Using Lignin to Modify Starch-Based Adhesive Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adhesive Preparation
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubé, M.A.; Salehpour, S. Applying the principles of green chemistry to polymer production technology. Macromol. React. Eng. 2014, 8, 7–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Cunningham, M.F.; Smeets, N.M.B.; Dubé, M.A. Starch nanoparticle incorporation in latex-based adhesives. Eur. Polym. J. 2018, 106, 128–138. [Google Scholar] [CrossRef]
- Ellis, R.P.; Cochrane, M.P.; Dale, M.F.B.; Duffus, C.M.; Lynn, A.; Morrison, I.M.; Prentice, R.D.M.; Swanston, J.S.; Tiller, S.A. Starch production and industrial use. J. Sci. Food Agric. 1998, 77, 289–311. [Google Scholar] [CrossRef]
- Emblem, A.; Hardwidge, M. (Eds.) Chapter 16: Adhesives for packaging. In Packaging Technology: Fundamentals, Materials and Processes; Woodhead Publishing: London, UK, 2012; ISBN 9781845696658. [Google Scholar]
- Thomas, D.J.; Atwell, W.A. (Eds.) Starch Structure: Chapter 1. In Starches; American Association of Cereal Chemists: St. Paul, MN, USA, 1999; ISBN 978-1-891127-01-4. [Google Scholar]
- ASI Adhesives and Sealants Industry Packaging Enduser: Starch and Dextrin Based Adhesives | 2005-08-01 | asi Magazine. Available online: https://www.adhesivesmag.com/articles/84472-packaging-enduser-starch--and-dextrin-based-adhesives (accessed on 2 July 2019).
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Improved thermal processing for food texture modification. Modif. Food Texture Nov. Ingred. Process. Tech. 2015, 1, 115–131. [Google Scholar] [CrossRef]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.C.; Åman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Bajpai, P. (Ed.) Chapter 12: Corrugated containers. In Biermann’s Handbook of Pulp and Paper; Elsevier: Cambridge, UK, 2018; ISBN 978-0-12-814238-7. [Google Scholar]
- Linke, K. Starch-Based Adhesives. U.S. Patent 4,272,295, 9 June 1981. [Google Scholar]
- 20 Mule Team Borax. Borates in Starch and Dextrin Adhesives; Rio Tinto: London, UK, 2011. [Google Scholar]
- Allen, L.A. Starch Based Adhesives and Method Therefor. U.S. Patent 4,359,341, 16 November 1982. [Google Scholar]
- Fitt, L.E.; Pienkowski, J.J.; Wallace, J.R. Starch-Hemicellulose Adhesive for High Speed Corrugating. U.S. Patent 5,358,559, 25 October 1994. [Google Scholar]
- Pizzi, A.; Mittal, K.L.; Conner, A.; Baumann, M. Chapter 15: Carbohydrate polymers as adhesives. In Handbook of Adhesive Technology; Pizzi, A., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1994; ISSN 0144-8617. [Google Scholar]
- Whistler, R.L.; BeMiller, J.N.; Paschall, E.F. (Eds.) Starch: Chemistry and Technology, 2nd ed.; Academic Press: London, UK, 1984; ISBN 0323139507. [Google Scholar]
- McElmury, D.E.; Fischer, A.C. Single Ungelatinized Starch-Component-Corrugating Adhesive. U.S. Patent 3,487,033, 30 December 1969. [Google Scholar]
- Leake, C.H.; Foran, M.T.; Jeffcoat, R.; Philbin, M.T.; Fannon, J.E. All Natural, Starch-Based, Waterresistant Corrugating Adhesive. U.S. Patent 5,405,437, 11 April 1995. [Google Scholar]
- Snyder, P.A. Starch-Based Corrugating Adhesive Containing Fibers. U.S. Patent 4,941,922, 17 July 1990. [Google Scholar]
- McPherson, R.; Antrim, R.L.; Schmidt, A.G. Corrugation Adhesive, Corrugated Board and Preparation Method Therefor. U.S. Patent 6,179,905 B1, 30 January 2001. [Google Scholar]
- The International Lignin Institute about Lignin. Available online: http://www.ili-lignin.com/aboutlignin.php (accessed on 22 July 2019).
