Factors That Determine the Adhesive Strength in a Bioinspired Bone Tissue Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization and Sample Preparation
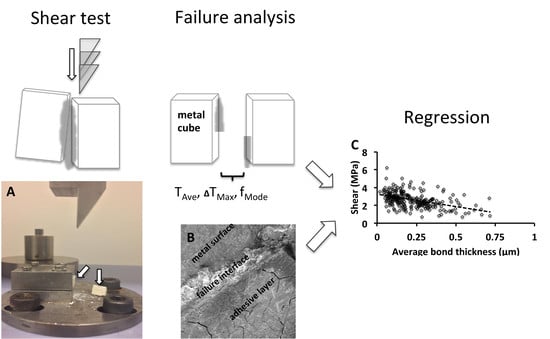
2.3. Mechanical Testing
2.4. Failure Analysis
2.5. Statistical Analysis
3. Results
3.1. Material Characterization
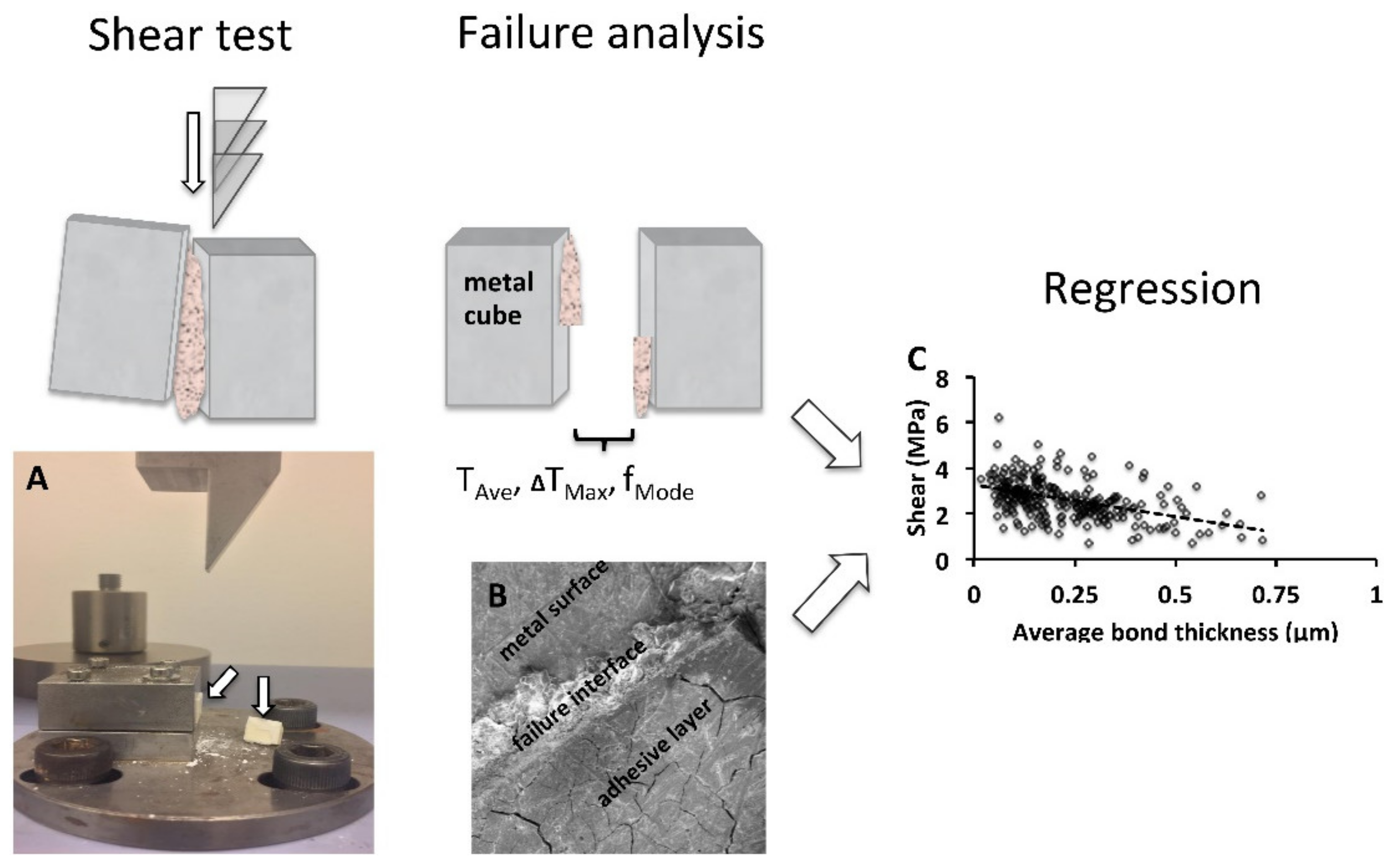
3.2. Surface Roughness of Test Cubes
3.3. Adhesive (Shear) Strength of PMC Adhesive
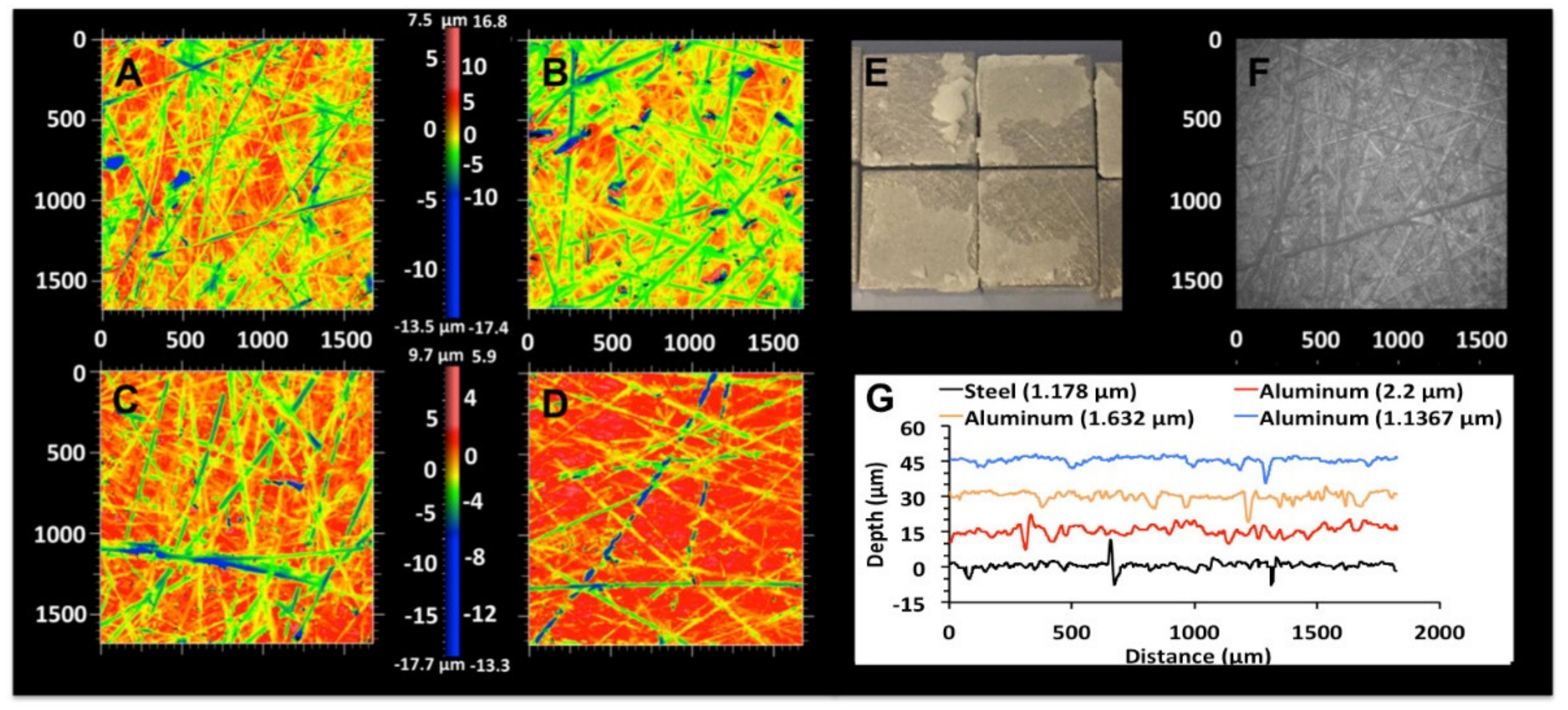
3.3.1. Effect of Curing/Handling Conditions on PMC Adhesive Strength
3.3.2. Effect of Surface Roughness on PMC Adhesive Strength
3.4. Failure Mode Analysis
3.5. Linear Regression and Scatter Plot Analysis
3.5.1. Scatter Plot Analysis
3.5.2. GLM, Linear Regression
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Group | Formula | Cure | Force (N) | Time | Mix | Cube | Rough (μm) |
|---|---|---|---|---|---|---|---|
| 1 | 27% | Humidity | 31 | 24h | Hand | Steel | 1.23 |
| 2 | 27% | Water | 31 | 24h | Hand | Steel | 1.23 |
| 3 | 27% | PBS | 31 | 24h | Hand | Steel | 1.23 |
| 4 | 27% | Humid | 31 | 5m | Hand | Steel | 1.23 |
| 5 | 27% | Water | 31 | 5m | Hand | Steel | 1.23 |
| 6 | 27% | PBS | 31 | 5m | Hand | Steel | 1.23 |
| 7 | 27% | PBS | 31 | 5m | Auto | Steel | 1.23 |
| 8 | 27% | PBS | 31 | 1m | Hand | Steel | 1.23 |
| 9 | 27% | PBS | 3 | 24h | Hand | Steel | 1.23 |
| 10 | 27% | PBS | 3 | 5m | Hand | Steel | 1.23 |
| 11 | 27% | PBS | 3 | 5m | Auto | Steel | 1.23 |
| 12 | 27% | PBS | 3 | 1m | Auto | Steel | 1.23 |
| 13 | 27% | Humid | 31 | 1m | Hand | Steel | 1.23 |
| 14 | 50% | PBS | 31 | 24h | Hand | Steel | 1.23 |
| 15 | 70% | PBS | 31 | 24h | Hand | Steel | 1.23 |
| 16 | 50% | Humid | 31 | 1m | Hand | Steel | 1.23 |
| 17 | 70% | Humid | 31 | 1m | Hand | Steel | 1.23 |
| 18 | 90% | Humid | 31 | 1m | Hand | Steel | 1.23 |
| 19 | 27% * | PBS | 31 | 24h | Hand | Steel | 1.23 |
| 20 | 50% * | PBS | 31 | 24h | Hand | Steel | 1.23 |
| 21 | 70% * | PBS | 31 | 24h | Hand | Steel | 1.23 |
| 22 | PMMA | PBS | 31 | 24h | Hand | Steel | 1.23 |
| 23 | PMMA | PBS | 31 | 1m | Hand | Steel | 1.23 |
| 24 | PMMA | PBS | 31 | 1m | Hand | Aluminum | 2.17 |
| 25 | PMMA | PBS | 31 | 1m | Hand | Aluminum | 1.72 |
| 26 | PMMA | PBS | 31 | 1m | Hand | Aluminum | 1.27 |
| 27 | 27% | PBS | 31 | 1m | Hand | Aluminum | 2.17 |
| 28 | 27% | PBS | 31 | 1m | Hand | Aluminum | 1.72 |
