Development of Heavy Metal-Free Photocatalytic RhB Decomposition System Using a Biodegradable Plastic Substrate
Abstract
1. Introduction
2. Materials
Preparation and Characterization of Photocatalysts
3. Methods
3.1. Characterization of Photocatalysts
3.2. Set up Photocatalytic RhB Degradation by Flow System and Batch System
4. Results and Discussion
4.1. Photocatalytic Characterization
4.2. Photocatalytic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, G.K. Solar power generation by pv (photovoltaic) technology: A review. Energy 2013, 53, 1–13. [Google Scholar] [CrossRef]
- Dao, V.D.; Vu, N.H.; Yun, S. Recent advances and challenges for solar-driven water evaporation system toward applications. Nano Energy 2020, 68, 104324. [Google Scholar] [CrossRef]
- Kudo, A. Development of photocatalyst materials for water splitting. Int. J. Hydrog. Energy 2006, 31, 197–202. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Photocatalytic water splitting into H2 and O2 over various tantalate photocatalysts. Catal. Today 2003, 78, 561–569. [Google Scholar] [CrossRef]
- McCullagh, C.; Robertson, J.M.C.; Bahnemann, D.W.; Robertson, P.K.J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef]
- Kahng, S.; Yoo, H.; Kim, J.H. Recent advances in earth-abundant photocatalyst materials for solar H2 production. Adv. Powder Technol. 2020, 31, 11–28. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.A. Mittal Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Rochat, J.; Demenge, P.; Rerat, J.C. Toxicologic study of a fluorescent tracer: Rhodamine b Toxicol. Eur. Res. 1978, 1, 23–26. [Google Scholar]
- Merouani, S.; Hamdaoui, Q.; Saoudi, F. Sonochemical degradation of Rhodamine B in aqueous phase: Effects of additives. Chem. Eng. J. 2010, 158, 550–557. [Google Scholar] [CrossRef]
- Byrne, J.A.; Eggins, B.R.; Brown, N.M.D.; Mckinney, B.; Rouse, M. Immobilisation of TiO2 Powder for the Treatment of Polluted Water. Appl. Catal. B Environ. 1998, 17, 25–36. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Baltanas, M.A.; Cassano, A.E. Supported Titanium Dioxide as Photocatalyst in Water Decontamination: State-of-Art. Catal. Today 1997, 39, 219–231. [Google Scholar] [CrossRef]
