Synthesis of a Very High Specific Surface Area Active Carbon and Its Electrical Double-Layer Capacitor Properties in Organic Electrolytes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis and Characterizations of the Electrode Materials
2.3. Formulations of the Electrolytes and Ionic Conductivity Measurement
2.4. Fabrication of EDLC Coin Cells
2.5. Electrochemical Measurements
3. Results and Discussion
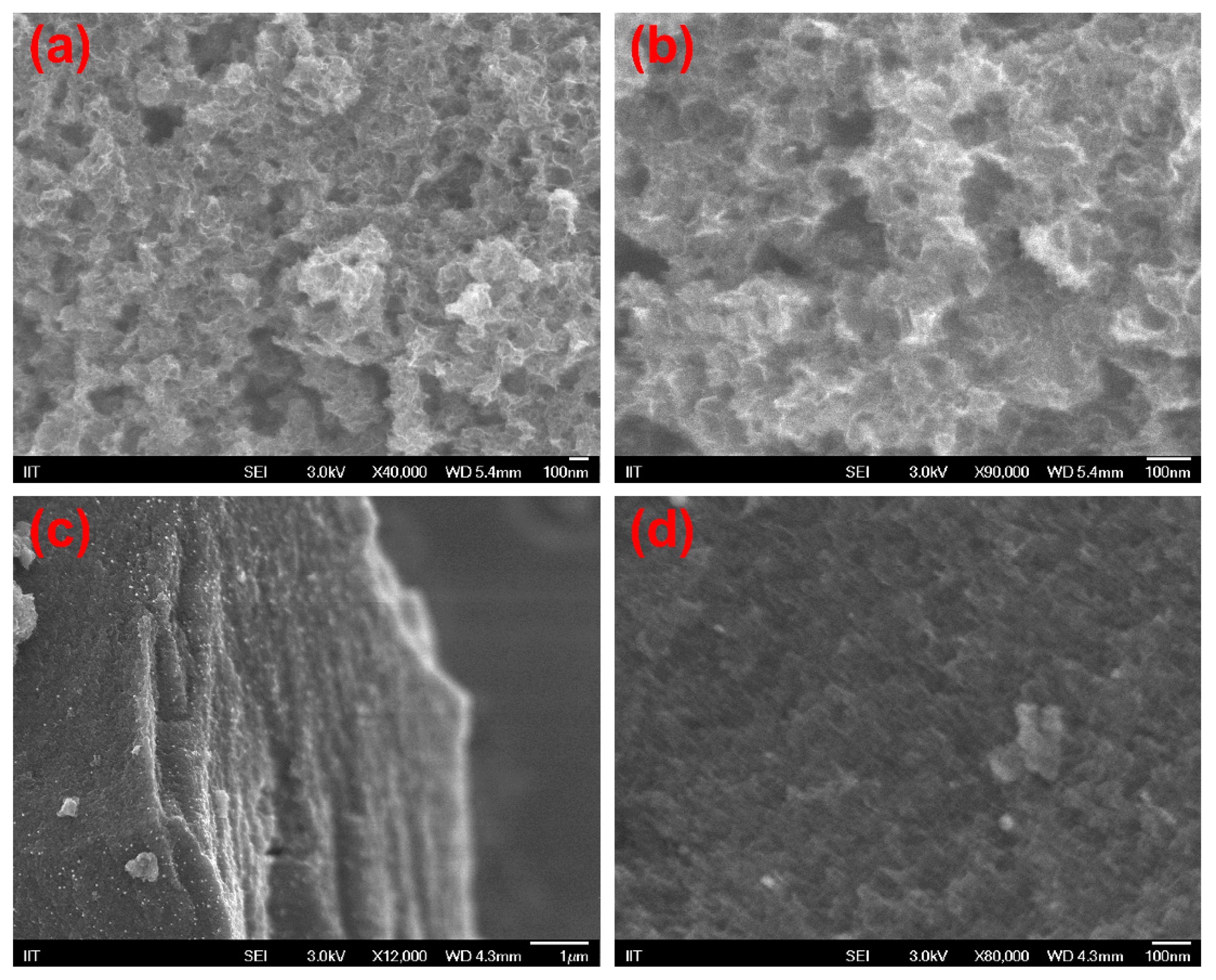
3.1. Synthesis and Properties of the Electrode Material
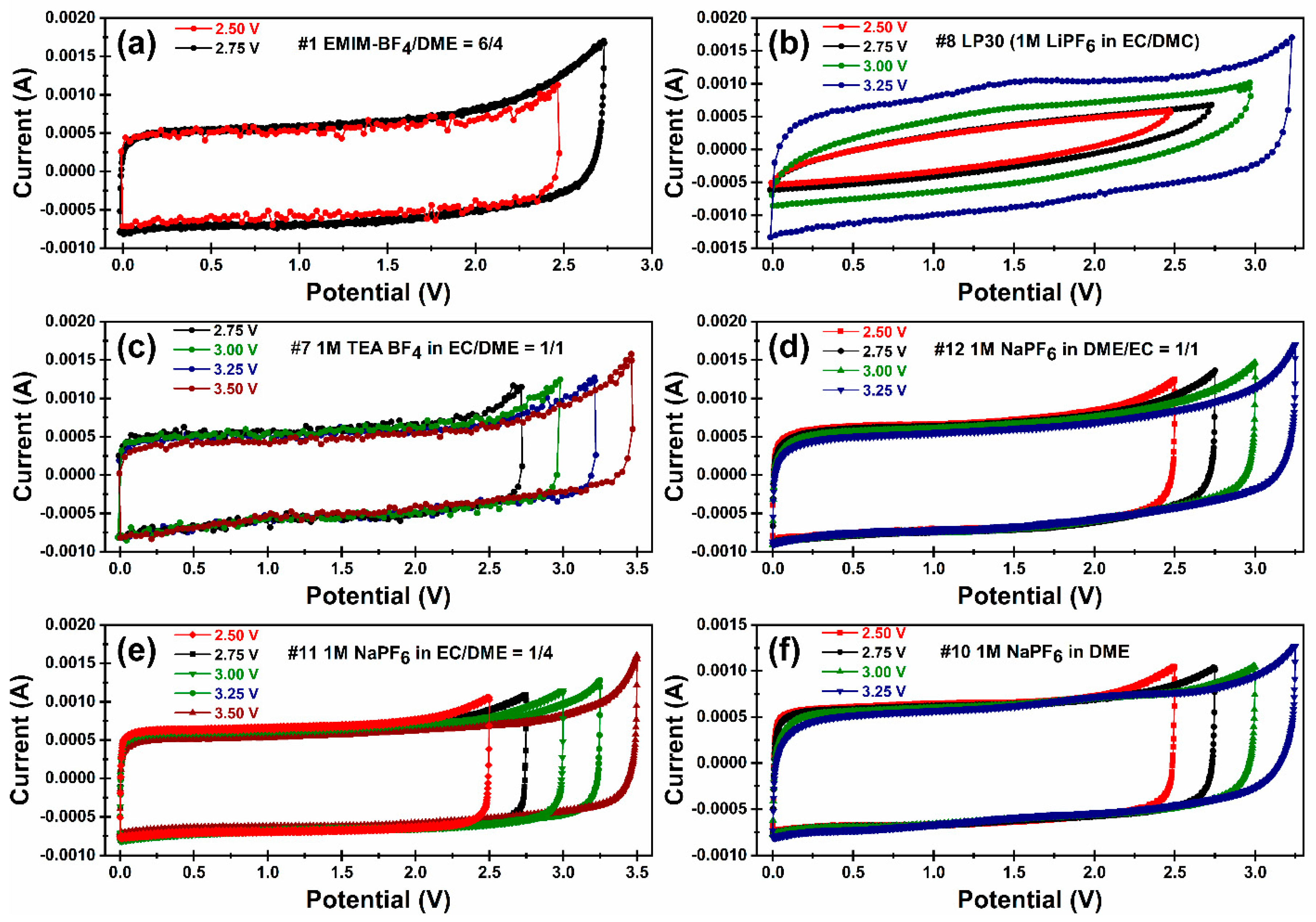
3.2. Electrolyte Formulations
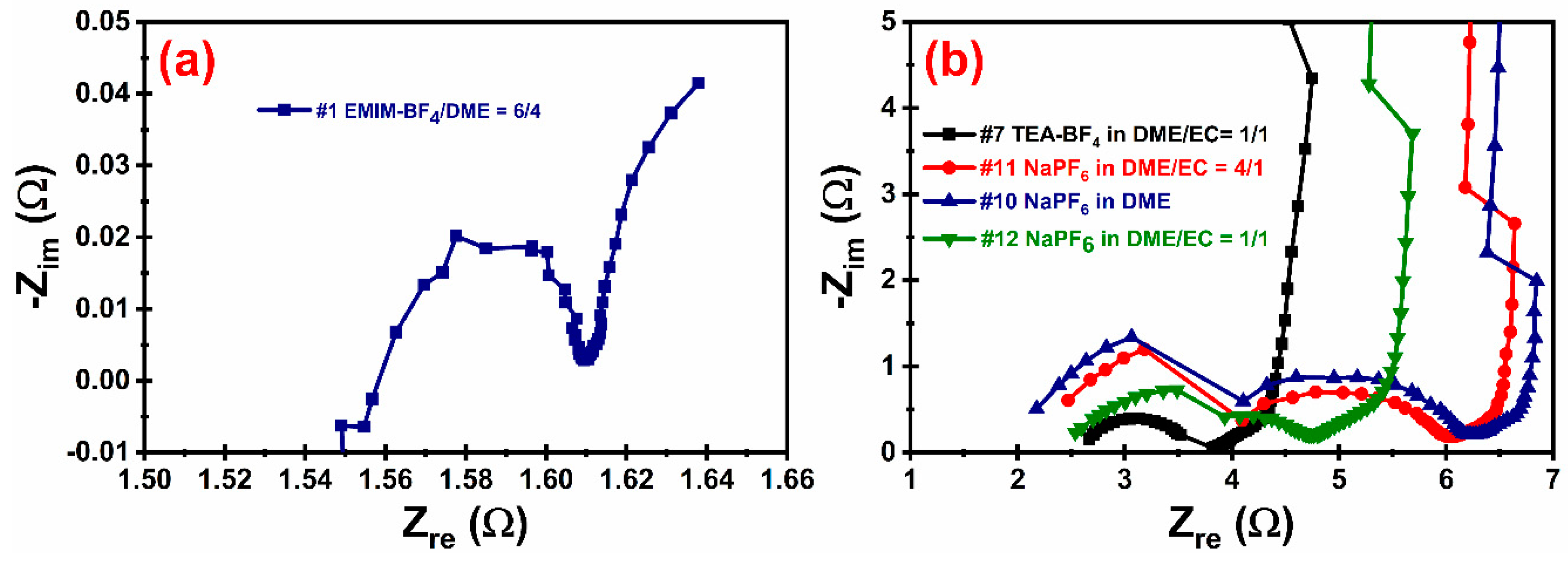
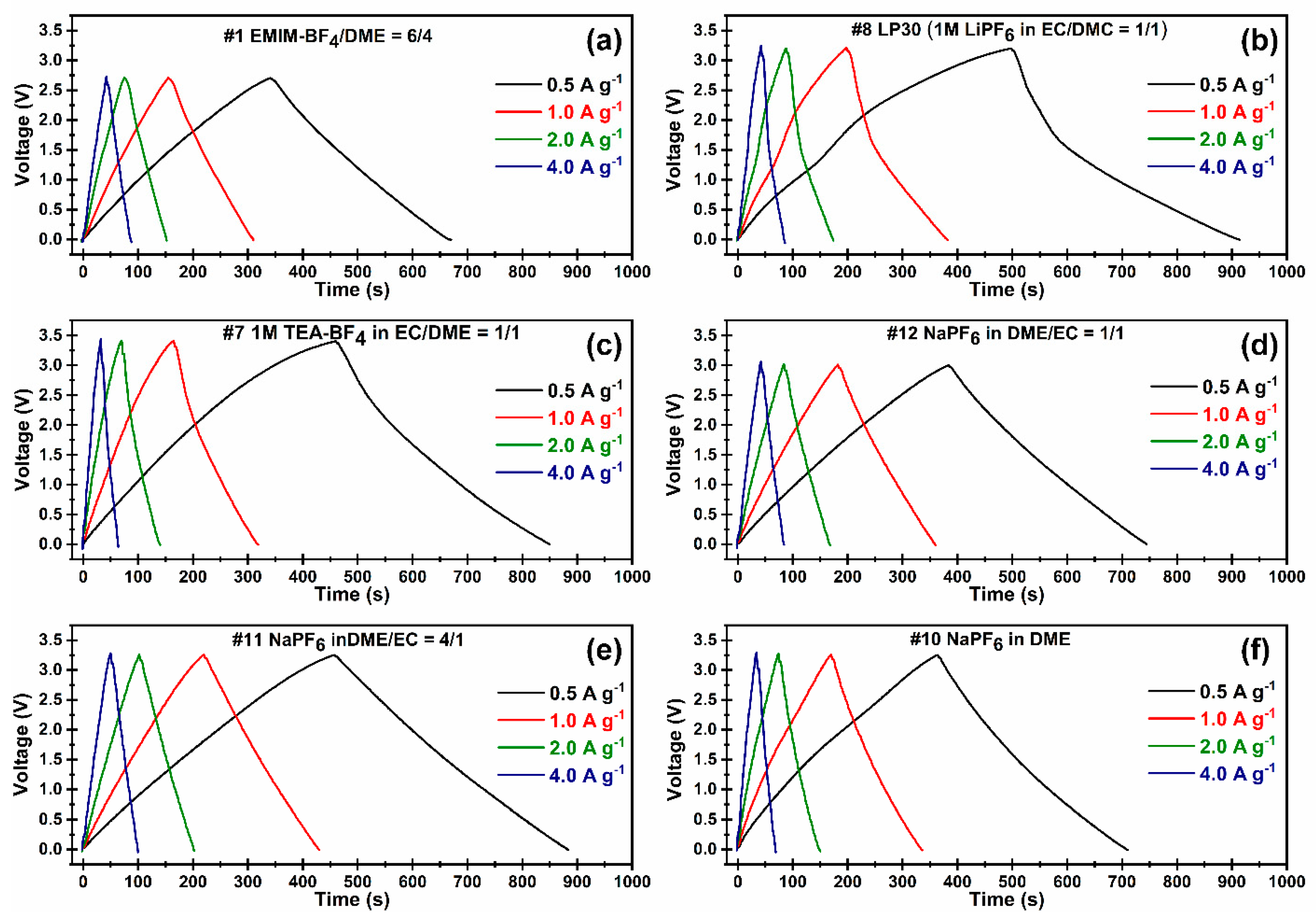
3.3. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suda, Y.; Mizutani, A.; Harigai, T.; Takikawa, H.; Ue, H.; Umeda, Y. Influences of internal resistance and specific surface area of electrode materials on characteristics of electric double layer capacitors. AIP Conf. Proc. 2017, 1807, 020022. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. WIREs Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Yan, J.-m.; Zhang, X.-b. Materials Design and System Construction for Conventional and New-Concept Supercapacitors. Adv. Sci. 2017, 4, 1600382. [Google Scholar] [CrossRef] [PubMed]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef]
- Bo, Z.; Li, C.; Yang, H.; Ostrikov, K.; Yan, J.; Cen, K. Design of Supercapacitor Electrodes Using Molecular Dynamics Simulations. Nano-Micro Lett. 2018, 10, 33. [Google Scholar] [CrossRef]
- Yang, H.; Bo, Z.; Yan, J.; Cen, K. Influence of wettability on the electrolyte electrosorption within graphene-like nonconfined and confined space. Int. J. Heat Mass Transf. 2019, 133, 416–425. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Yang, J.; Bo, Z.; Hu, M.; Yan, J.; Cen, K. Molecular Origin of Electric Double-Layer Capacitance at Multilayer Graphene Edges. J. Phys. Chem. Lett. 2017, 8, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, K.; Liu, Y.; Tan, Y.; Zhao, X.; Wu, J.; Niu, X.; Ran, F. Novel Hybrid Nanoparticles of Vanadium Nitride/Porous Carbon as an Anode Material for Symmetrical Supercapacitor. Nano-Micro Lett. 2016, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Huang, Y.; Xie, J.; Zhao, X.; Li, C.; Qu, J.; Zhang, Q.; Sun, J.; He, B.; et al. 3D Printing Fiber Electrodes for an All-Fiber Integrated Electronic Device via Hybridization of an Asymmetric Supercapacitor and a Temperature Sensor. Adv. Sci. 2018, 5, 1801114. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Zhang, X.; Shi, H.; Zeng, W.; Zhang, H.; Liu, Q.; Li, C.; Liu, Q.; Duan, H. Porous ultrathin carbon nanobubbles formed carbon nanofiber webs for high-performance flexible supercapacitors. J. Mater. Chem. A 2017, 5, 14801–14810. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, T.; Li, M.; Yu, M.; Luo, Y.; Tong, Y.; Li, Y. Multiscale Pore Network Boosts Capacitance of Carbon Electrodes for Ultrafast Charging. Nano Lett. 2017, 17, 3097–3104. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Han, Y.; Lai, Z.; Wang, Z.; Yu, M.; Tong, Y.; Lu, X. Designing Carbon Based Supercapacitors with High Energy Density: A Summary of Recent Progress. Chem. Eur. J. 2018, 24, 7312–7329. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Ding, F.; Sang, L.; Ma, Z.; Shao, G. Template-free synthesis of ultrathin porous carbon shell with excellent conductivity for high-rate supercapacitors. Carbon 2017, 111, 419–427. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Liu, N.; Shen, J.; Liu, D. Activated high specific surface area carbon aerogels for EDLCs. Microporous Mesoporous Mater. 2013, 167, 176–181. [Google Scholar] [CrossRef]
- Jung, S.; Myung, Y.; Kim, B.N.; Kim, I.G.; You, I.-K.; Kim, T. Activated Biomass-derived Graphene-based Carbons for Supercapacitors with High Energy and Power Density. Sci. Rep. 2018, 8, 1915. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.; Olejniczak, A.; Galinski, M.; Stepniak, I. Performance of carbon–carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes. J. Power Sources 2010, 195, 5814–5819. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-Y.; Lin, R.; Murali, S.; Li Zhang, L.; McDonough, J.K.; Ruoff, R.S.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Outstanding performance of activated graphene based supercapacitors in ionic liquid electrolyte from −50 to 80°C. Nano Energy 2013, 2, 403–411. [Google Scholar] [CrossRef]
- Van Aken, K.L.; Beidaghi, M.; Gogotsi, Y. Formulation of Ionic-Liquid Electrolyte To Expand the Voltage Window of Supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 4806–4809. [Google Scholar] [CrossRef]
