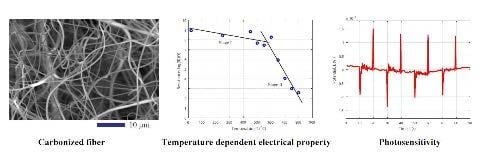
Effect of Pyrolysis Temperature on the Electrical Property and Photosensitivity of a PAN-PMMA Derived Carbon Fiber
Abstract
1. Introduction
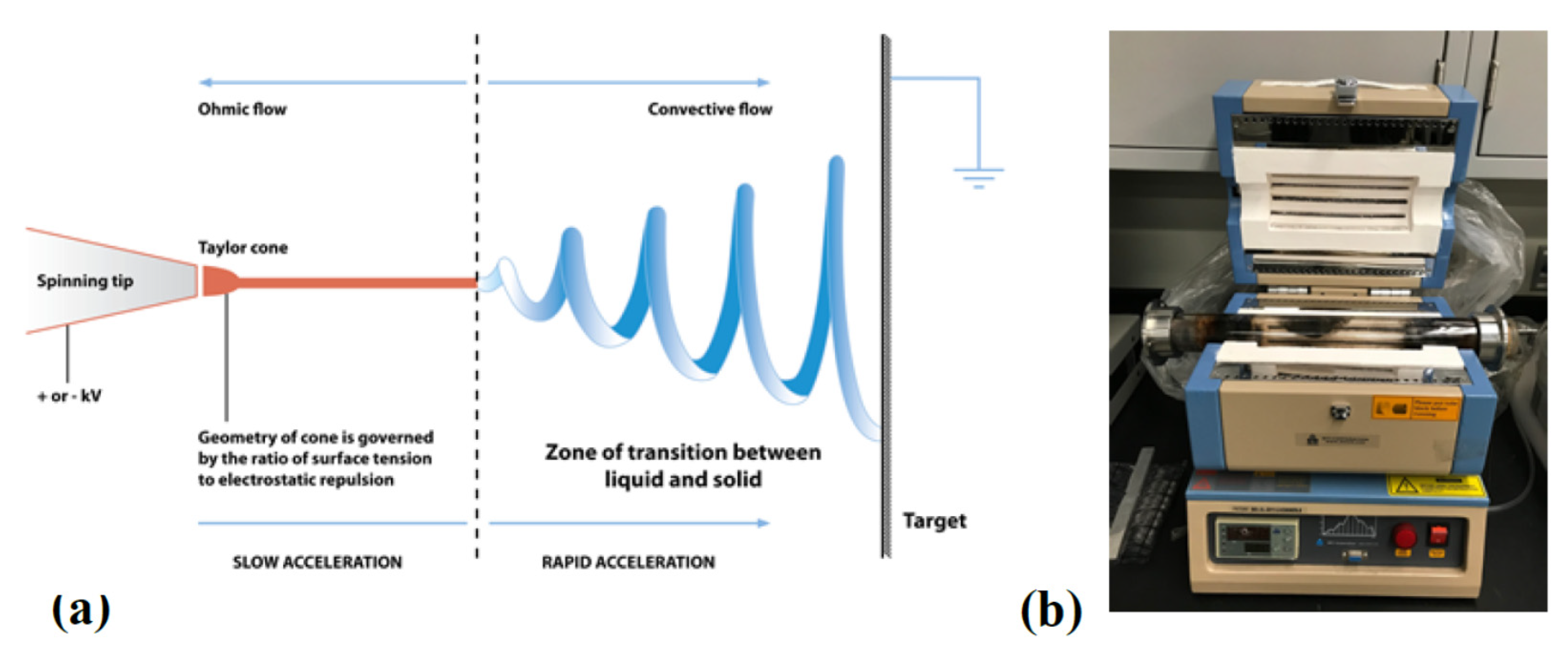
2. Materials and Experimental Methods
3. Results and Discussion
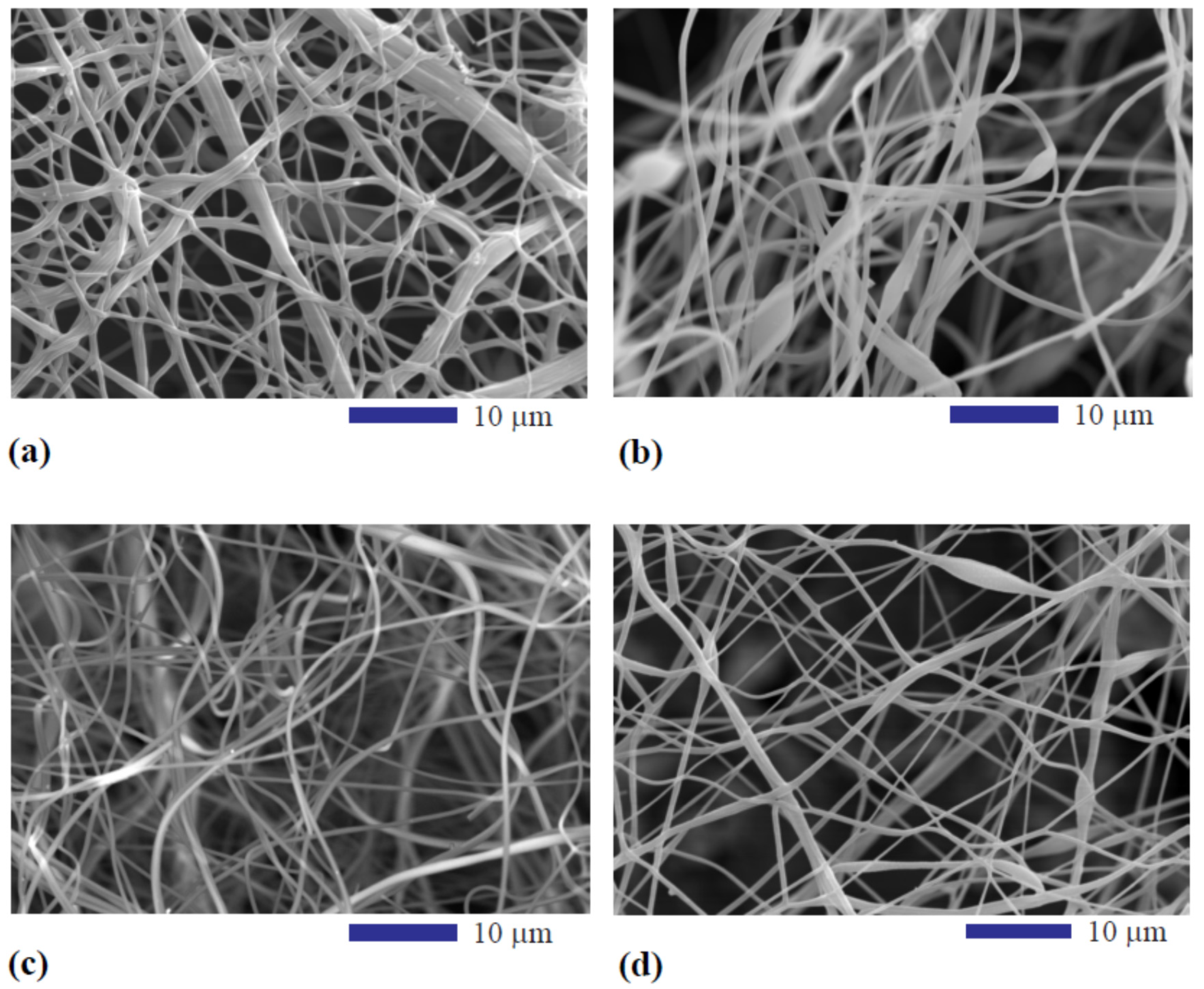
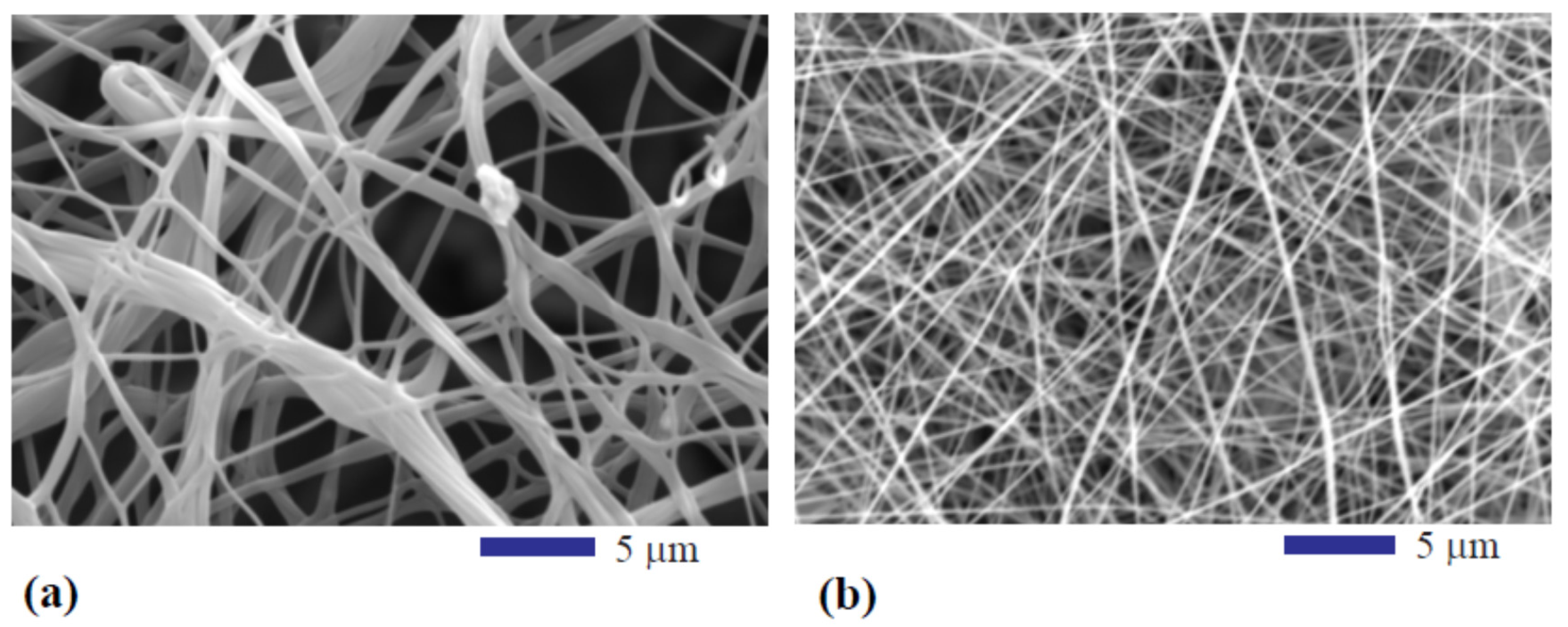
3.1. Effect of Pyrolysis Temperature on Structure
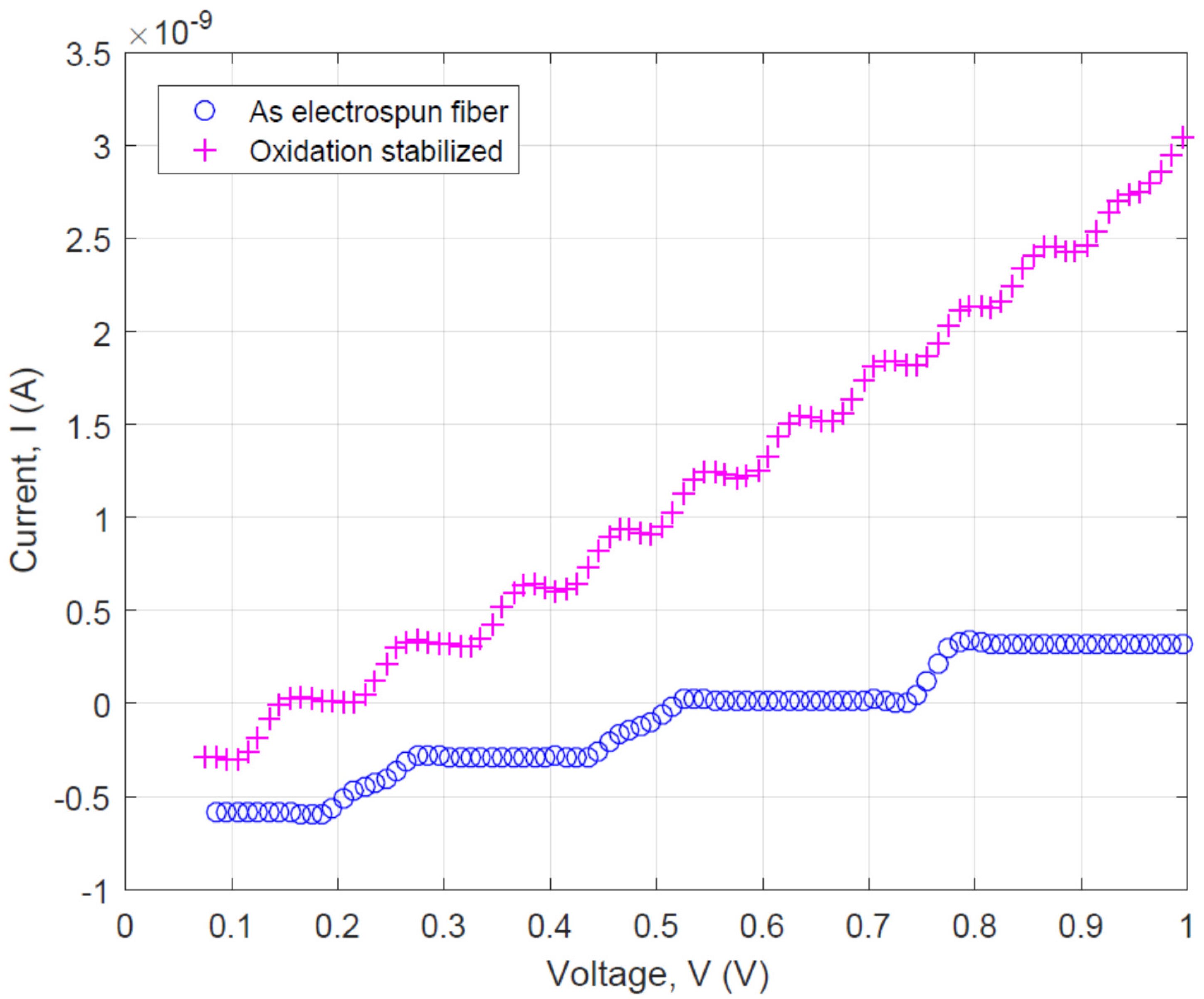
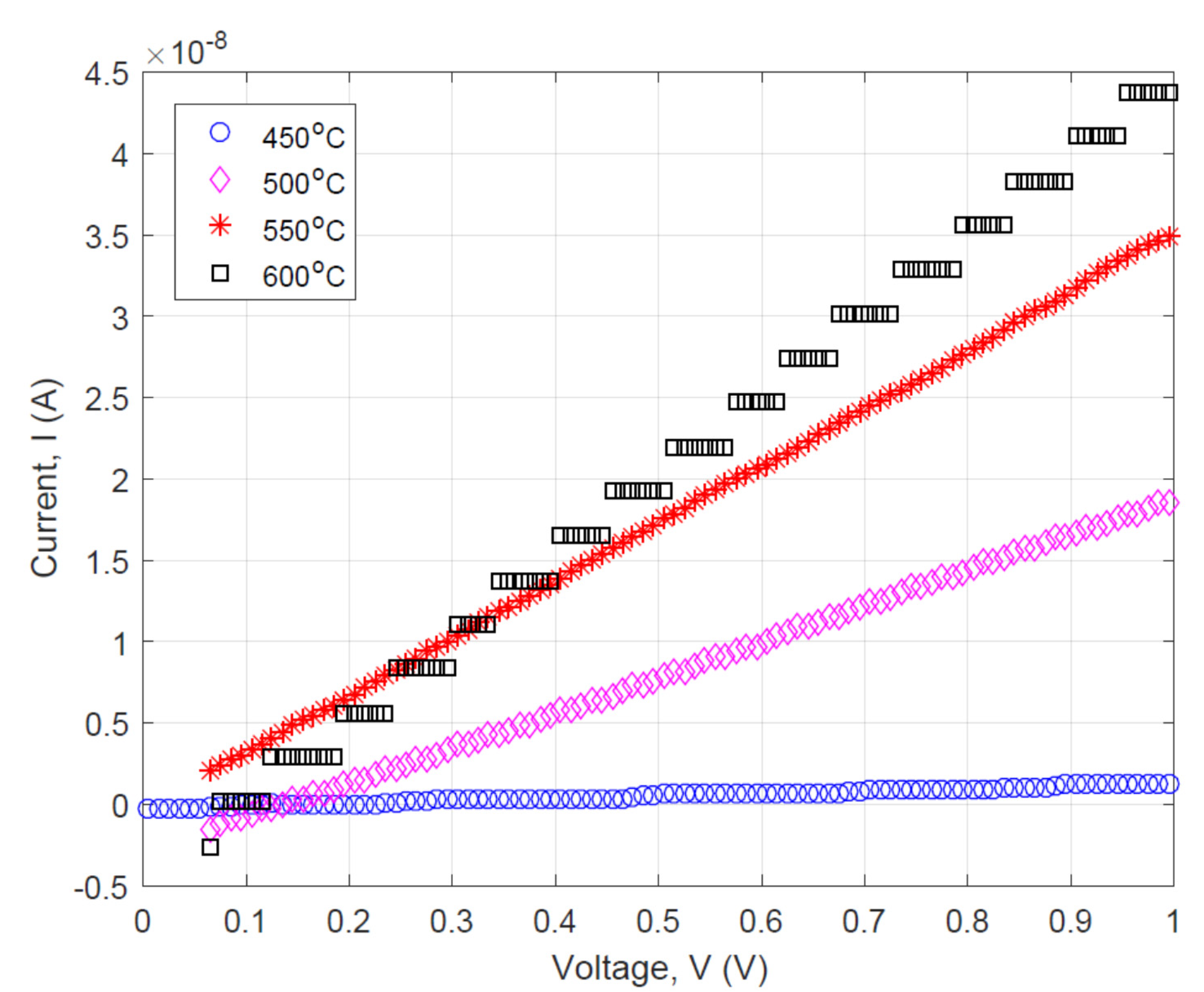
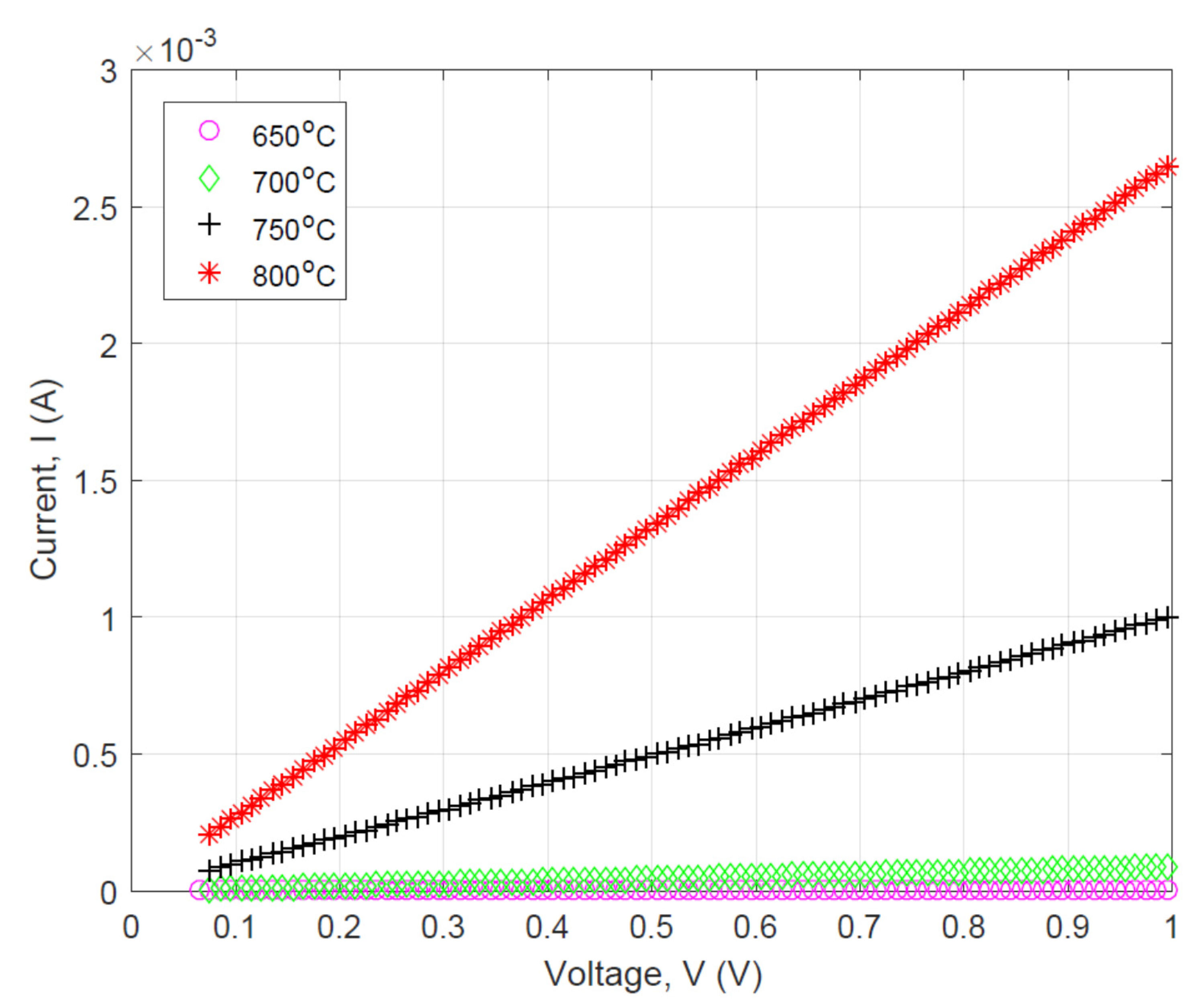
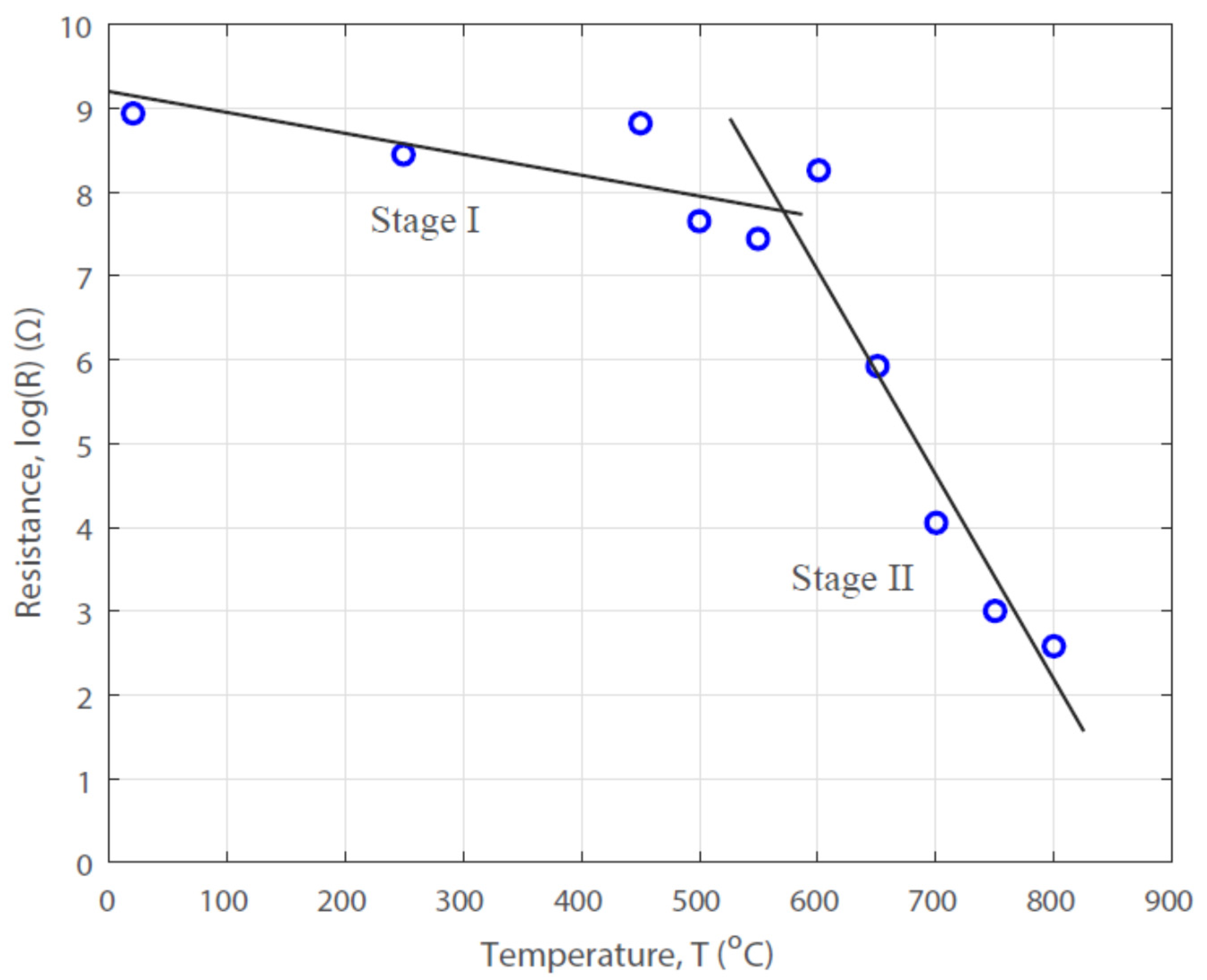
3.2. Effect of Pyrolysis Temperature on the Electrical Property
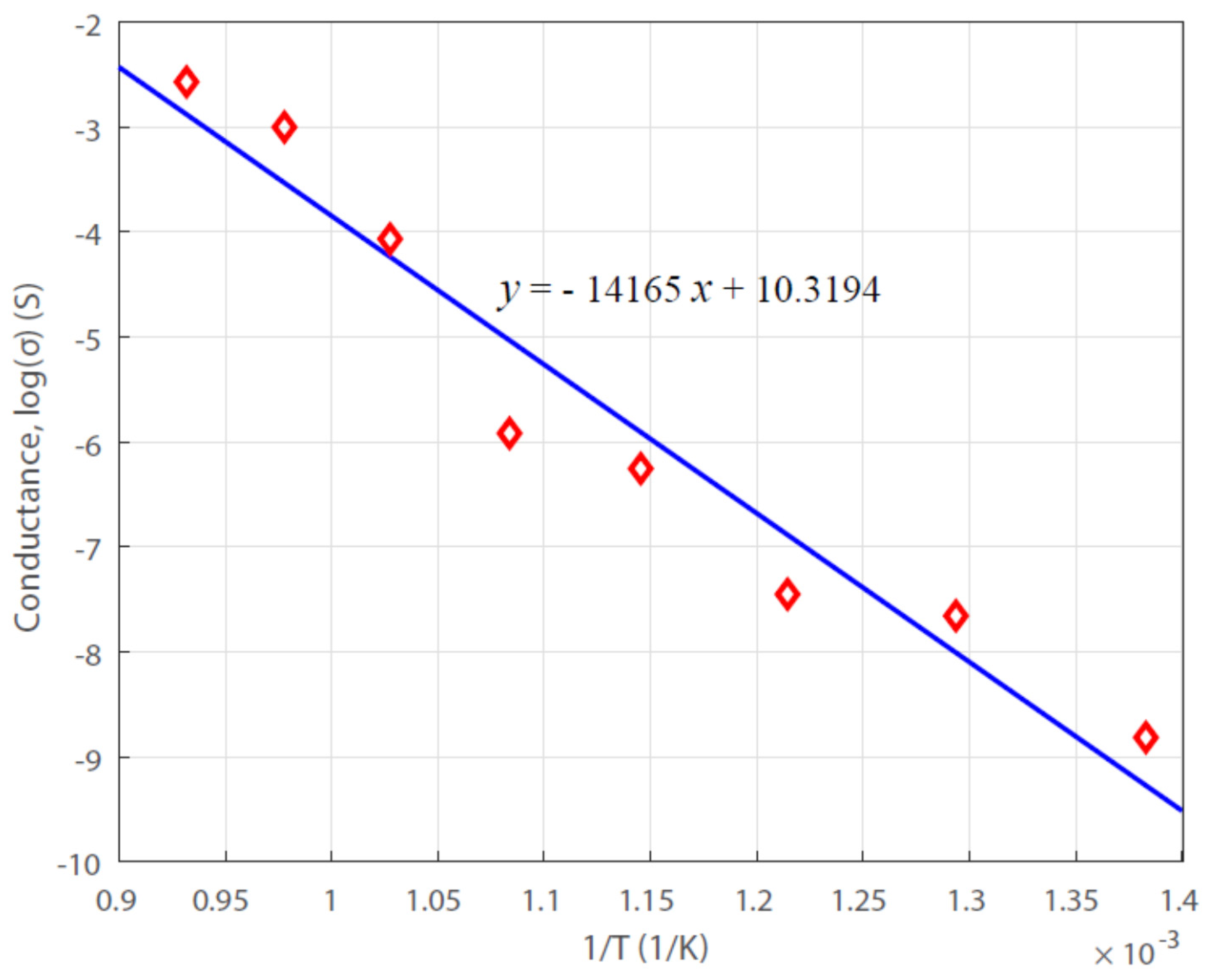
3.3. Activation Energy of the Carbonization Reaction
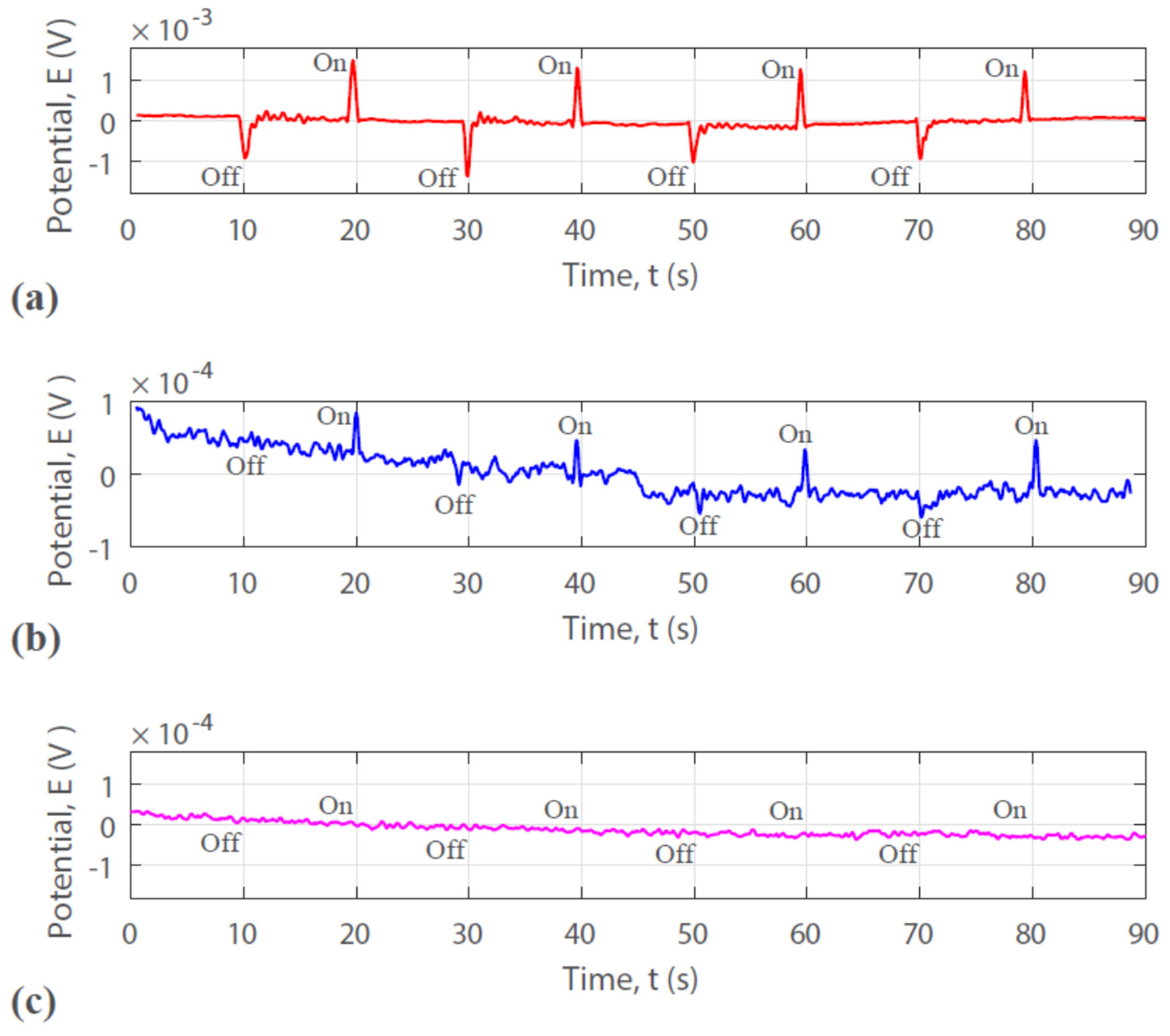
3.4. Photosensitive Property
3.5. Role of PMMA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, S.; Park, J.; Jo, Y.; Kim, Y.-B.; Jang, J.; Kim, S.-K.; Jeong, S.; Park, J.-U. Haze-free transparent electrodes using metal nanofibers with carbon shells for high-temperature stability. Appl. Surf. Sci. 2019, 483, 1101–1109. [Google Scholar] [CrossRef]
- Sun, X.; Li, C.; Bai, J. Amorphous cobalt carbon nanofibers decorated with conductive Ag as free-standing flexible electrode material for high-performance supercapacitors. J. Electron. Mater. 2019, 48, 2754–2760. [Google Scholar] [CrossRef]
- Ravindren, R.; Mondal, S.; Nath, K.; Das, N.C. Prediction of electrical conductivity, double percolation limit and electromagnetic interference shielding effectiveness of copper nanowire filled flexible polymer blend nanocomposites. Compos. Part B Eng. 2019, 164, 559–569. [Google Scholar] [CrossRef]