- Bideau, M.; Claudel, B.; Dubien, C.; Faure, L.; Kazouan, H. On the immobilization of titanium dioxide in the photocatalytic oxidation of spent waters. J. Photochem. Photobiol. A 1995, 91, 137–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Crittenden, J.C.; Hand, D.W.; Parram, D.L. Fixed-bed photocatalysts for solar decontamination of water Environ. Sci. Technol. 1994, 28, 435–442. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.C. Photocatalyst TiO2 Supported on Glass Fiber for Indoor Air Purification: Effect of NO on the Photodegradation of CO and NO2. J. Photochem. Photobiol. A 2003, 156, 171–177. [Google Scholar] [CrossRef]
- Rao, N.N.; Chaturvedi, V. Photoactivity of TiO2 Coated Pebbles. Ind. Eng. Chem. Res. 2007, 46, 4406–4414. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.T. Preparation and Catalytic Property Study of a Heavy Kind of Suspended Photocatalyst of TiO2 activated Carbon Immobilized on Silicone Rubber Film. Mater. Chem. Phys. 2005, 92, 604–608. [Google Scholar] [CrossRef]
- Fernandez, A.; Lasaletta, G.; Jimenez, V.M.; Justo, A.; Gonzallez-Elipe, A.R.; Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y. Preparation and Characterization of TiO2 Photocatalysts Supported on Various Rigid Supports (Glass, Quartz and Stainless Steel). Comparative Studies of Photocatalytic Activity in Water Purification. Appl. Catal. B 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Oh, H.J.; Dao, V.D.; Choi, H.S. Electromagnetic shielding effectiveness of a thin silver layer deposited onto PET film via atmospheric pressure plasma reduction. Appl. Surf. Sci. 2018, 435, 7–15. [Google Scholar] [CrossRef]
- Mani, T.; Primpke, S.; Lorenz, C.; Gerdts, G.; Burkhardt-Holm, P. Microplastic pollution in benthic mid-stream sediments of the Rhine River//Microplastic Pollution in Benthic Midstream Sediments of the Rhine River. Environ. Sci. Technol. 2019, 53, 6053–6062. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, H. Adsorption of organic pollutants on (degradable) microplastics and the influences on their bioavailability. Environ. Chem. 2018, 37, 375–382. [Google Scholar]
