Modeling of Copper Adsorption on Mesoporous Carbon CMK-3: Response Surface Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. SBA-15 and Mesoporous Carbon CMK-3 Synthesis
2.3. SBA-15 and CMK-3 Characterization
2.4. Adsorption in Batch Mode
2.5. Response Surface Modeling
0.59BC − 6.01A2 + 0.59B2 − 6.20C2.
3.76 × 10−4CM − 7.89 × 10−3TM − 3.84 × 10−4C2 + 5.86 × 10−3T2 − 0.11M2.
3. Results and Discussion
3.1. Physicochemical Characterizations
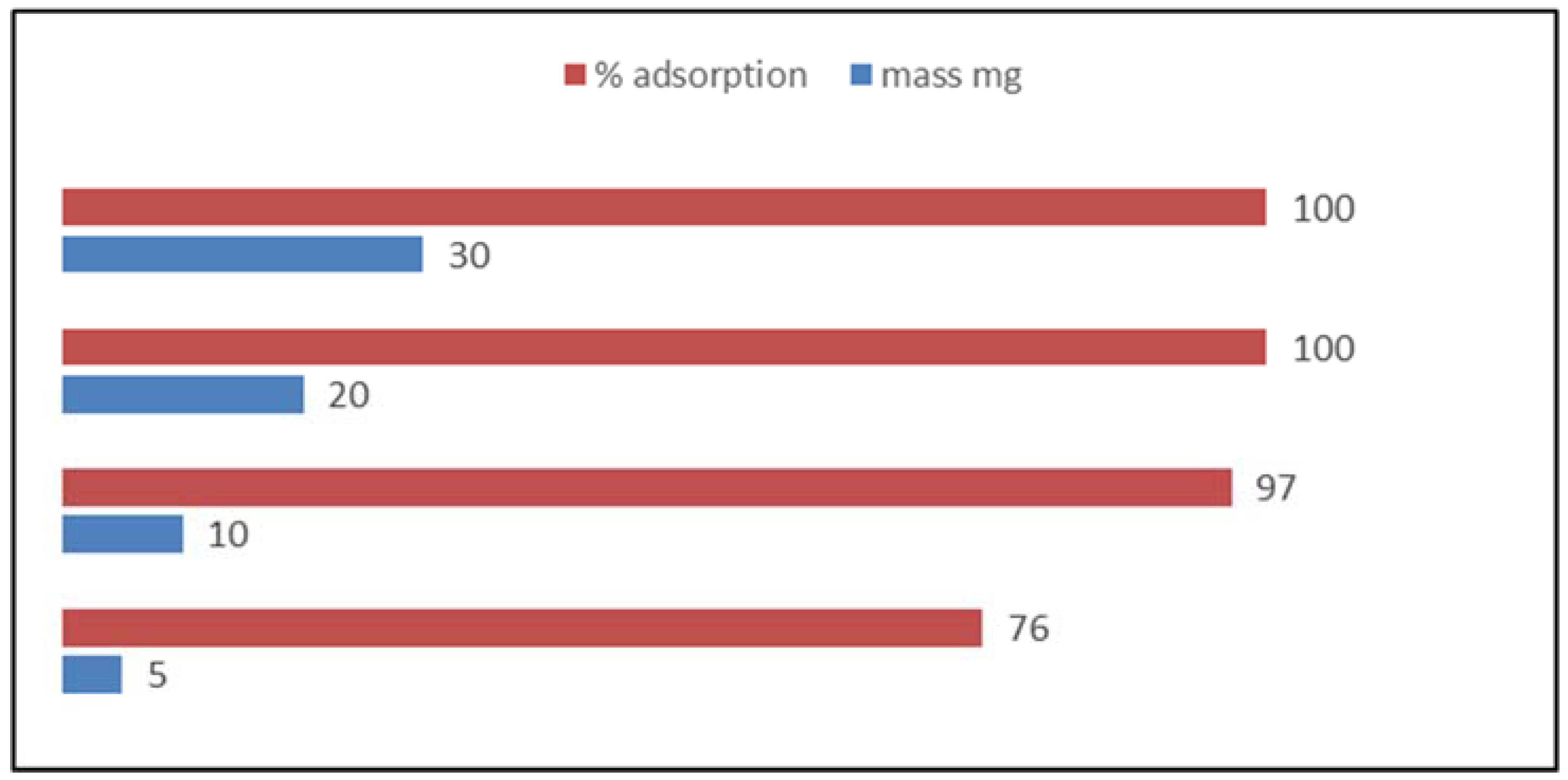
3.2. Effect of Sorbent Mass
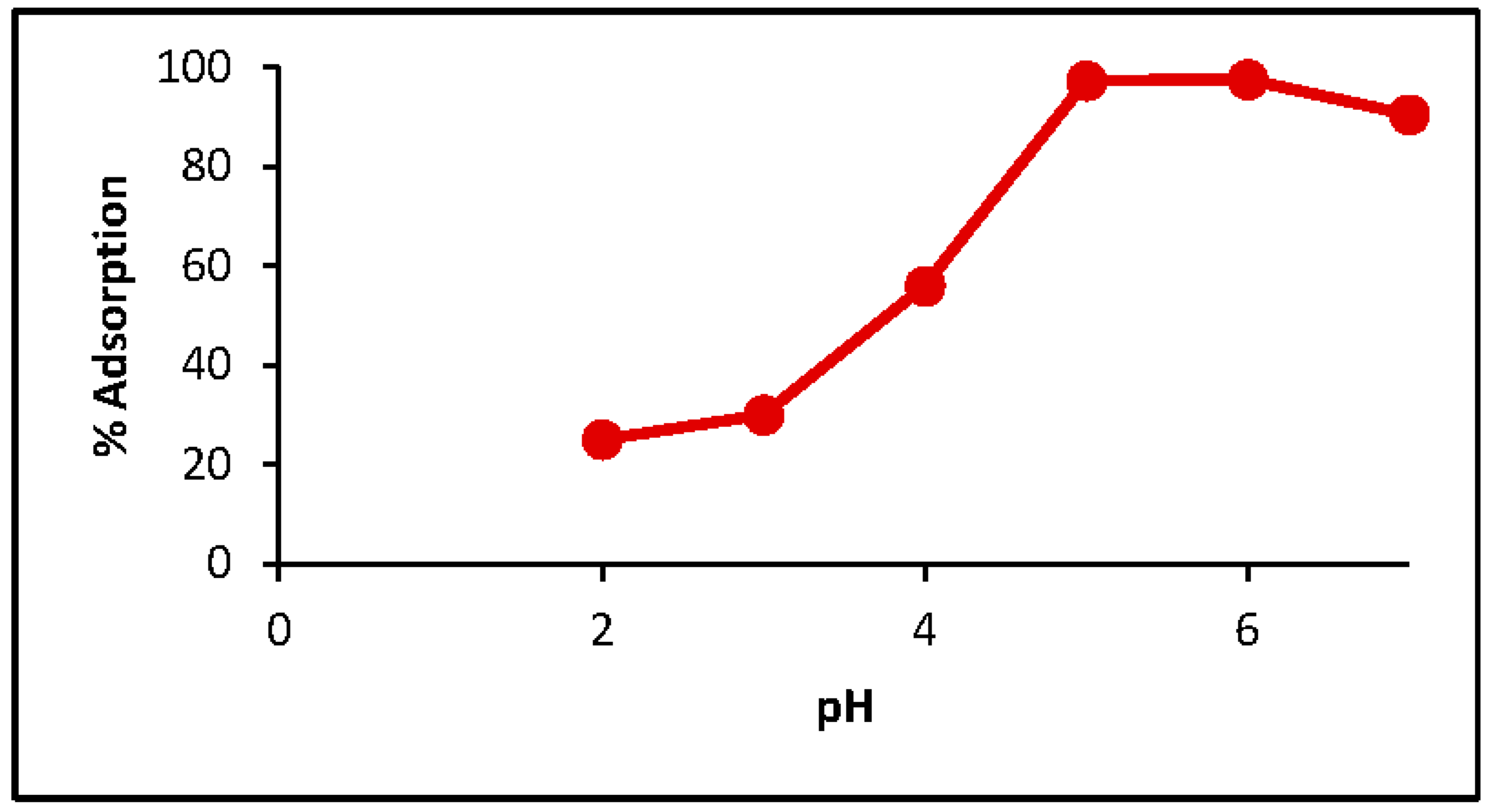
3.3. pH Effect
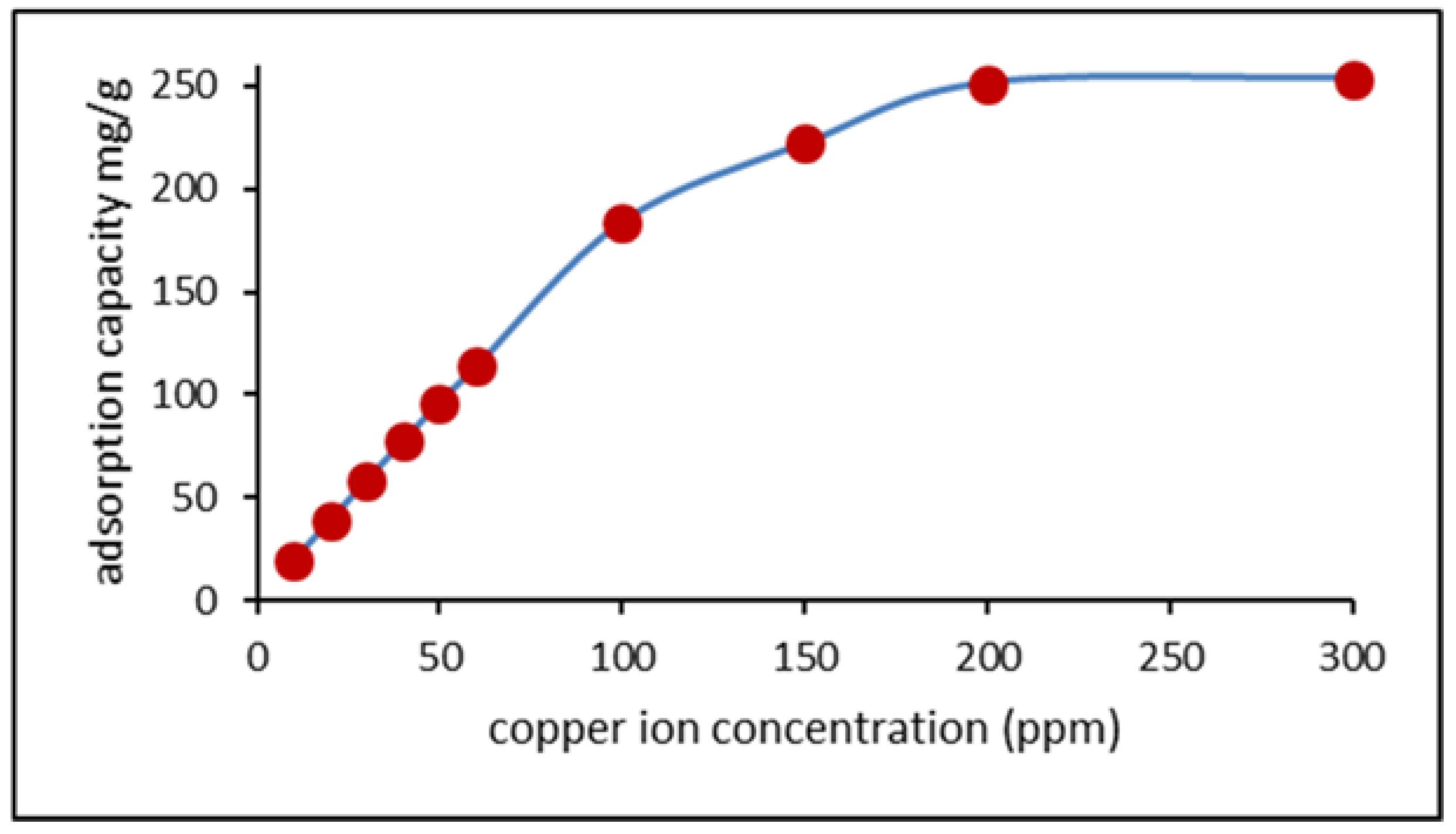
3.4. Effect of Copper Concentration
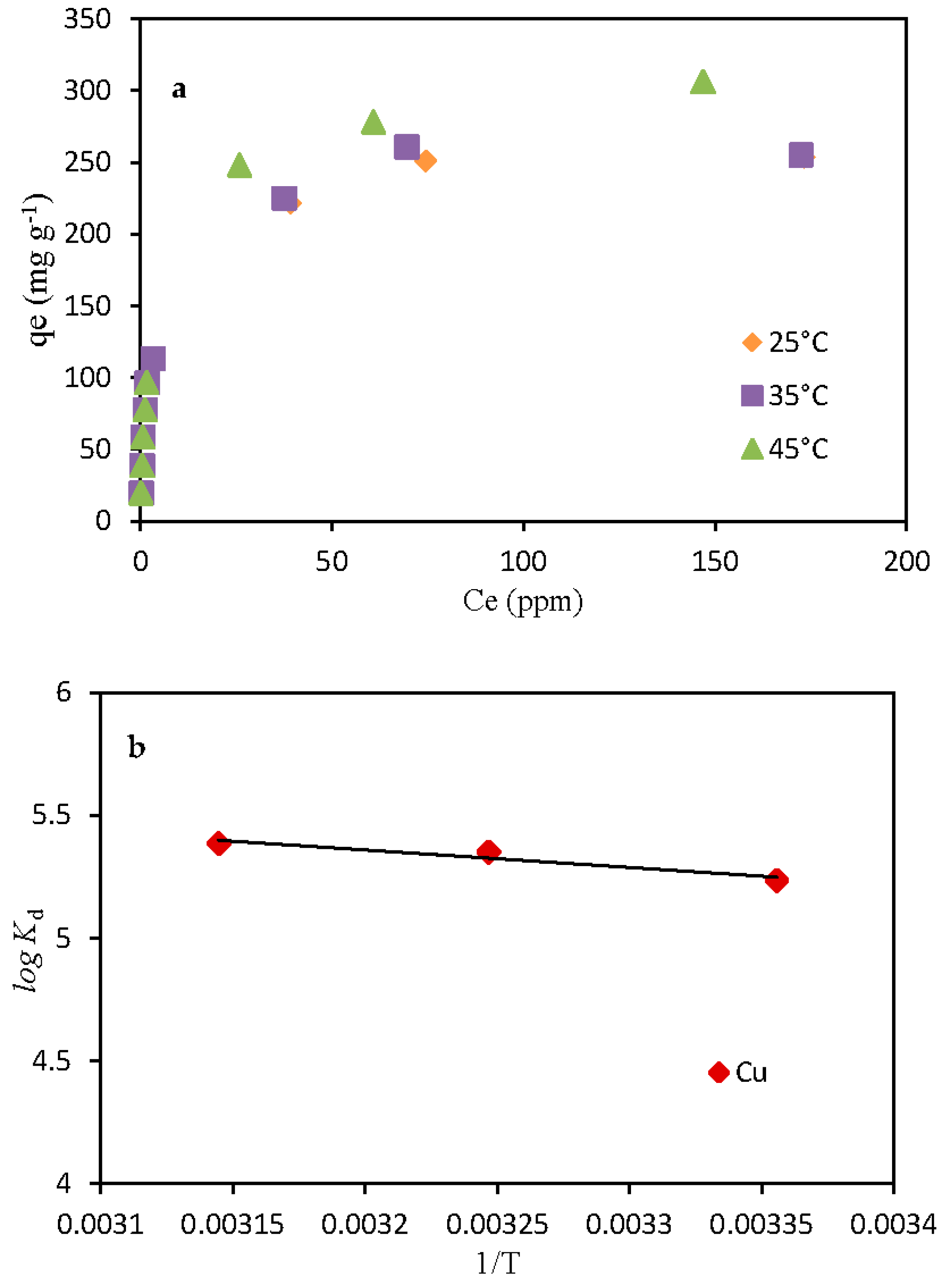
3.5. Temperature Effect
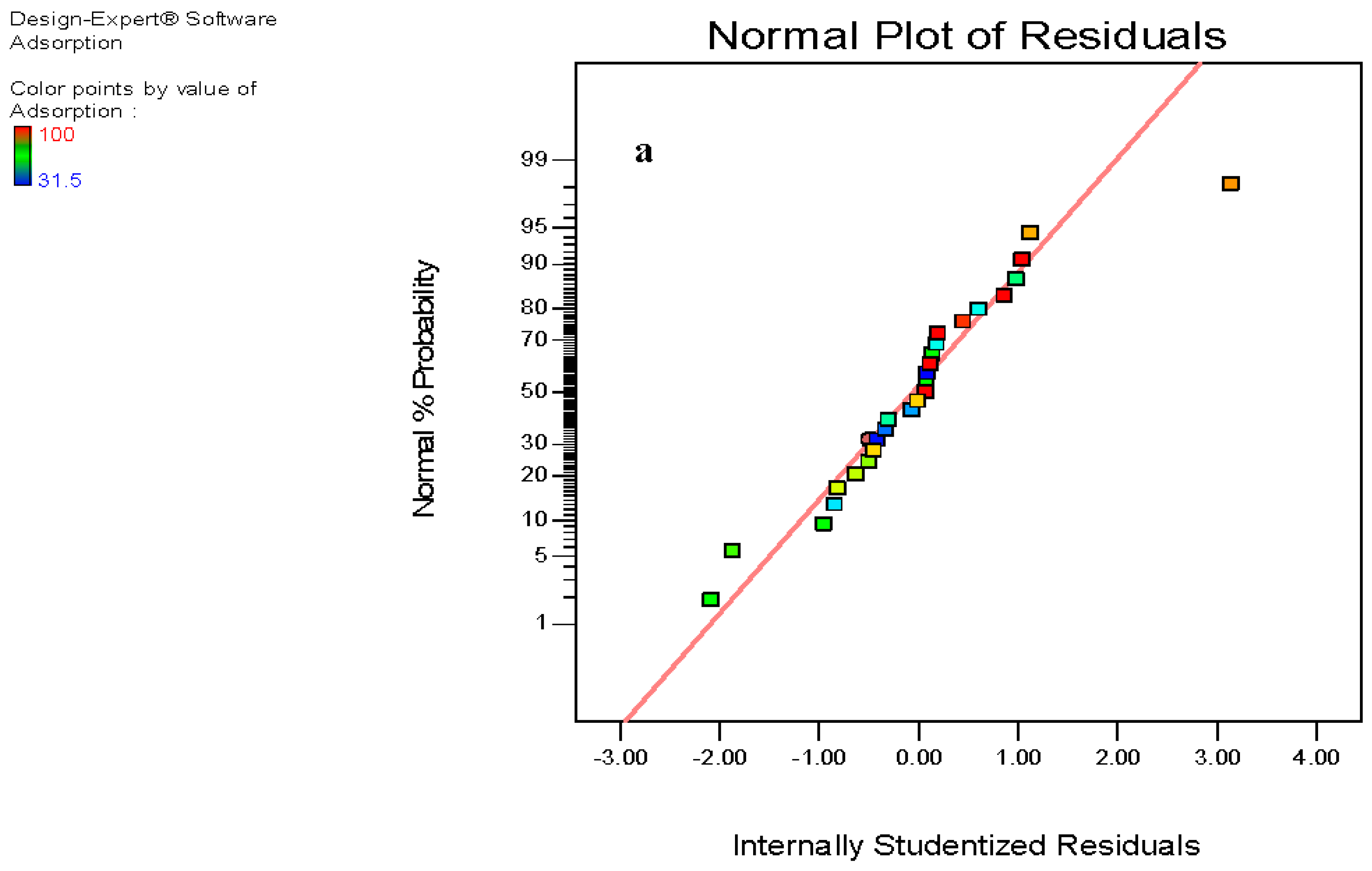
