Selective Recovery of Manganese from Anode Sludge Residue by Reductive Leaching
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Leaching Experiments
3. Results and Discussion
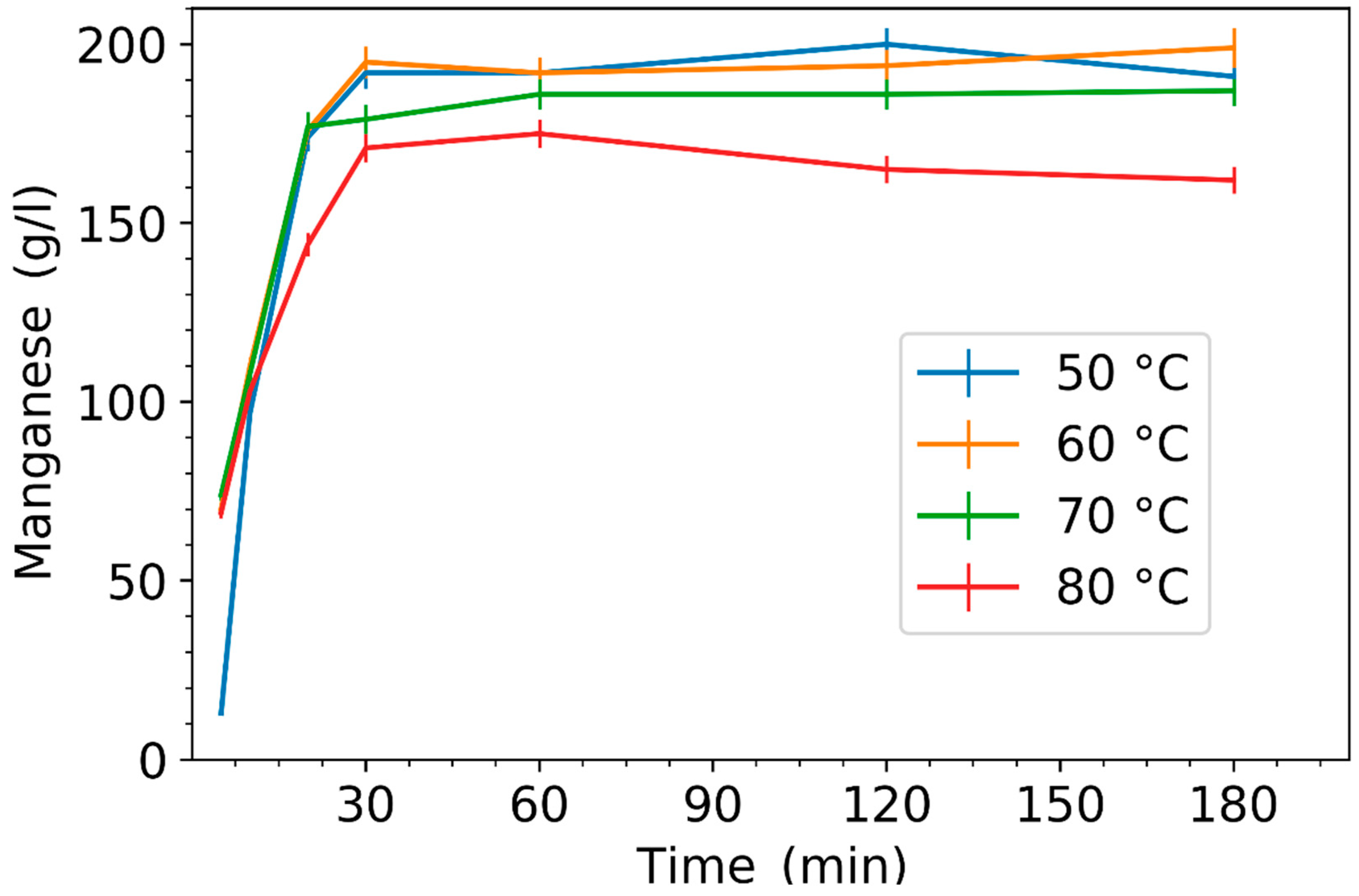
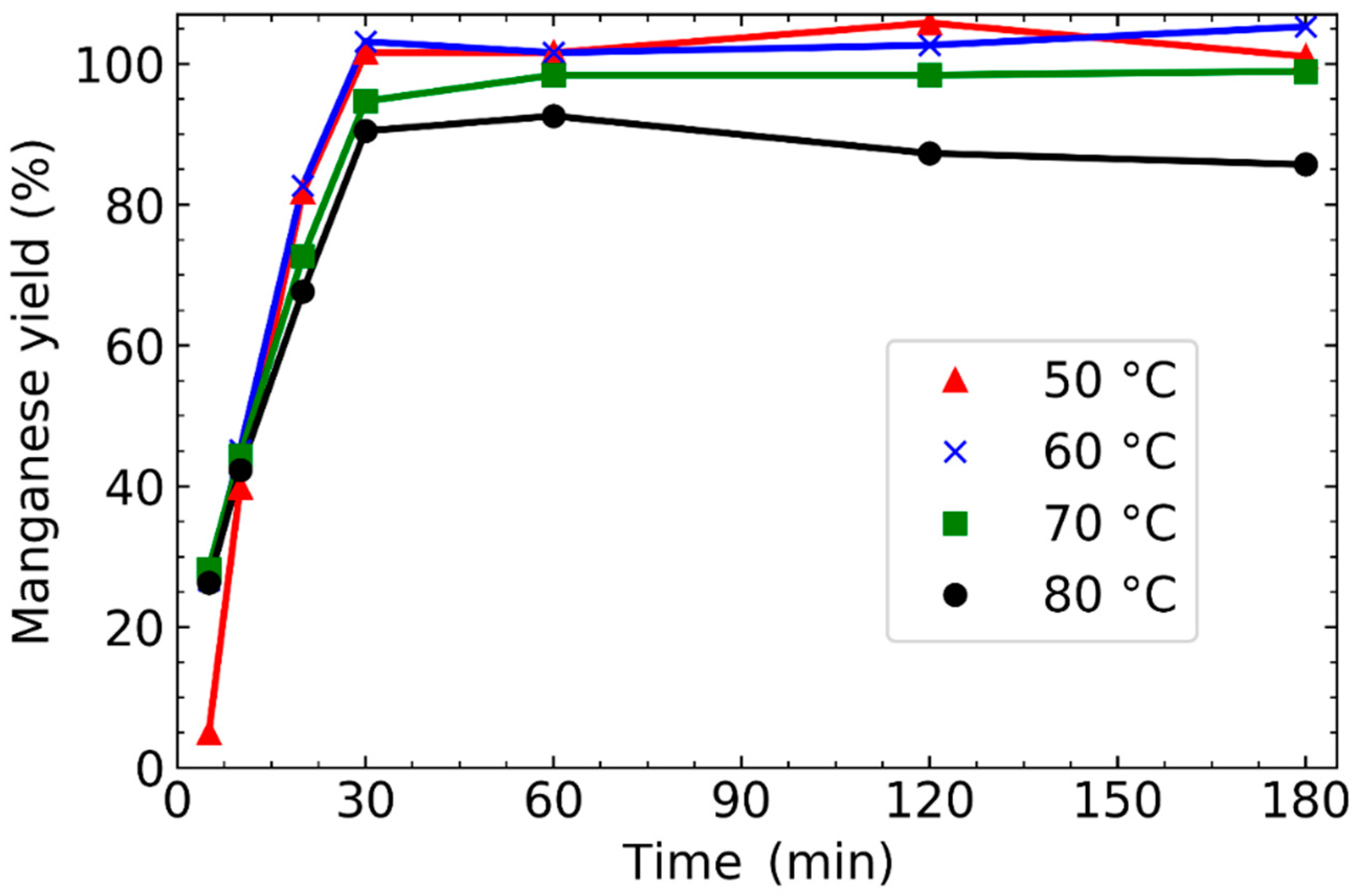
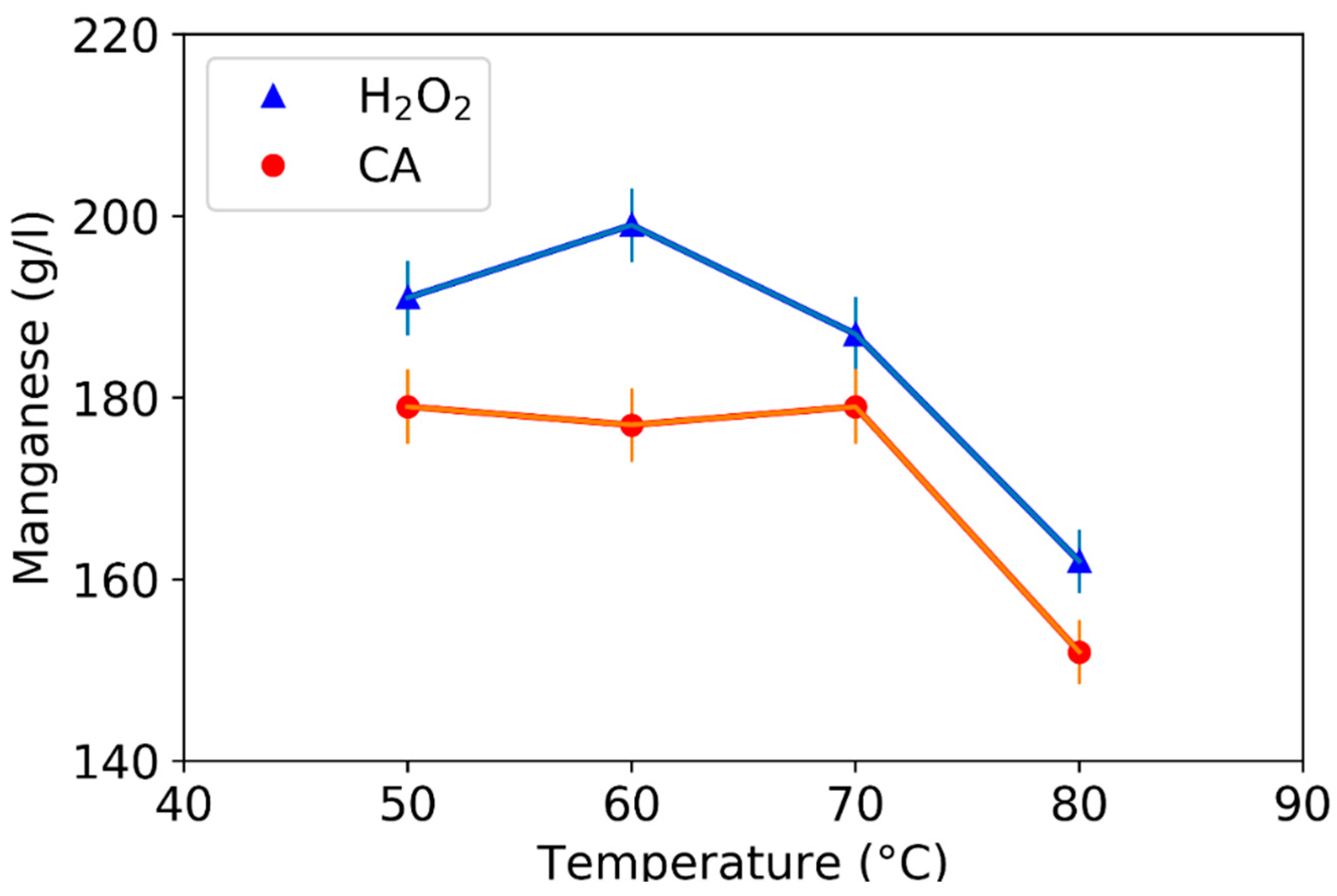
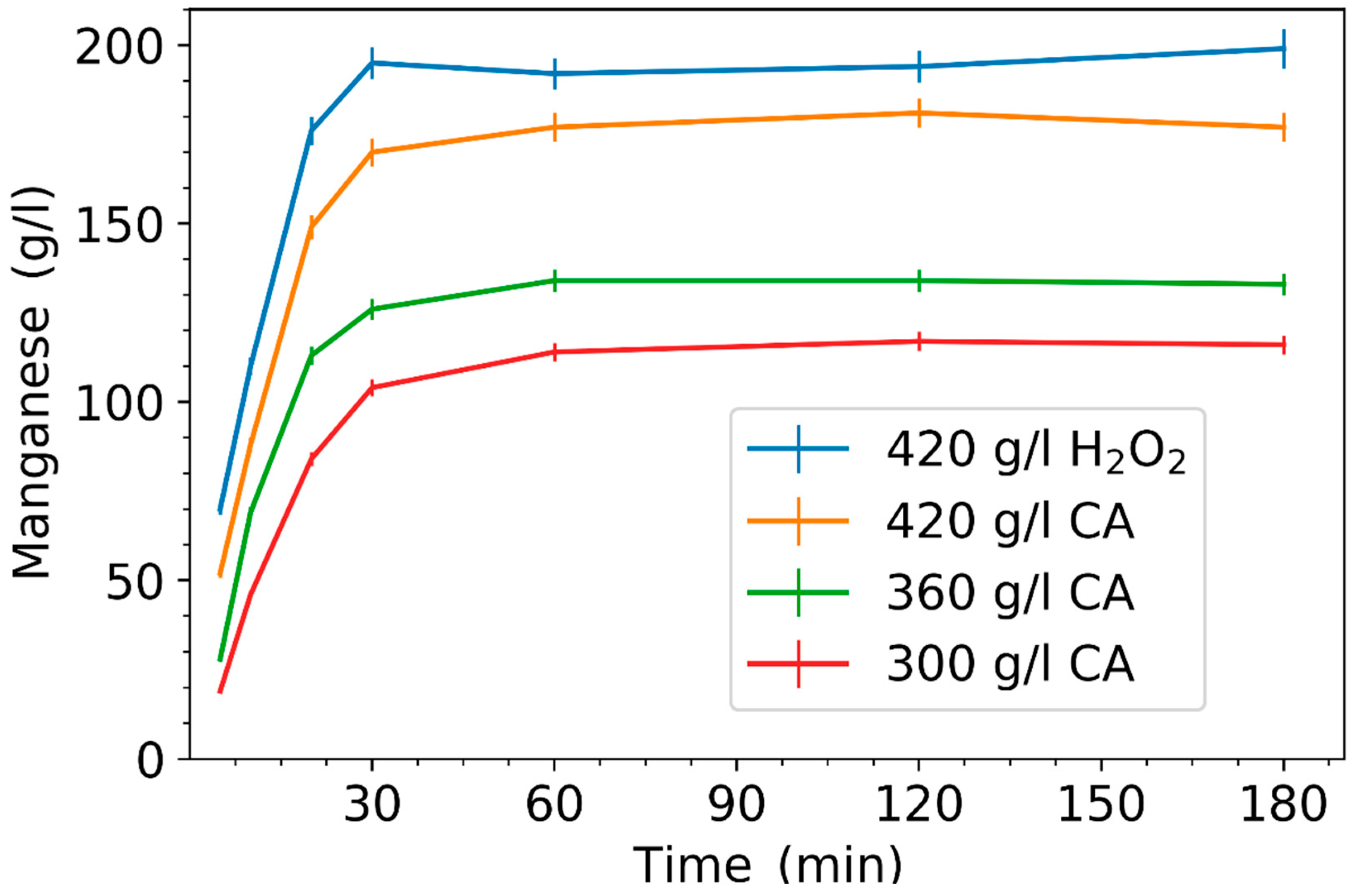
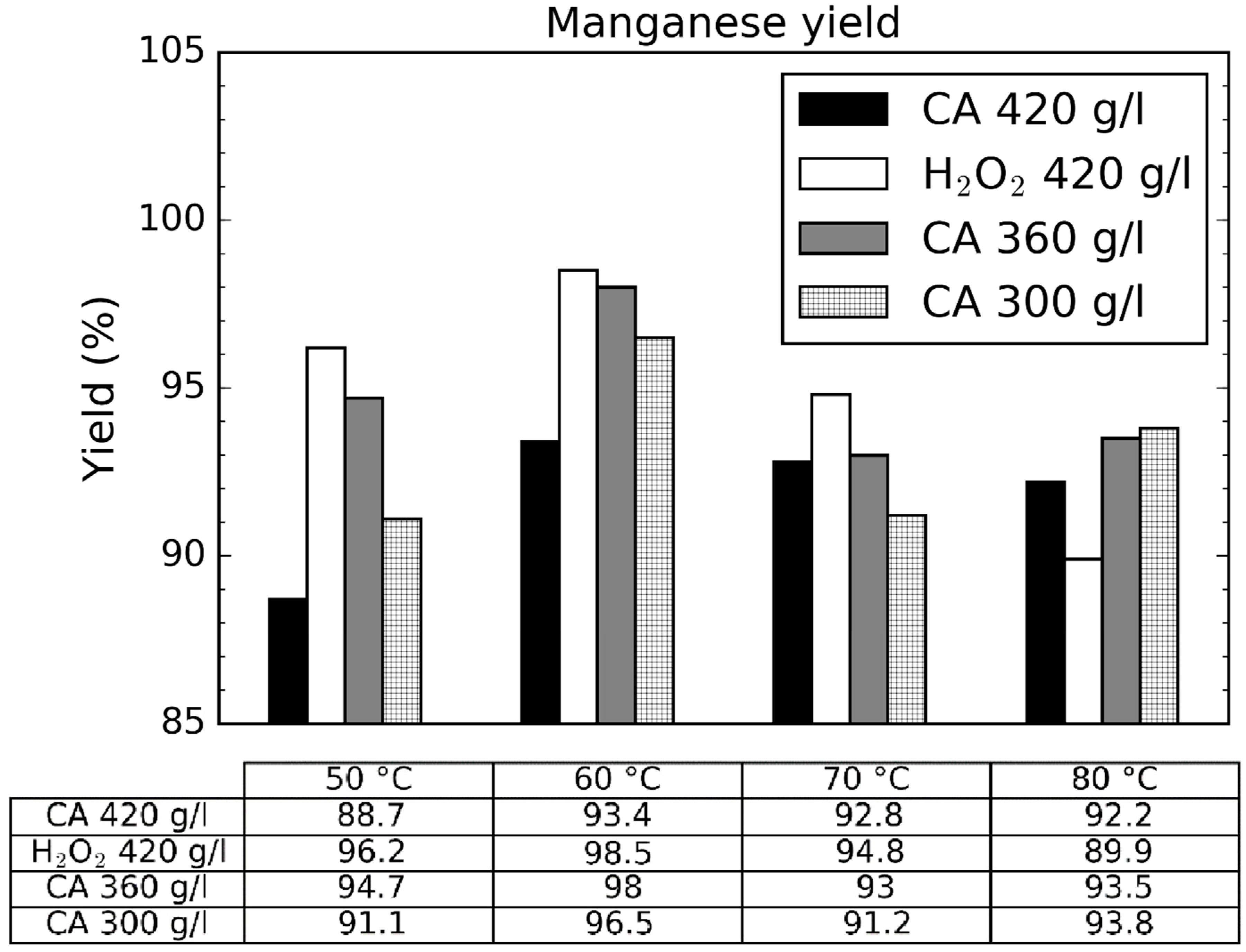
3.1. Effect of Leaching Parameters on Manganese Concentration and Yield
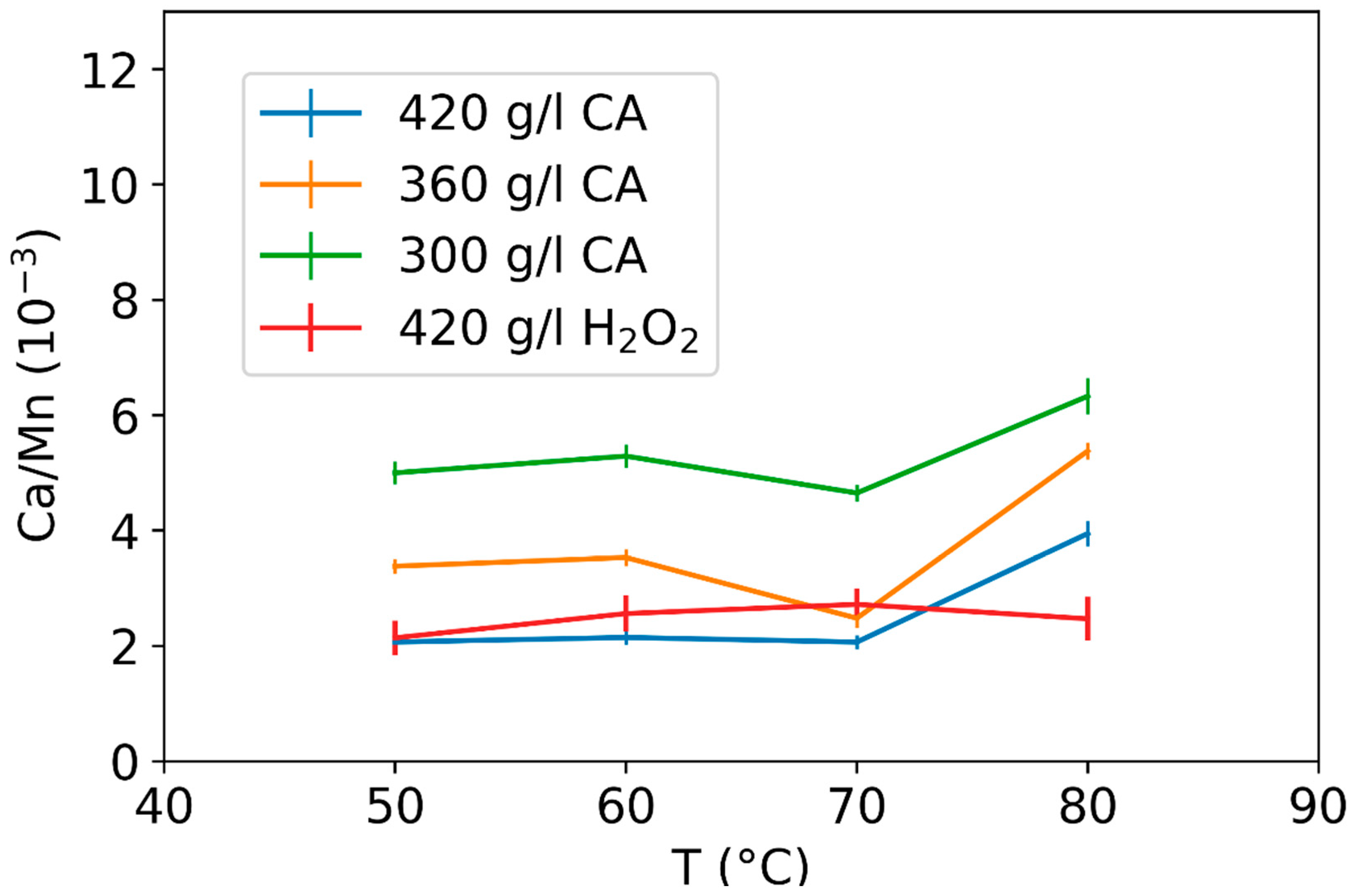
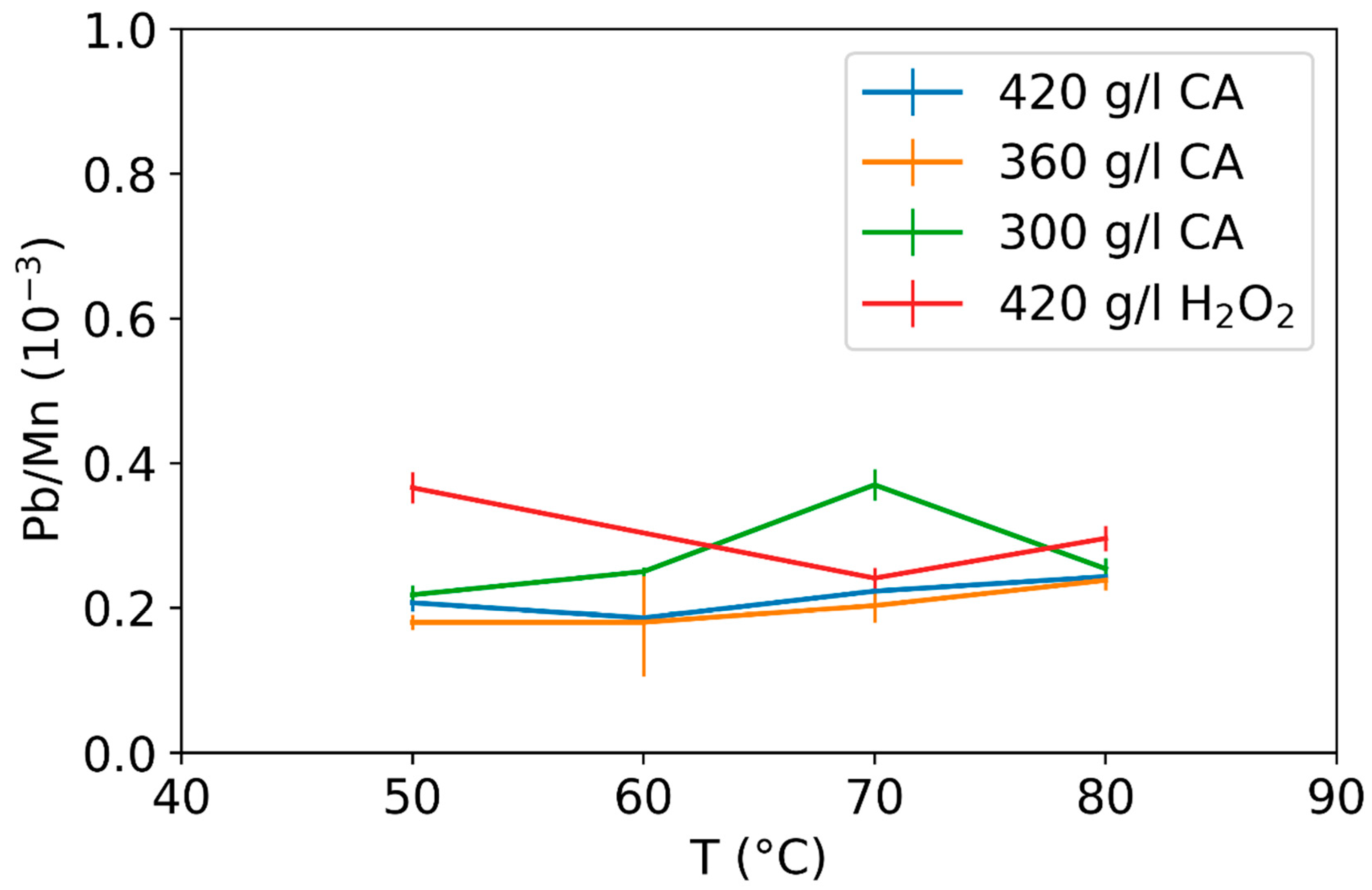
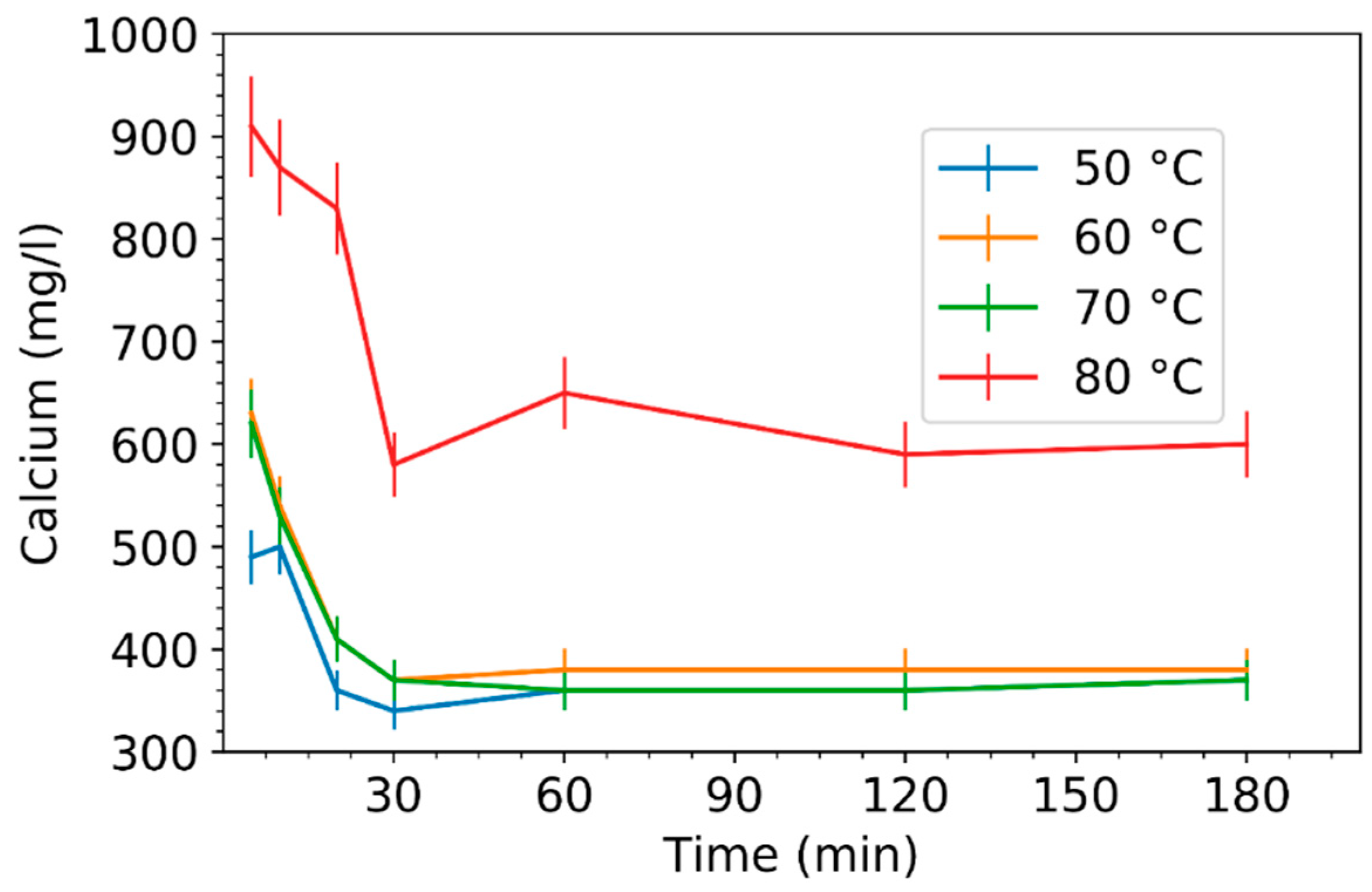
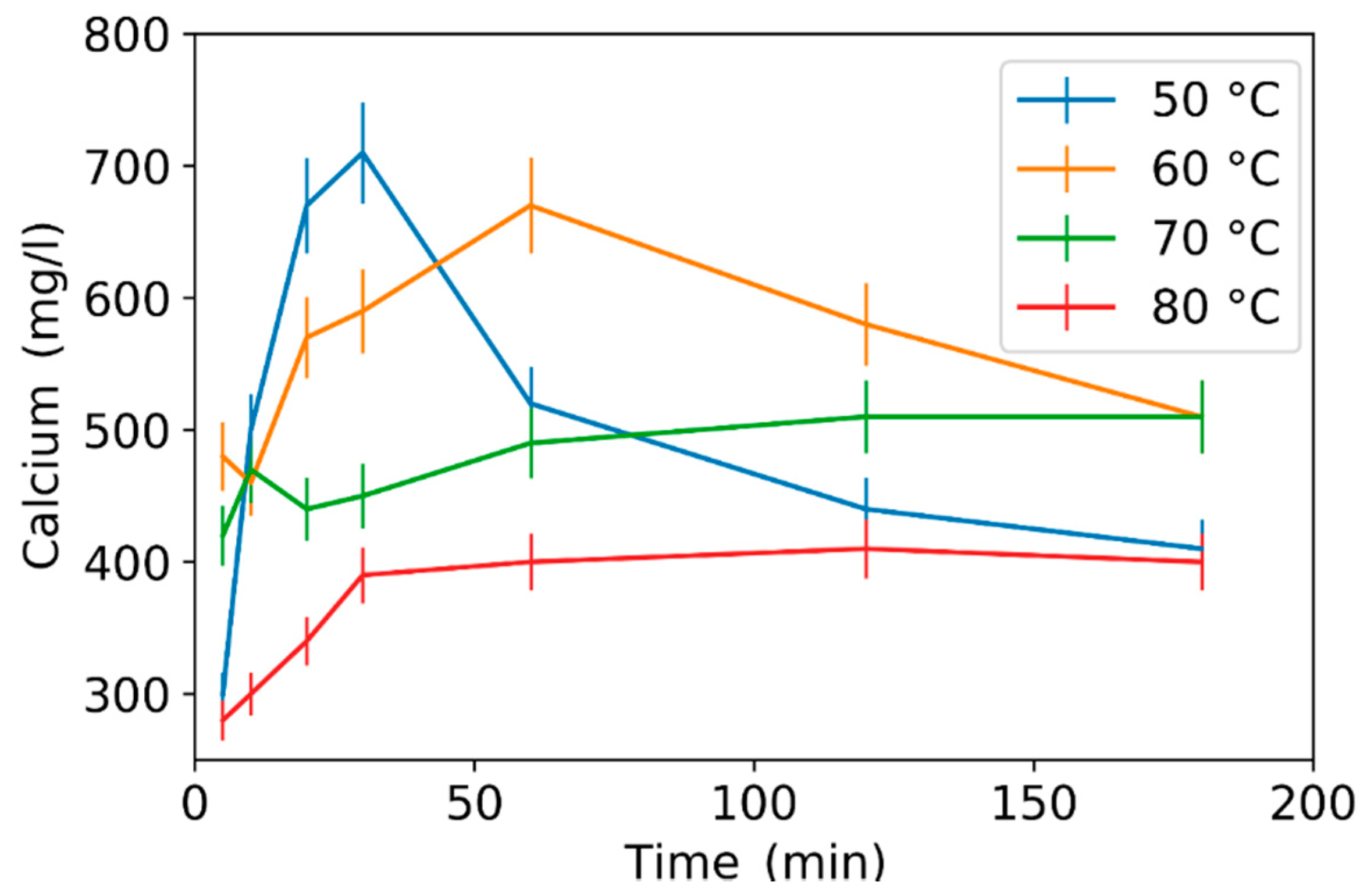
3.2. Effect of Leaching Parameters on Calcium and Lead Concentrations
3.3. Purity of Leachate
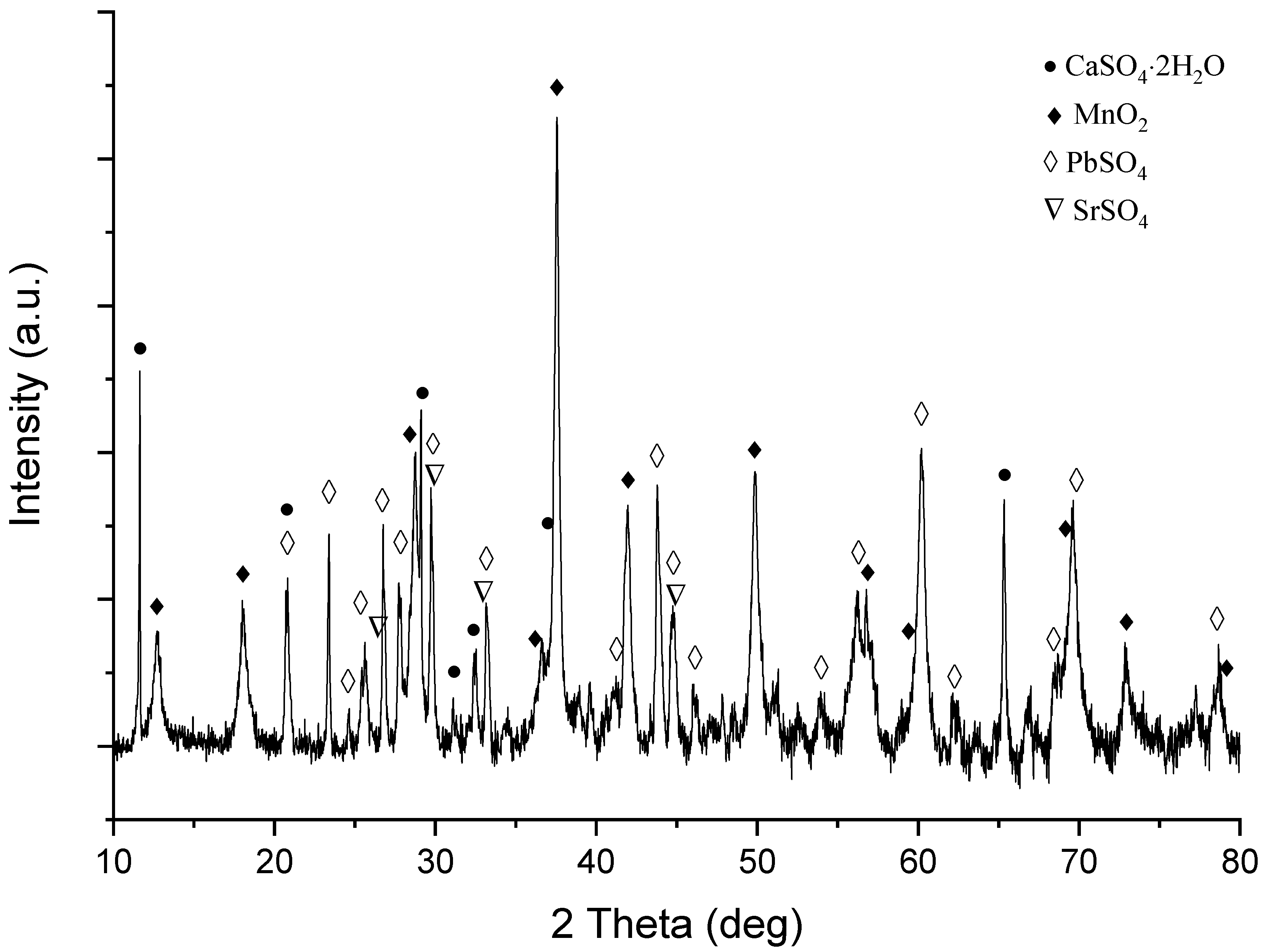
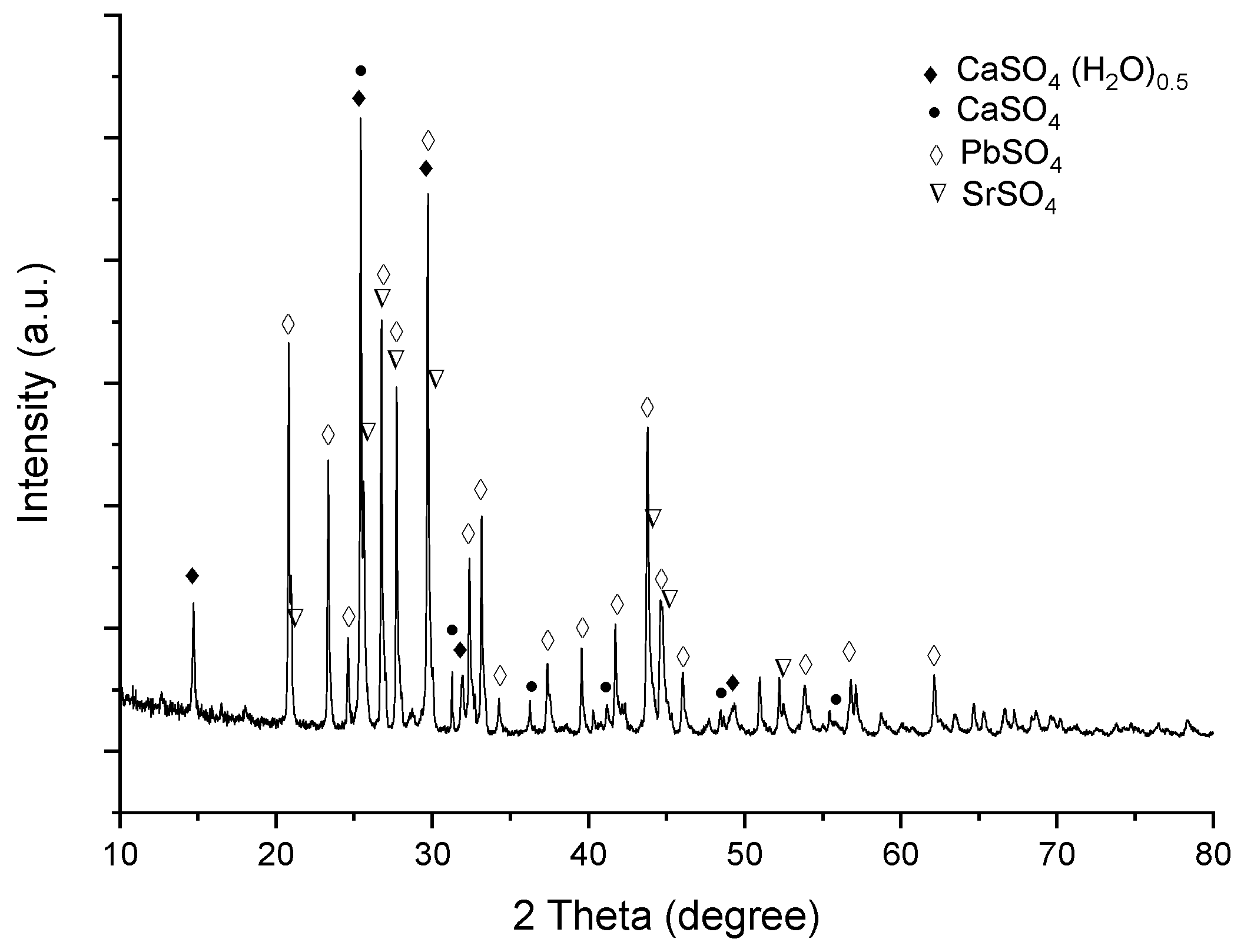
