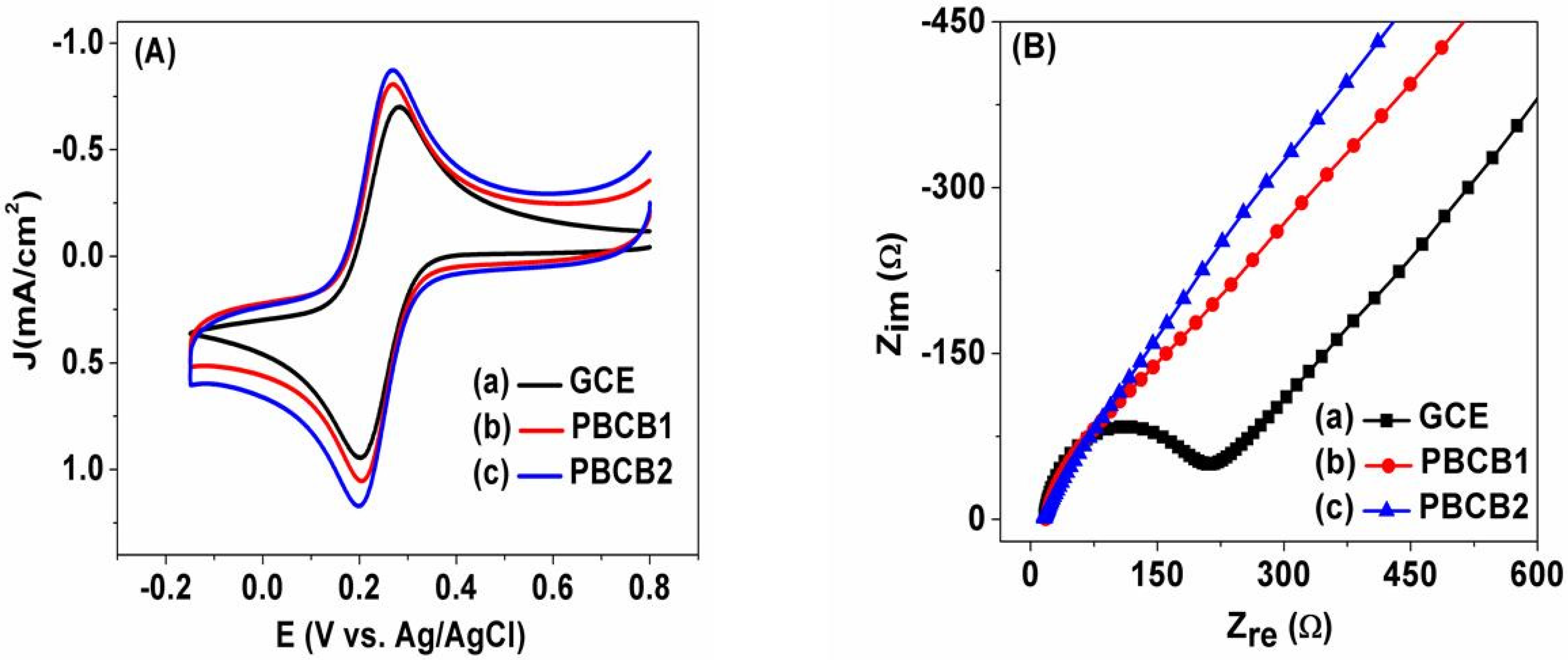
3.2. Surface Characterization of PBCB1 and PBCB2
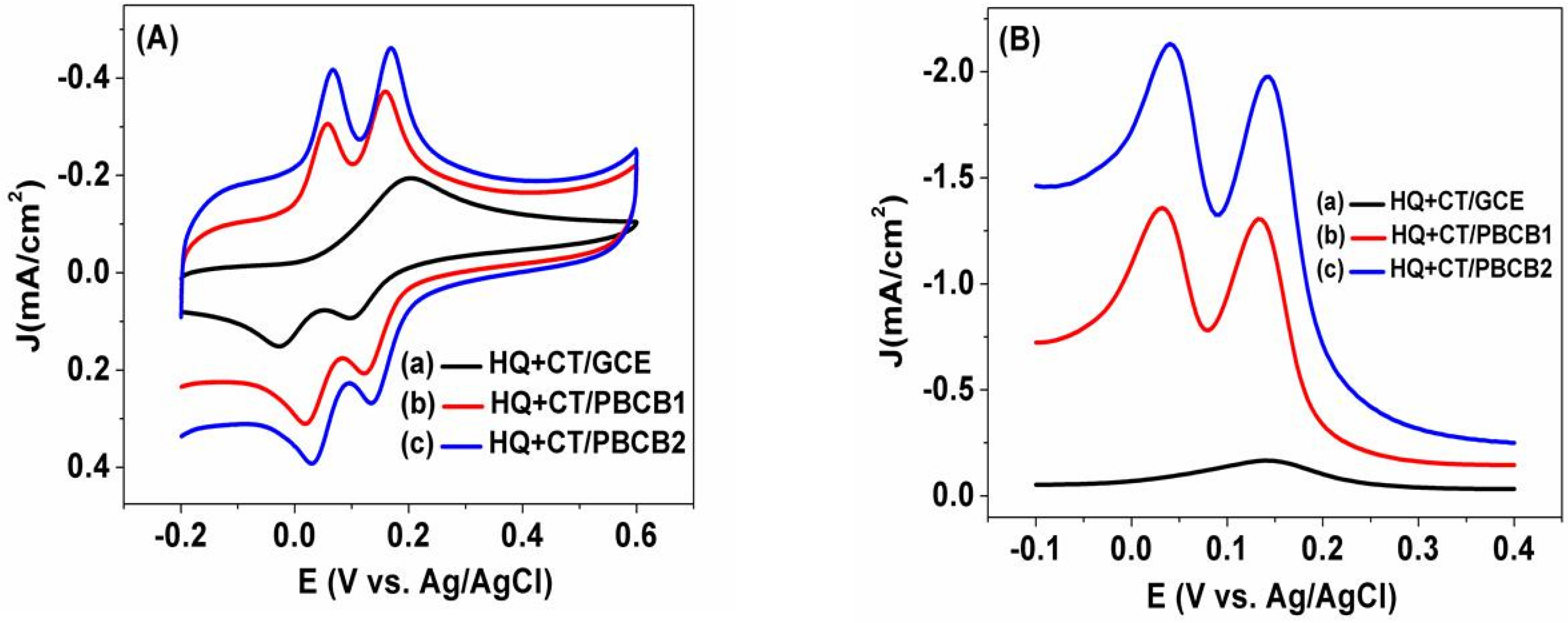
3.3. Electrochemical Behavior of HQ and CT
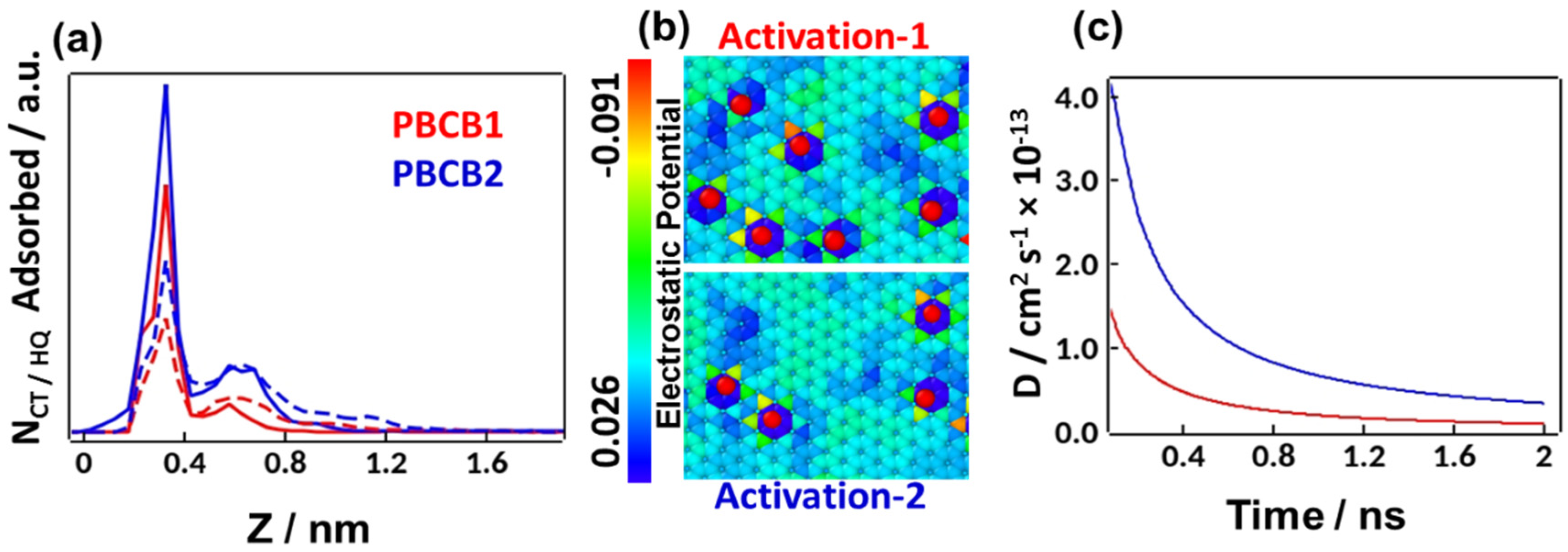
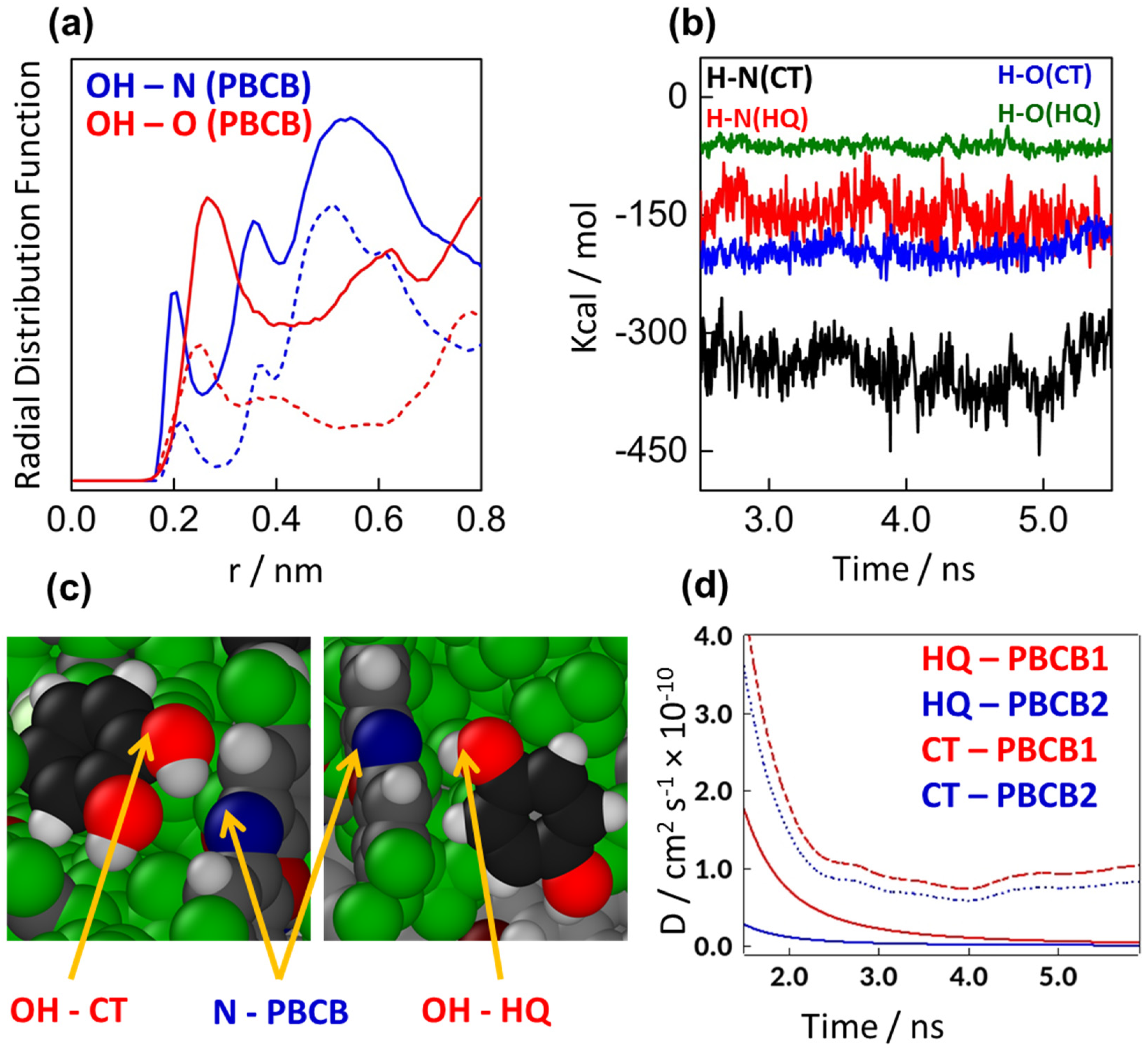
3.4. Computational Analysis through MD Simulations
3.5. Simultaneous Determination of HQ and CT
3.6. Interference Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ahammad, A.J.S.; Rahman, M.M.; Xu, G.R.; Kim, S.; Lee, J.J. Highly sensitive and simultaneous determination of hydroquinone and catechol at poly(thionine) modified glassy carbon electrode. Electrochim. Acta 2011, 56, 5266. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Sarker, S.; Rahman, M.A.; Lee, J.J. Simultaneous determination of hydroquinone and catechol at an activated glassy carbon electrode. Electroanalysis 2010, 22, 694. [Google Scholar] [CrossRef]
- Li, M.; Ni, F.; Wang, Y.; Xu, S.; Zhang, D.; Chen, S.; Wang, L. Sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of resorcinol with A Zn/Al layered double hydroxide film modified glassy carbon electrode. Electroanalysis 2009, 21, 1521. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Du, Y.; Lu, D.; Zhang, Y.; Wang, C. Simultaneous determination of catechol and hydroquinone based on poly (diallyldimethylammonium chloride) functionalized graphene-modified glassy carbon electrode. J. Solid State Electrochem. 2012, 16, 1323. [Google Scholar] [CrossRef]
- Nagaraja, P.; Vasantha, R.A.; Sunitha, K.R. A new sensitive and selective spectrophotometric method for the determination of catechol derivatives and its pharmaceutical preparations. J. Pharm. Biomed. Anal. 2001, 25, 417. [Google Scholar] [CrossRef]
- Wang, L.H.; Kuo, Y.P. Simultaneous quantitative determination of resorcinol and 1-Naphthol in haircolor products by high-performance liquid chromatography. Chromatographia 1999, 49, 208. [Google Scholar] [CrossRef]