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Baumberger, S.; Lapierre, C.; Monties, B.; Lourdin, D.; Colonna, P. Preparation and properties of thermally moulded and cast lignosulfonates-starch blends. Ind. Crops Prod. 1997, 6, 253–258. [Google Scholar] [CrossRef]
- Baumberger, S.; Lapierre, C.; Monties, B. Utilization of pine kraft lignin in starch composites: Impact of structural heterogeneity. J. Agric. Food Chem. 1998, 46, 2234–2240. [Google Scholar] [CrossRef]
- Lepifre, S.; Froment, M.; Cazaux, F.; Houot, S.; Lourdin, D.; Coqueret, X.; Lapierre, C.; Baumberger, S. Lignin incorporation combined with electron-beam irradiation improves the surface water resistance of starch films. Biomacromolecules 2004, 5, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Measuring the Bond Strength of a Glued Manufacturer’s Joint. In TAPPI T 837; TAPPI: Atlanta, GA, USA, 1995.
- Kong, F.; Wang., S.; Price, J.T.; Konduri, M.K.R.; Fatehi, P. Water soluble kraft lignin-acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, D.; Guo, W.; Qiu, X. Investigation of grafted sulfonated alkali lignin polymer as dispersant in coal-water slurry. J. Ind. Eng. Chem. 2015, 27, 192–200. [Google Scholar] [CrossRef]
- Alwadani, N.; Fatehi, P. Lignin Modzification to Produce hydrOphobic Products. Master’s Thesis, Lakehead University, Thunder Bay, ON, Canada, 2017. [Google Scholar]
- Baumberger, S.; Lapierre, C.; Monties, B.; Della Valle, G. Use of kraft lignin as filler for starch films. Polym. Degrad. Stab. 1998, 59, 273–277. [Google Scholar] [CrossRef]
