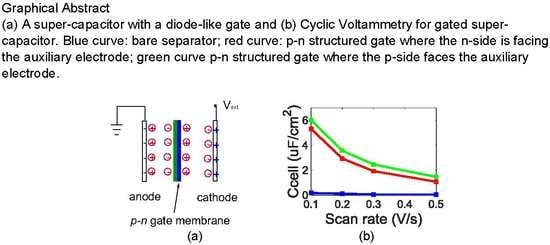
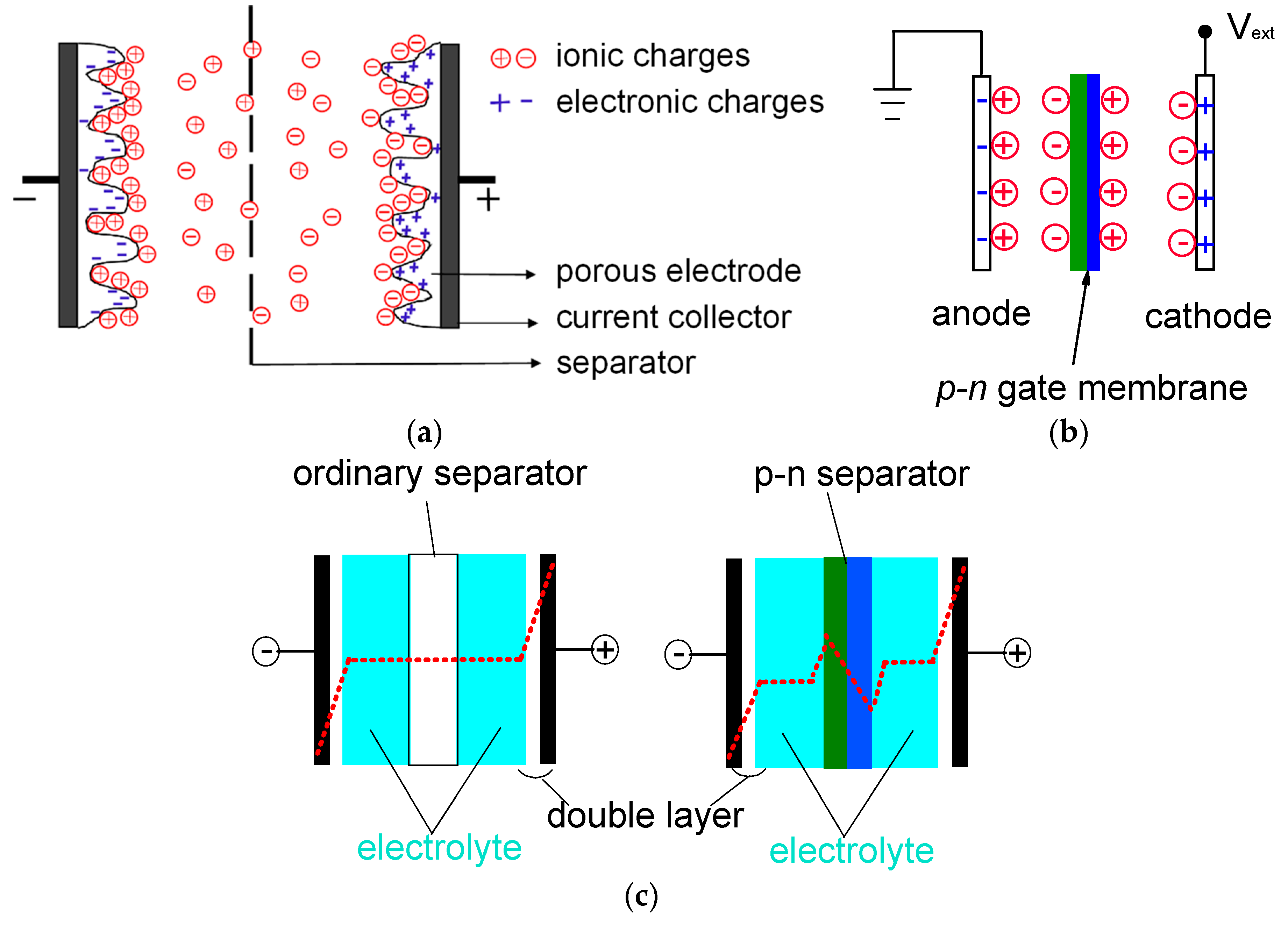
Ion-Liquid Based Supercapacitors with Inner Gate Diode-Like Separators
Abstract
1. Introduction
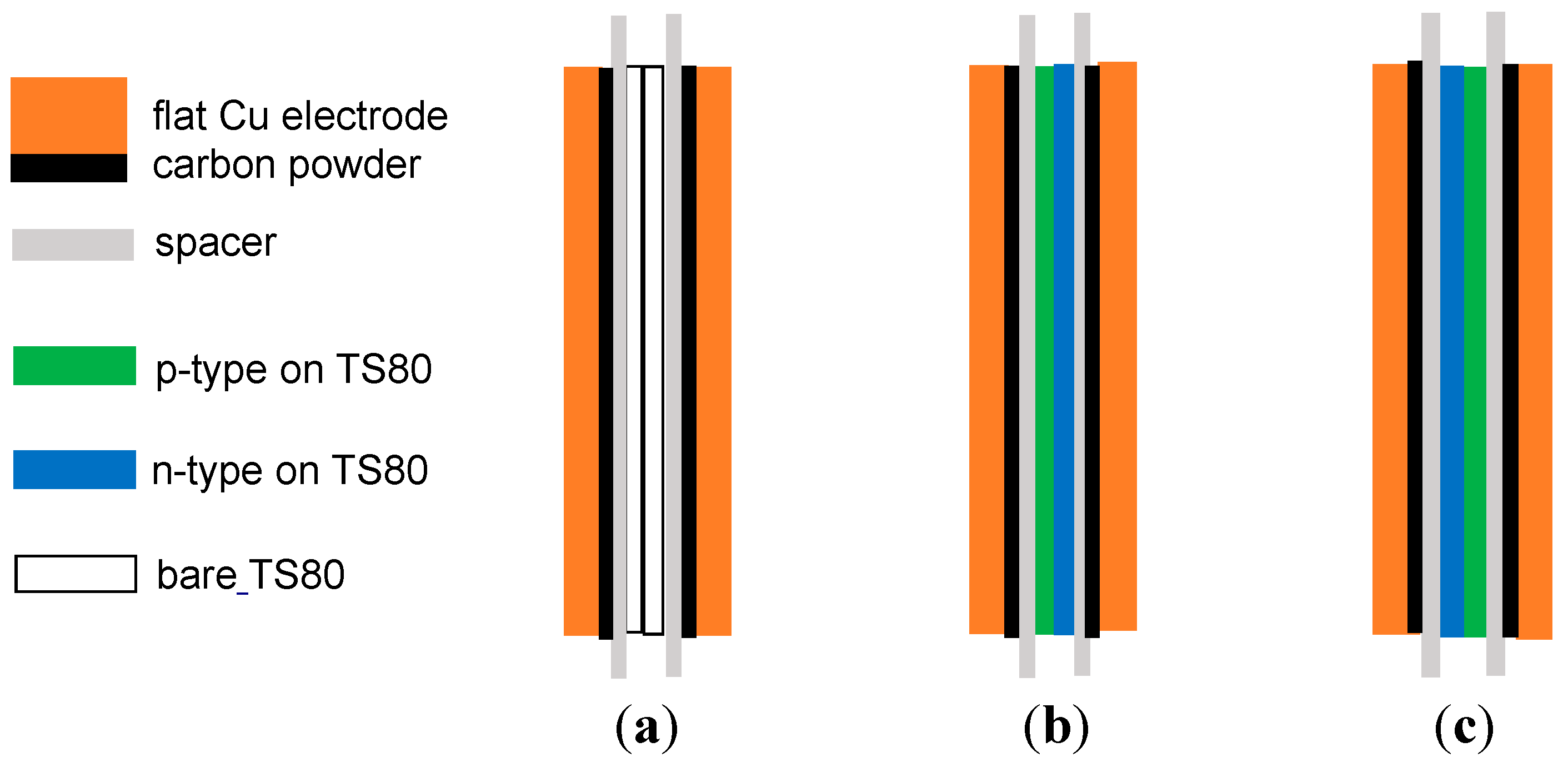
2. Experiment and Methods
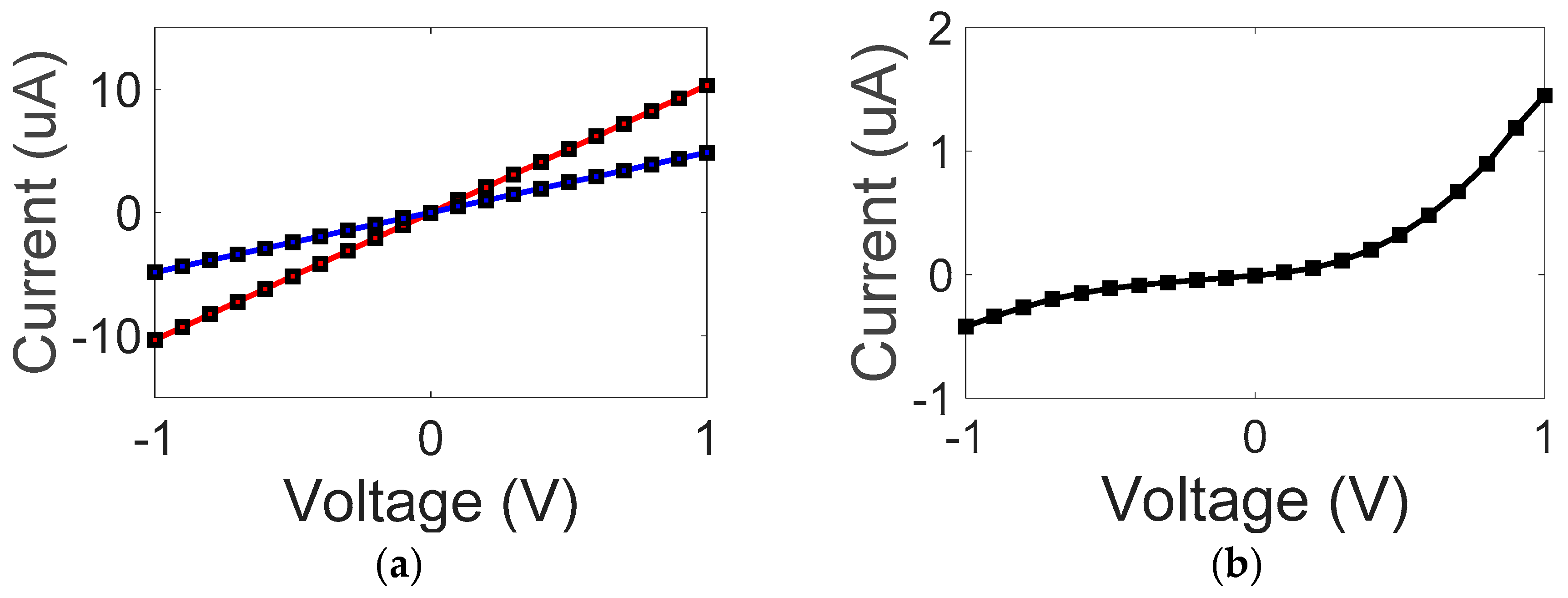
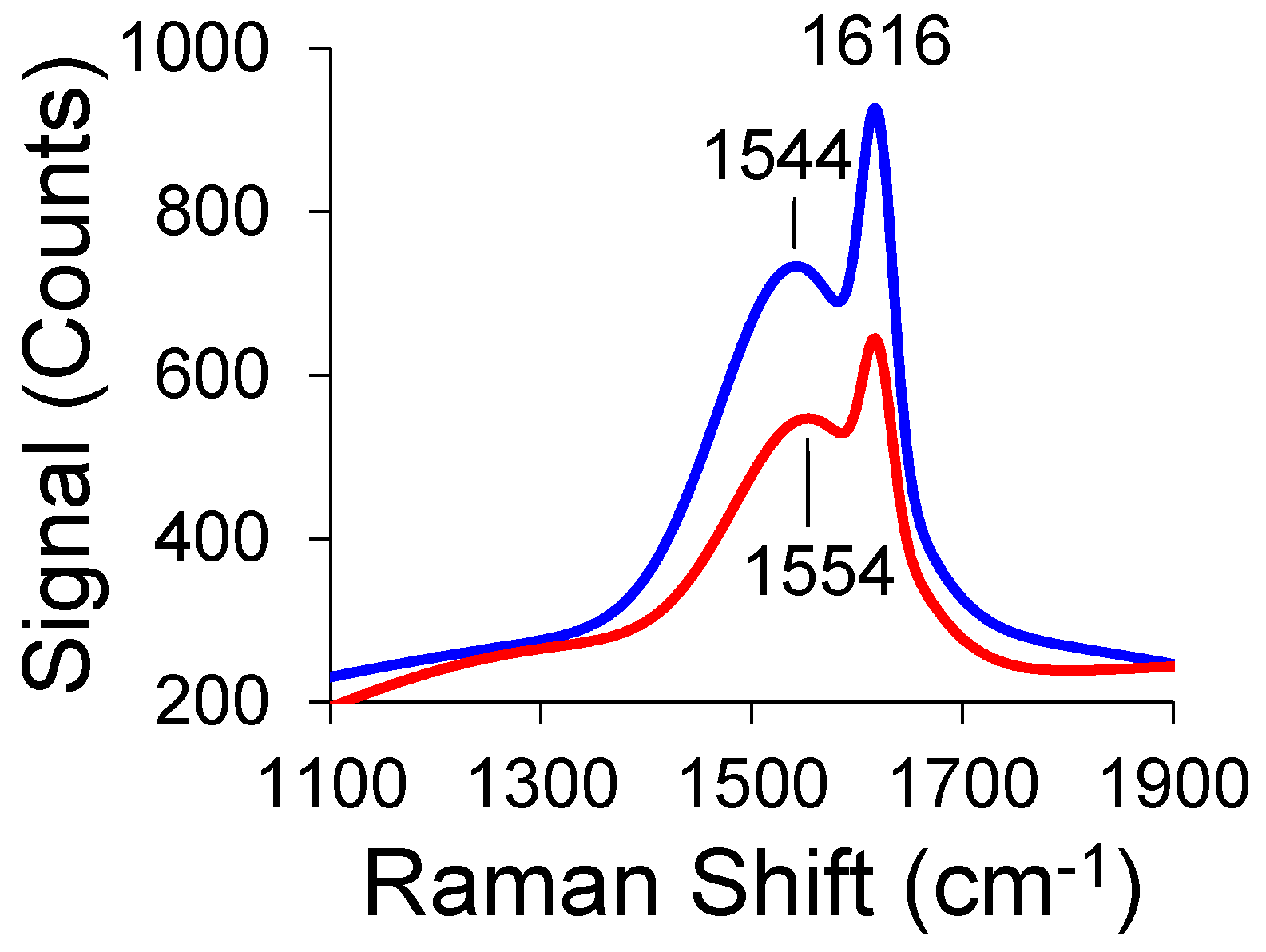
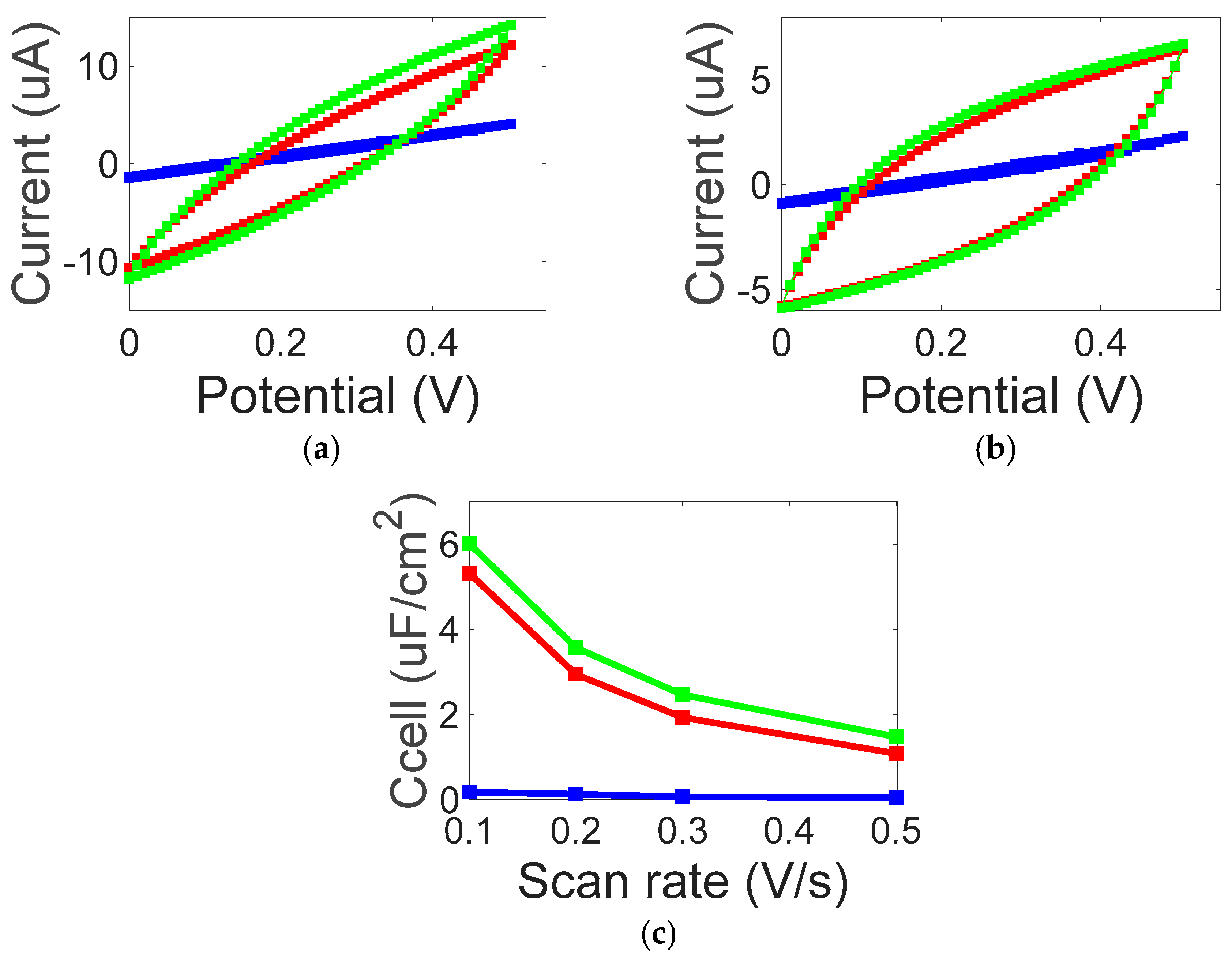
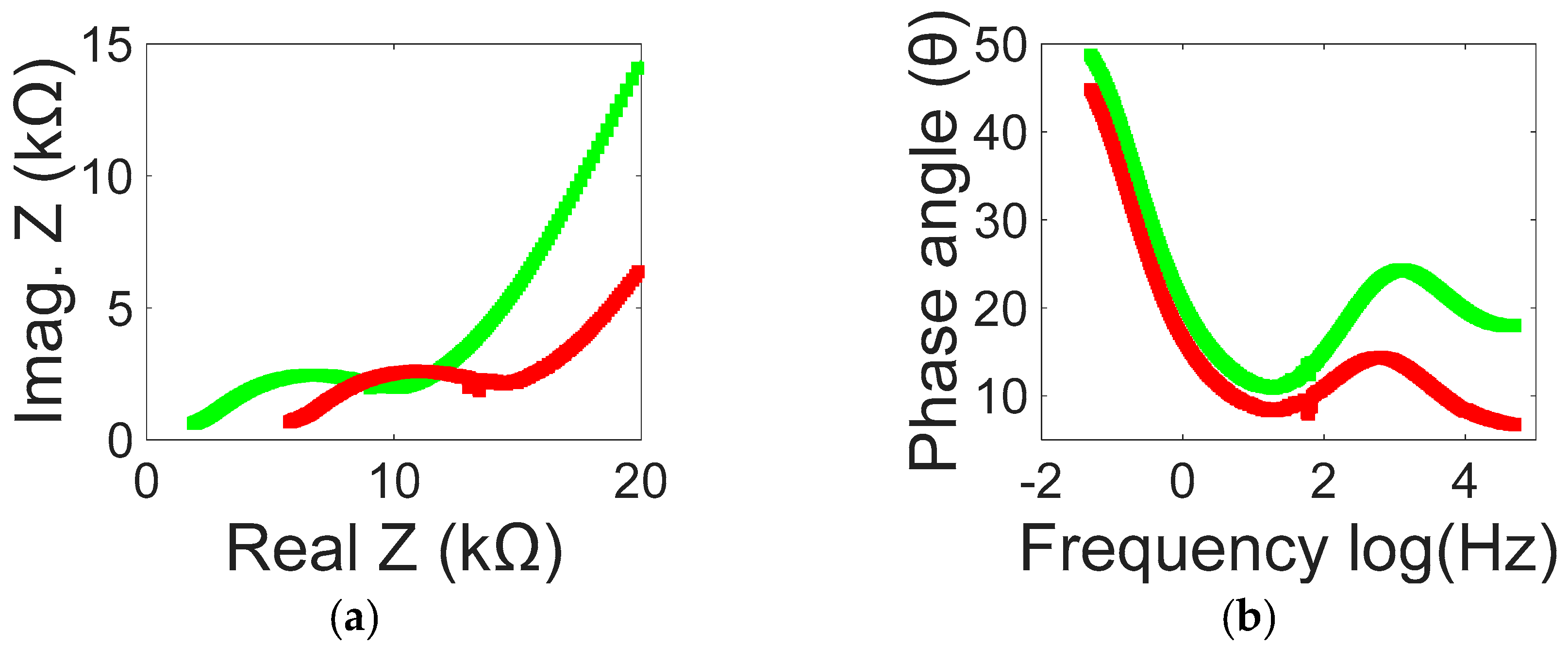
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Cessa, R.; Grebel, H.; Jiang, Z.; Fukuda, C.; Pita, H.; Chowdhury, T.S.; Dong, Z.; Wan, Y. Integration of alternative energy sources into digital micro-grids. Environ. Prog. Sustain. Energy 2018, 37, 155–164. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Kostoglou, N.; Koczwara, C.; Prehal, C.; Terziyska, V.; Babic, B.; Matovic, B.; Constantinides, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; et