- Isobe, A.; Iwasaki, S.; Uchida, K.; Tokai, T. Abundance of non-conservative microplastics in the upper ocean from 1957–2066. Nat. Commun. 2019, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; Van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef]
- Eling, B.; Gogolewski, S.; Pennings, A.J. Biodegadable materials of poly (l-lactic acid). 1. Melt-spun and solution spun fibers. Polymer 1982, 23, 1587–1593. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Wu, Y.L.; Wang, H.; Qiu, Y.K.; Loh, X.J. PLA-based thermogel for the sustained delivery of chemotherapeutics in a mouse model of hepatocellular carcinoma. RSC Adv. 2016, 6, 44506–44513. [Google Scholar] [CrossRef]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Glotova, V.N.; Bikmullina, T.N.; Lukianov, A.E.; Novikov, V.T. Lactide and Lactic Acid Oligomer Solubility in Certain Solvents. Petrol. Coal 2016, 58, 585–589. [Google Scholar]
- Lee, G.-J.; Wu, J.J. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—A review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Gou, X.L.; Cheng, F.Y.; Shi, Y.H.; Zhang, L.; Peng, S.J.; Chen, J.; Shen, P.W. Shape-controlled synthesis of ternary chalcogenide ZnIn2S4 and CuIn(S,Se)2 nano-/microstructures via facile solution route. J. Am. Chem. Soc. 2006, 128, 7222–7229. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Martha, S.; Parida, K. Facile Synthesis of Au/g-C3N4 Nanocomposites: An Inorganic/Organic Hybrid Plasmonic Photocatalyst with Enhanced Hydrogen Gas Evolution Under Visible-Light Irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar]
- Zhang, R.; Li, P.; Wang, F.; Ye, L.; Gaur, A.; Huang, Z.; Zhao, Z.; Bai, Y.; Zhou, Y. Atomically dispersed Mo atoms on amorphous g-C3N4 promotes visible-light absorption and charge carriers transfer. Appl. Catal. B Environ. 2019, 250, 273–279. [Google Scholar] [CrossRef]
- Zhu, B.; Wageh, S.; Al-Ghamdi, A.; Yang, S.; Tian, Z.; Yu, J. Adsorption of CO2, O2, NO and CO on s-triazine-based g-C3N4 surface. Catal. Today 2019, 335, 117–127. [Google Scholar] [CrossRef]