| 29 | 27% | PBS | 31 | 1m | Hand | Aluminum | 1.27 |
| 30 | 90% | PBS | 31 | 1m | Hand | Aluminum | 2.17 |
| 31 | 90% | PBS | 31 | 1m | Hand | Aluminum | 1.72 |
| 32 | 90% | PBS | 31 | 1m | Hand | Aluminum | 1.27 |
| 33 | 27% | PBS | 31 | 1m | Hand | Bone | |
| 34 | 27% | PBS | 3 | 1m | Hand | Bone |
References
- Granskog, V.; García-Gallego, S.; von Kieseritzky, J.; Rosendahl, J.; Stenlund, P.; Zhang, Y.; Petronis, S.; Lyvén, B.; Arner, M.; Håkansson, J.; et al. High-Performance Thiol–Ene Composites Unveil a New Era of Adhesives Suited for Bone Repair. Adv. Funct. Mater. 2018, 28, 1800372. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.W.; Zhang, H.; et al. Mussel-Inspired Tissue-Adhesive Hydrogel Based on the Polydopamine-Chondroitin Sulfate Complex for Growth-Factor-Free Cartilage Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef] [PubMed]
- Hofman, A.H.; van Hees, I.A.; Yang, J.; Kamperman, M. Bioinspired Underwater Adhesives by Using the Supramolecular Toolbox. Adv. Mater. 2018, 30, 1704640. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Huang, J.; Lee, Y.; Dutta, S.; Yoo, H.Y.; Jung, Y.M.; Jho, Y.; Zeng, H.; Hwang, D.S. Complexation and coacervation of like-charged polyelectrolytes inspired by mussels. Proc. Natl. Acad. Sci. USA 2016, 113, E847–E853. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, Y.; Yang, C.; Wang, M. Silica Nanoparticles as Adhesives for Biological Tissues? Re-Examining the Effect of Particles Size, Particle Shape, and the Unexpected Role of Base. Part. Part. Syst. Charact. 2017, 34, 1700286. [Google Scholar] [CrossRef]
- Rose, S.; Prevoteau, A.; Elziere, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef]
- Stewart, R.J.; Wang, C.S.; Shao, H. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci. 2011, 167, 85–93. [Google Scholar] [CrossRef]
- Kirillova, A.; Kelly, C.; von Windheim, N.; Gall, K. Bioinspired Mineral–Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2018, 7, 1800467. [Google Scholar] [CrossRef]
- Liu, X.; Pujari-Palmer, M.; Wenner, D.; Procter, P.; Insley, G.; Engqvist, H. Adhesive Cements That Bond Soft Tissue Ex Vivo. Materials 2019, 12, 2473. [Google Scholar] [CrossRef]
- Pujari-Palmer, M.; Guo, H.; Wenner, D.; Autefage, H.; Spicer, D.C.; Stevens, M.M.; Omar, O.; Thomsen, P.; Edén, M.; Insley, G.; et al. A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials. Materials 2018, 11, 2492. [Google Scholar] [CrossRef]