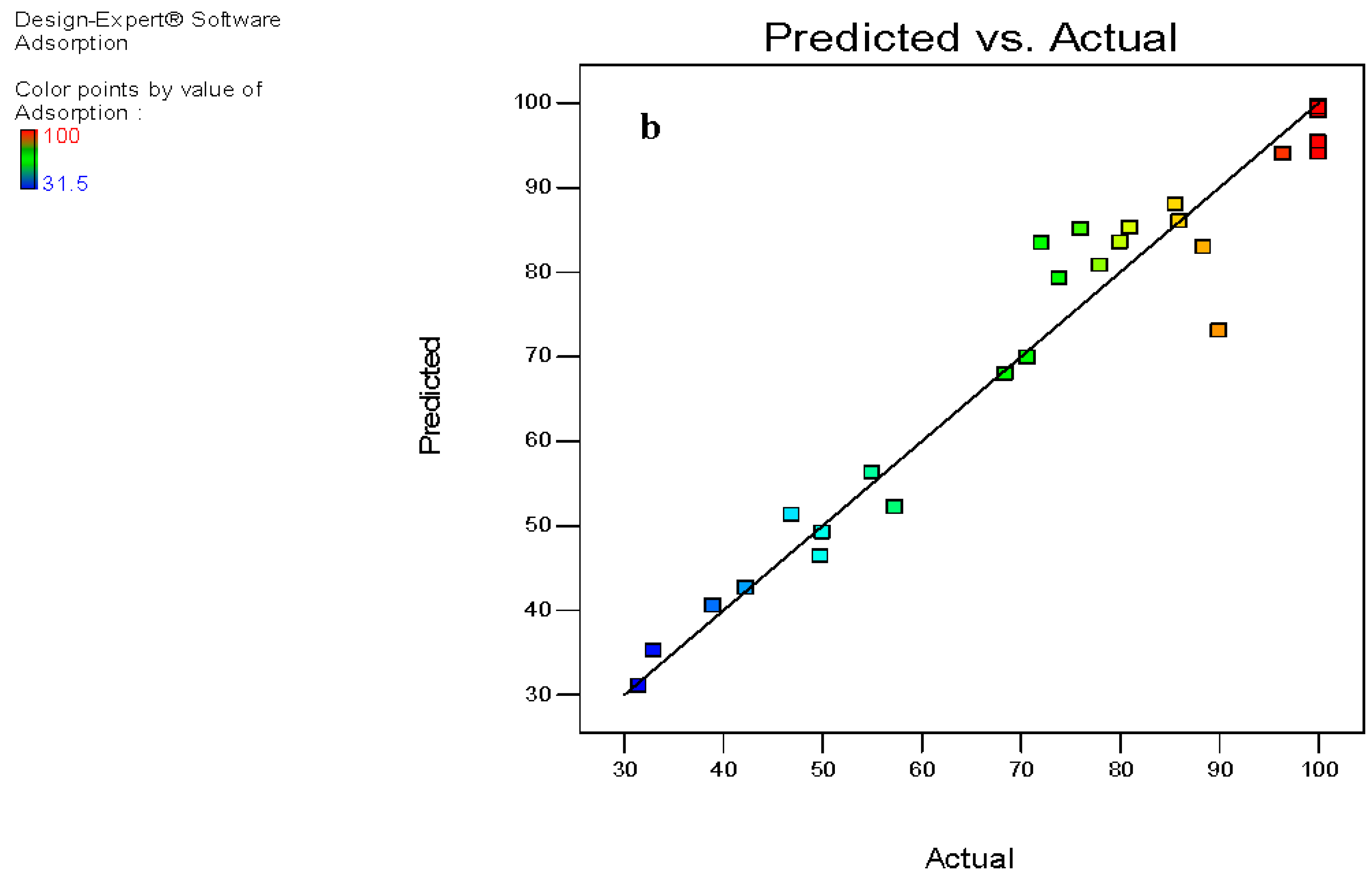
3.6. Composite Surface Design Methods
3.7. Analysis of Variance (ANOVA)
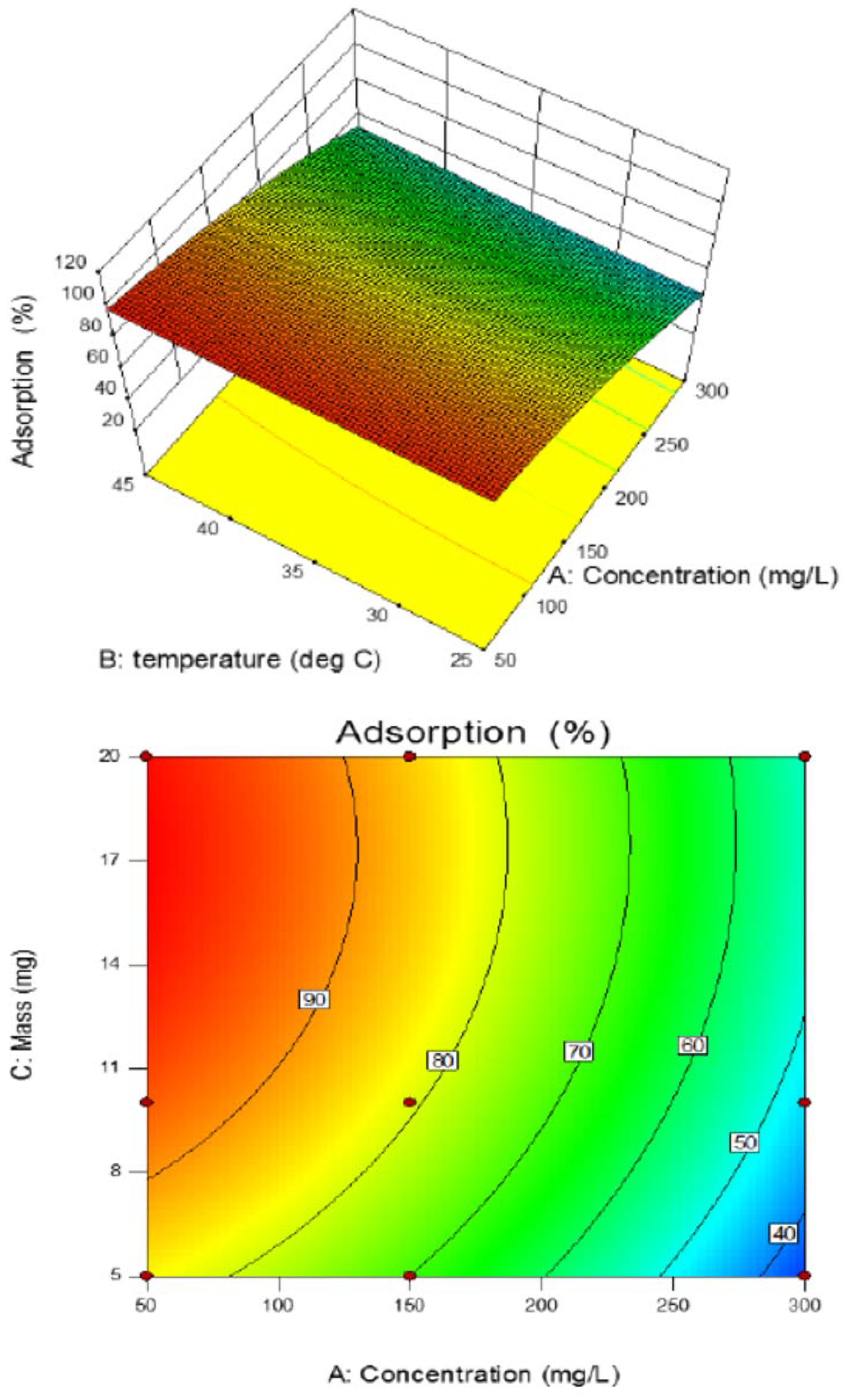
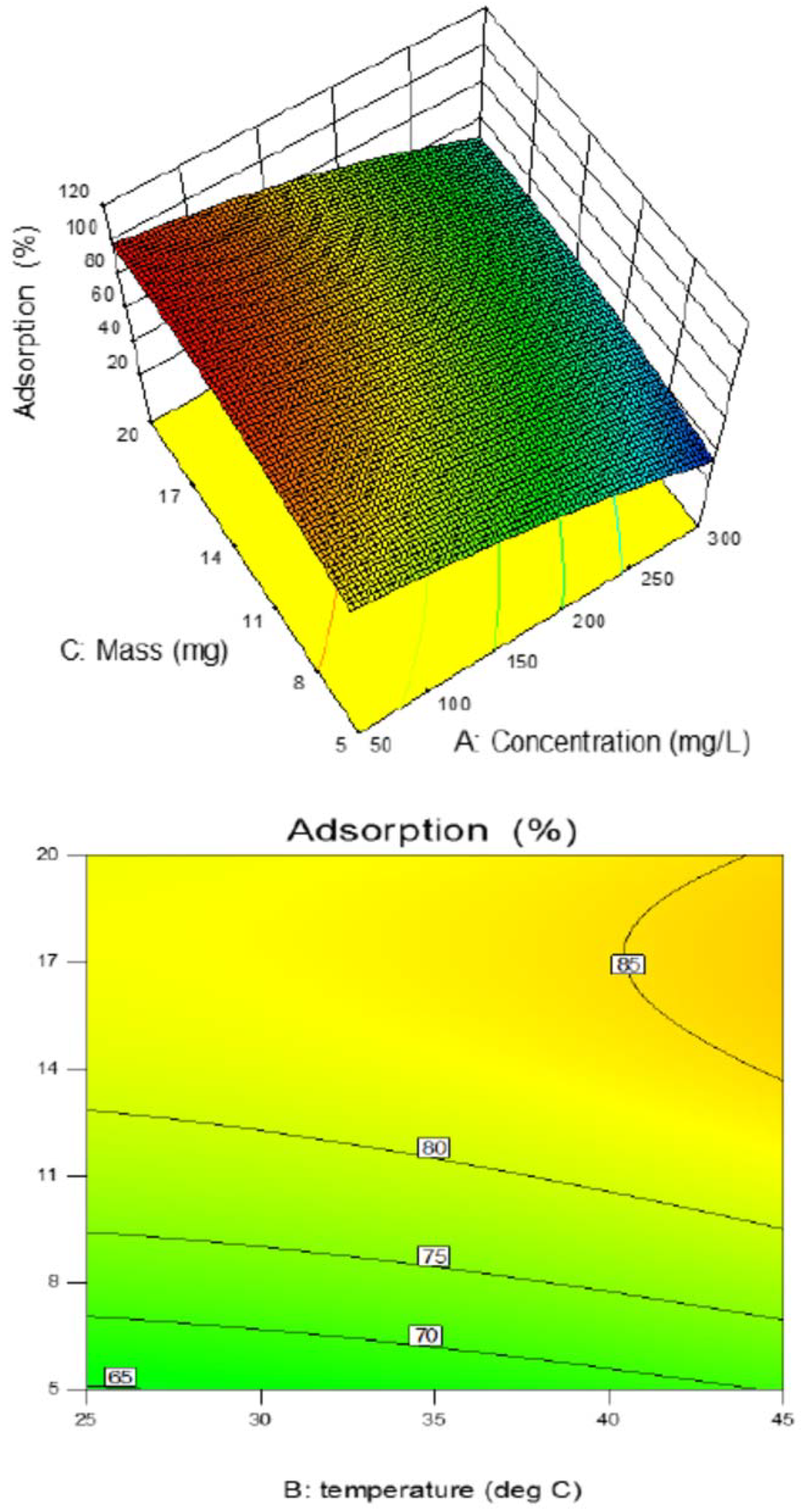
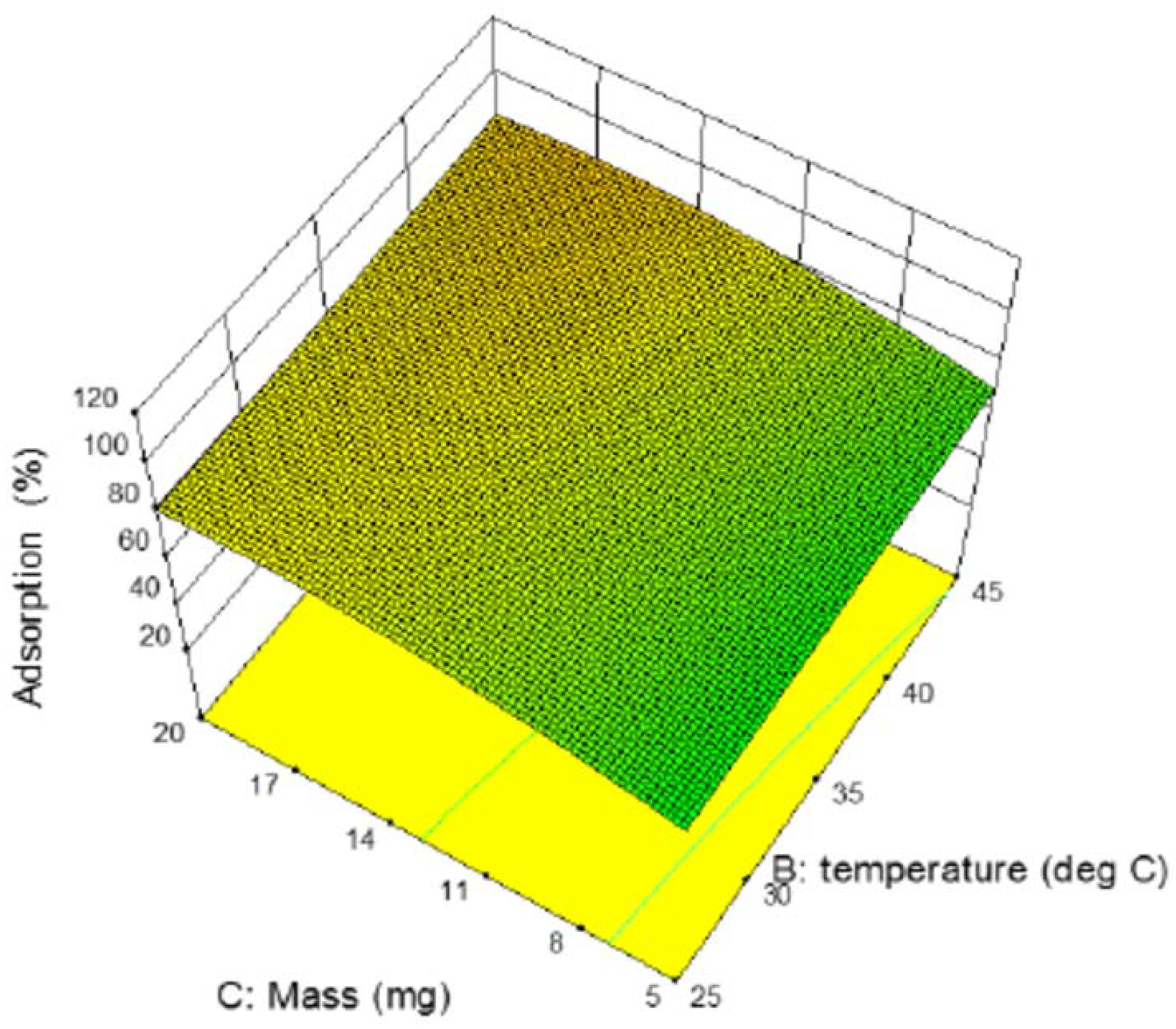
3.8. Effect of the Studied Parameters on Copper Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H.; Saad, Z. Synthetic Nax Zeolite as a Very Efficient Heavy Metals Sorbent in Batch and Dynamic Conditions. Colloids Interfaces 2018, 2, 22. [Google Scholar] [CrossRef]
- Cheng, H.P.; Su, L.C.; Chen, C.Y. Thermodynamics and kinetics of adsorption of Cu (II) onto waste iron oxide. J. Hazard. Mater. 2007, 144, 406–411. [Google Scholar] [CrossRef]