al. Nanoporous activated carbon cloth as a versatile material for hydrogen adsorption, selective gas separation and electrochemical energy storage. Nano Energy 2017, 40, 49–64. [Google Scholar] [CrossRef]
- Lee, J.; Jäckel, N.; Kim, D.; Widmaier, M.; Sathyamoorthi, S.; Srimuk, P.; Kim, C.; Fleischmann, S.; Zeiger, M.; Presser, V. Porous carbon as a quasi-reference electrode in aqueous electrolytes. Electrochim. Acta 2016, 222, 1800–1805. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Multisegmented Au-MnO2/Carbon Nanotube Hybrid Coaxial Arrays for High-Power Supercapacitor Applications. J. Phys. Chem. 2009, 114, 658–663. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Fan, Z.; Qian, W.; Zhang, M.; Shen, X.; Wei, F. Preparation of graphene nanosheet/carbon nanotube/polyaniline composite as electrode material for supercapacitors. J. Power Sources 2010, 195, 3041–3045. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.J.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Letts. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, G.; Yin, L.; Ren, W.; Li, F.; Cheng, H. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Varzi, A.; Täubert, C.; Wohlfahrt-Mehrens, M.; Kreis, M.; Schütz, W. Study of multi-walled carbon nanotubes for lithium-ion battery electrodes. J. Power Sources 2011, 196, 3303–3309. [Google Scholar] [CrossRef]
- Obreja, V.V.N. On the performance of supercapacitors with electrodes based on carbon nanotubes and carbon activated material. Physica E 2008, 40, 2596–2605. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. J. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Shulga, Y.M.; Baskakov, S.A.; Baskakova, Y.V.; Volfkovich, Y.M.; Shulga, N.Y.; Skryleva, E.A.; Parkhomenko, Y.N.; Belay, K.G.; Gutsev, G.L.; Rychagov, A.Y.; et al. Supercapacitors with graphene oxide separators and reduced graphite oxide electrodes. J. Power Sources 2015, 279, 722–730. [Google Scholar] [CrossRef]
- Karabelli, D.; Leprêtre, J.C.; Alloin, F.; Sanchez, J.Y. Poly (vinylidene fluoride) based macroporous separators for supercapacitors. Electrochim. Acta 2011, 57, 98–103. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, J.; Cai, S.; Zhou, X.; Mansour, A. Development of all-solid-state mediator-enhanced supercapacitors with polyvinylidene fluoride/lithium trifluoromethanesulfonate separators. J. Power Sources 2011, 196, 10479–10483. [Google Scholar] [CrossRef]
- Choi, B.G.; Hong, J.; Hong, W.; Hammond, P.; Park, H. Facilitated ion transport in all-solid-state flexible supercapacitors. Am. Chem. Soc. Nano 2011, 5, 7205–7213. [Google Scholar] [CrossRef]
- Banerjee, A.; Grebel, H. On the stopping potential of ionic currents. Electrochem. Commun. 2010, 12, 274. [Google Scholar] [CrossRef]
- Grebel, J.; Banerjee, A.; Grebel, H. Towards bi-carrier ion transistor: DC and optically induced effects in electrically controlled electrochemical cell. Electrochim. Acta 2013, 95, 308–312. [Google Scholar] [CrossRef]
- Grebel, H.; Patel, A. Electrochemical cells with intermediate capacitor elements. Chem. Phys. Lett. 2015, 640, 36–39. [Google Scholar] [CrossRef][Green Version]
- Sreevatsa, S.; Grebel, H. Graphene as a permeable ionic barrier. ECS Trans. 2009, 19, 259–264. [Google Scholar]
