Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization
2.3. Photocatalytic Activity
3. Results and Discussion
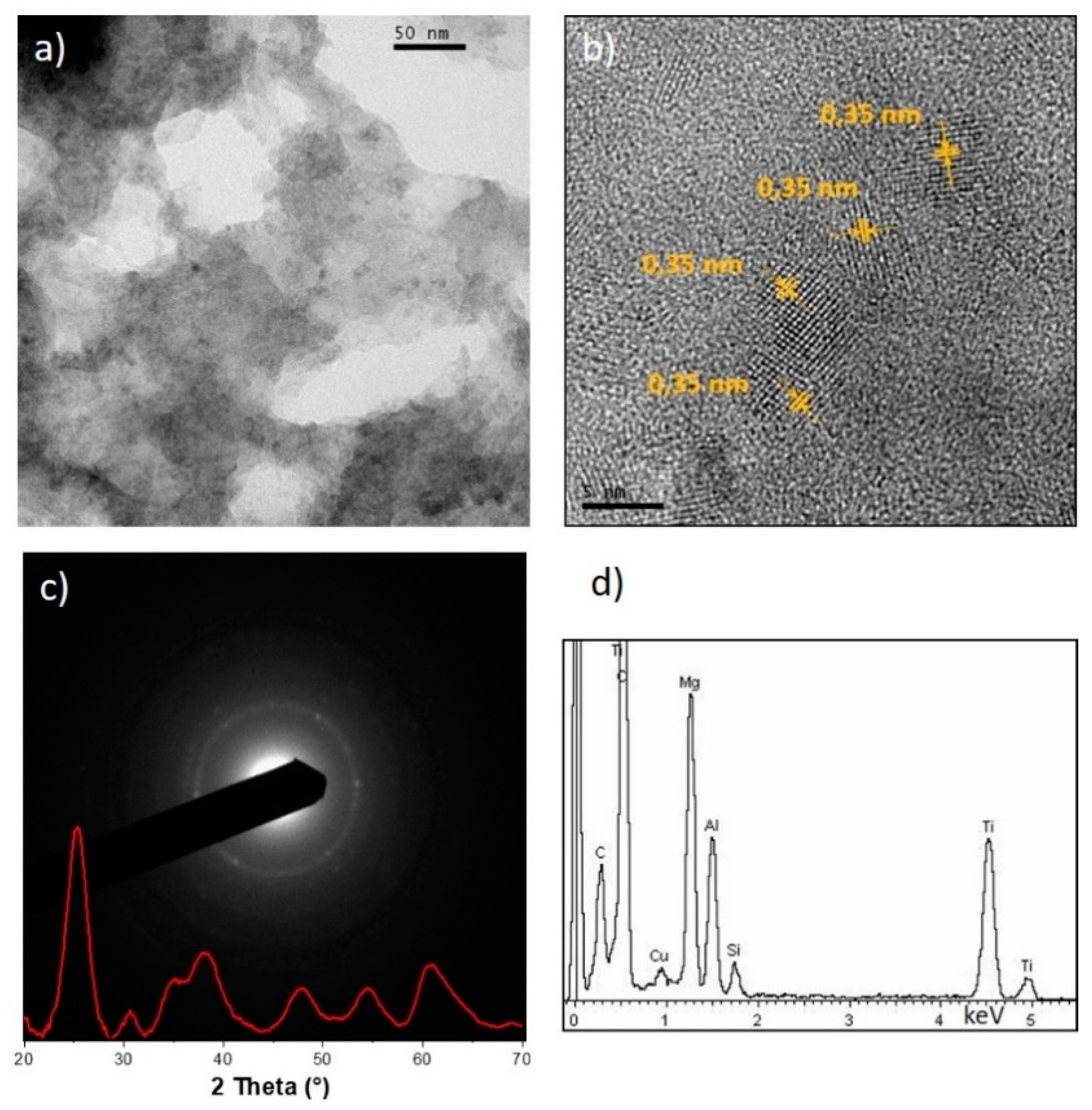
3.1. TiO2 Colloidal Dispersion
3.2. LDH/TiO2 Nanocomposite Preparation and Characterization
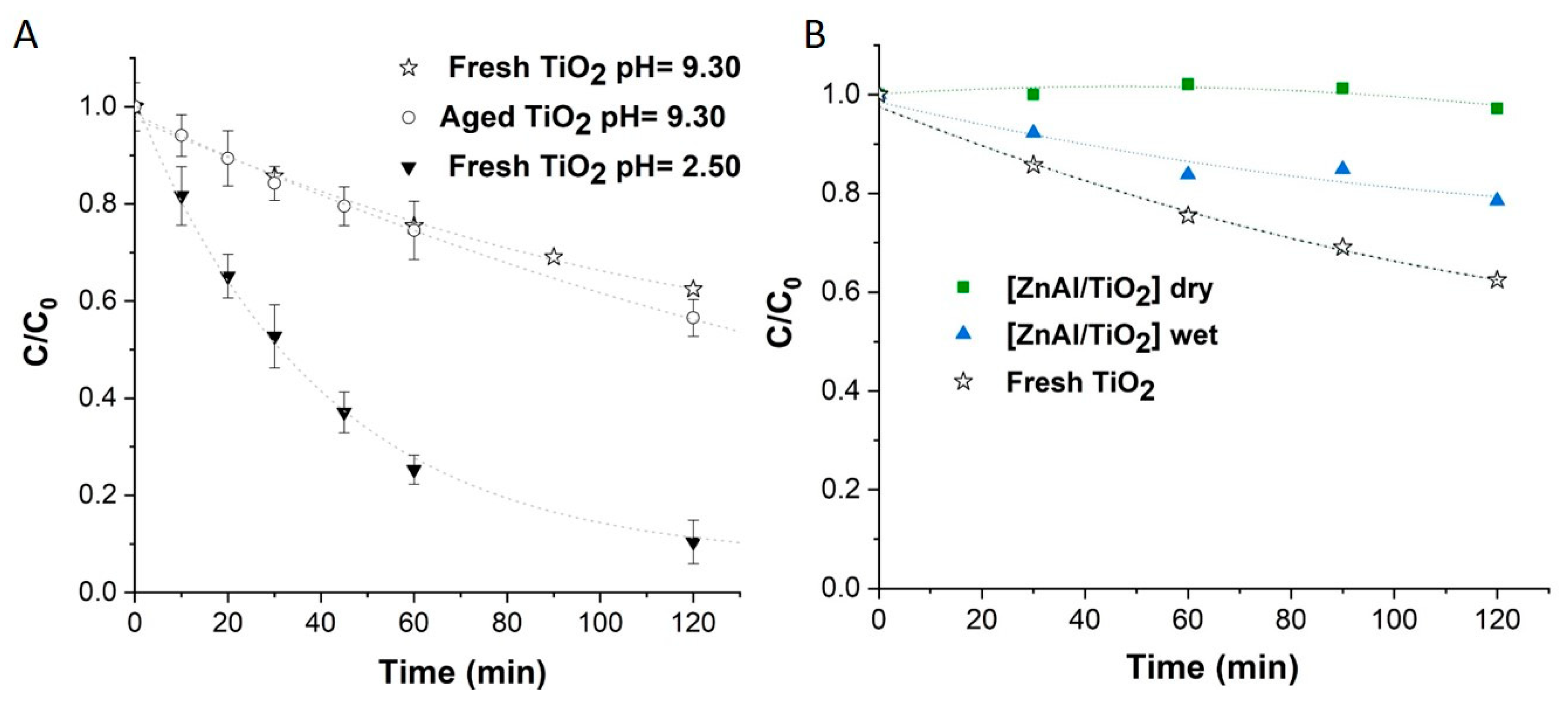
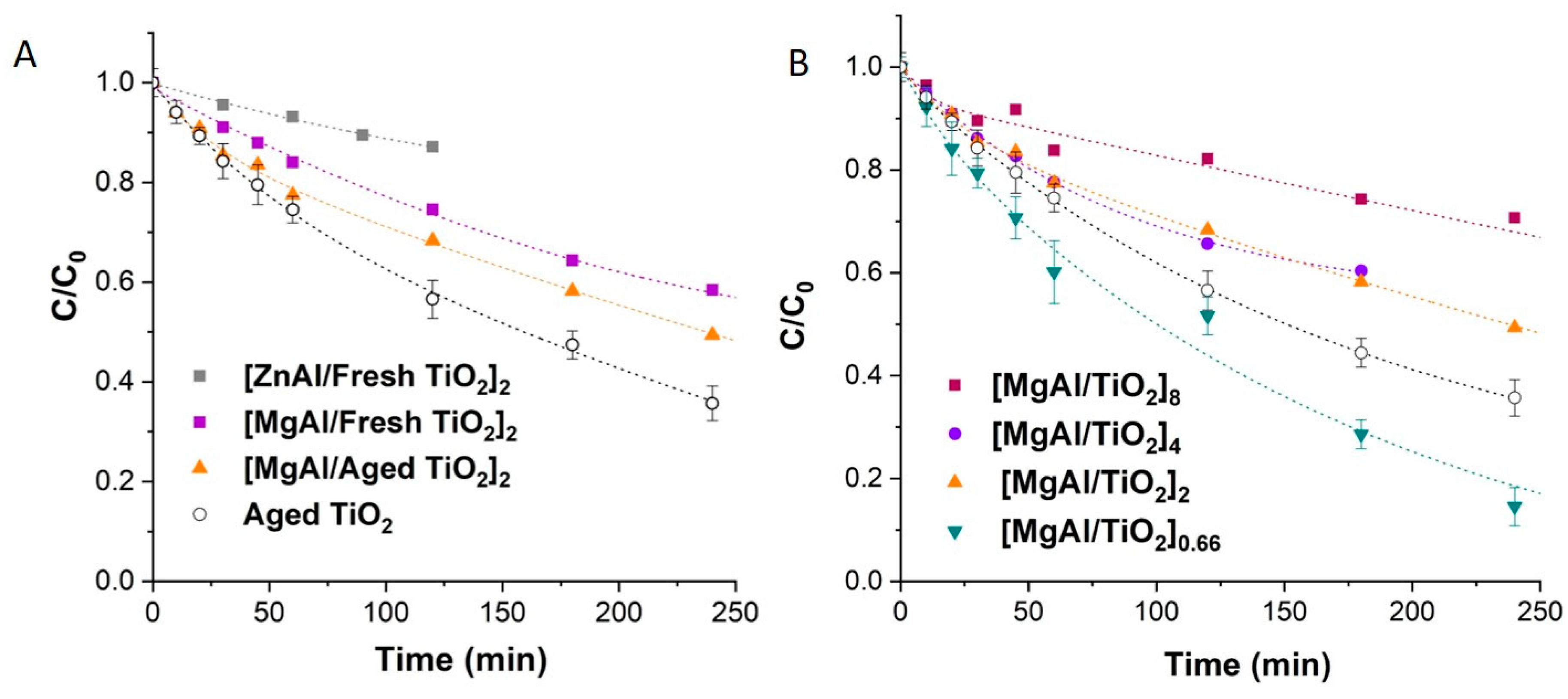
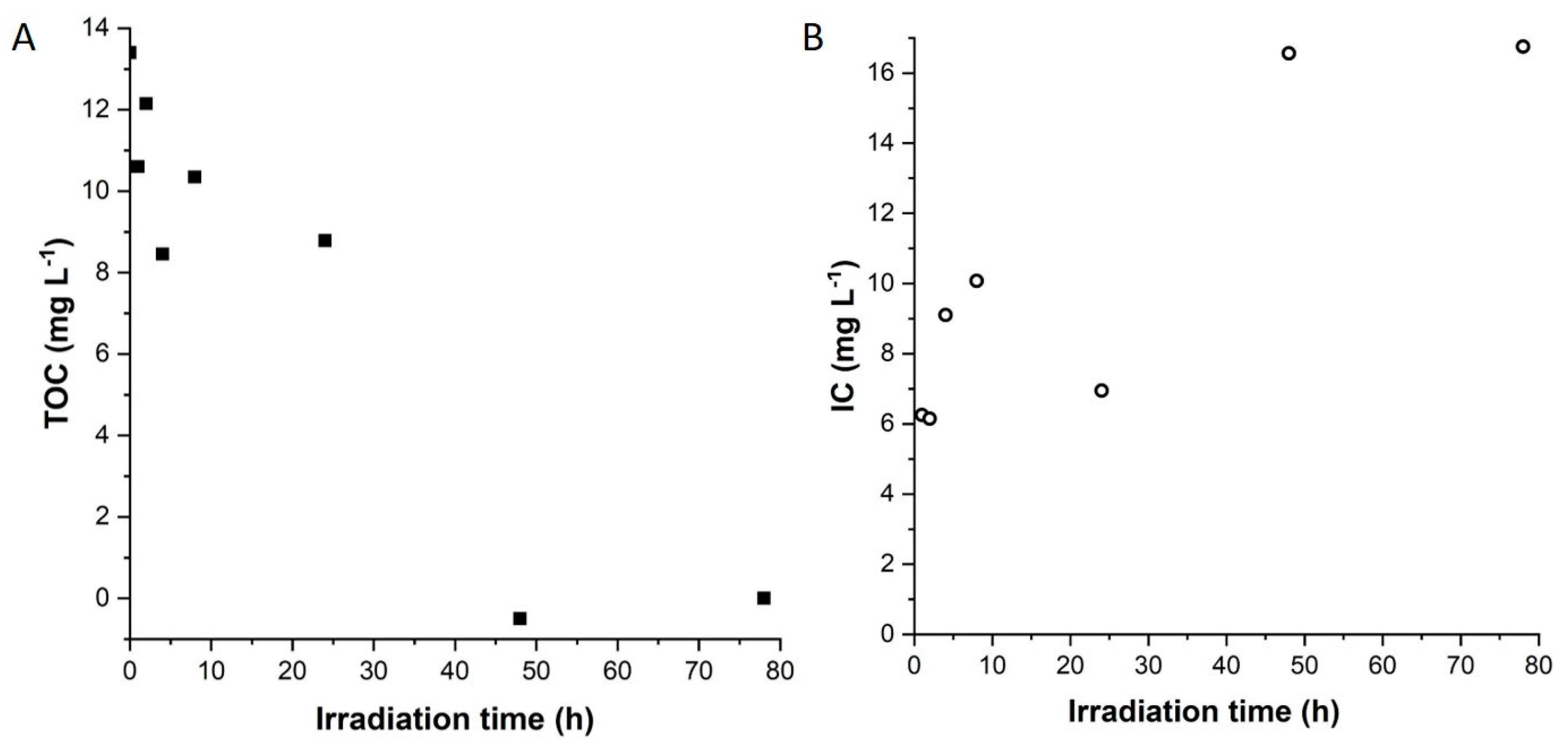
3.3. Photodegradation of OII in the Presence of Colloidal TiO2 and LDH Based Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Anal. Chem. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Anisuzzaman, S.M.; Bono, A.; Sarbatly, R. Adsorption of 2,4,6-trichlorophenol (TCP) onto activated carbon. J. King Saud Univ. Sci. 2013, 25, 251–255. [Google Scholar] [CrossRef]
- Sanghi, R.; Bhattacharya, B. Review on decolorisation of aqueous dye solutions by low cost adsorbents. Coloration Technology 2002, 118, 256–269. [Google Scholar] [CrossRef]
- Kuśmierek, K. The removal of chlorophenols from aqueous solutions using activated carbon adsorption integrated with H2O2 oxidation. React. Kinet. Mech. Catal. 2016, 119, 19–34. [Google Scholar] [CrossRef]
- Khorsandi, H.; Ghochlavi, N.; Aghapour, A.A. Biological Degradation of 2,4,6-Trichlorophenol by a Sequencing Batch Reactor. Environ. Process. 2018, 5, 907–917. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, S.-P.; Thiruvenkatachari, R.; Shim, W.-G.; Moon, H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigm. 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Secula, M.S.; Creţescu, I.; Petrescu, S. An experimental study of indigo carmine removal from aqueous solution by electrocoagulation. Desalination 2011, 277, 227–235. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Delgado-Regalado, A. Removal of Carmine Indigo Dye with Moringa oleifera Seed Extract. Ind. Eng. Chem. Res. 2009, 48, 6512–6520. [Google Scholar] [CrossRef]