- Stevens, E.S.; Klamczynski, A.; Glenn, G.M. Starch-lignin foams. Express Polym. Lett. 2010, 4, 311–320. [Google Scholar] [CrossRef]
- Baumberger, S. Chapter 1: Starch-lignin films. In Chemical Modification, Properties and Usage of Lignin; Hu, T.Q., Ed.; Springer: Boston, MA, USA, 2002; ISBN 978-1-4615-0643-0. [Google Scholar]
- Spiridon, I.; Teaca, C.A.; Bodirlau, R. Preparation and characterization of adipic acid-modified starch microparticles/plasticized starch composite films reinforced by lignin. J. Mater. Sci. 2011, 46, 3241–3251. [Google Scholar] [CrossRef]
- Vengal, J.C.; Srikumar, M. Processing and study of novel lignin-starch and lignin-gelatin biodegradable polymeric films. Trends Biomater. Artif. Organs 2005, 18, 237–241. [Google Scholar]
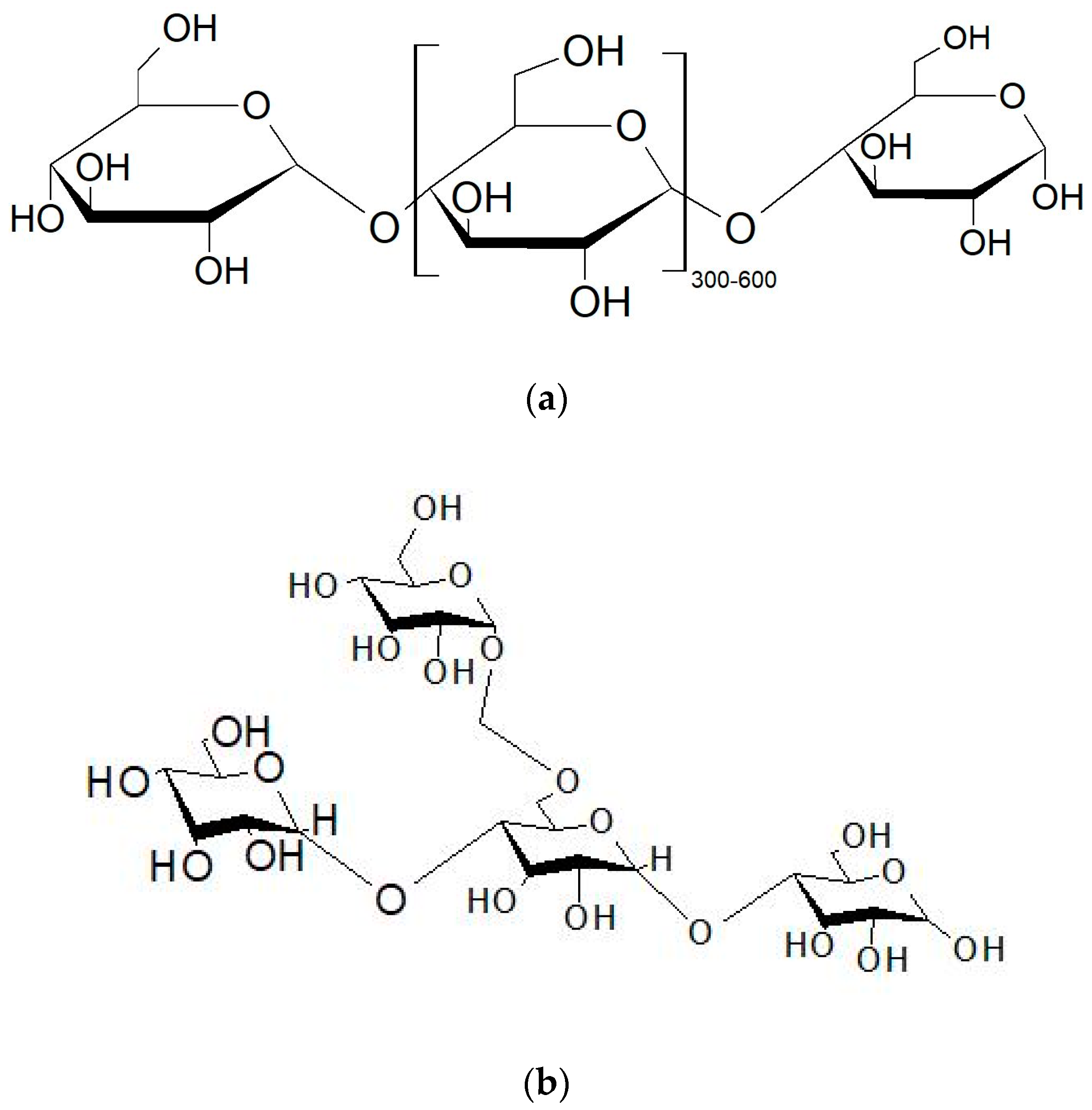

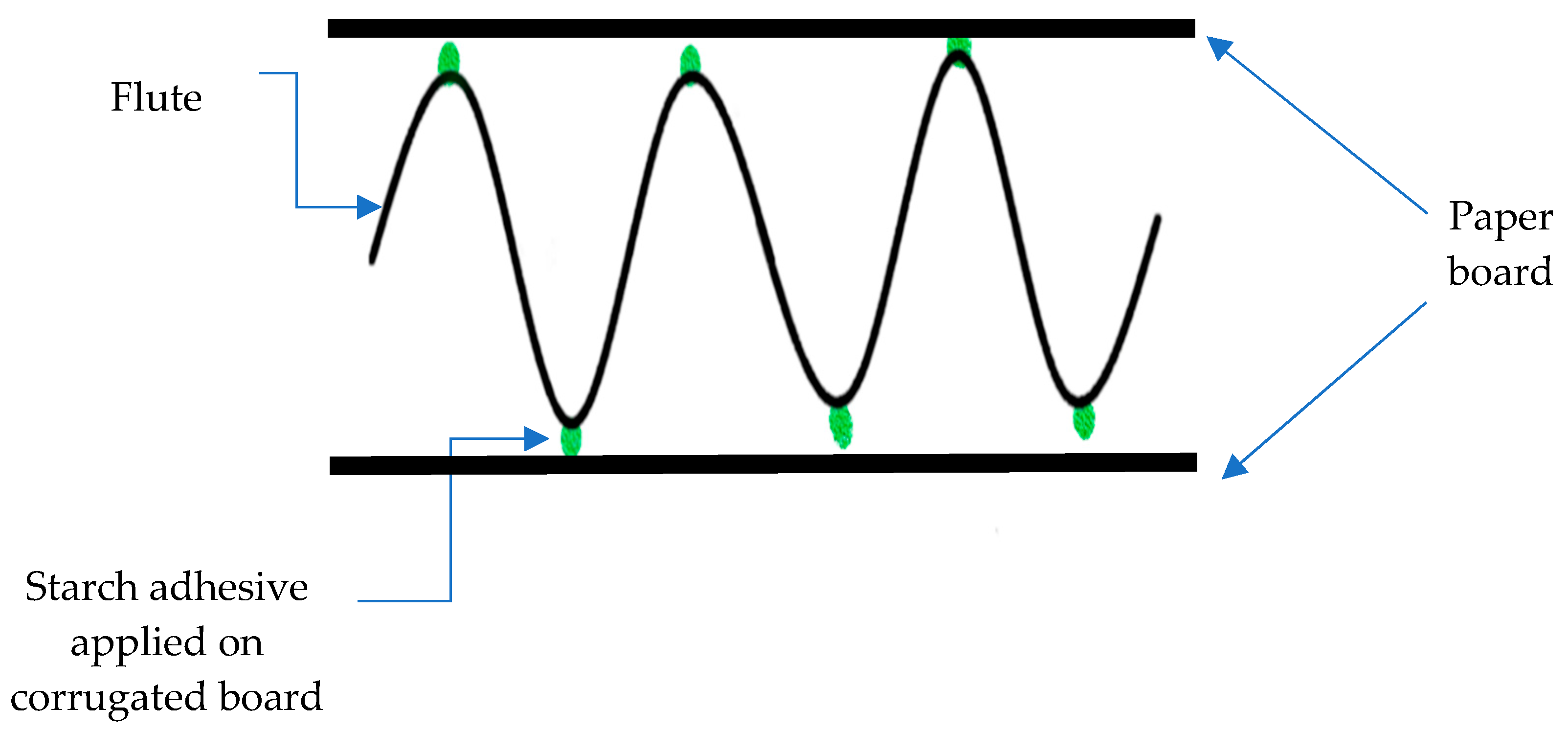



| Starch Source | Amylose (wt%) | Gelation Temperature (°C) |
|---|---|---|
| Corn | 20–28 | 62–72 |
| Wheat | 17–34 | 58–64 |
| Oat | 18–29 | 56–61 |
| Rice | 17–29 | 68–78 |
| Potato | 25–31 | 59–68 |
| Property | Units | Range |
|---|---|---|
| pH | 2.2–5.5 | |
| Ash | wt% | 0.2–1.5 |
| Sodium | wt% | 0.1–0.6 |
| Sulfur | wt% | 1.2–2.4 |
| Carbohydrates | wt% | 1.2–2.4 |
| Hydroxyl Number | mg KOH/g | 250–275 |
| Glass Transition Temperature | °C | 150–175 |
| Molecular Weight | Daltons | 5000–8000 |
| Polydispersity | 3.0–4.5 |
| Adhesive No. | Percentage of Starch Substituted with Lignin (wt%) | Adhesive No. | Percentage of Starch Substituted with Lignin (wt%) | ||