3.4. Leaching Residue
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pakarinen, J. Recovery and Refining of Manganese as By-Product from Hydrometallurgical Processes. Ph.D. Thesis, Lappeenranta University of Technology, Lappeenranta, Finland, 28 October 2011. [Google Scholar]
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide. Hydrometallurgy 2007, 89, 137–159. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries 2016:, U.S. Geological Survey; Government Printing Office: Washington, DC, USA, 2016; 202p. [CrossRef]
- Zhang, X.; Liu, Z.; Wu, X.; Du, J.; Tao, C. Electric field enhancement in leaching of manganese from low-grade manganese dioxide ore: Kinetics and mechanism study. J. Electroanal. Chem. 2017, 788, 165–174. [Google Scholar] [CrossRef]
- Chandra, N.; Amritphale, S.S.; Pal, D. Manganese recovery from secondary resources: A green process for carbothermal reduction and leaching of manganese bearing hazardous waste. J. Hazard Mater. 2011, 186, 293–299. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Off. J. Eur. Union 2008, 51, 3–30. [Google Scholar]
- Finnish Ministry of the Environment. Waste Act 646/2011, Jätelaki 646/2011. 2016. Available online: http://www.finlex.fi/fi/laki/ajantasa/2011/20110646 (accessed on 3 March 2020). (In Finnish).
- Sinclair, J.R. The Extractive Metallurgy of Zinc; Spectrum Series; Australasian Institute of Mining and Metallurgy: Melbourne, VIC, Australia, 2005; p. 13. [Google Scholar]
