The Formation of Layered Double Hydroxide Phases in the Coprecipitation Syntheses of [Ni0.80Co0.15](1−x)/0.95Alx(OH)2(anionn−)x/n (x = 0–0.2, n = 1, 2)
Abstract
:1. Introduction
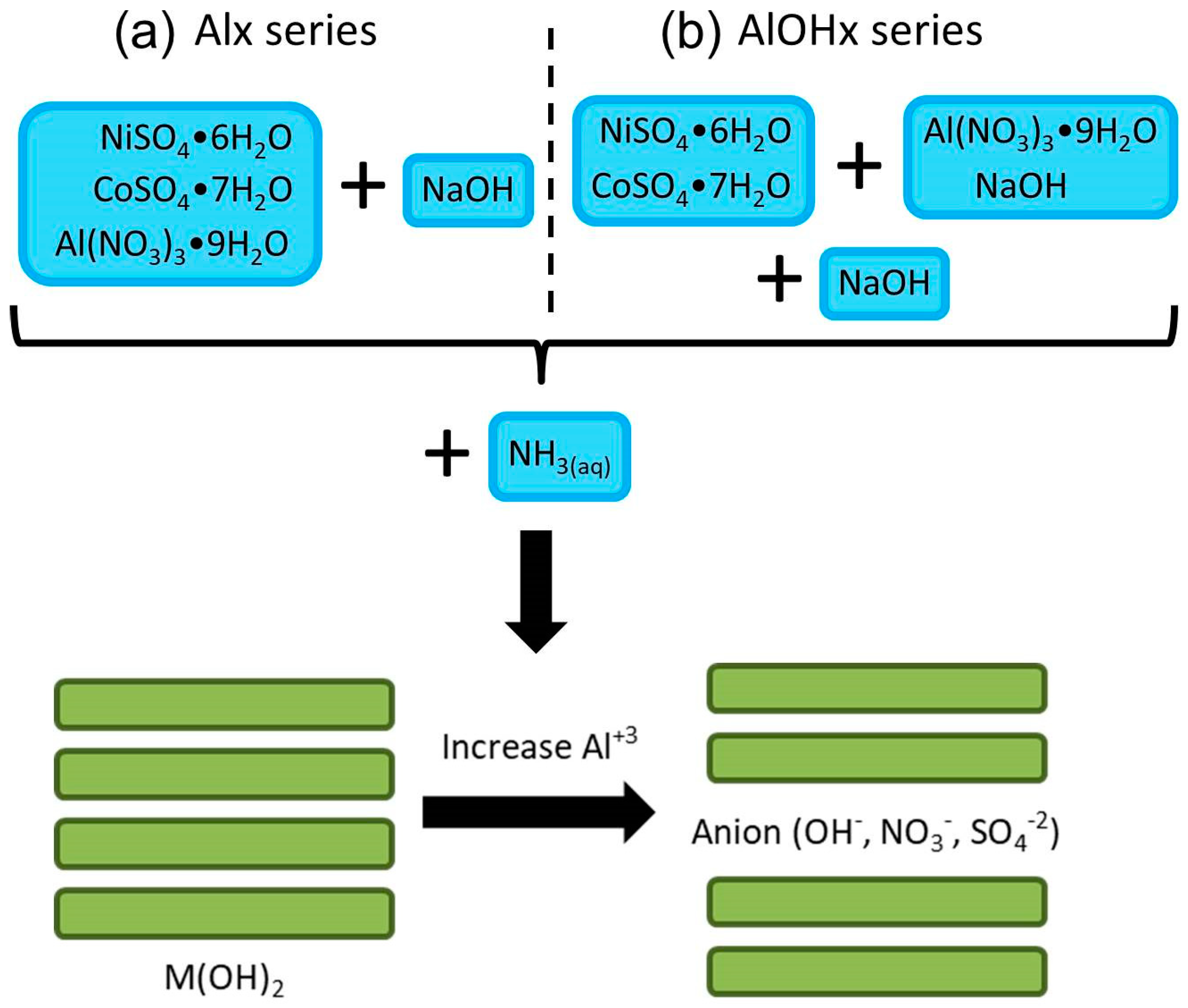
2. Experimental
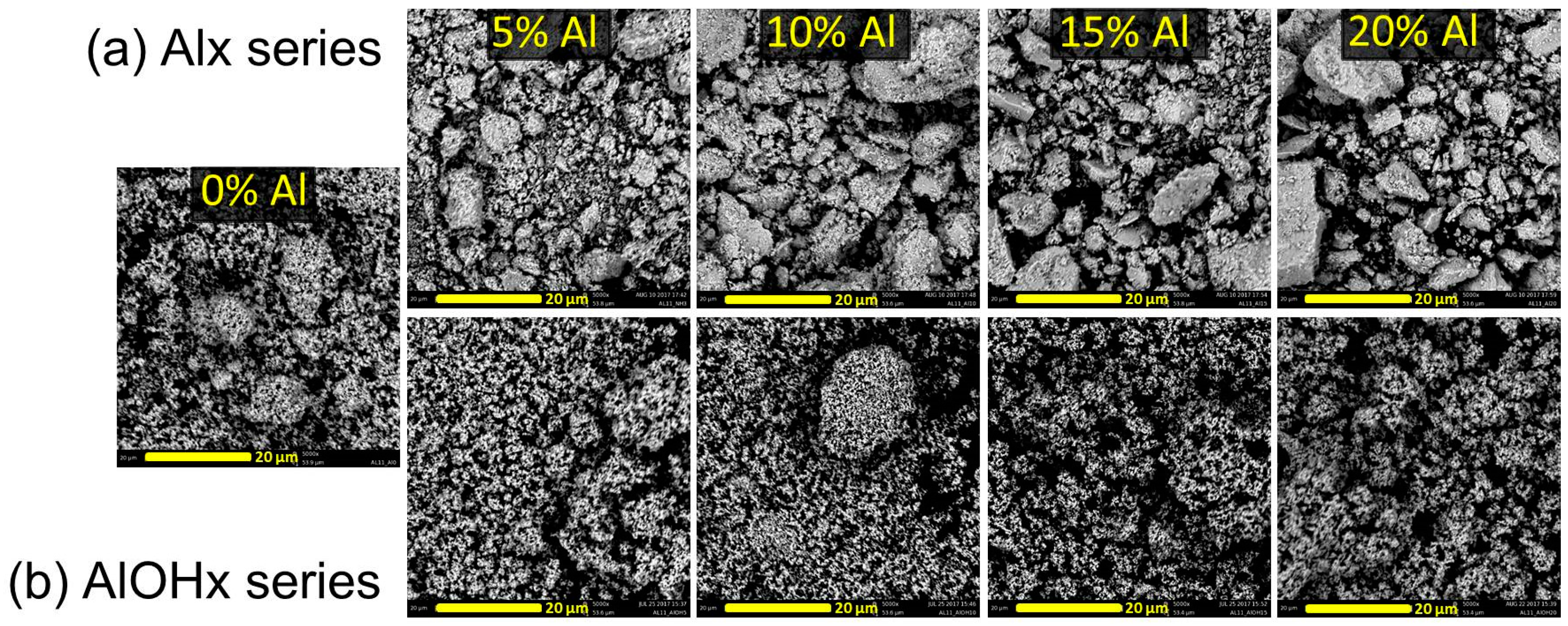
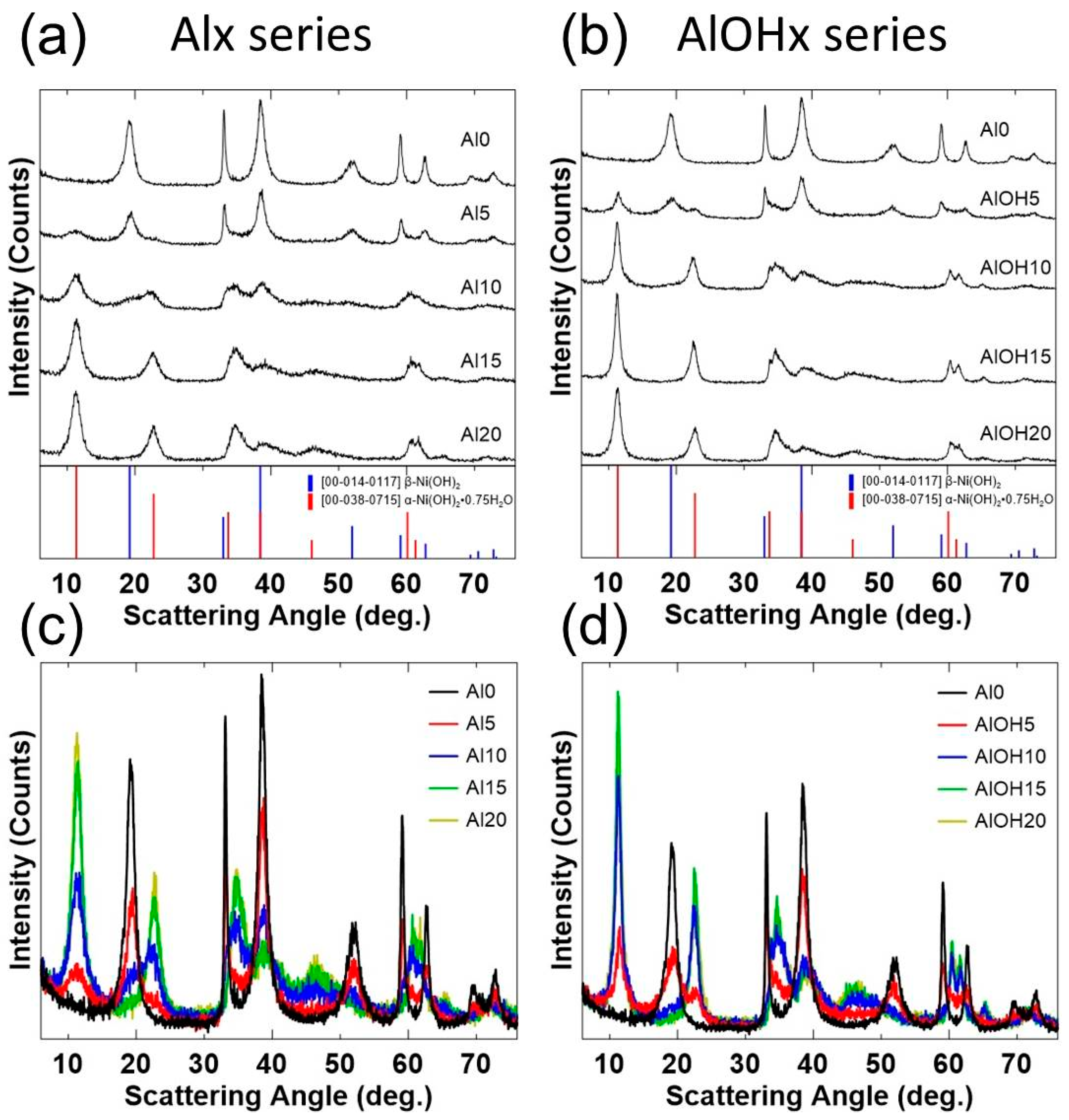
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered Li[NixCo1−2xMnx]O2 Cathode Materials for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2001, 4, A200–A203. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Lu, Z.; Dahn, J.R. Structure and Electrochemistry of Li[NixCo1−2xMnx]O2 (0≤x≤1/2). J. Electrochem. Soc. 2002, 149, A1332–A1336. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. (Eds.) Linden’s Handbook of Batteries, 4th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Rozier, P.; Tarascon, J.M. Review—Li-Rich Layered Oxide Cathodes for Next-Generation Li-Ion Batteries: Chances and Challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A review of Ni-based layered oxides for rechargeable Li-ion batteries. J. Mater. Chem. A 2017, 5, 874–901. [Google Scholar]
- Radin, M.D.; Hy, S.; Sina, M.; Fang, C.; Liu, H.; Vinckeviciute, J.; Zhang, M.; Whittingham, M.S.; Meng, Y.S.; Van der Ven, A. Narrowing the Gap between Theoretical and Practical Capacities in Li-Ion Layered Oxide Cathode Materials. Adv. Energy Mater. 2017, 1602888, 1–33. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and Reality of Ni-Rich Cathode for Commercialization. Adv. Energy Mater. 2018, 8, 1–25. [Google Scholar]