- Liu, J.; Liang, Y.; Wang, L.; Wang, B.; Zhang, T.; Yi, F. Fabrication and photosensitivity of CdS photoresistor on silica nanopillars substrate. Mater. Sci. Semicond. Process. 2016, 56, 217–221. [Google Scholar] [CrossRef]
- Gan, Y.X.; Yu, C.; Panahi, N.; Gan, J.B.; Cheng, W. Processing iron oxide nanoparticle-loaded composite carbon fiber and the photosensitivity characterization. Fibers 2019, 7, 25. [Google Scholar] [CrossRef]
- Su, L.; Gan, Y.X. Experimental study on synthesizing TiO2 nanotube/polyaniline (PANI) nanocomposites and their thermoelectric and photosensitive property characterization. Compos. Part B Eng. 2012, 43, 170–182. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Wang, J.; Zhang, Q.; Zhang, B. Design and preparation of self-driven BSA surface imprinted tubular carbon nanofibers and their specific adsorption performance. Chem. Eng. J. 2019, 373, 923–934. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Long, L.; Fu, X.; Sui, G.; Yang, X. An optimal carbon fiber interlayer integrated with bio-based gel polymer electrolyte enabling trapping-diffusion-conversion of polysulfides in lithium-sulfur batteries. Chem. Eng. J. 2019, 370, 1068–1076. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Ge, C.; Zhou, W.; Zhu, Y.; Xu, B. Branched carbon nanotube/carbon nanofiber composite for supercapacitor electrodes. Mater. Lett. 2019, 246, 174–177. [Google Scholar] [CrossRef]