- Biso, M.; Mastragostino, M.; Montanino, M.; Passerini, S.; Soavi, F. Electropolymerization of poly(3-methylthiophene) in pyrrolidinium-based ionic liquids for hybrid supercapacitors. Electrochim. Acta 2008, 53, 7967–7971. [Google Scholar] [CrossRef]
- Mousavi, M.P.S.; Wilson, B.E.; Kashefolgheta, S.; Anderson, E.L.; He, S.; Bühlmann, P.; Stein, A. Ionic Liquids as Electrolytes for Electrochemical Double-Layer Capacitors: Structures that Optimize Specific Energy. ACS Appl. Mater. Interfaces 2016, 8, 3396–3406. [Google Scholar] [CrossRef]
- Wolff, C.; Jeong, S.; Paillard, E.; Balducci, A.; Passerini, S. High power, solvent-free electrochemical double layer capacitors based on pyrrolidinium dicyanamide ionic liquids. J. Power Sources 2015, 293, 65–70. [Google Scholar] [CrossRef]
- Ue, M. Electrochemical Properties of Organic Liquid Electrolytes Based on Quaternary Onium Salts for Electrical Double-Layer Capacitors. J. Electrochem. Soc. 1994, 141, 2989. [Google Scholar] [CrossRef]
- Brandt, A.; Isken, P.; Lex-Balducci, A.; Balducci, A. Adiponitrile-based electrochemical double layer capacitor. J. Power Sources 2012, 204, 213–219. [Google Scholar] [CrossRef]
- Brandt, A.; Balducci, A. The Influence of Pore Structure and Surface Groups on the Performance of High Voltage Electrochemical Double Layer Capacitors Containing Adiponitrile-Based Electrolyte. J. Electrochem. Soc. 2012, 159, A2053–A2059. [Google Scholar] [CrossRef]
- Chiba, K.; Ueda, T.; Yamaguchi, Y.; Oki, Y.; Shimodate, F.; Naoi, K. Electrolyte Systems for High Withstand Voltage and Durability I. Linear Sulfones for Electric Double-Layer Capacitors. J. Electrochem. Soc. 2011, 158, A872. [Google Scholar] [CrossRef]
- Laheäär, A.; Jänes, A.; Lust, E. Electrochemical Behavior of Carbide Derived Carbons in LiPF6 and LiCF3SO3 Nonaqueous Electrolytes. ECS Trans. 2019, 28, 65–75. [Google Scholar] [CrossRef]
- Jänes, A.; Eskusson, J.; Thomberg, T.; Romann, T.; Lust, E. Ionic liquid-1,2-dimethoxyethane mixture as electrolyte for high power density supercapacitors. J. Energy Chem. 2016, 25, 609–614. [Google Scholar] [CrossRef]