- Bystrom, L.J.; Pujari-Palmer, M. Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro. J. Funct. Biomater. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.; Lux, R.; Proff, P.; Lauer, G.; Pradel, W.; Leonhardt, H.; Reinstorf, A.; Gelinsky, M.; Jung, R.; Eckelt, U.; et al. O-phospho-L-serine: A modulator of bone healing in calcium-phosphate cements. Biomed. Tech. (Berl) 2008, 53, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Offer, L.; Veigel, B.; Pavlidis, T.; Heiss, C.; Gelinsky, M.; Reinstorf, A.; Wenisch, S.; Lips, K.S.; Schnettler, R. Phosphoserine-modified calcium phosphate cements: Bioresorption and substitution. J. Tissue Eng. Regen. Med. 2011, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Reinstorf, A.; Hempel, U.; Olgemöller, F.; Domaschke, H.; Schneiders, W.; Mai, R.; Stadlinger, B.; Rösen-Wolff, A.; Rammelt, S.; Gelinsky, M.; et al. O-phospho-L-serine modified calcium phosphate cements—Material properties, in vitro andin vivo investigations. Mater. Werkst. 2006, 37, 491–503. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates as bioceramics: State of the art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef]
- Procter, P.; Pujari-Palmer, M.; Hulsart-Billström, G.; Wenner, D.; Insley, G.; Larsson, S.; Engqvist, H. A biomechanical test model for evaluating osseous and osteochondral tissue adhesives. BMC Biomed. Eng. 2019, 1, 11. [Google Scholar] [CrossRef]
- Adnan, A.; Sun, C.T. Effect of Adhesive Thickness on Joint Strength: A Molecular Dynamics Perspective. J. Adhes. 2008, 84, 401–420. [Google Scholar] [CrossRef]
- Armstrong, S.; Geraldeli, S.; Maia, R.; Raposo, L.H.; Soares, C.J.; Yamagawa, J. Adhesion to tooth structure: A critical review of "micro" bond strength test methods. Dent. Mater. 2010, 26, e50–e62. [Google Scholar] [CrossRef]
- Boutar, Y.; Naïmi, S.; Mezlini, S.; da Silva, L.F.M.; Hamdaoui, M.; Ben Sik Ali, M. Effect of adhesive thickness and surface roughness on the shear strength of aluminium one-component polyurethane adhesive single-lap joints for automotive applications. J. Adhes. Sci. Technol. 2016, 30, 1913–1929. [Google Scholar] [CrossRef]
- Klocke, A.; Kahl-Nieke, B. Influence of cross-head speed in orthodontic bond strength testing. Dent. Mater. 2005, 21, 139–144. [Google Scholar] [CrossRef]