- Hamad, H.; Ezzeddine, Z.; Kanaan, S.; Lakis, F.; Hijazi, A.; Moussawi, M.A. A Novel Modification and Selective Route for the Adsorption of Pb2+ by oak charcoal functionalized with Glutaraldehyde. Adv. Powder Technol. 2016, 27, 631–637. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Hamad, H.; Ezzeddine, Z.; Lakis, F.; Rammal, H.; Srour, M.; Hijazi, A. An Insight into the Removal of Cu (II) and Pb (II) by Aminopropyl Modified Mesoporous Carbon CMK-3: Adsorption capacity and mechanism. Mater. Chem. Phys. 2016, 178, 57–64. [Google Scholar] [CrossRef]
- Liang, C.D.; Li, Z.J.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H. Removal of Methylene Blue by Mesoporous CMK-3: Kinetics, Isotherms and Thermodynamics. J. Mol. Liq. 2016, 223, 763–770. [Google Scholar] [CrossRef]
- Qiang, Z.; Gurkan, B.; Ma, J.; Liu, X.; Guo, Y.; Cakmak, M.; Cavicchi, K.A.; Vogt, B.D. Roll-to-roll fabrication of high surface area mesoporous carbon with process-tunable pore texture for optimization of adsorption capacity of bulky organic dyes. Micropor. Mesopor. Mater. 2016, 227, 57–64. [Google Scholar] [CrossRef]
- Qiang, Z.; Guo, Y.; Liu, H.; Cheng, S.Z.D.; Cakmak, M.; Cavicchi, K.A.; Vogt, B.D. Large-Scale Roll-to-Roll Fabrication of Ordered Mesoporous Materials using Resol-Assisted Cooperative Assembly. ACS Appl. Mater. Interfaces. 2015, 7, 4306–4310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiang, Z.; Vogt, B.D. Relationship between crosslinking and ordering kinetics for the fabrication of soft templated (FDU-16) mesoporous carbon thin films. RSC Adv. 2014, 4, 44858–44867. [Google Scholar] [CrossRef]
