Nickel Nanofibers Manufactured via Sol-Gel and Electrospinning Processes for Electrically Conductive Adhesive Applications
Abstract
:1. Introduction
2. Materials and Methods
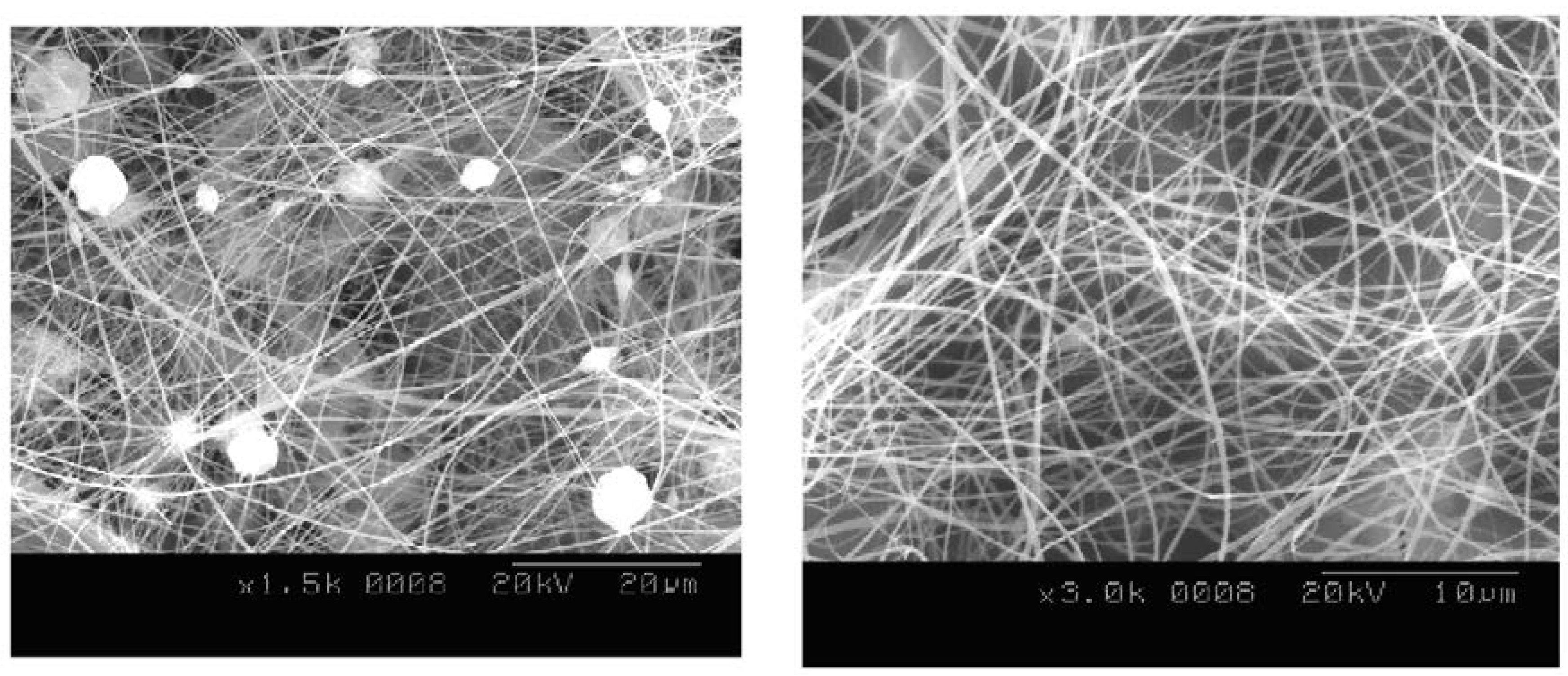
2.1. Ni Nanofibers via Electrospinning
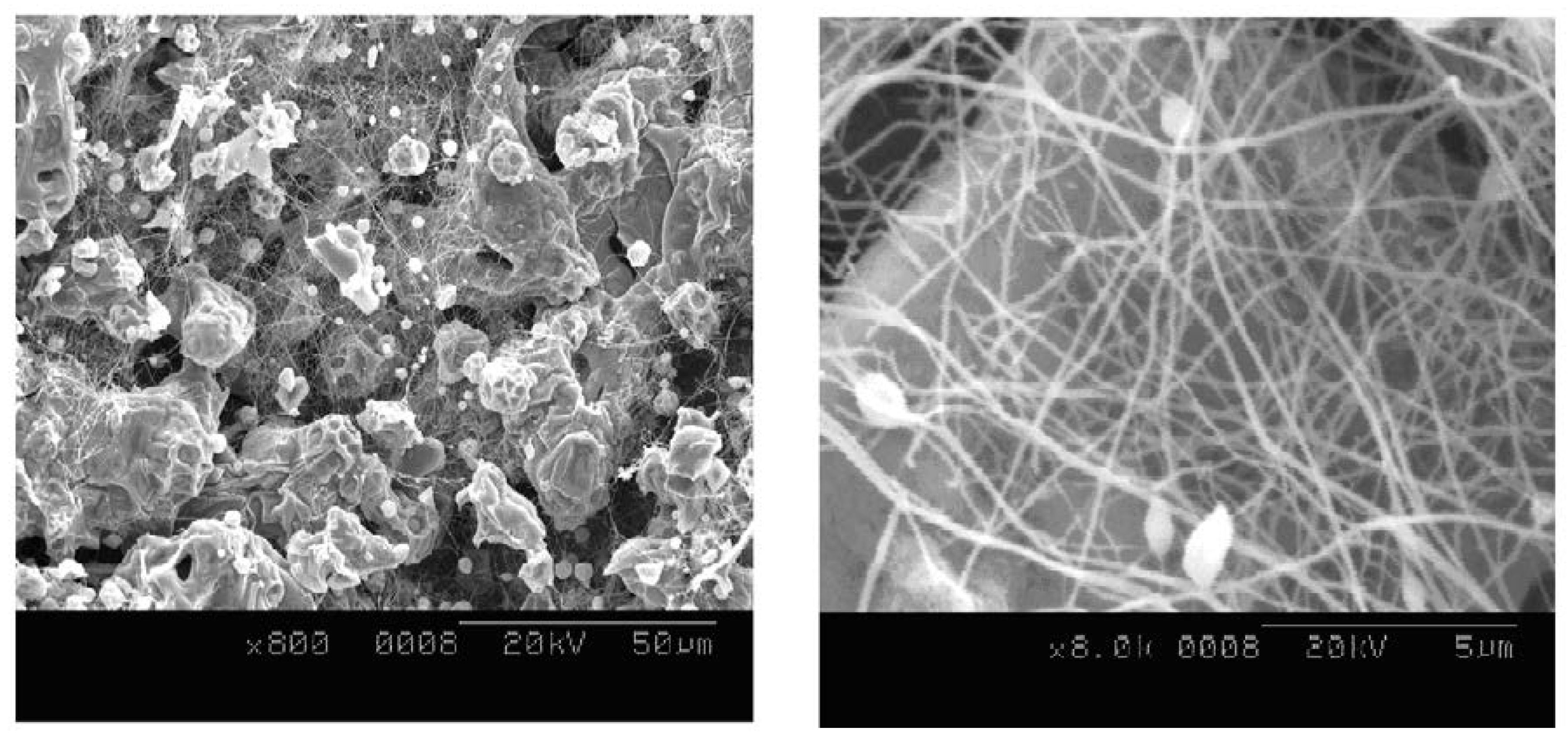
2.2. Ag-Coated Ni Nanofibers
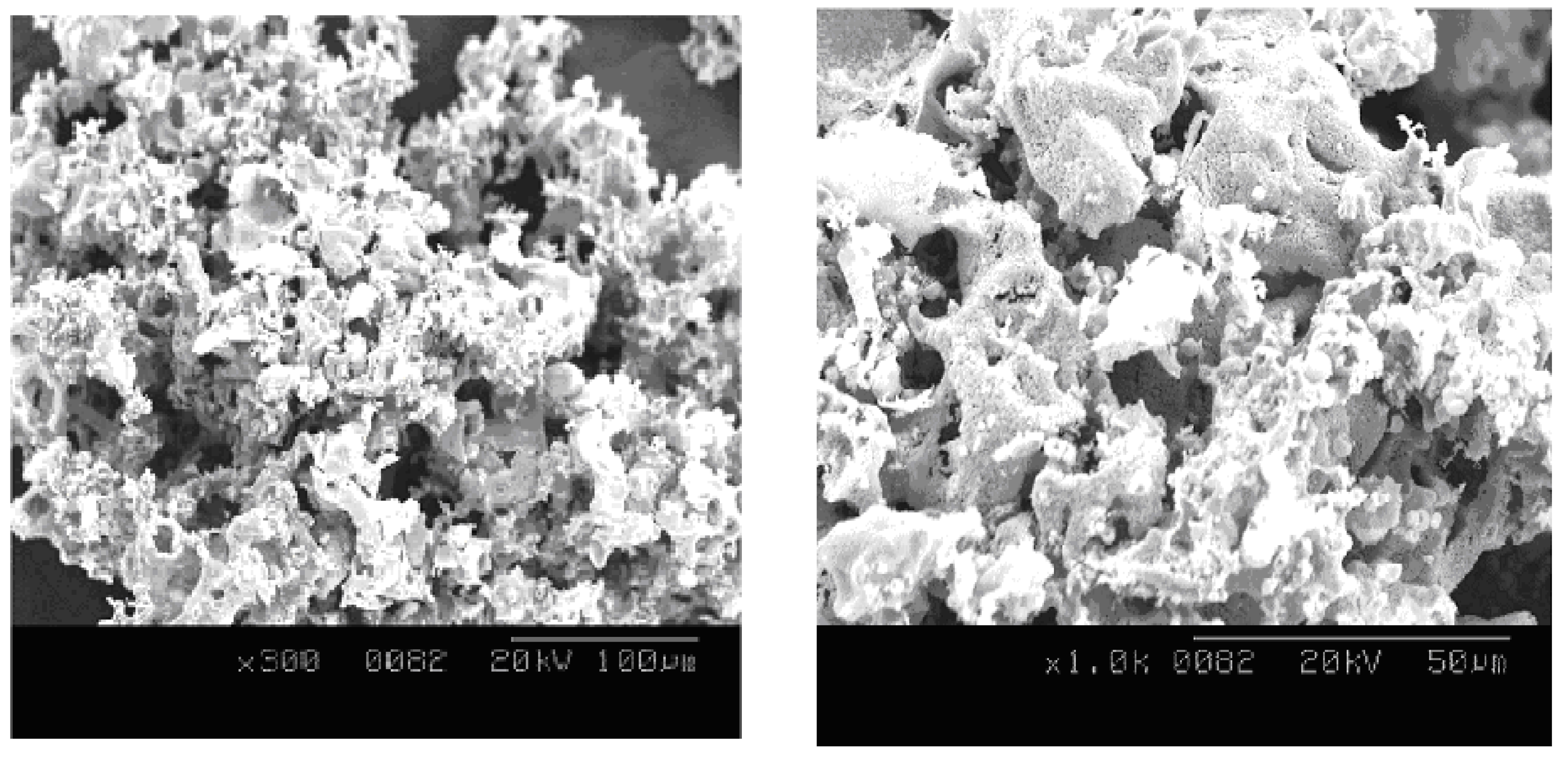
2.3. Preparation of Nanocomposites with Epoxy
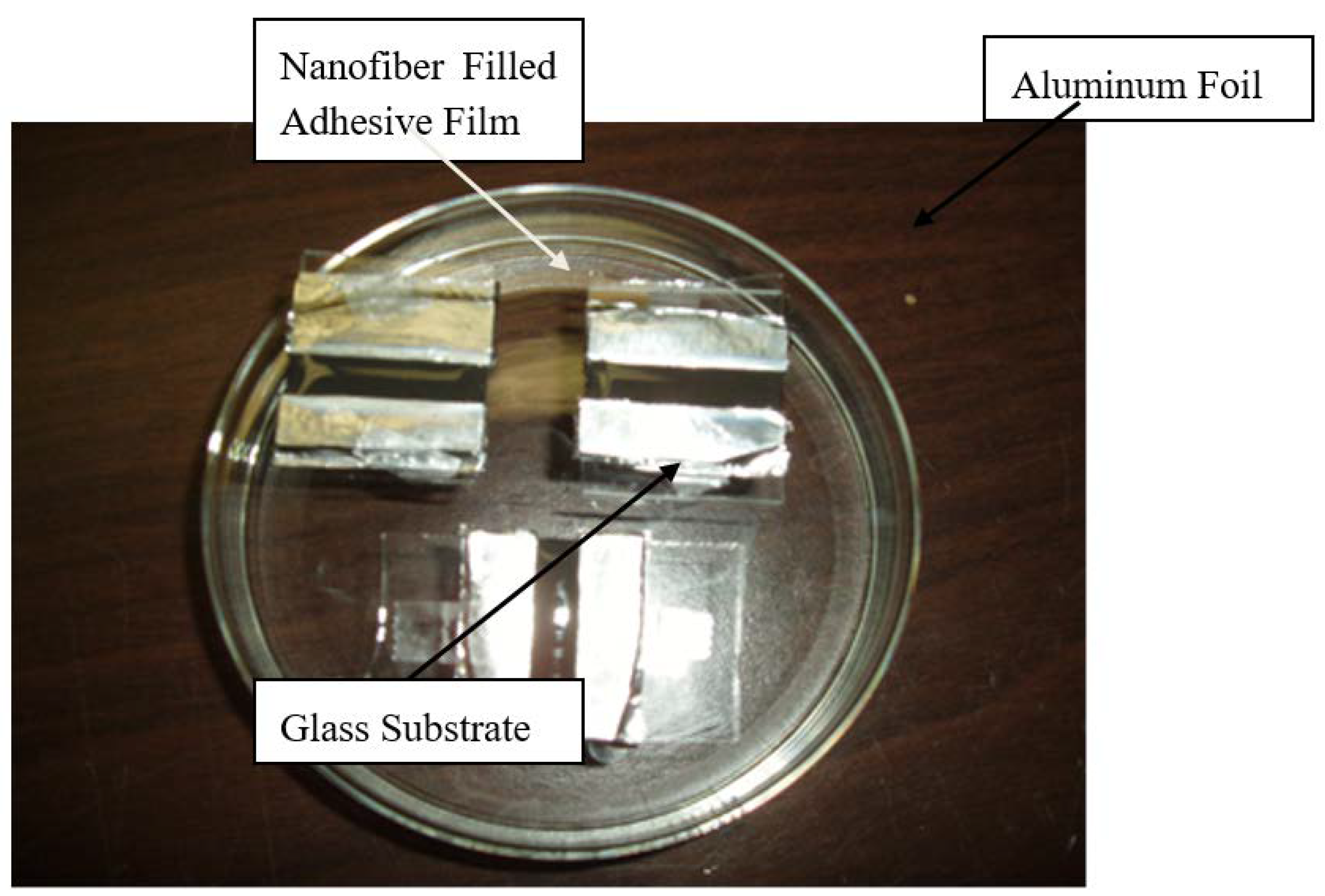
2.4. Characterization
2.4.1. Scanning Electron Microscope (SEM)
2.4.2. Thermogravimetric Analyzer (TGA)
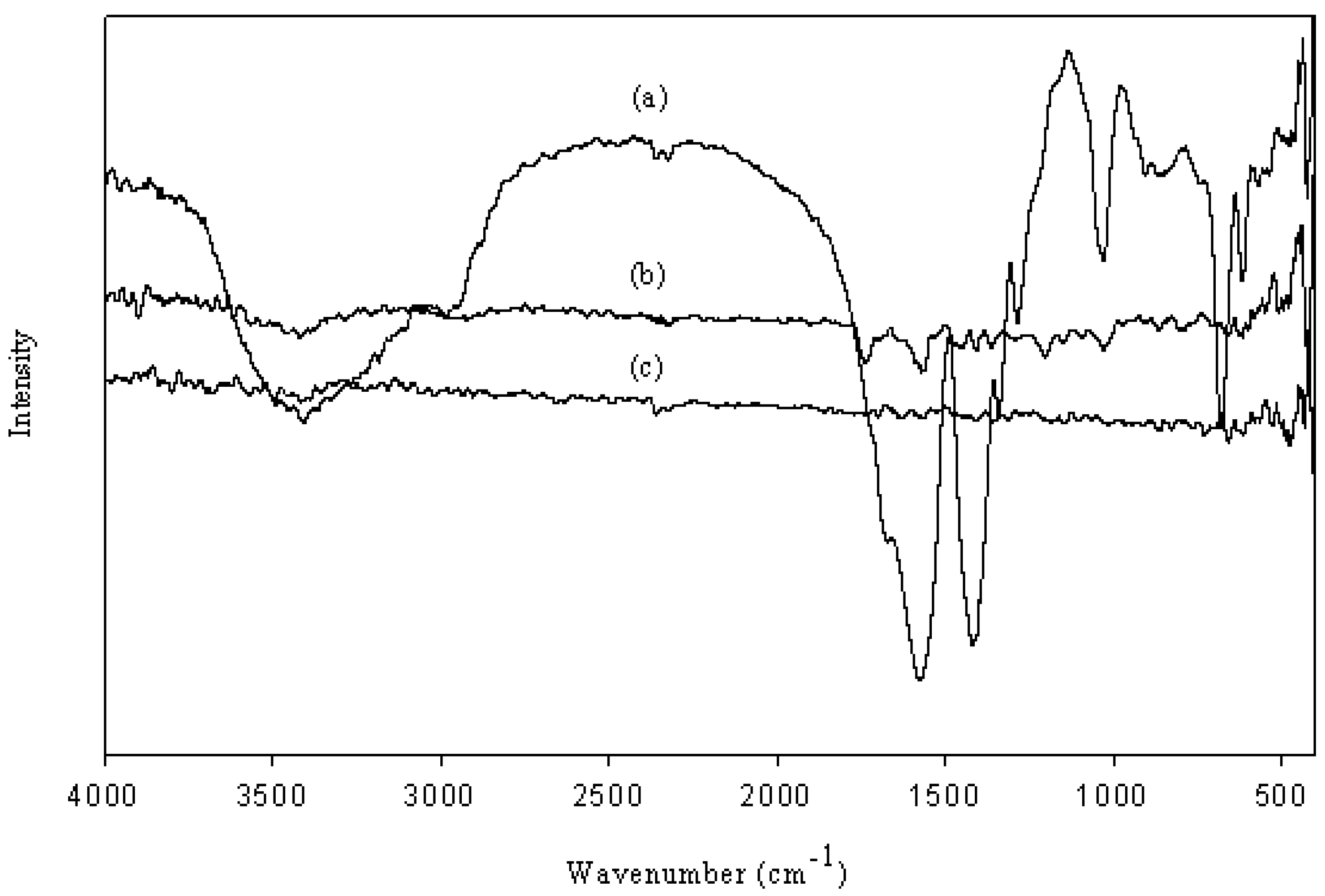
2.4.3. Fourier Transform Infrared Spectrometer (FT-IR)
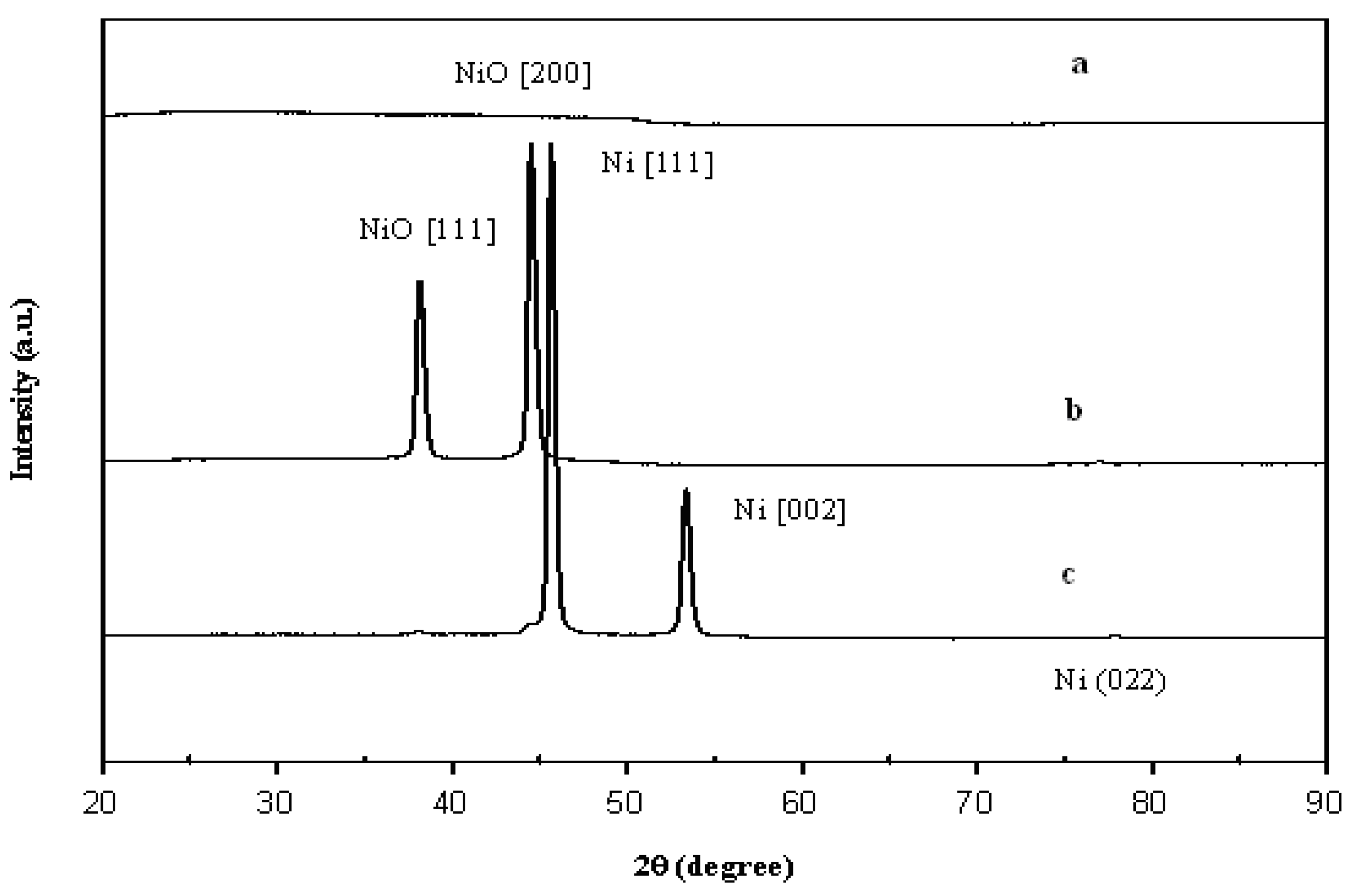
2.4.4. Wide-Angle X-Ray Diffractometer (WAXD)
2.4.5. Resistivity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sancaktar, E.; Aussawasathien, D. Nanocomposites of epoxy with electrospun carbon nanofibers: Mechanical behaviour. J. Adhes. 2009, 85, 160–179. [Google Scholar] [CrossRef]