- Aromaa, J.; Barker, M.H.; Lagerstedt, A.; Torkkeli, J.; Forsén, O. Evaluation of Lead Anodes and their Tendecy to Manganese Dioxide Deposition. In Proceedings of the European Metallurgical Conference EMC 2009, Clausthal-Zellerfeld, Germany, 28 June–1 July 2009. [Google Scholar]
- Bratt, G.C.; Smith, W.N. The effects of strontium compounds and related materials in the electrolytic production of zinc. Electrochemistry 1965, 7, 939–948. [Google Scholar]
- Morrison, R.M.; MacKinnon, D.J.; Uceda, D.A.; Warren, P.E.; Mouland, J.E. The effect of some trace metal impurities on the electrowinning of zinc from Kidd Creek electrolyte. Hydrometallurgy 1992, 29, 413–430. [Google Scholar] [CrossRef]
- Mishra, D.; Srivastava, R.R.; Sahu, K.K.; Singh, T.B.; Jana, R.K. Leaching of roast-reduced manganese nodules in NH3-(NH4)2CO3 medium. Hydrometallurgy 2011, 109, 215–220. [Google Scholar] [CrossRef]
- Welham, N.J. Activation of the carbothermic reduction of manganese ore. Int. J. Miner. Process. 2002, 69, 187–198. [Google Scholar] [CrossRef]
- Paixao, J.M.M.; Amaral, J.C.; Memoria, L.E.; Freitas, L.R. Sulphation of Carajas manganese ore. Hydrometallurgy 1995, 39, 215–222. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, G.; Zhao, Y. Study in reduction-roast leaching manganese from low-grade manganese dioxide ores using cornstalk as reductant. Hydrometallurgy 2009, 96, 176–179. [Google Scholar] [CrossRef]
- You, Z.; Li, G.; Zhang, Y.; Peng, Z.; Jiang, T. Extraction of manganese from iron rich MnO2 ores via selective sulfation roasting with SO2 followed by water leaching. Hydrometallurgy 2015, 156, 225–231. [Google Scholar] [CrossRef]