- Virtanen, M.T.; Hattula, M.-L. The fate of 2,4,6-trichlorophenol in an aquatic continuous-flow system. Chemosphere 1982, 11, 641–649. [Google Scholar] [CrossRef]
- O’Neill, C.; Hawkes, F.R.; Hawkes, D.L.; Lourenço, N.D.; Pinheiro, H.M.; Delée, W. Colour in textile effluents—Sources, measurement, discharge consents and simulation: A review. J. Chem. Technol. Biotechnol. 1999, 74, 1009–1018. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, J. Hierarchical-Structured Anatase-Titania/Cellulose Composite Sheet with High Photocatalytic Performance and Antibacterial Activity. Chem. Eur. J. 2015, 21, 2568–2575. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Huang, C.; Yao, W.; Wu, Q.; Xu, Q. Synthesis and photocatalytic activity of ultrafine Ag3PO4 nanoparticles on oxygen vacated TiO2. Appl. Catal., B 2017, 205, 489–497. [Google Scholar] [CrossRef]
- Gutiérrez-Segura, E.; Solache-Ríos, M.; Colín-Cruz, A. Sorption of indigo carmine by a Fe-zeolitic tuff and carbonaceous material from pyrolyzed sewage sludge. J. Hazard. Mater. 2009, 170, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.D. The Surface of Titanium Dioxide. In Progress in Surface and Membrane Science; Cadenhead, D.A., Danielli, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1976; Volume 11, pp. 181–226. [Google Scholar]
- Augustynski, J. Aspects of photo-electrochemical and surface behaviour of titanium (IV) oxide. In Solid Materials; Springer: Berlin/Heidelberg, Germany, 1988; pp. 1–61. [Google Scholar]
- Ohtani, B.; Nishimoto, S. Effect of surface adsorptions of aliphatic alcohols and silver ion on the photocatalytic activity of titania suspended in aqueous solutions. J. Phys. Chem. 1993, 97, 920–926. [Google Scholar] [CrossRef]