- Ruther, R.E.; Sun, C.-N.; Holliday, A.; Cheng, S.; Delnick, F.M.; Zawodzinski, T.A.; Nanda, J. Stable Electrolyte for High Voltage Electrochemical Double-Layer Capacitors. J. Electrochem. Soc. 2016, 164, A277–A283. [Google Scholar] [CrossRef]
- Väli, R.; Laheäär, A.; Jänes, A.; Lust, E. Characteristics of non-aqueous quaternary solvent mixture and Na-salts based supercapacitor electrolytes in a wide temperature range. Electrochim. Acta 2014, 121, 294–300. [Google Scholar] [CrossRef]
- Laheäär, A.; Jänes, A.; Lust, E. NaClO4 and NaPF6 as potential non-aqueous electrolyte salts for electrical double layer capacitor application. Electrochim. Acta 2012, 82, 309–313. [Google Scholar] [CrossRef]
- Mei, X.; Yue, Z.; Tufts, J.; Dunya, H.; Mandal, B.K. Synthesis of new fluorine-containing room temperature ionic liquids and their physical and electrochemical properties. J. Fluor. Chem. 2018, 212, 26–37. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Yin, J.; Bai, C.; Zhang, J.; Li, Y.; Yang, Y.; Ge, Z.; Zhang, M.; Wei, L.; et al. Mesoporous activated carbon materials with ultrahigh mesopore volume and effective specific surface area for high performance supercapacitors. Carbon 2017, 124, 64–71. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yang, X.; Leng, K.; Huang, Y.; Chen, Y. High-Performance Supercapacitor Electrode Materials Prepared from Various Pollens. Small 2013, 9, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Lou, F.; Melandsø Buan, M.E.; Sheridan, E.; Chen, D. Enhancing capacitance of supercapacitor with both organic electrolyte and ionic liquid electrolyte on a biomass-derived carbon. RSC Adv. 2017, 7, 23859–23865. [Google Scholar] [CrossRef]
- Simon, P.; Brousse, T.; Favier, F. Supercapacitors Based on Carbon or Pseudocapacitive Materials; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Kameda, Y.; Umebayashi, Y.; Takeuchi, M.; Wahab, M.A.; Fukuda, S.; Ishiguro, S.-i.; Sasaki, M.; Amo, Y.; Usuki, T. Solvation Structure of Li+ in Concentrated LiPF6−Propylene Carbonate Solutions. J. Phys. Chem. B 2007, 111, 6104–6109. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, Q.; Zhang, S.; Tang, H.; Li, L.; Pang, H. Macroporous Activated Carbon Derived from Rapeseed Shell for Lithium–Sulfur Batteries. Appl. Sci. 2017, 7, 1036. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Łasińska, A.; Florjańczyk, Z.; Zygadło-Monikowska, E.; Affek, A. Crystallization and melting of PEO:LiTFSI polymer electrolytes investigated simultaneously by impedance spectroscopy and polarizing microscopy. Electrochim. Acta 2005, 50, 3969–3977. [Google Scholar] [CrossRef]