- Yan, X.; Gao, Q.; Qin, J.; Hui, X.; Ye, Z.; Li, J.; Ma, Z. A facile method for fabricating TiO2/g-C3N4 hollow nanotube heterojunction and its visible light photocatalytic performance. Mater. Lett. 2018, 217, 1–4. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F.; Wang, J.; Li, S.; Zhang, H. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2018, 225, 307–313. [Google Scholar] [CrossRef]
- Huy, B.T.; Thao, C.T.B.; Dao, V.D.; Phuong, N.T.K.; Lee, Y.I. A mixed-metal oxide/graphitic carbon nitride: High visible light photocatalytic activity for efficient mineralization of Rhodamine B. Adv. Mater. Interfaces 2018, 4, 1700128. [Google Scholar] [CrossRef]
- Wang, S.M.; Li, D.L.; Sun, C.; Yang, S.G.; Guan, Y.; He, H. Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenyl methane dye degradation. Appl. Catal. B Environ. 2014, 144, 885–892. [Google Scholar] [CrossRef]
- Chang, F.; Xie, Y.; Li, C.; Chen, J.; Luo, J.; Hu, X.; Shen, J. A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl. Surf. Sci. 2013, 280, 967–974. [Google Scholar] [CrossRef]
- Han, C.C.; Ge, L.; Chen, C.F.; Li, Y.J.; Xiao, X.L.; Zhang, Y.N.; Guo, L.L. Heavy visible light induced Co3O4-g-C3N4 heterojunction photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2014, 147, 546–553. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbail, P.; Ekicibil, A.; Metin, Ö. Monodisperse cobalt ferrite nanoparticles assembled on mesoporousgraphitic carbon nitride (CoFe2O4/mpg-C3N4): A magnetically recoverable nanocomposite for the photocatalytic degradation of organic dyes. J. Magn. Magn. Mater. 2018, 456, 400–412. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, X.; Zhu, Y.; Fu, X.H.; Zhang, Y.M. 3D-2D-3D BiOI/porous g-C3N4/graphene hydrogel composite photocatalyst with synergy of adsorption-photocatalysis in static and flow systems. J. Alloy. Compd. 2021, 850, 156778. [Google Scholar] [CrossRef]
- Jain, P.; Kumar, A.; Verma, N.; Gupta, R.K. In-situ synthesis of TiO2 nanoparticles in ACF: Photocatalytic degradation under continuous flow. Sol. Energy 2019, 189, 35–44. [Google Scholar] [CrossRef]
- Wang, C.; Luo, S.; Liu, C.; Chen, C. WO3 quantum dots enhanced the photocatalytic performances of graphene oxide/TiO2 films under flowing dye solution. Inorg. Chem. Commun. 2020, 115, 107875. [Google Scholar] [CrossRef]