- Ju, J.; Lv, Y.; An, X.; Liu, W.; Li, Z.; Kang, W.; Cheng, B. The stereoscopic honeycomb-like porous carbon nanofibers as a carrier of TiO2 nanoparticles for high-performance Li-ion capacitor. J. Alloys Compd. 2019, 791, 1248–1256. [Google Scholar] [CrossRef]
- Bian, Z.; Yuan, T.; Xu, Y.; Pang, Y.; Yao, H.; Li, J.; Yang, J.; Zheng, S. Boosting Li–S battery by rational design of freestanding cathode with enriched anchoring and catalytic N-sites carbonaceous host. Carbon 2019, 150, 216–223. [Google Scholar] [CrossRef]
- Gan, Y.X.; Dong, J.; Gan, J.B. Carbon network/aluminum composite made by powder metallurgy and its corrosion behavior in seawater. Mater. Chem. Phys. 2017, 202, 190–196. [Google Scholar] [CrossRef]
- Gan, Y.X.; Draper, C.W.; Gan, J.B. Carbon nanofiber network made by electrohydrodynamic casting immiscible fluids. Mater. Today Commun. 2017, 13, 248–254. [Google Scholar] [CrossRef]
- Niu, Q.; Zhao, S.; Gao, K.; Wang, L. Polyacrylonitrile-based nitrogen-doped carbon materials with different micro-morphology prepared by electrostatic field for supercapacitors. J. Electron. Mater. 2019, 48, 5264–5272. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, X.; Xu, H.; Wen, Y.; Lu, H.; Meng, X. Salting-out and salting-in of protein: A novel approach toward fabrication of hierarchical porous carbon for energy storage application. J. Alloys Compd. 2019, 788, 397–406. [Google Scholar] [CrossRef]
- Ho, D.H.; Cheon, S.; Hong, P.; Park, J.H.; Suk, J.W.; Kim, D.H.; Han, J.T.; Cho, J.H. Multifunctional smart textronics with blow-spun nonwoven fabrics. Adv. Funct. Mater. 2019, 29, 1900025. [Google Scholar] [CrossRef]