- Zhao, L.; Lv, B.; Yuan, H.; Zhou, Z.; Xiao, D. A sensitive chemiluminescence method for determination of hydroquinone and catechol. Sensors 2007, 7, 578. [Google Scholar] [CrossRef] [Green Version]
- Pistonesi, M.F.; Nezio, M.S.D.; Centurion, M.E.; Palomeque, M.E.; Lista, A.G.; Band, B.S.F. Determination of phenol, resorcinol and hydroquinone in air samples by synchronous fluorescence using partial least-squares (PLS). Talanta 2006, 69, 1265. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ye, J.; Zhang, J.; Huang, X.; Yu, C. A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J. Electroanal. Chem. 2011, 650, 209. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Ru, P.L.; Chen, S.M. Electrochemically synthesized Pt–MnO2 composite particles for simultaneous determination of catechol and hydroquinone. Sens. Actuators B Chem. 2012, 169, 235. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Nath, N.C.D.; Xu, G.R.; Kim, S.; Lee, J.J. Interference-free determination of dopamine at the poly(thionine)-modified glassy carbon electrode. J. Electrochem. Soc. 2011, 158, F106. [Google Scholar] [CrossRef]
- Yu, J.; Du, W.; Zhao, F.; Zeng, B. High sensitive simultaneous determination of catechol and hydroquinone at mesoporous carbon CMK-3 electrode in comparison with multi-walled carbon nanotubes and Vulcan XC-72 carbon electrodes. Electrochim. Acta 2009, 54, 984. [Google Scholar] [CrossRef]
- Li, D.W.; Li, Y.T.; Song, W.; Long, Y.T. Simultaneous determination of dihydroxybenzene isomers using disposable screen-printed electrode modified by multiwalled carbon nanotubes and gold nanoparticles. Anal. Methods 2010, 2, 837. [Google Scholar] [CrossRef]