- Madhavi, S.; Rao, G.V.S.; Chowdari, B.V.R.; Li, S.F.Y. Effect of aluminum doping on cathodic behaviour of LiNi0.7Co0.3O2. J. Power Sources 2001, 93, 156–162. [Google Scholar] [CrossRef]
- Guilmard, M.; Croguennec, L.; Delmas, C. Thermal Stability of Lithium Nickel Oxide Derivatives. Part II: LixNi0.70Co0.15Al0.15O2 and LixNi0.90Mn0.10O2 (x = 0.50 and 0.30). Comparison with LixNi1.02O2 and LixNi0.89Al0.16O2. Chem. Mater. 2003, 15, 4484–4493. [Google Scholar] [CrossRef]
- Lee, K.S.; Myung, S.T.; Moon, J.S.; Sun, Y.K. Particle size effect of Li[Ni0.5Mn0.5]O2 prepared by co-precipitation. Electrochim. Acta 2008, 53, 6033–6037. [Google Scholar] [CrossRef]
- Van Bommel, A.; Dahn, J.R. Synthesis of Spherical and Dense Particles of the Pure Hydroxide Phase Ni1/3Mn1/3Co1/3(OH)2. J. Electrochem. Soc. 2009, 156, A362–A365. [Google Scholar] [CrossRef]
- Van Bommel, A.; Dahn, J.R. Analysis of the growth mechanism of coprecipitated spherical and dense nickel, manganese, and cobalt-containing hydroxides in the presence of aqueous ammonia. Chem. Mater. 2009, 21, 1500–1503. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, F.; Dahn, J.R. Phases Formed in Al-Doped Ni1/3Mn1/3Co1/3(OH)2 Prepared by Coprecipitation: Formation of Layered Double Hydroxide. J. Electrochem. Soc. 2008, 155, A642–A647. [Google Scholar] [CrossRef]
- Luo, W.; Dahn, J.R. Preparation of Co1− zAlz(OH)2(NO3)z Layered Double Hydroxides and Li(Co1− zAlz)O2. Chem. Mater. 2009, 21, 56–62. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Wei, F. Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides - Properties, synthesis, and applications. Adv. Funct. Mater. 2012, 22, 675–694. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D. Synthesis of high-density nickel cobalt aluminum hydroxide by continuous coprecipitation method. ACS Appl. Mater. Interfaces 2012, 4, 586–589. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Li, J.; Casagrande, T.; Butcher, C.; Martinez, J.; Korinek, A.; Botton, G.; Dahn, J.R. Investigating the Removal of Layered Double Hydroxides in [Ni0.80Co0.15]0.95-xAl0.05+x(OH)2 (x = 0, 0.05) Prepared by Coprecipitation. J. Electrochem. Soc. 2018, 165, A2781–A2791. [Google Scholar] [CrossRef]
- Tessier, C.; Guerlou-Demourgues, L.; Faure, C.; Basterreix, M.; Nabias, G.; Delmas, C. Structural and textural evolution of zinc-substituted nickel hydroxide electrode materials upon ageing in KOH and upon redox cycling. Solid State Ionics 2000, 133, 11–23. [Google Scholar] [CrossRef]
- Huang, J.; Lei, T.; Wei, X.; Liu, X.; Liu, T.; Cao, D.; Yin, J.; Wang, G. Effect of Al-doped β-Ni(OH)2 nanosheets on electrochemical behaviors for high performance supercapacitor application. J. Power Sources 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Su, L.; Ma, C.; Hou, T.; Han, W. Selective synthesis and capacitive characteristics of CoNiAl three-component layered double hydroxide platelets. RSC Adv. 2013, 3, 19807–19811. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A 2014, 471, 20140792. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y.; Su, Y.; Zhang, B.; Li, C.; Wang, H.; Wang, L. Design and synthesis of ternary-component layered double hydroxides for high-performance supercapacitors: Understanding the role of trivalent metal ions. Electrochim. Acta 2017, 225, 263–271. [Google Scholar] [CrossRef]