- Pitkänen, O.; Tolvanen, J.; Szenti, I.; Kukovecz, Á.; Hannu, J.; Jantunen, H.; Kordás, K. Lightweight hierarchical carbon nanocomposites with highly efficient and tunable electromagnetic interference shielding properties. ACS Appl. Mater. Interfaces 2019, 11, 19331–19338. [Google Scholar] [CrossRef]
- Ren, H.; Zheng, L.; Wang, G.; Gao, X.; Tan, Z.; Shan, J.; Cui, L.; Li, K.; Jian, M.; Zhu, L.; et al. Transfer-medium-free nanofiber-reinforced graphene film and applications in wearable transparent pressure sensors. ACS Nano 2019, 13, 5541–5548. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Lin, L.; Wang, H.; Huang, X.; Xue, H.; Gao, J. Highly stretchable, anti-corrosive and wearable strain sensors based on the PDMS/CNTs decorated elastomer nanofiber composite. Chem. Eng. J. 2019, 362, 89–98. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, L.; Mao, Z.; Wang, R.; He, B.; Gong, Y.; Hu, X. Mesopore-induced ultrafast Na+-Storage in T-Nb2O5/carbon nanofiber films toward flexible high-power Na-ion capacitors. Small 2019, 15, 1804539. [Google Scholar] [CrossRef]
- Serdaroğlu, D.Ç.; Korkusuz, H.K.; Karakaya, M.; Donmez, I.; Ünal, M.A.; Gunasekaran, S. Nanoparticle embedded nanofiber synthesis and evaluation of usability on biomedical applications. MRS Adv. 2018, 3, 233–240. [Google Scholar] [CrossRef]
- Cipriani, E.; Zanetti, M.; Bracco, P.; Brunella, V.; Luda, M.P.; Costa, L. Crosslinking and carbonization processes in PAN films and nanofibers. Polym. Degrad. Stab. 2016, 123, 178–188. [Google Scholar] [CrossRef]