- Xu, W.; Wei, Y. Strength and interface failure mechanism of adhesive joints. Int. J. Adhes. Adhes. 2012, 34, 80–92. [Google Scholar] [CrossRef]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Arenas, J.M.; Narbón, J.J.; Alía, C. Optimum adhesive thickness in structural adhesives joints using statistical techniques based on Weibull distribution. Int. J. Adhes. Adhes. 2010, 30, 160–165. [Google Scholar] [CrossRef]
- Shekhawat, V.K.; Laurent, M.P.; Muehleman, C.; Wimmer, M.A. Surface topography of viable articular cartilage measured with scanning white light interferometry. Osteoarthr. Cartil. 2009, 17, 1197–1203. [Google Scholar] [CrossRef]
- Jo, Y.K.; Choi, B.-H.; Zhou, C.; Ahn, J.-S.; Jun, S.H.; Cha, H.J. Bioengineered mussel glue incorporated with a cell recognition motif as an osteostimulating bone adhesive for titanium implants. J. Mater. Chem. B 2015, 3, 8102–8114. [Google Scholar] [CrossRef]
- Kandalam, U.; Bouvier, A.J.; Casas, S.B.; Smith, R.L.; Gallego, A.M.; Rothrock, J.K.; Thompson, J.Y.; Huang, C.Y.; Stelnicki, E.J. Novel bone adhesives: A comparison of bond strengths in vitro. Int. J. Oral. Maxillofac. Surg. 2013, 42, 1054–1059. [Google Scholar] [CrossRef]
- Schreader, K.J.; Bayer, I.S.; Milner, D.J.; Loth, E.; Jasiuk, I. A polyurethane-based nanocomposite biocompatible bone adhesive. J. Appl. Polym. Sci. 2013, 127, 4974–4982. [Google Scholar] [CrossRef]
- Young, A.M.; Man Ho, S.; Abou Neel, E.A.; Ahmed, I.; Barralet, J.E.; Knowles, J.C.; Nazhat, S.N. Chemical characterization of a degradable polymeric bone adhesive containing hydrolysable fillers and interpretation of anomalous mechanical properties. Acta Biomater. 2009, 5, 2072–2083. [Google Scholar] [CrossRef]
- Kelly, J.R.; Benetti, P.; Rungruanganunt, P.; Bona, A.D. The slippery slope: Critical perspectives on in vitro research methodologies. Dent. Mater. 2012, 28, 41–51. [Google Scholar] [CrossRef]
- Spicer, C.; Pujari-Palmer, M.; Autefage, H.; Insley, G.; Procter, P.; Engqvist, H.; Stevens, M. Synthesis of Phospho-Amino Acid Analogues as Tissue Adhesive Cement Additives. Cent. Sci. 2020, 6, 226–231. [Google Scholar] [CrossRef]