- Umasankar, Y.; Periasamy, A.P.; Chen, S.M. Electrocatalysis and simultaneous determination of catechol and quinol by poly(malachite green) coated multiwalled carbon nanotube film. Anal. Biochem. 2011, 411, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Z.; Cao, Y.; Jing, Y.; Liu, Y. High sensitive simultaneous determination of hydroquinone and catechol based on graphene/BMIMPF6 nanocomposite modified electrode. Sens. Actuators B Chem. 2011, 157, 540. [Google Scholar] [CrossRef]
- Huo, Z.; Zhou, Y.; Liu, Q.; He, X.; Liang, Y.; Xu, M. Sensitive simultaneous determination of catechol and hydroquinone using a gold electrode modified with carbon nanofibers and gold nanoparticles. Microchim. Acta 2011, 173, 119. [Google Scholar] [CrossRef]
- Yuan, Y.; Ahammad, A.J.S.; Xu, G.R.; Kim, S.; Lee, J.J. Poly(thionine) modified GC electrode for simultaneous detection of dopamine and uric Acid in the presence of ascorbic acid. Bull. Korean Chem. Soc. 2008, 29, 1883. [Google Scholar]
- Zhao, D.M.; Zhang, X.H.; Feng, L.J.; Jia, L.; Wang, S.F. Simultaneous determination of hydroquinone and catechol at PASA/MWNTs composite film modified glassy carbon electrode. Colloids Surf. B 2009, 74, 317. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Liu, H.; Liu, R.; Wang, H.; Liu, J. Electrochemical determination of diphenols and their mixtures at the multiwall carbon nanotubes/poly (3-methylthiophene) modified glassy carbon electrode. Microchim. Acta 2010, 169, 277. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Q.; Chen, Y.; Wang, F. Simultaneous determination of hydroquinone and catechol using poly(p-aminobenzoic acid) modified glassy carbon electrode. J.Appl. Polym. Sci. 2009, 113, 2881. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, H.; Lu, H. Electrocatalytic oxidation of hydroquinone at poly(crystal-violet) film–modified electrode and its selective determination in the presence of o-hydroquinone and m-hydroquinone. Anal. Lett. 2009, 42, 339. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Saha, S.K.; Bakshi, P.K.; Hussain, A.; Ahammad, A.J.S. Poly(brilliant cresyl blue)-modified electrode for highly sensitive and simultaneous determination of hydroquinone and catechol. J. Electrochem. Soc. 2013, 160, B37. [Google Scholar] [CrossRef]