- Schierholz, R.; Kröger, D.; Weinrich, H.; Gehring, M.; Tempel, H.; Kungl, H.; Mayer, J.; Eichel, R.-A. The carbonization of polyacrylonitrile-derived electrospun carbon nanofibers studied by in situ transmission electron microscopy. RSC Adv. 2019, 9, 6267–6277. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Khan, W.S.; Asmatulu, R. Synthesis of electrospun polyacrylonitrile- derived carbon fibers and comparison of properties with bulk form. PLoS ONE 2018, 13, e0201345. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Alharbi, A.; Khan, W.S.; Rahman, A.S.; Asmatulu, R. Mechanical and thermal properties of carbonized PAN nanofibers cohesively attached to surface of carbon fiber reinforced composites. Macromol. Symp. 2016, 365, 140–150. [Google Scholar] [CrossRef]
- Ghorpade, R.V.; Cho, D.W.; Hong, S.C. Effect of controlled tacticity of polyacrylonitrile (co)polymers on their thermal oxidative stabilization behaviors and the properties of resulting carbon films. Carbon 2017, 121, 502–511. [Google Scholar] [CrossRef]
- Hameed, N.; Sharp, J.; Nunna, S.; Creighton, C.; Magniez, K.; Jyotishkumar, P.; Salim, N.V.; Fox, B. Structural transformation of polyacrylonitrile fibers during stabilization and low temperature carbonization. Polym. Degrad. Stab. 2016, 128, 39–45. [Google Scholar] [CrossRef]
- Jing, M.; Wang, C.G.; Wang, Q.; Bai, Y.; Zhu, B. Chemical structure evolution and mechanism during pre-carbonization of PAN-based stabilized fiber in the temperature range of 350–600 °C. Polym. Degrad. Stab. 2007, 92, 1737–1742. [Google Scholar] [CrossRef]