- Sarfraz, M.; Shakir, I. Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J. Energy Storage 2017, 13, 103–122. [Google Scholar] [CrossRef]
- Latorre-Sanchez, M.; Atienzar, P.; Abellán, G.; Puche, M.; Fornés, V.; Ribera, A.; García, H. The synthesis of a hybrid graphene-nickel/manganese mixed oxide and its performance in lithium-ion batteries. Carbon 2012, 50, 518–525. [Google Scholar] [CrossRef]
- Seo, J.S.; Lee, J.W. Fast growth of the precursor particles of Li(Ni0.8Co0.16Al0.04)O2 via a carbonate co-precipitation route and its electrochemical performance. J. Alloys Compd. 2017, 694, 703–709. [Google Scholar] [CrossRef]
- He, K.; Ruan, Z.; Teng, X.; Zhu, Y. Facile synthesis and electrochemical properties of spherical LiNi0.85−xCo0.15AlxO2 with sodium aluminate via co-precipitation. Mater. Res. Bull. 2017, 90, 131–137. [Google Scholar] [CrossRef]
- Guilmard, M.; Pouillerie, C.; Croguennec, L.; Delmas, C. Structural and electrochemical properties of LiNi0.70Co0.15Al0.15O2. Solid State Ionics 2003, 160, 39–50. [Google Scholar] [CrossRef]
- Wu, S.H.; Yang, C.W. Preparation of LiNi0.8Co0.2O2-based cathode materials for lithium batteries by a co-precipitation method. J. Power Sources 2005, 146, 270–274. [Google Scholar] [CrossRef]
- Muto, S.; Tatsumi, K.; Kojima, Y.; Oka, H.; Kondo, H.; Horibuchi, K.; Ukyo, Y. Effect of Mg-doping on the degradation of LiNiO2-based cathode materials by combined spectroscopic methods. J. Power Sources 2012, 205, 449–455. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhu, Y.; Teng, X. Effect of pre-thermal treatment on the lithium storage performance of LiNi0.8Co0.15Al0.05O2. J. Mater. Sci. 2016, 51, 1400–1408. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Liu, C.; Song, D.W.; Zhang, L.Q.; Bie, L.J. A new synthesis strategy towards enhancing the structure and cycle stabilities of the LiNi0.80Co0.15Al0.05O2 cathode material. J. Mater. Chem. A 2017, 5, 835–841. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Wang, Z.; Guo, H.; Xiong, X. Synthesis of Mg-doped LiNi0.8Co0.15Al0.05O2 oxide and its electrochemical behavior in high-voltage lithium-ion batteries. Ceram. Int. 2014, 40, 13223–13230. [Google Scholar] [CrossRef]
- Liu, B.S.; Wang, Z.B.; Yu, F.D.; Xue, Y.; Wang, G.J.; Zhang, Y.; Zhou, Y.X. Facile strategy of NCA cation mixing regulation and its effect on electrochemical performance. RSC Adv. 2016, 6, 108558–108565. [Google Scholar] [CrossRef]
- Tang, Z.F.; Bao, J.J.; Du, Q.X.; Shao, Y.; Gao, M.H.; Zou, B.K.; Chen, C.H. Surface Surgery of the Nickel-Rich Cathode Material LiNi0.815Co0.15Al0.035O2: Toward a Complete and Ordered Surface Layered Structure and Better Electrochemical Properties. ACS Appl. Mater. Interfaces 2016, 8, 34879–34887. [Google Scholar] [CrossRef]
- Tang, Z.F.; Wu, R.; Huang, P.F.; Wang, Q.S.; Chen, C.H. Improving the electrochemical performance of Ni-rich cathode material LiNi0.815Co0.15Al0.035O2 by removing the lithium residues and forming Li3PO4 coating layer. J. Alloys Compd. 2017, 693, 1157–1163. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, C.W.; Bae, Y.S.; Hwang, I.; Park, Y.K.; Song, J.H. Method of Preparation for Particle Growth Enhancement of LiNi0.8Co0.15Al0.05O2. Electrochem. Solid State Lett. 2009, 12, A211–A214. [Google Scholar] [CrossRef]