- Laheäär, A.; Kurig, H.; Jänes, A.; Lust, E. LiPF6 based ethylene carbonate–dimethyl carbonate electrolyte for high power density electrical double layer capacitor. Electrochim. Acta 2009, 54, 4587–4594. [Google Scholar] [CrossRef]
- Borodin, O.; Behl, W.; Jow, T.R. Oxidative Stability and Initial Decomposition Reactions of Carbonate, Sulfone, and Alkyl Phosphate-Based Electrolytes. J. Phys. Chem. C 2013, 117, 8661–8682. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.-W.; Wu, Y.-X.; Wei, T.-C.; Geng, D.; Mei, J.; Liu, H.; Lau, W.-M.; Liu, L.-M. Three-dimensional hierarchical interwoven nitrogen-doped carbon nanotubes/CoxNi1-x-layered double hydroxides ultrathin nanosheets for high-performance supercapacitors. Electrochim. Acta 2016, 203, 21–29. [Google Scholar] [CrossRef]
- Kulandaivalu, S.; Suhaimi, N.; Sulaiman, Y. Unveiling high specific energy supercapacitor from layer-by-layer assembled polypyrrole/graphene oxide|polypyrrole/manganese oxide electrode material. Sci. Rep. 2019, 9, 4884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Pei, L.; Ying, D.; Xu, X.; Zhao, L.; Jia, J.; Cao, X. Converting Ni-loaded biochars into supercapacitors: Implication on the reuse of exhausted carbonaceous sorbents. Sci. Rep. 2017, 7, 41523. [Google Scholar] [CrossRef] [PubMed]
- Sedlakova, V.; Sikula, J.; Majzner, J.; Sedlak, P.; Kuparowitz, T.; Buergler, B.; Vasina, P. Supercapacitor equivalent electrical circuit model based on charges redistribution by diffusion. J. Power Sources 2015, 286, 58–65. [Google Scholar] [CrossRef]
- Sedlakova, V.; Sikula, J.; Valsa, J.; Majzner, J.; Dvorak, P. Supercapacitor charge and self-discharge analysis. In Proceedings of the Passive Space Component Days, ESA/ESTEC, Noordwijk, The Netherlands, 24–26 September 2013. [Google Scholar]
- Zubieta, L.; Bonert, R. Characterization of double-layer capacitors for power electronics applications. IEEE Trans. Ind. Appl. 2000, 36, 199–205. [Google Scholar] [CrossRef]