- Zhang, Y.; Tajaddod, N.; Song, K.; Minus, M.L. Low temperature graphitization of interphase polyacrylonitrile (PAN). Carbon 2015, 91, 479–493. [Google Scholar] [CrossRef]
- Chai, X.; Mi, H.; Zhu, C.; He, C.; Xu, J.; Zhou, X.; Liu, J. Low-temperature thermal stabilization of polyacrylontrile-based precursor fibers towards efficient preparation of carbon fibers with improved mechanical properties. Polymer 2015, 76, 131–139. [Google Scholar] [CrossRef]
- Korobeinyk, A.V.; Whitby, R.L.; Mikhalovsky, S.V. High temperature oxidative resistance of polyacrylonitrile-methylmethacrylate copolymer powder converting to a carbonized monolith. Eur. Polym. J. 2012, 48, 97–104. [Google Scholar] [CrossRef]
- Joo, S.; Park, J.Y.; Seo, S.; Moon, S.; Lee, K.J.; An, J.; Lee, C.-S.; Bae, J. Study on peculiar carbon pattern formation from polymer blend thin films under electric fields. Thin Solid Films 2018, 660, 846–851. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Yarin, A.; Pourdeyhimi, B. The production of 100/400 nm inner/outer diameter carbon tubes by solution blowing and carbonization of core-shell nanofibers. Carbon 2010, 48, 3575–3578. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Xu, R.-S.; Chen, M.; Wang, X.; Ling, L.-C.; Zhang, R.; Yang, J.-H. Hollow carbon nanospheres prepared by carbonizing polymethylmethacrylate/polyacrylonitrile core/shell polymer particles. New Carbon Mater. 2008, 23, 205–208. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Zhu, P.; Yan, C.; Jia, H.; Kiyak, Y.; Zang, J.; He, J.; Dirican, M.; Zhang, X. Glass fiber separator coated by porous carbon nanofiber derived from immiscible PAN/PMMA for high-performance lithium-sulfur batteries. J. Membr. Sci. 2018, 552, 31–42. [Google Scholar] [CrossRef]