- Mihaylov, B.V.; Hendrix, J.L.; Nelson, J.H. Comparative catalytic activity of selected metal oxides and sulfides for the photo-oxidation of cyanide. J. Photochem. Photobiol. A 1993, 72, 173–177. [Google Scholar] [CrossRef]
- Karakitsou, K.E.; Verykios, X.E. Effects of altervalent cation doping of titania on its performance as a photocatalyst for water cleavage. J. Phys. Chem. 1993, 97, 1184–1189. [Google Scholar] [CrossRef]
- Goel, M.; Chovelon, J.-M.; Ferronato, C.; Bayard, R.; Sreekrishnan, T.R. The remediation of wastewater containing 4-chlorophenol using integrated photocatalytic and biological treatment. J. Photochem. Photobiol. B 2010, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Mirzamohammady, M.; Vahid, B.; Behnajady, B. Increasing photoactivity of titanium dioxide immobilized on glass plate with optimization of heat attachment method parameters. J. Hazard. Mater. 2008, 160, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.R.; Pons, M.N.; Zahraa, O. Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation: Influence of dye molecular structure. J. Hazard. Mater. 2009, 168, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, D.; Lianos, P.; Yianoulis, P.; Koutsoukos, P. Sol-Gel Derived TiO2 Microemulsion Gels and Coatings. Langmuir 1994, 10, 1684–1689. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.F.; Brodie, D.E. Photoconducting TiO2 prepared by spray pyrolysis using TiCl4. Thin Solid Films 1992, 213, 265–270. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 2019, 219, 804–825. [Google Scholar] [CrossRef] [PubMed]
- Carreon, M.A.; Choi, S.Y.; Mamak, M.; Chopra, N.; Ozin, G.A. Pore architecture affects photocatalytic activity of periodic mesoporous nanocrystalline anatase thin films. J. Mater. Chem. 2007, 17, 82–89. [Google Scholar] [CrossRef]
- Carreon, M.L.; Carreon, H.G.; Espino-Valencia, J.; Carreon, M.A. Photocatalytic degradation of organic dyes by mesoporous nanocrystalline anatase. Mater. Chem. Phys. 2011, 125, 474–478. [Google Scholar] [CrossRef]