|---|---|---|---|---|---|
| Carrier Portion | Slurry Portion | Carrier Portion | Slurry Portion | ||
| 0-0 | 0 | 0 | 5-20 | 5 | 20 |
| 0-10 | 0 | 10 | 5-30 | 5 | 30 |
| 0-20 | 0 | 20 | 10-0 | 10 | 0 |
| 0-30 | 0 | 30 | 10-10 | 10 | 10 |
| 5-0 | 5 | 0 | 10-20 | 10 | 20 |
| 5-10 | 5 | 10 | 10-30 | 10 | 30 |
| Adhesive No. | Viscosity (Stein Hall Seconds) | Adhesive No. | Viscosity (Stein Hall Seconds) |
|---|---|---|---|
| 0-0 | 90 | 5-20 | 20 |
| 0-10 | 90 | 5-30 | 20 |
| 0-20 | 90 | 10-0 | 15 |
| 0-30 | 90 | 10-10 | 15 |
| 5-0 | 20 | 10-20 | 15 |
| 5-10 | 17 | 10-30 | 15 |
| Adhesive No. | Water Contact Angle |
|---|---|
| 0-20 | 14° |
| 0-30 | 21° |
| 5-20 | 22° |
| 5-30 | 24° |
| 10-20 | 26° |
| 10-30 | 33° |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri, A.; Wearing, J.; Dubé, M.A. Using Lignin to Modify Starch-Based Adhesive Performance. ChemEngineering 2020, 4, 3. https://doi.org/10.3390/chemengineering4010003
Nasiri A, Wearing J, Dubé MA. Using Lignin to Modify Starch-Based Adhesive Performance. ChemEngineering. 2020; 4(1):3. https://doi.org/10.3390/chemengineering4010003
Chicago/Turabian StyleNasiri, Anahita, Jim Wearing, and Marc A. Dubé. 2020. "Using Lignin to Modify Starch-Based Adhesive Performance" ChemEngineering 4, no. 1: 3. https://doi.org/10.3390/chemengineering4010003
APA StyleNasiri, A., Wearing, J., & Dubé, M. A. (2020). Using Lignin to Modify Starch-Based Adhesive Performance. ChemEngineering, 4(1), 3. https://doi.org/10.3390/chemengineering4010003