- Gong, J.; Chen, X.; Tang, T. Recent progress in controlled carbonization of (waste) polymers. Prog. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Rao, M.; Geng, X.; Liao, Y.; Hu, S.; Li, W. Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J. Membr. Sci. 2012, 399, 37–42. [Google Scholar] [CrossRef]
- Nataraj, S.; Yang, K.; Aminabhavi, T. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Liu, W.; Wang, W.; Xing, Z.; Qi, Y.; Wu, G. Radiation-induced crosslinking of polyacrylonitrile fibers and the subsequent regulative effect on the peroxidation process. Radiat. Phys. Chem. 2012, 81, 622–627. [Google Scholar] [CrossRef]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 1710–1719. [Google Scholar] [CrossRef]
- Lin, S.; Cai, Q.; Ji, J.; Sui, G.; Yu, Y.; Yang, X.; Ma, Q.; Wei, Y.; Deng, X. Electrospun nanofiber reinforced and toughened composites through in situ nano-interface formation. Compos. Sci. Technol. 2008, 68, 3322–3329. [Google Scholar] [CrossRef]
- Xue, T.J.; McKinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: An Introduction; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; pp. 725–777. [Google Scholar]
- Shackelford, J.F. Introduction to Materials Science for Engineers; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009; pp. 451–452. [Google Scholar]
- Mangonon, P.L. The Principles of Materials Selection for Engineering Design; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 1999; pp. 109–117. [Google Scholar]