- Katti, A.; Venna, S.R.; Carreon, M.A. Self-assembly hydrothermal assisted synthesis of mesoporous anatase in the presence of ethylene glycol. Catal. Commun. 2009, 10, 2036–2040. [Google Scholar] [CrossRef]
- Khammar, S.; Bahramifar, N.; Younesi, H. Preparation and surface engineering of CM-β-CD functionalized Fe3O4@TiO2 nanoparticles for photocatalytic degradation of polychlorinated biphenyls (PCBs) from transformer oil. J. Hazard. Mater. 2020, 394, 122422. [Google Scholar] [CrossRef] [PubMed]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Chapter 14.1—Layered Double Hydroxides (LDH). In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar]
- Rives, V. (Ed.) Layered Double Hydroxides: Present and Future; Nova Science Publishers, Inc.: New York, NY, USA, 2001; p. 439. [Google Scholar]
- Chubar, N.; Gilmour, R.; Gerda, V.; Micusik, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.; Gao, J.; Wu, F.; Zhang, X. High performance NiFe layered double hydroxide for methyl orange dye and Cr(VI) adsorption. Chemosphere 2016, 152, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, B.; Bantignies, J.-L.; Le-Parc, R.; Prélot, B.; Zajac, J.; Layrac, G.; Tichit, D.; Martin-Gassin, G. Multiscale Mechanistic Study of the Adsorption of Methyl Orange on the External Surface of Layered Double Hydroxide. J. Phys. Chem. C 2019, 123, 22212–22220. [Google Scholar] [CrossRef]
- Bouhent, M.; Derriche, Z.; Denoyel, R.; Prevot, V.; Forano, C. Thermodynamical and structural insights of orange II adsorption by MgRAlNO3 layered double hydroxides. J. Solid State Chem. 2011, 184, 1016–1024. [Google Scholar] [CrossRef]