- Moreno-Tovara, R.; Terrésb, E.; Rene Rangel-Mendez, J. Oxidation and EDX elemental mapping characterization of an ordered mesoporous carbon: Pb(II) and Cd(II) removal. Appl. Surf. Sci. 2014, 303, 373–380. [Google Scholar] [CrossRef]
- Moradi, S.E.; Baniamerian, M.J. Applications of chemically modified ordered mesoporous carbon as solid phase extraction sorbent for preconcentration of trace lead ion in water samples. Chem. Ind. Chem. Eng. Q. 2011, 17, 397–408. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.; Stucky, G. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Vinu, A.; Srinivasu, P.; TakahashI, M.; Mori, T.; Balasubramanian, V.V.; Ariga, K. Controlling the textural parameters of mesoporous carbon materials. Micropor. Mesopor. Mater. 2007, 100, 20–26. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Thuan, T.V.; Phuong Quynh, B.T.; Nguyen, T.D.; Thanh Ho, V.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surfaces Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chem. Senses 2006, 31, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Liua, F.Q.; Yanga, X.; Linga, P.; Li, L.J.; Long, C.; Li, A.M. Adsorption performances and mechanisms of the newly synthesized N,N-di(carboxymethyl) dithiocarbamate chelating resin toward divalent heavy metal ions from aqueous media. J. Hazard. Mater. 