- Graydon, J.W.; Panjehshahi, M.; Kirk, D.W. Charge redistribution and ionic mobility in the micropores of supercapacitors. J. Power Sources 2014, 245, 822–829. [Google Scholar] [CrossRef]
- Faranda, R. A new parameters identification procedure for simplified double layer capacitor two-branch model. Electr. Power Syst. Res. 2010, 80, 363–371. [Google Scholar] [CrossRef]
- Sedlakova, V.; Majzner, J.; Josef, S.; Sedlak, P.; Kuparowitz, T.; Buergler, B.; Vasina, P. Supercapacitor Degradation Assesment by Power Cycling and Calendar Life Tests. Metrol. Meas. Syst. 2016, 23, 345–358. [Google Scholar] [CrossRef]
- Kreczanik, P.; Venet, P.; Hijazi, A.; Clerc, G. Study of Supercapacitor Aging and Lifetime Estimation According to Voltage, Temperature, and RMS Current. IEEE Trans. Ind. Electron. 2014, 61, 4895–4902. [Google Scholar] [CrossRef]
- Murray, D.B.; Hayes, J.G. Cycle Testing of Supercapacitors for Long-Life Robust Applications. IEEE Trans. Power Electron. 2015, 30, 2505–2516. [Google Scholar] [CrossRef]
- Rasines, G.; Lavela, P.; Macías, C.; Zafra, M.C.; Tirado, J.L.; Parra, J.B.; Ania, C.O. N-doped monolithic carbon aerogel electrodes with optimized features for the electrosorption of ions. Carbon 2015, 83, 262–274. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Lieder, M.; Jablonska, M. N-doped mesoporous carbon nanosheets obtained by pyrolysis of a chitosan–melamine mixture for the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 44969–44977. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Demir, M.; Saraswat, S.K.; Gupta, R.B. Hierarchical nitrogen-doped porous carbon derived from lecithin for high-performance supercapacitors. RSC Adv. 2017, 7, 42430–42442. [Google Scholar] [CrossRef]
- Kim, K.-S.; Park, S.-J. Synthesis and high electrochemical capacitance of N-doped microporous carbon/carbon nanotubes for supercapacitor. J. Electroanal. Chem. 2012, 673, 58–64. [Google Scholar] [CrossRef]


| Solvent | Abbr. | MW (g·mol−1) | Density (g cm−3) | m.p. (°C) | b.p. (°C) | Permittivity (25 °C) | Viscosity (25 °C) (cPs) | DN Number (kcal·mol−1) |
|---|---|---|---|---|---|---|---|---|
| Acetonitrile | AN | 41.05 | 0.786 | −46 | 81 | 37.5 | 0.34 | 14.1 |
| Adiponitrile | ADN | 108.14 | 0.951 | 1–3 | 295.1 | 30 [23] | 5.8 | --- |