- Askeland, D.R.; Wright, W.J. Essentials of Materials Science and Engineering; Cengage Learning: Stamford, CT, USA, 2014; pp. 47–48. [Google Scholar]
- Zeng, W.R.; Li, S.F.; Chow, W.K. Preliminary studies on burning behavior of polymerthylmethacrylate (PMMA). J. Fire Sci. 2002, 20, 297–317. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Nguyen, A.; Rosas, N.; Flores, I.; Chen, C.; Gan, J.B.; Hamdan, A.S.; Gan, Y.X. Effect of Pyrolysis Temperature on the Electrical Property and Photosensitivity of a PAN-PMMA Derived Carbon Fiber. ChemEngineering 2019, 3, 86. https://doi.org/10.3390/chemengineering3040086
Xu T, Nguyen A, Rosas N, Flores I, Chen C, Gan JB, Hamdan AS, Gan YX. Effect of Pyrolysis Temperature on the Electrical Property and Photosensitivity of a PAN-PMMA Derived Carbon Fiber. ChemEngineering. 2019; 3(4):86. https://doi.org/10.3390/chemengineering3040086
Chicago/Turabian StyleXu, Tyler, Antonino Nguyen, Noe Rosas, Isidro Flores, Cindy Chen, Jeremy B. Gan, Anan S. Hamdan, and Yong X. Gan. 2019. "Effect of Pyrolysis Temperature on the Electrical Property and Photosensitivity of a PAN-PMMA Derived Carbon Fiber" ChemEngineering 3, no. 4: 86. https://doi.org/10.3390/chemengineering3040086
APA StyleXu, T., Nguyen, A., Rosas, N., Flores, I., Chen, C., Gan, J. B., Hamdan, A. S., & Gan, Y. X. (2019). Effect of Pyrolysis Temperature on the Electrical Property and Photosensitivity of a PAN-PMMA Derived Carbon Fiber. ChemEngineering, 3(4), 86. https://doi.org/10.3390/chemengineering3040086