- Aussawasathien, D.; Sancaktar, E. Effect of non-woven carbon nanofiber mat presence on cure kinetics of epoxy nanocomposites. Macromol. Symp. 2008, 264, 26–33. [Google Scholar] [CrossRef]
- Aussawasathien, D.; Sancaktar, E. Electrospun polyacrylonitrile-based carbon nanofibers and their silver modifications: Surface morphologies and properties. Curr. Nanosci. 2008, 4, 130–137. [Google Scholar] [CrossRef]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, D.; Lee, D.; Kim, S.; Morris, M. Preparation and morphology of niobium oxide fibres by electrospinning. Chem. Phys. Lett. 2003, 374, 79–84. [Google Scholar] [CrossRef]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, H.Y.; Cha, D.I.; Lee, D.R. Preparation and morphology of palladium oxide fibers via electrospinning. Mater. Lett. 2004, 58, 3368–3372. [Google Scholar] [CrossRef]
- Shao, C.L.; Kim, H.Y.; Gong, J.; Ding, B.; Lee, D.R. A novel method for making silica nanofibres by using electrospun fibres of polyvinylalcohol/silica composite as precursor. Nanotechnology 2002, 13, 635–637. [Google Scholar] [CrossRef]
- Shao, C.L.; Kim, H.Y.; Gong, J.; Ding, B.; Lee, D.R.; Park, S.J. Fiber mats of poly(vinyl alcohol)/silica composite via electrospinning. Mater. Lett. 2003, 57, 1579–1584. [Google Scholar] [CrossRef]
- Guan, H.; Shao, C.; Wen, S.; Chen, B.; Gong, J.; Yang, X. A novel method for preparing Co3O4 nanofibers by using electrospun PVA/cobalt acetate composite fibers as precursor. Mate. Chem. Phys. 2003, 82, 1002–1006. [Google Scholar] [CrossRef]
- Guan, H.; Shao, C.; Wen, S.; Chen, B.; Gong, J.; Yang, X. Preparation and characterization of NiO nanofibres via an electrospinning technique. Inorg. Chem. Comm. 2003, 6, 1302–1303. [Google Scholar] [CrossRef]