- Xie, H.; Du, K.; Hu, G.; Duan, J.; Peng, Z.; Zhang, Z.; Cao, Y. Synthesis of LiNi0.8Co0.15Al0.05O2 with 5-sulfosalicylic acid as a chelating agent and its electrochemical properties. J. Mater. Chem. A 2015, 3, 20236–20243. [Google Scholar] [CrossRef]
- Duan, J.; Hu, G.; Cao, Y.; Tan, C.; Wu, C.; Du, K.; Peng, Z. Enhanced electrochemical performance and storage property of LiNi0.815Co0.15Al0.035O2 via Al gradient doping. J. Power Sources 2016, 326, 322–330. [Google Scholar] [CrossRef]
- Xie, H.; Hu, G.; Du, K.; Peng, Z.; Cao, Y. An improved continuous co-precipitation method to synthesize LiNi0.80Co0.15Al0.05O2 cathode material. J. Alloys Compd. 2016, 666, 84–87. [Google Scholar] [CrossRef]
- Duan, J.; Wu, C.; Cao, Y.; Huang, D.; Du, K.; Peng, Z.; Hu, G. Enhanced compacting density and cycling performance of Ni-riched electrode via building mono dispersed micron scaled morphology. J. Alloys Compd. 2017, 695, 91–99. [Google Scholar] [CrossRef]
- Purwanto, A.; Yudha, C.S.; Ubaidillah, U.; Widiyandari, H.; Ogi, T.; Haerudin, H. NCA cathode material: Synthesis methods and performance enhancement efforts. Mater. Res. Express 2018, 5, 122001. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Shi, E.; Zhao, S.; Jiang, K.; Wang, D.; Wang, P.; Guo, S.; He, P.; Zhou, H. In situ X-ray diffraction and thermal analysis of LiNi0.8Co0.15Al0.05O2 synthesized via co-precipitation method. J. Energy Chem. 2018, 27, 1655–1660. [Google Scholar] [CrossRef]