| Ethylene carbonate | EC | 88.06 | 1.321 | 34–37 | 243 | 90.5 (40 °C) | 1.919 (40 °C) | 16.4 |
| Dimethyl carbonate | DMC | 90.08 | 1.073 | 2–4 | 90.1 | 3.20 | 0.664 | 17.2 |
| Dimethoxy ethane | DME | 90.12 | 0.867 | −58 | 85 | 7.2 | 0.46 | 20 |
| Propylene carbonate | PC | 102.09 | 1.205 | −48.8 | 242 | 65.5 | 2.50 | 15.1 |
| Solute | Solvent Composition | χ (25 °C) (mS cm−1) | Maximum OPV (V) | Ref. |
|---|---|---|---|---|
| 1 M LiPF6 | AN | 50 | 2.7 | [30] |
| 1 M TEA-BF4 | AN | 56 | 2.7 | |
| 1 M LiBF4 | AN | 18 | 2.7 | |
| 1 M LiPF6 | PC | 5.8 | 2.7 | |
| 1 M TEA-BF4 | PC | 13 | 2.7 | |
| 1 M LiBF4 | PC | 3.4 | 2.7 | |
| 0.7 M TEA-BF4 | ADN | 4.3 | 3.75 | [31] |
| 1 M SBP-BF4 | EiPS (ethyl isopropyl sulfone) | 3.7 | [33] | |
| 1 M LiPF6 | EC/DMC 1/1 v/v | ~9 | 3.2 | [34] |
| 1 M LiCF3SO3 | EC/DMC 1/1 v/v | Low stability | ||
| 1 M NaPF6 | DME | 12 | 3.5 | [36] |
| 1 M NaPF6 | EC/PC/DMC/EA 1/1/1/0.5 v/v | ~11 | 3.4 | [37] |
| 1 M NaPF6 | EC/DMC | 3.4 | [38] | |
| 1 M NaClO4 | EC/DMC | 3.2 | ||
| 60% v/v EMIM-BF4 | DME 40% v/v | 24.2 | 2.7 | [35] |
| Carbon Precursor | Carbonization Yield from ~15 g Precursor | BET before KOH Activation | BET after KOH Activation | ||||
|---|---|---|---|---|---|---|---|
| SSA (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | SSA (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | ||
| PC–Starch | 1.18 g | 676.202 | 1.679 | 12.069 | --- | --- | --- |
| PC–MF | 2.81 g | 823.343 | 1.634 | 3.723 | 3193.395 | 3.372 | 3.535 |
| Electrolyte | χ (25 °C) (mS cm−1) a | Our Measurement | Literature |
|---|---|---|---|
| #1 | EMIM–BF4/DME = 6/4 | 24.7 | 24.2 |
| #2 | EMIM–BF4/DME = 4/6 | Not miscible | |
| #3 | EMIM–BF4/EC/DME = 4/1/5 | 25.8 | |
| #4 | EMIM–BF4/EC/DME = 4/3/3 | 26.8 | |
| #5 | 1M TEA–BF4 in DME | Not miscible | |
| #6 | 1M TEA–BF4 in EC/DME = 1/4 | Not miscible | |
| #7 | 1M TEA–BF4 in EC/DME = 1/1 | 13.7 | |
| #8 | 1M LiPF6 in EC/DMC = 1/1 (LP30) | Commercial sample | ~9 |
| #9 | 1M NaBF4 in EC/DME = 1/1 | Not miscible | |
| #10 | 1M NaPF6 in DME | 12.8 | |
| #11 | 1M NaPF6 in EC/DME = 1/4 | 16.6 | |
| #12 | 1M NaPF6 in EC/DME = 1/1 | 12.3 |
| #1 | #7 | #8 | #10 | #11 | #12 | |
|---|---|---|---|---|---|---|
| Electrolyte formulation | EMIM-BF4/DME = 6/4 v/v | 1 M TEA-BF4 in EC/DME = 1/1 v/v | 1 M LiPF6 in EC/DMC = 1/1 v/v (LP30) | 1 M NaPF6 in DME | 1 M NaPF6 in EC/DME = 1/4 v/v | 1 M NaPF6 in EC/DME = 1/1 v/v |