- Chivu, V.; Gilea, D.; Cioatera, N.; Carja, G.; Mureseanu, M. Heterostructures of Ce-Ti/layered double hydroxides and the derived MMOs for photoenergy applications. Appl. Surf. Sci. 2020, 513, 145853. [Google Scholar] [CrossRef]
- Egambaram, O.P.; Pillai, S.K.; Lategan, M.; Ray, S.S. Nanostructured Zn-Ti layered double hydroxides with reduced photocatalytic activity for sunscreen application. J. Nanopart. Res. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Cao, M. NiFe Layered Double Hydroxide on Nitrogen Doped TiO2 Nanotube Arrays toward Efficient Oxygen Evolution. ACS Appl. Energy Mater. 2019, 2, 5960–5967. [Google Scholar] [CrossRef]
- Pourradi, H.; Ghani, K.; Mahdavi, M. Advanced Interface Engineering of CH3NH3PbI3 Perovskite Solar Cells: The Unique Role of Layered Double Hydroxide Precursor. ACS Appl. Energy Mater. 2020, 3, 1476–1483. [Google Scholar] [CrossRef]
- Ran, L.; Yin, L. Ternary Hierarchical Cu7S4/TiO2/CoCr-LDH Heterostructured Nanorod Arrays with Multiphase Reaction Interfaces for More Efficient Photoelectrochemical Water Splitting. Adv. Mater. Interfaces 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Vucetic, S.B.; Rudic, O.L.; Markov, S.L.; Bera, O.J.; Vidakovic, A.M.; Skapin, A.S.S.; Ranogajec, J.G. Antifungal efficiency assessment of the TiO2 coating on facade paints. Environ. Sci. Pollut. Res. 2014, 21, 11228–11237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: A review. Chem. Eng. J. (Amsterdam Neth.) 2020, 392, 123684. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Q.; Li, Z.; Liu, J.; Jing, X.; Zhang, H.; Wang, J. Synthesis of exfoliated titanium dioxide nanosheets/nickel-aluminum layered double hydroxide as a novel electrode for supercapacitors. RSC Adv. 2015, 5, 49204–49210. [Google Scholar] [CrossRef]
- Ziarati, A.; Badiei, A.; Grillo, R.; Burgi, T. 3D Yolk@Shell TiO2-x/LDH Architecture: Tailored Structure for Visible Light CO2 Conversion. ACS Appl. Mater. Interfaces 2019, 11, 5903–5910. [Google Scholar] [CrossRef] [PubMed]