- Zhang, Y.; You, Z.; Li, G.; Jiang, T. Manganese extraction by sulfur-based reduction roasting-acid leaching from low-grade manganese oxide ores. Hydrometallurgy 2013, 133, 126–132. [Google Scholar] [CrossRef]
- Li, C.; He, L.; Li, S.; Wang, Z. A study on the preparation of manganese sulfate by co-calcining pyrolysite and pyrite. Chem. World 2000, 4, 66–69. [Google Scholar]
- Nayl, A.A.; Ismail, I.M.; Aly, H.F. Recovery of pure MnSO4∙H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int. J. Miner. Process. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Rodrigues, S.; Munichndraiah, N.; Shukla, A.K. A cyclic voltammetric study of the kinetics and mechanism of electrodeposition of manganese dioxide. J. Appl. Electrochem. 1998, 28, 1235–1241. [Google Scholar] [CrossRef]
- Petitpierre, J.-P.; Comninellis, C.; Plattner, E. Oxydation Du MnSO4 en dioxyde de manganese dans H2SO4 30%. Electrochim. Acta 1990, 35, 281–287. [Google Scholar] [CrossRef]
- Lee, J.A.; Maskell, W.C.; Tye, F.L. The electrochemical reduction of manganese dioxide in acidic solutions: Part I. J. Electroanal. Chem. 1977, 79, 79–104. [Google Scholar] [CrossRef]
- El Hazek, M.N.; Lasheen, T.A.; Helal, A.S. Reductive leaching of manganese from low grade Sinai ore in HCl using H2O2 as reductant. Hydrometallurgy 2006, 84, 187–191. [Google Scholar] [CrossRef]
- Do, S.-H.; Batchelor, B.; Lee, H.-K.; Kong, S.-H. Hydrogen peroxide decomposition on manganese oxide (pyrolusite): Kinetics, intermediates, and mechanism. Chemosphere 2009, 75, 8–12.