| Maximum OPV, (V) | 2.5–2.75 | 3.25–3.5 | 3.0–3.25 | 3.0–3.25 | 3.0–3.25 | 2.75–3.0 |
| Internal resistance, (Ohm) | ~1.6 | ~3.7 | ~620 | ~4.6 | ~5.9 | ~6.1 |
| Coulombic efficiency a Cd/Cc (%) | 96.5 | 92.2 | 91.9 | 97.1 | 95.4 | 97.3 |
| Initial specific discharge capacitance a, C (F g−1) | 112.7 | 87.9 | 113.2 | 102.8 | 130.5 | 119.0 |
| Initial energy density a, E (Wh kg−1) | 29.6 | 37.4 | 46.0 | 34.8 | 47.9 | 37.2 |
| Electrolyte Formulation | Initial (50th) Cd (F g−1) | 1500th Cd (F g−1) | Cd Retention of the First 1500 Cycles (%) | 4000th Cd (F g−1) | Cd Retention of the Next 2500 Cycles (%) |
|---|---|---|---|---|---|
| 1 M NaPF6 in DME (#10) | 102.8 | 67.7 | 65.9% | 57.8 | 85.4% |
| 1 M NaPF6 in EC/DME = 1/4 v/v (#11) | 130.5 | 76.3 | 58.5% | 71.4 | 93.6% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Z.; Dunya, H.; Ashuri, M.; Kucuk, K.; Aryal, S.; Antonov, S.; Alabbad, B.; Segre, C.U.; Mandal, B.K. Synthesis of a Very High Specific Surface Area Active Carbon and Its Electrical Double-Layer Capacitor Properties in Organic Electrolytes. ChemEngineering 2020, 4, 43. https://doi.org/10.3390/chemengineering4030043
Yue Z, Dunya H, Ashuri M, Kucuk K, Aryal S, Antonov S, Alabbad B, Segre CU, Mandal BK. Synthesis of a Very High Specific Surface Area Active Carbon and Its Electrical Double-Layer Capacitor Properties in Organic Electrolytes. ChemEngineering. 2020; 4(3):43. https://doi.org/10.3390/chemengineering4030043
Chicago/Turabian StyleYue, Zheng, Hamza Dunya, Maziar Ashuri, Kamil Kucuk, Shankar Aryal, Stoichko Antonov, Bader Alabbad, Carlo U. Segre, and Braja K. Mandal. 2020. "Synthesis of a Very High Specific Surface Area Active Carbon and Its Electrical Double-Layer Capacitor Properties in Organic Electrolytes" ChemEngineering 4, no. 3: 43. https://doi.org/10.3390/chemengineering4030043
APA StyleYue, Z., Dunya, H., Ashuri, M., Kucuk, K., Aryal, S., Antonov, S., Alabbad, B., Segre, C. U., & Mandal, B. K. (2020). Synthesis of a Very High Specific Surface Area Active Carbon and Its Electrical Double-Layer Capacitor Properties in Organic Electrolytes. ChemEngineering, 4(3), 43. https://doi.org/10.3390/chemengineering4030043