- Kun, R.; Balazs, M.; Dekany, I. Photooxidation of organic dye molecules on TiO2 and zinc-aluminum layered double hydroxide ultrathin multilayers. Colloids Surf. A 2005, 265, 155–162. [Google Scholar] [CrossRef]
- Mourid, E.H.; El Mouchtari, E.M.; El Mersly, L.; Benaziz, L.; Rafqah, S.; Lakraimi, M. Development of a new recyclable nanocomoposite LDH-TiO2 for the degradation of antibiotic sulfamethoxazole under UVA radiation: An approach towards sunlight. J. Photochem. Photobiol. A 2020, 396, 112530. [Google Scholar] [CrossRef]
- Seftel, E.M.; Niarchos, M.; Vordos, N.; Nolan, J.W.; Mertens, M.; Mitropoulos, A.C.; Vansant, E.F.; Cool, P. LDH and TiO2/LDH-type nanocomposite systems: A systematic study on structural characteristics. Microporous Mesoporous Mater. 2015, 203, 208–215. [Google Scholar] [CrossRef]
- Paušová, Š.; Krýsa, J.; Jirkovský, J.; Forano, C.; Prevot, V.; Mailhot, G. Photocatalytic properties of aqueous systems containing TiO2 nanoparticles. Catal. Today 2011, 161, 140–146. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of chloroform and other organic molecules in aqueous titanium dioxide suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Pausova, S.; Krysa, J.; Jirkovsky, J.; Mailhot, G.; Prevot, V. Photocatalytic behavior of nanosized TiO2 immobilized on layered double hydroxides by delamination/restacking process. Environ. Sci. Pollut. Res. 2012, 19, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Onam Onyatta, J.; Kimutai Tum, P.; Kithure, J.G.N.; Oduor, F.D.O. Photocatalytic degradation of acid Orange II dye on selected commercial titanium dioxide catalysts. Int. J. Adv. Res. 2016, 4, 1149–1155. [Google Scholar]
- Paušová, Š.; Krýsa, J.; Jirkovský, J.; Forano, C.; Mailhot, G.; Prevot, V. Insight into the photocatalytic activity of ZnCr–CO3 LDH and derived mixed oxides. Appl. Catal. B 2015, 170–171, 25–33. [Google Scholar] [CrossRef]