- Rao, Y.K.; Rashed, A.H. Kinetics of reduction of nickel oxide with helium-hydrogen gas mixtures in the range 300-400 degrees C. Trans. Instn. Min. Metall. Sect. C Mineral Process Extr. Metall. 2001, 110, C1–C6. [Google Scholar]
- Dilsiz, N.; Partch, R.; Matijevic, E.; Sancaktar, E. Silver coating of spindle- and filament- type magnetic particles for conductive adhesive applications. J. Adhe. Sci. Tech. 1997, 11, 1105–1118. [Google Scholar] [CrossRef]
- Sancaktar, E.; Dilsiz, N. Thickness dependent conduction behavior of various particles for conductive adhesive applications. J. Adhe. Sci. Tech. 1999, 13, 763–771. [Google Scholar] [CrossRef]
- Sancaktar, E.; Dilsiz, N. Pressure dependent conduction behavior of various particles for conductive adhesive applications. J. Adhe. Sci. Tech. 1999, 13, 679–693. [Google Scholar] [CrossRef]
- Sancaktar, E.; Wei, Y. The effect of pressure on the initial establishment of conductive paths in electronically conductive adhesives. J. Adhe. Sci. Tech. 1996, 10, 1221–1235. [Google Scholar] [CrossRef]
- Sancaktar, E.; Dilsiz, N. Anisotropic alignment of nickel particles in magnetic field for electronically conductive adhesives. J. Adhe. Sci. Tech. 1997, 11, 155–166. [Google Scholar] [CrossRef]
- Sarkar, A.; Kapoor, S.; Yashwant, G.; Salunke, H.G.; Mukherjee, T. Preparation and characterization of ultrafine Co and Ni particles in a polymer matrix. J. Phys. Chem. B 2005, 109, 7203–7207. [Google Scholar] [CrossRef]
- ASTM International. ASTM F43-99, Standard Test Methods for Resistivity of Semiconductor Materials (Withdrawn 2003); ASTM International: West Conshohocken, PA, USA, 1999. [Google Scholar]
- Sancaktar, E.; Zhang, P. Nonlinear Viscoelastic Modeling of the Fiber Matrix Interphase in Composite Materials. J. Mech. Des. 1990, 112, 605–619. [Google Scholar] [CrossRef]
- Shao, C.; Yang, X.; Guan, H.; Liu, Y.; Gong, J. Electrospun nanofibers of NiO/ZnO composite. Inorg. Chem. Comms. 2004, 7, 625–627. [Google Scholar] [CrossRef]