- da Silva, A.R.L.; dos Santos, A.J.; Martinez-Huitle, C.A. Electrochemical measurements and theoretical studies for understanding the behavior of catechol, resorcinol and hydroquinone on the boron doped diamond surface. RSC Adv. 2018, 8, 3483. [Google Scholar] [CrossRef] [Green Version]
- Faraezi, S.; Khan, M.S.; Ohba, T. Dehydration of cations inducing fast ion transfer and high electrical capacitance performance on graphene electrode in aqueous electrolytes. Ind. Eng. Chem. Res. 2020, 59, 5768. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Faraudo, J.; Figueras, A.; Alibes, R.; Busque, F.; Ruiz-Molina, D. Switchable self-assembly of a bioinspired alkyl catechol at a solid/liquid interface: Competitive interfacial, noncovalent, and solvent interactions. Chem. Eur. J. 2012, 18, 3056. [Google Scholar] [CrossRef]
- Li, Y.; Liao, M.; Zhou, J. Catechol and its derivatives adhesion on graphene: Insights from molecular dynamics simulations. J. Phys. Chem. C 2018, 122, 22965. [Google Scholar] [CrossRef]
- Yeh, I.; Lenhart, J.L.; Rinderspacher, B.C. Molecular dynamics simulations of adsorption of catechol and related phenolic compounds to alumina surfaces. J. Phys. Chem. C 2015, 119, 7721. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Alam, M.K.; Islam, T.; Hasan, M.M.; Karim, R.; Anju, A.N.; Mozumder, M.N.I. Poly (brilliant cresyl blue)-reduced graphene oxide modified activated GCE for nitrite detection: Analyzing the synergistic interactions through experimental and computational study. Electrochim. Acta 2020, 349, 136375. [Google Scholar] [CrossRef]