- Zhang, Y.; Grebel, H. Controlling ionic currents with transistor-like structure. ECS Trans. 2007, 2, 1–18. [Google Scholar]
- Grebel, H. Capacitor-within-capacitor. SN Appl. Sci. 2019, 1, 48. [Google Scholar] [CrossRef]
- Mirza, S.M.; Grebel, H. Crisscrossed and co-aligned single-wall carbon based films. Appl. Phys. Lett. 2007, 91, 183102. [Google Scholar] [CrossRef]
- Mirza, S.M.; Grebel, H. Thermoelectric properties of aligned carbon nanotubes. Appl. Phys. Lett. 2008, 92, 203116. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Boul, P.; Ericson, L.M.; Huffman, C.; Wang, Y.; Haroz, E.; Kuper, C.; Tour, J.; Ausman, K.D.; Smalley, R.E. Reversible water solubilization of single wall carbon-nanotubes by polymer wrapping. Chem. Phys. Lett. 2001, 342, 265–271. [Google Scholar] [CrossRef]
- Shim, M.; Javey, A.; Kam, N.W.S.; Dai, H. Polymer Functionalization for Air-Stable n-Type Carbon Nanotube Field-Effect Transistors. J. Am. Chem. Soc. 2001, 123, 11512–11513. [Google Scholar] [CrossRef] [PubMed]
- Mastragostino, M.; Arbizzani, C. Polymer-based supercapacitors. J. Power Sources 2001, 97, 812–815. [Google Scholar] [CrossRef]
- Kim, I.H.; Kim, K.B. Ruthenium oxide thin film electrodes for supercapacitors. Electrochem. Solid State Lett. 2001, 4, A62–A64. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Chowdhury, T.S.; Grebel, H. Supercapacitors with electrical gates. Electrochem. Acta 2019, 307, 459–464. [Google Scholar] [CrossRef]
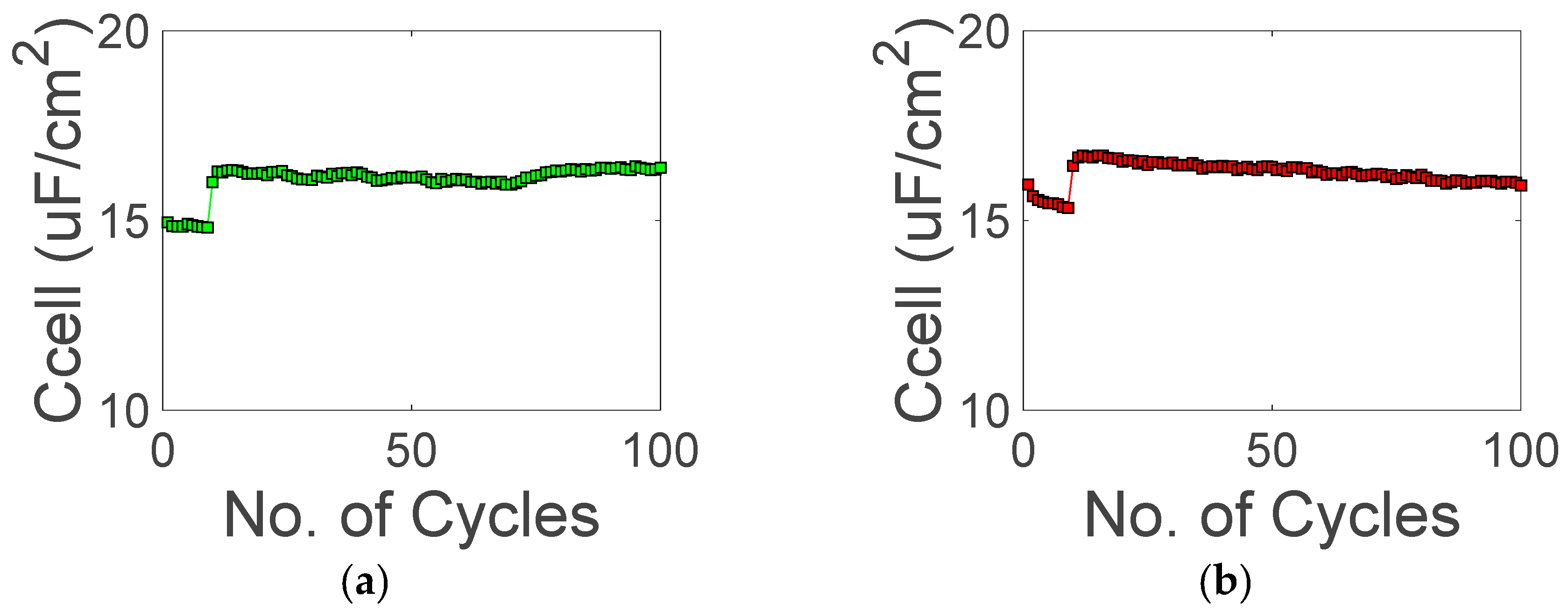
| Type | ΔV at 57 °C | ΔV at 68 °C |
|---|---|---|
| n-type | +0.8 mV | +1.2 mV |
| p-type | −1.8 mV | −2.5 mV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, T.S.; Grebel, H. Ion-Liquid Based Supercapacitors with Inner Gate Diode-Like Separators. ChemEngineering 2019, 3, 39. https://doi.org/10.3390/chemengineering3020039
Chowdhury TS, Grebel H. Ion-Liquid Based Supercapacitors with Inner Gate Diode-Like Separators. ChemEngineering. 2019; 3(2):39. https://doi.org/10.3390/chemengineering3020039
Chicago/Turabian StyleChowdhury, Tazima S., and Haim Grebel. 2019. "Ion-Liquid Based Supercapacitors with Inner Gate Diode-Like Separators" ChemEngineering 3, no. 2: 39. https://doi.org/10.3390/chemengineering3020039
APA StyleChowdhury, T. S., & Grebel, H. (2019). Ion-Liquid Based Supercapacitors with Inner Gate Diode-Like Separators. ChemEngineering, 3(2), 39. https://doi.org/10.3390/chemengineering3020039