- Li, H.; Cormier, M.; Zhang, N.; Inglis, J.; Li, J.; Dahn, J.R. Is Cobalt Needed in Ni-Rich Positive Electrode Materials for Lithium Ion Batteries? J. Electrochem. Soc. 2019, 166, A429–A439. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Castro-García, S.; Castro-Couceiro, A.; Señarís-Rodríguez, M.A.; Soulette, F.; Julien, C.M. Influence of aluminum doping on the properties of LiCoO2 and LiNi0.5Co0.5O2 oxides. Solid State Ionics 2003, 156, 15–26. [Google Scholar] [CrossRef]
- Albrecht, S.; Kümpers, J.; Kruft, M.; Malcus, S.; Vogler, C.; Wahl, M.; Wohlfahrt-Mehrens, M. Electrochemical and thermal behavior of aluminum- and magnesium-doped spherical lithium nickel cobalt mixed oxides Li1-x(Ni1-y-zCoyMz)O2 (M = Al, Mg). J. Power Sources 2003, 119–121, 178–183. [Google Scholar] [CrossRef]
- Apte, N.G.; Kiran, E.; Chernosky, J.V. Thermal decomposition of aluminium-bearing compounds. J. Therm. Anal. 1988, 34, 975–981. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Mozejko, J. Thermodynamic functions of activated complexes created in thermal decomposition processes of sulphates. Thermochim. Acta 2000, 344, 73–79. [Google Scholar] [CrossRef]
- Wang, L.; Maxisch, T.; Ceder, G. A first-principles approach to studying the thermal stability of oxide cathode materials. Chem. Mater. 2007, 19, 543–552. [Google Scholar] [CrossRef]
- Tian, C.; Lin, F.; Doeff, M.M. Electrochemical Characteristics of Layered Transition Metal Oxide Cathode Materials for Lithium Ion Batteries: Surface, Bulk Behavior, and Thermal Properties. Acc. Chem. Res. 2018, 51, 89–96. [Google Scholar] [CrossRef]
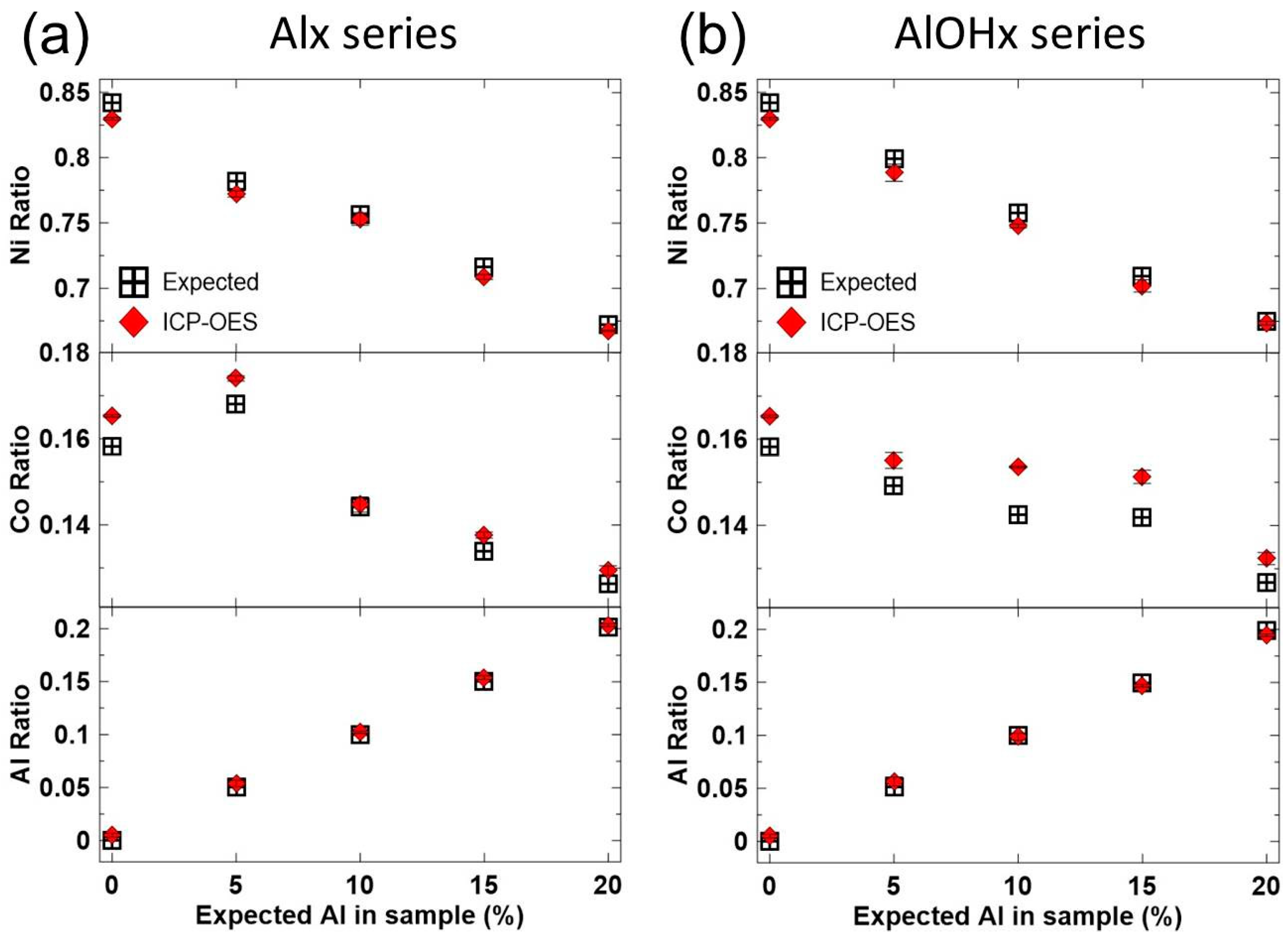
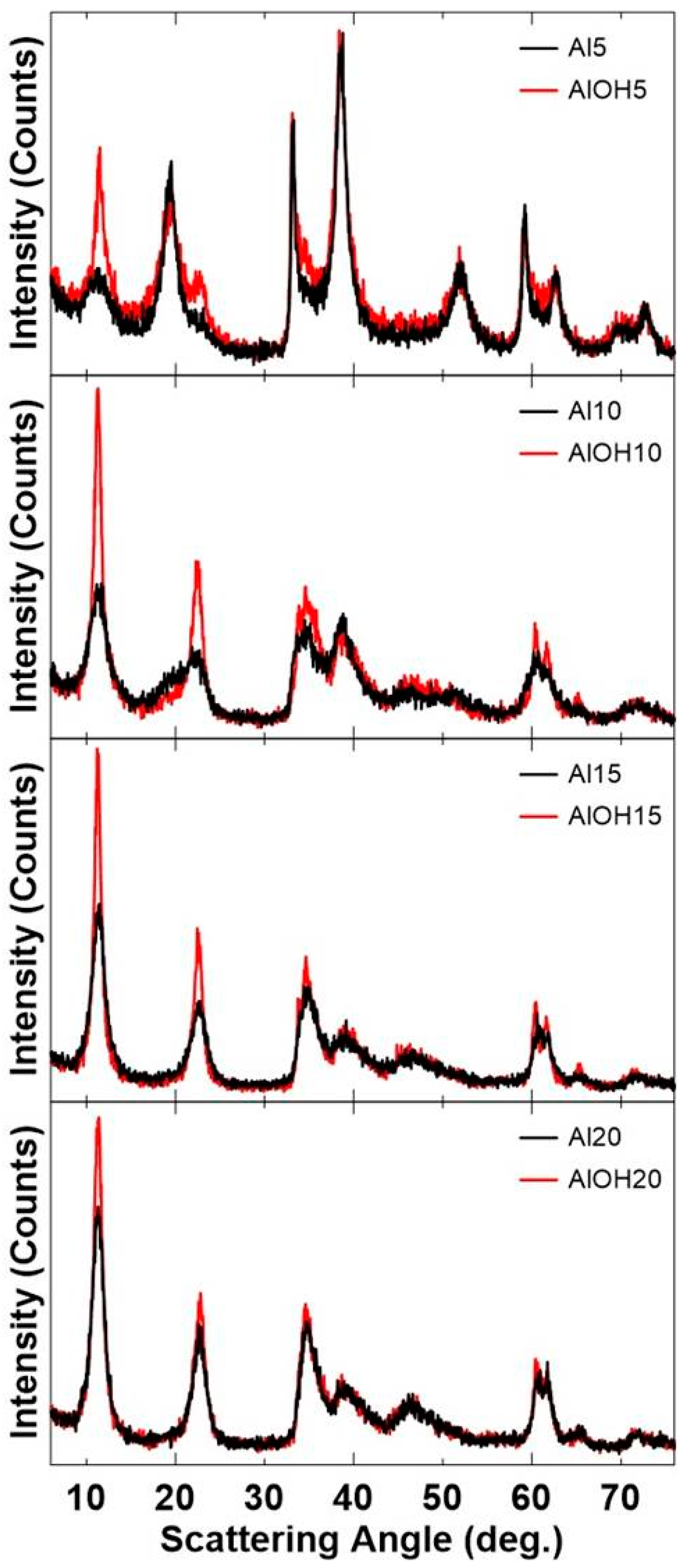