- Li, H.; Zhang, Z.; Tang, S.; Li, Y.; Zhang, Y. Ultrasonically assisted acid extraction of manganese from slag. Ultrason. Sonochem. 2008, 15, 339–343. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Cao, Z.-F.; Zhong, H. Two-stage leaching of manganese and silver from manganese–silver ores by reduction with calcium sulfide and oxidation with copper(II). Hydrometallurgy 2018, 175, 240–249. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Cao, Z.-F.; Zhong, H. A novel process for the separation and recovery of value-added metals from manganese–silver ores by EDTA/EDTA–2Na and thiosulfate. Hydrometallurgy 2018, 178, 256–263. [Google Scholar] [CrossRef]
- Vu, H.; Jandova, J.; Lisa, K.; Vranka, F. Leaching of manganese deep ocean nodules in FeSO4–H2SO4-H2O solutions. Hydrometallurgy 2005, 77, 147–153. [Google Scholar] [CrossRef]
- Chen, W.-S.; Liao, C.-T.; Lin, K.-Y. Recovery of Zinc and Manganese from Spent Battery Powder by Hydrometallurgical Route. Energy Procedia 2017, 107, 167–174. [Google Scholar]
- Pagnanelli, F.; Furlani, G.; Valentini, P.; Veglio, F.; Toro, L. Leaching of low-grade manganese ores by using nitric acid and glucose: Optimization of the operating conditions. Hydrometallurgy 2004, 75, 157–167. [Google Scholar] [CrossRef]
- Tian, Q.-H.; Jiao, C.-Y.; Guo, X.-Y. Extraction of valuable metals from manganese-silver ore. Hydrometallurgy 2012, 119–120, 8–15. [Google Scholar] [CrossRef]
- Petrie, L.M. Molecular interpretation for SO2 dissolution kinetics of pyrolusite, manganite and hematite. Appl. Geochem. 1995, 10, 253–267. [Google Scholar] [CrossRef]
- Kobylin, P.M.; Taskinen, P.A. Thermodynamic modelling of aqueous Mn(II) sulfate solutions. Calphad 2012, 38, 146–154. [Google Scholar] [CrossRef]
- Field, T.B.; Coburn, J.; McCourt, J.L.; McBryde, W.A.E. Composition and stability of some metal citrate and diglycolate complexes in aqueous solution. Anal. Chim. Acta 1975, 74, 101–106. [Google Scholar] [CrossRef]
- Meyer, J.L. Formation constants for interaction of citrate with calcium and magnesium lons. Anal. Biochem. 1974, 62, 295–300. [Google Scholar] [CrossRef]
- Singh, R.P.; Yeboah, Y.D.; Pambid, E.R.; Debayle, P. Stability constant of the calcium-citrate(3-) ion pair complex. J. Chem. Eng. Data 1991, 36, 52–54. [Google Scholar] [CrossRef]


| Mn (wt%) | Pb (wt%) | Ca (wt%) | S (wt%) | Sr (wt%) | Zn (wt%) |
|---|---|---|---|---|---|
| 41.9 | 6.5 | 1.2 | 4.2 | 0.92 | 1.00 |
| K (wt%) | Mg (wt%) | Na (wt%) | Fe (wt%) | Al (wt%) | Si (wt%) |
| 1.4 | 0.17 | 0.21 | 0.015 | 0.04 | 0.23 |
| S/L (g/L) | Anode Sludge (g) | H2SO4 (M) | H2SO4 (mL) | Reducing Agent (mL) |
|---|---|---|---|---|
| 420 | 210 | 5.18 | 332 | 168 |
| 360 | 180 | 4.14 | 356 | 144 |
| 300 | 150 | 3.22 | 380 | 120 |
| Mn (g/L) | Zn (g/L) | K (g/L) | S (g/L) | Ca (mg/L) | Pb (mg/L) | Fe (mg/L) | Al (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| 420 g/L, 50 °C, H2O2 | 191 | 4.06 | 3.9 | 119 | 410 | 70 | 44 | 27 |
| 420 g/L, 70 °C, H2O2 | 187 | 4.09 | 3.8 | 120 | 510 | 45 | 46 | 60 |
| 420 g/L, 70 °C, CA | 179 | 4.09 | 3.7 | 107 | 370 | 40 | 44 | 27 |
| 360 g/L, 50 °C, CA | 139 | 4.05 | 2.6 | 93 | 470 | 25 | 41 | 11 |
| 300 g/L, 50 °C, CA | 116 | 3.07 | 2.2 | 79 | 620 | 29 | 30 | 13 |
| As (mg/kg) | Hg (mg/kg) | Cd (mg/kg) | Cr (mg/kg) | Cu (mg/kg) | Pb (mg/kg) | Ni (mg/kg) | Zn (mg/kg) | |
|---|---|---|---|---|---|---|---|---|
| Field | 25 | 1 | 2.5 | 300 | 600 | 100 | 100 | 1500 |
| Forest | 40 | 1 | 2.5 | 300 | 700 | 150 | 150 | 4500 |
| This work | - | - | - | - | 1.44 | 76 | - | 9314 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauppinen, T.; Vielma, T.; Salminen, J.; Lassi, U. Selective Recovery of Manganese from Anode Sludge Residue by Reductive Leaching. ChemEngineering 2020, 4, 40. https://doi.org/10.3390/chemengineering4020040
Kauppinen T, Vielma T, Salminen J, Lassi U. Selective Recovery of Manganese from Anode Sludge Residue by Reductive Leaching. ChemEngineering. 2020; 4(2):40. https://doi.org/10.3390/chemengineering4020040
Chicago/Turabian StyleKauppinen, Toni, Tuomas Vielma, Justin Salminen, and Ulla Lassi. 2020. "Selective Recovery of Manganese from Anode Sludge Residue by Reductive Leaching" ChemEngineering 4, no. 2: 40. https://doi.org/10.3390/chemengineering4020040
APA StyleKauppinen, T., Vielma, T., Salminen, J., & Lassi, U. (2020). Selective Recovery of Manganese from Anode Sludge Residue by Reductive Leaching. ChemEngineering, 4(2), 40. https://doi.org/10.3390/chemengineering4020040