- Khan, S.M.; Kitayama, H.; Yamada, Y.; Gohda, S.; Ono, H.; Umeda, D.; Abe, K.; Hata, K.; Ohba, T. High CO2 sensitivity and reversibility on nitrogen-containing polymer by remarkable CO2 adsorption on nitrogen sites. J. Phys. Chem. C 2018, 122, 24143. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwall, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Peng, Y.; Qi, H.; Gao, Q.; Zhang, C. Application of multielectrode array modified with carbon nanotubes to simultaneous amperometric determination of dihydroxybenzene isomers. Sens. Actuators B Chem. 2009, 136, 113. [Google Scholar] [CrossRef]
- Ding, Y.P.; Liu, W.L.; Wu, Q.S.; Wang, X.G. Direct simultaneous determination of dihydroxybenzene isomers at C-nanotube-modified electrodes by derivative voltammetry. J. Electroanal. Chem. 2005, 575, 275. [Google Scholar] [CrossRef]
- Yang, P.; Wei, W.; Tao, C.; Zeng, J. Simultaneous voltammetry determination of dihydroxybenzene isomers by poly-bromophenol blue/carbon nanotubes composite modified electrode. Bull. Environ. Contam. Toxicol. 2007, 79, 5. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, R.; Chai, Y.; Hu, F. Simultaneous determination of hydroquinone, catechol, resorcinol and nitrite using gold nanoparticles loaded on poly-3-amino-5-mercapto-1,2,4-triazole-MWNTs film modified electrode. Anal. Methods 2012, 4, 1626. [Google Scholar] [CrossRef]
- Hu, F.; Chen, S.; Wang, C.; Yuan, R.; Yuan, D.; Wang, C. Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite. Anal. Chim. Acta 2012, 724, 40. [Google Scholar] [CrossRef]
- Deng, K.; Li, C.; Li, X.; Xu, G. Simultaneous electrochemical determination of hydroquinone and catechol at MWNTs and Cobalt(II) tetrakisphenylporphyrin modified electrode. Anal. Lett. 2012, 45, 883. [Google Scholar] [CrossRef]
- Yang, C.; Chai, Y.; Yuan, R.; Xu, W.; Chen, S. Gold nanoparticle–graphene nanohybrid bridged 3-amino-5-mercapto-1,2,4-triazole-functionalized multiwall carbon nanotubes for the simultaneous determination of hydroquinone, catechol, resorcinol and nitrite. Anal. Methods 2013, 5, 666. [Google Scholar] [CrossRef]