- Paušová, Š.; Krýsa, J.; Jirkovský, J.; Prevot, V.; Mailhot, G. Preparation of TiO2-SiO2 composite photocatalysts for environmental applications. J. Chem. Technol. Biotechnol. 2014, 89, 1129–1135. [Google Scholar] [CrossRef]
- Li, T.; Wang, T.; Qu, G.; Liang, D.; Hu, S. Synthesis and photocatalytic performance of reduced graphene oxide–TiO2 nanocomposites for orange II degradation under UV light irradiation. Environ. Sci. Pollut. Res. 2017, 24, 12416–12425. [Google Scholar] [CrossRef] [PubMed]




| Sample | Mg/Al a | %TiO2 a | w/w LDH/TiO2 | %TiO2 Immobilization | nH2O b | %Weight Loss | BET Surface Area (m2 g−1) |
|---|---|---|---|---|---|---|---|
| [MgAl] | 1.9 | 0 | - | - | 2.6 | 46.2 | 87 |
| [MgAl/TiO2]8 | 1.8 | 8.9 | 10.1 | 81 | 1.5 | 41.6 | 126 |
| [MgAl/TiO2]4 | 1.8 | 18.6 | 4.2 | 93 | 1.6 | 41.4 | 145 |
| [MgAl/TiO2]2 | 1.9 | 28.0 | 2.7 | 84 | 2.3 | 40.1 | 190 |
| [MgAl/TiO2]0.66 | 2.1 | 47.6 | 1.1 | 80 | 2.8 | 27.0 | 253 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djeda, R.; Mailhot, G.; Prevot, V. Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II. ChemEngineering 2020, 4, 39. https://doi.org/10.3390/chemengineering4020039
Djeda R, Mailhot G, Prevot V. Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II. ChemEngineering. 2020; 4(2):39. https://doi.org/10.3390/chemengineering4020039
Chicago/Turabian StyleDjeda, Rodrigue, Gilles Mailhot, and Vanessa Prevot. 2020. "Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II" ChemEngineering 4, no. 2: 39. https://doi.org/10.3390/chemengineering4020039
APA StyleDjeda, R., Mailhot, G., & Prevot, V. (2020). Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II. ChemEngineering, 4(2), 39. https://doi.org/10.3390/chemengineering4020039