- Carbonaro, S.; Sugihara, M.N.; Strathmann, T.J. Continuous-flow photocatalytic treatment of pharmaceutical micropollutants: Activity, inhibition, and deactivation of TiO2 photocatalysts in wastewater effluent. Appl. Catal. B Environ. 2013, 129, 1–12. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]

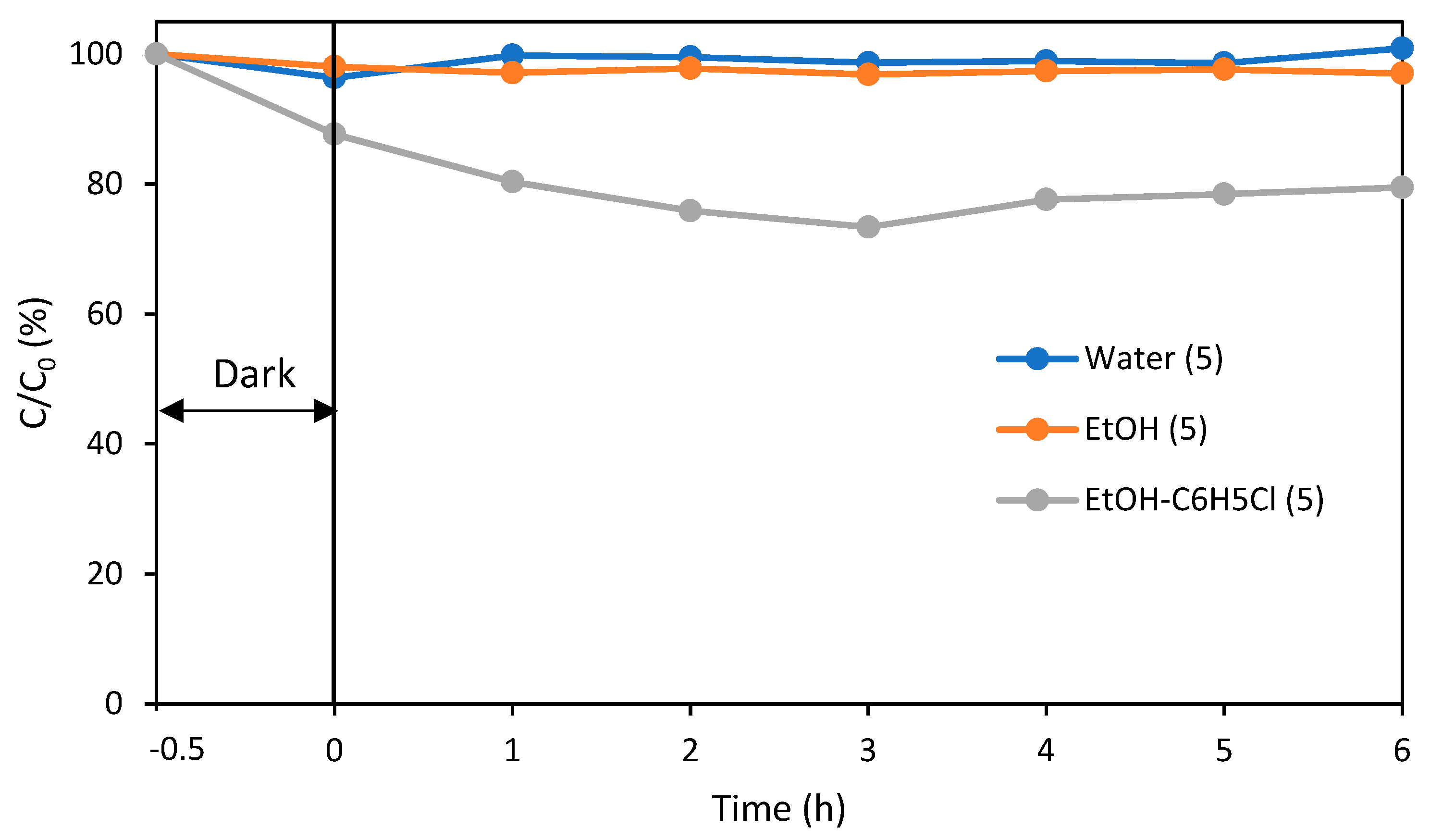

| Sample | Rhodamine B (5 ppm, 42 mL) |
| Temperature | Room temperature (25 °C) |
| Photocatalyst | PLA rings/NT-500 (3 g) |
| Flow rate | 0.700 mL/min |
| Light source | Xenon lamp with cut filter (6000–7000 μW/cm2, λ ≥ 420 nm) |
| Irradiation time | 0–6 h |
| Analysis | UV-visible spectrometer (554 nm) |
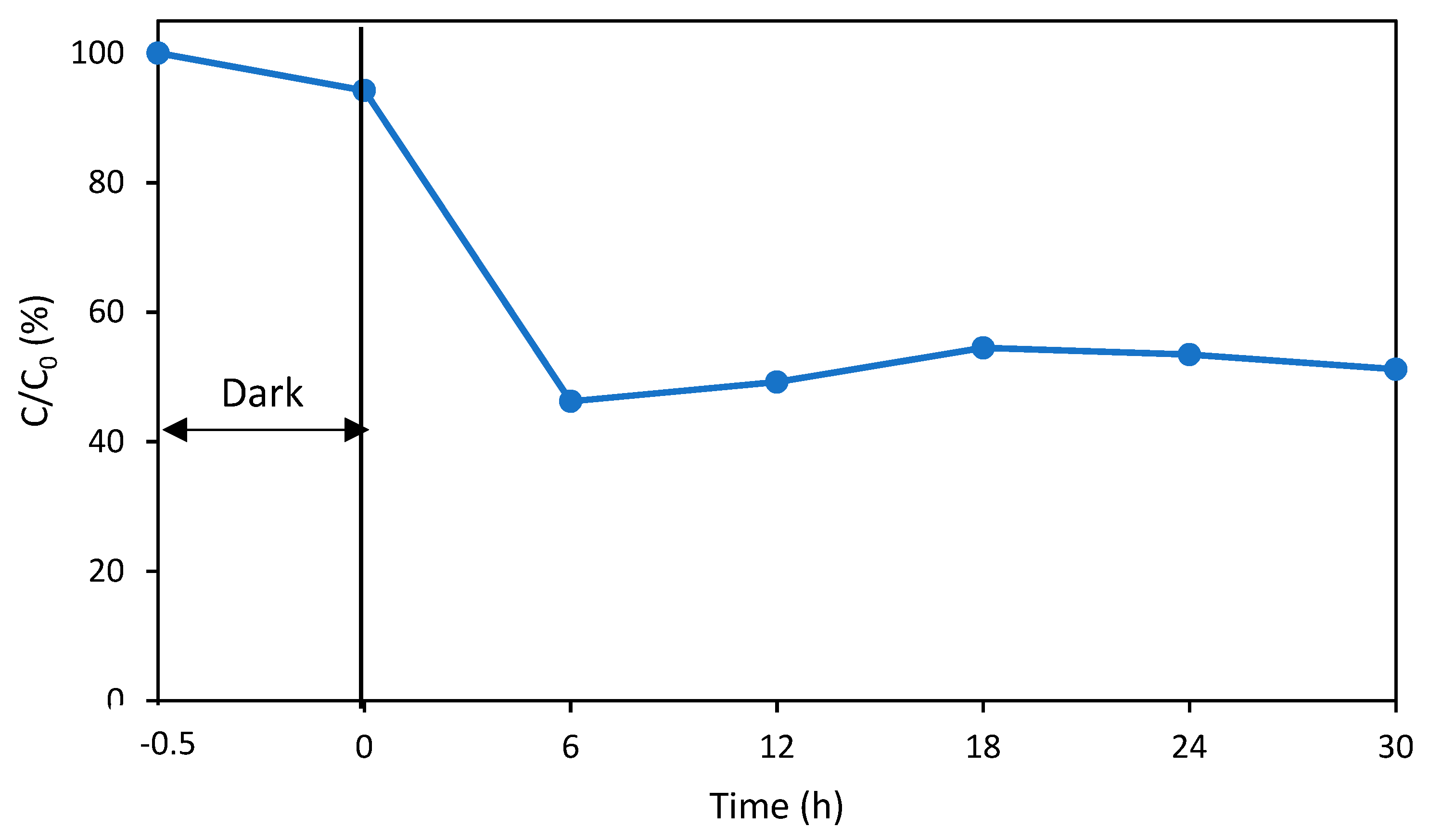
| Sample | Rhodamine B (5 ppm, 42 mL) |
| Temperature | Room temperature (25 °C) |
| Photocatalyst | Polylactic acid rings/NT-500 (3 g) |
| Flow rate | 0.350 mL/min |
| Light source | Xenon lamp (15,000–16,000 μW/cm2) |
| Irradiation time | 0–6 h or 30 h |
| Analysis | UV-visible spectrometer (554 nm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tateishi, I.; Furukawa, M.; Katsumata, H.; Kaneco, S. Development of Heavy Metal-Free Photocatalytic RhB Decomposition System Using a Biodegradable Plastic Substrate. ChemEngineering 2021, 5, 11. https://doi.org/10.3390/chemengineering5010011
Tateishi I, Furukawa M, Katsumata H, Kaneco S. Development of Heavy Metal-Free Photocatalytic RhB Decomposition System Using a Biodegradable Plastic Substrate. ChemEngineering. 2021; 5(1):11. https://doi.org/10.3390/chemengineering5010011
Chicago/Turabian StyleTateishi, Ikki, Mai Furukawa, Hideyuki Katsumata, and Satoshi Kaneco. 2021. "Development of Heavy Metal-Free Photocatalytic RhB Decomposition System Using a Biodegradable Plastic Substrate" ChemEngineering 5, no. 1: 11. https://doi.org/10.3390/chemengineering5010011
APA StyleTateishi, I., Furukawa, M., Katsumata, H., & Kaneco, S. (2021). Development of Heavy Metal-Free Photocatalytic RhB Decomposition System Using a Biodegradable Plastic Substrate. ChemEngineering, 5(1), 11. https://doi.org/10.3390/chemengineering5010011