| Interferences | Current Ratios [HQ] | Current Ratios [CT] |
|---|---|---|
| Resorcinol | 1.11 | 0.98 |
| Phenol | 1.02 | 0.96 |
| 2-Nitrophenol | 1.06 | 0.96 |
| 4-Nitrophenol | 1.06 | 1.09 |
| NH4+ | 1.04 | 0.94 |
| Ca2+ | 1.06 | 0.94 |
| Mg2+ | 1.14 | 1.03 |
| K+ | 1.05 | 0.96 |
| Fe2+ | 0.95 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraezi, S.; Khan, M.S.; Monira, F.Z.; Mamun, A.A.; Akter, T.; Mamun, M.A.; Rabbani, M.M.; Uddin, J.; Ahammad, A.J.S. Sensitivity Control of Hydroquinone and Catechol at Poly(Brilliant Cresyl Blue)-Modified GCE by Varying Activation Conditions of the GCE: An Experimental and Computational Study. ChemEngineering 2022, 6, 27. https://doi.org/10.3390/chemengineering6020027
Faraezi S, Khan MS, Monira FZ, Mamun AA, Akter T, Mamun MA, Rabbani MM, Uddin J, Ahammad AJS. Sensitivity Control of Hydroquinone and Catechol at Poly(Brilliant Cresyl Blue)-Modified GCE by Varying Activation Conditions of the GCE: An Experimental and Computational Study. ChemEngineering. 2022; 6(2):27. https://doi.org/10.3390/chemengineering6020027
Chicago/Turabian StyleFaraezi, Sharifa, Md Sharif Khan, Ferzana Zaman Monira, Abdullah Al Mamun, Tania Akter, Mohammad Al Mamun, Mohammad Mahbub Rabbani, Jamal Uddin, and A. J. Saleh Ahammad. 2022. "Sensitivity Control of Hydroquinone and Catechol at Poly(Brilliant Cresyl Blue)-Modified GCE by Varying Activation Conditions of the GCE: An Experimental and Computational Study" ChemEngineering 6, no. 2: 27. https://doi.org/10.3390/chemengineering6020027
APA StyleFaraezi, S., Khan, M. S., Monira, F. Z., Mamun, A. A., Akter, T., Mamun, M. A., Rabbani, M. M., Uddin, J., & Ahammad, A. J. S. (2022). Sensitivity Control of Hydroquinone and Catechol at Poly(Brilliant Cresyl Blue)-Modified GCE by Varying Activation Conditions of the GCE: An Experimental and Computational Study. ChemEngineering, 6(2), 27. https://doi.org/10.3390/chemengineering6020027