- Perrin, B.R.; Dupeux, M.; Tozzi, P.; Delay, D.; Gersbach, P.; von Segesser, L.K. Surgical glues: Are they really adhesive? Eur. J. Cardiothorac. Surg. 2009, 36, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Sirisha, K.; Rambabu, T.; Shankar, Y.; Ravikumar, P. Validity of bond strength tests: A critical review: Part I. J. Conserv. Dent. 2014, 17, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Cho, B. Effects of the acetone content of single solution dentin bonding agents on the adhesive layer thickness and the microtensile bond strength. Dent. Mater. 2004, 20, 107–115. [Google Scholar] [CrossRef]
- Kim, W.-S.; Yun, I.-H.; Lee, J.-J.; Jung, H.-T. Evaluation of mechanical interlock effect on adhesion strength of polymer–metal interfaces using micro-patterned surface topography. Int. J. Adhes. Adhes. 2010, 30, 408–417. [Google Scholar] [CrossRef]
- Sun, F.; Li, H.; Lindberg, H.; Leifer, K.; Gamstedt, E.K. Polymer fracture and deformation during nanosectioning in an ultramicrotome. Eng. Fract. Mech. 2017, 182, 595–606. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation. Comput. Mater. Sci. 2016, 112, 161–169. [Google Scholar] [CrossRef]
- Von Fraunhofer, J.A. Adhesion and cohesion. Int. J. Dent. 2012, 2012, 951324. [Google Scholar] [CrossRef]
- Krishnan, A.; Xu, L.R. Systematic Evaluation of Bonding Strengths and Fracture Toughnesses of Adhesive Joints. J. Adhes. 2011, 87, 53–71. [Google Scholar] [CrossRef]
- Shimizu, K.; Malmos, K.; Holm, A.H.; Pedersen, S.U.; Daasbjerg, K.; Hinge, M. Improved adhesion between PMMA and stainless steel modified with PMMA brushes. ACS Appl. Mater. Interfaces 2014, 6, 21308–21315. [Google Scholar] [CrossRef]
- Castagnetti, D.; Spaggiari, A.; Dragoni, E. Effect of Bondline Thickness on the Static Strength of Structural Adhesives Under Nearly-Homogeneous Shear Stresses. J. Adhes. 2011, 87, 780–803. [Google Scholar] [CrossRef]
- Akhavan-Safar, A.; Ayatollahi, M.R.; da Silva, L.F.M. Strength prediction of adhesively bonded single lap joints with different bondline thicknesses: A critical longitudinal strain approach. Int. J. Solids Struct. 2017, 109, 189–198. [Google Scholar] [CrossRef]
- Azari, S.; Papini, M.; Spelt, J.K. Effect of adhesive thickness on fatigue and fracture of toughened epoxy joints—Part I: Experiments. Eng. Fract. Mech. 2011, 78, 153–162. [Google Scholar] [CrossRef]
- Bascom, W.D.; Timmons, C.O.; Jones, R.L. Apparent interfacial failure in mixed-mode adhesive fracture. J. Mater. Sci. 1975, 10, 1037–1048. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]