- Xiang, L.; Deng, X.Y.; Jin, Y. Experimental study on synthesis of NiO nano-particles. Sci. Mater. 2002, 47, 219–224. [Google Scholar] [CrossRef]
- Fu, R.; Baumann, T.F.; Cronin, S.; Dresselhaus, G.; Dresselhaus, M.S.; Satcher, J.H., Jr. Formation of graphitic structures in cobalt- and nickel-doped carbon aerogels. Langmuir 2005, 21, 2647–2651. [Google Scholar] [CrossRef]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of polychrome silver nanoparticles in different solvents. J. Mater. Chem. 2002, 12, 3783–3786. [Google Scholar] [CrossRef]





| Sample | Dosage (wt%) | Volume Resistivity (Ω·cm) |
|---|---|---|
| Short Ni nanofiber filled-epoxy resin | 10 | 607 |
| Ag-coated short Ni nanofiber filled-epoxy resin | 10 | 173 |
| Ni nanofiber mat filled-epoxy resin | 40.3 | 0.0145 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aussawasathien, D.; Sancaktar, E. Nickel Nanofibers Manufactured via Sol-Gel and Electrospinning Processes for Electrically Conductive Adhesive Applications. ChemEngineering 2020, 4, 26. https://doi.org/10.3390/chemengineering4020026
Aussawasathien D, Sancaktar E. Nickel Nanofibers Manufactured via Sol-Gel and Electrospinning Processes for Electrically Conductive Adhesive Applications. ChemEngineering. 2020; 4(2):26. https://doi.org/10.3390/chemengineering4020026
Chicago/Turabian StyleAussawasathien, Darunee, and Erol Sancaktar. 2020. "Nickel Nanofibers Manufactured via Sol-Gel and Electrospinning Processes for Electrically Conductive Adhesive Applications" ChemEngineering 4, no. 2: 26. https://doi.org/10.3390/chemengineering4020026
APA StyleAussawasathien, D., & Sancaktar, E. (2020). Nickel Nanofibers Manufactured via Sol-Gel and Electrospinning Processes for Electrically Conductive Adhesive Applications. ChemEngineering, 4(2), 26. https://doi.org/10.3390/chemengineering4020026