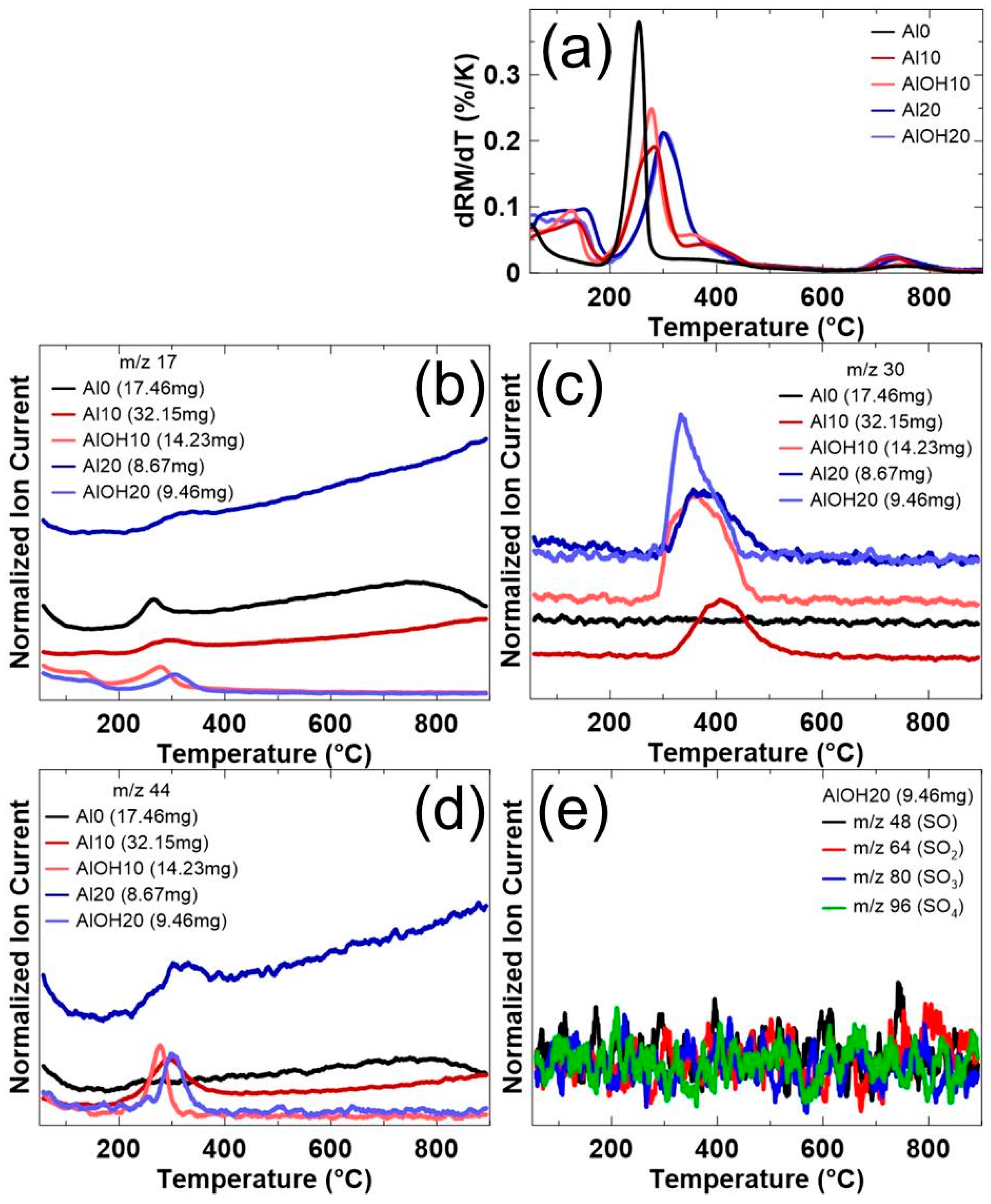
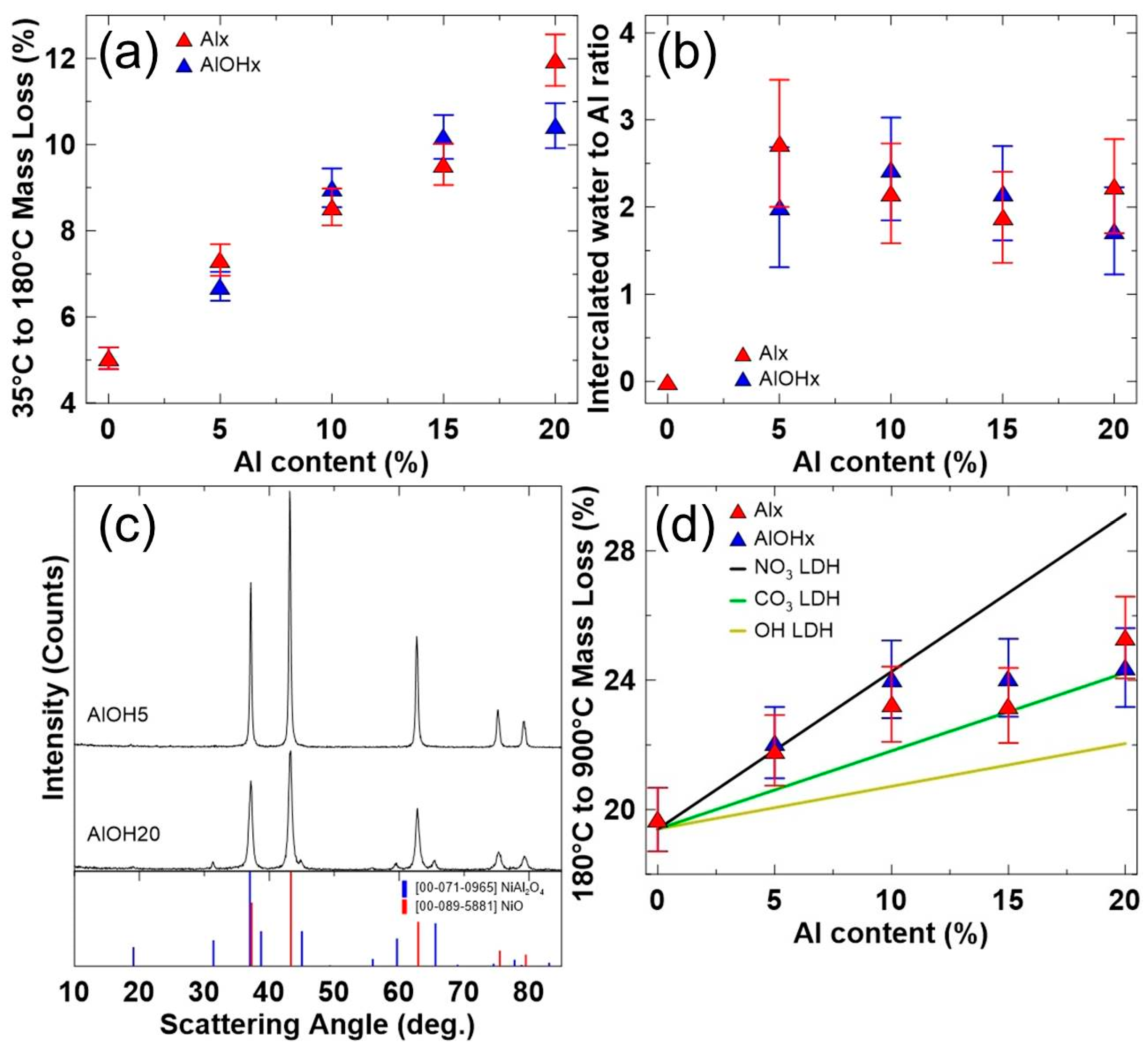
| Sample | Target Molar Ratios | |||||
|---|---|---|---|---|---|---|
| x in Alx or AlOHx | Ni | Co | Al | Alx method | AlOHx method | |
| NaOH | NaOH in Al solution | NaOH | ||||
| 0 | 0.842 | 0.158 | 0.000 | 2.200 | 0.000 | 2.200 |
| 5 | 0.800 | 0.150 | 0.050 | 2.200 | 0.250 | 1.950 |
| 10 | 0.758 | 0.142 | 0.100 | 2.200 | 0.500 | 1.700 |
| 15 | 0.716 | 0.134 | 0.150 | 2.200 | 0.750 | 1.450 |
| 20 | 0.674 | 0.126 | 0.200 | 2.200 | 1.000 | 1.200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Dahn, J.R. The Formation of Layered Double Hydroxide Phases in the Coprecipitation Syntheses of [Ni0.80Co0.15](1−x)/0.95Alx(OH)2(anionn−)x/n (x = 0–0.2, n = 1, 2). ChemEngineering 2019, 3, 38. https://doi.org/10.3390/chemengineering3020038
Liu A, Dahn JR. The Formation of Layered Double Hydroxide Phases in the Coprecipitation Syntheses of [Ni0.80Co0.15](1−x)/0.95Alx(OH)2(anionn−)x/n (x = 0–0.2, n = 1, 2). ChemEngineering. 2019; 3(2):38. https://doi.org/10.3390/chemengineering3020038
Chicago/Turabian StyleLiu, Aaron, and J. R. Dahn. 2019. "The Formation of Layered Double Hydroxide Phases in the Coprecipitation Syntheses of [Ni0.80Co0.15](1−x)/0.95Alx(OH)2(anionn−)x/n (x = 0–0.2, n = 1, 2)" ChemEngineering 3, no. 2: 38. https://doi.org/10.3390/chemengineering3020038
APA StyleLiu, A., & Dahn, J. R. (2019). The Formation of Layered Double Hydroxide Phases in the Coprecipitation Syntheses of [Ni0.80Co0.15](1−x)/0.95Alx(OH)2(anionn−)x/n (x = 0–0.2, n = 1, 2). ChemEngineering, 3(2), 38. https://doi.org/10.3390/chemengineering3020038