| Factor | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| Cure conditions | 100% humid | water | PBS | |
| Grip force (N) | 31 N | 3 N | ||
| Grip time (min) | 1 min | 5 min | 1440 min | |
| Formula (mole%) | 27% | 50% | 70% | 90% |
| Mix type | manual | automated | ||
| Apply time (sec) * Ave. thick (μm) | ||||
| * Thickest (μm) | ||||
| * Thinnest (μm) | ||||
| * Skew (%) | ||||
| * Failure mode | adhesive | mixed | combined | cohesive |
| Material | D(0.9) μm | D(0.5) μm | αTCP (wt%) | HA (wt%) | bTCP (wt%) |
|---|---|---|---|---|---|
| Surface | 12.8 | 2.08 | |||
| Volume | 33.9 | ||||
| XRD | 97.66 | 1.87 | 0.47 |
| Group | Sa (um) | Ra (um) | Sq (um) | Sku |
|---|---|---|---|---|
| Steel (US80 grit) | 1.23 ± 0.21 | 1.30 ± 0.26 | 1.69 ± 0.33 | 5.75 ± 0.78 |
| Aluminum (US80 grit) | 2.17 ± 0.44 | 2.17 ± 0.58 | 2.94 ± 0.48 | 6.99 ± 3.18 |
| Aluminum (US320 grit) | 1.72 ± 0.19 | 1.78 ± 0.31 | 2.30 ± 0.28 | 6.12 ± 1.61 |
| Aluminum (US1200 grit) | 1.27 ± 0.32 | 1.33 ± 0.38 | 1.74 ± 0.42 | 9.60 ± 5.96 |
| R2 | * R2 | Cure | Mix | Force | Time | Formula | Thick | Skew | Fail | |
|---|---|---|---|---|---|---|---|---|---|---|
| Shear1 | 0.358 | 0.316 | 0.518 | 0.317 | 0.248 | 0.404 | - | <0.001 | 0.114 | 0.004 |
| Shear2 | 0.800 | 0.782 | - | - | - | 0.930 | <0.001 | <0.001 | 0.687 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujari-Palmer, M.; Giró, R.; Procter, P.; Bojan, A.; Insley, G.; Engqvist, H. Factors That Determine the Adhesive Strength in a Bioinspired Bone Tissue Adhesive. ChemEngineering 2020, 4, 19. https://doi.org/10.3390/chemengineering4010019
Pujari-Palmer M, Giró R, Procter P, Bojan A, Insley G, Engqvist H. Factors That Determine the Adhesive Strength in a Bioinspired Bone Tissue Adhesive. ChemEngineering. 2020; 4(1):19. https://doi.org/10.3390/chemengineering4010019
Chicago/Turabian StylePujari-Palmer, Michael, Roger Giró, Philip Procter, Alicja Bojan, Gerard Insley, and Håkan Engqvist. 2020. "Factors That Determine the Adhesive Strength in a Bioinspired Bone Tissue Adhesive" ChemEngineering 4, no. 1: 19. https://doi.org/10.3390/chemengineering4010019
APA StylePujari-Palmer, M., Giró, R., Procter, P., Bojan, A., Insley, G., & Engqvist, H. (2020). Factors That Determine the Adhesive Strength in a Bioinspired Bone Tissue Adhesive. ChemEngineering, 4(1), 19. https://doi.org/10.3390/chemengineering4010019