2009, 167, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Da’na, E.; Sayari, A. Effect of regeneration conditions on the cyclic performance of amine-modified SBA-15 for removal of copper from aqueous solutions: Composite surface design methodology. Desalination 2011, 277, 54–60. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Madhava Rao, M.; Ramesh, A.; Purna Chandra Rao, G.; Seshaiah, K. Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls. J. Hazard. Mater. 2006, 129, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]


| Variables | Code | Symbol | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Cu2+ concentration (ppm) | A | C | 50 | 150 | 300 |
| Temperature (°C) | B | T | 25 | 35 | 45 |
| CMK-3 mass (mg) | C | M | 5 | 10 | 20 |
| Sample | SBET a (m2·g−1) | Pore Size b (nm) | Mesopore Volume c (cm3·g−1) | Micropore Volume (cm3·g−1) |
|---|---|---|---|---|
| SBA-15 | 896 | 5.6 | 0.706 | 0.115 |
| CMK-3 | 621 | 4 | 0.4 | 0.033 |
| Metal | ΔH° (kJ·mol−1) | ΔS° (J·K−1·mol−1) | T (K) | ΔG° (kJ·mol−1) | R2 |
|---|---|---|---|---|---|
| Cu2+ | 5.9 | 63.5 | 298 | −12.9 | 0.923 |
| 308 | −13.7 | ||||
| 318 | −14.2 |
| Variable 1 | Variable 2 | Variable 3 | Response | ||
|---|---|---|---|---|---|
| Run | Concentration | Temperature | Mass | Adsorption % | |
| mg/L | deg. C | mg | Actual | Predicted | |
| 1 | 0 | 1 | 1 | 85.6 | 87.95 |
| 2 | −1 | −1 | 0 | 96.4 | 94 |
| 3 | 1 | −1 | 1 | 50 | 49.21 |
| 4 | −1 | 0 | 1 | 100 | 98.95 |
| 5 | 1 | −1 | 0 | 42.26 | 42.62 |
| 6 | 1 | −1 | −1 | 31.5 | 31.07 |
| 7 | 0 | −1 | 0 | 73.9 | 79.22 |
| 8 | 1 | 1 | 0 | 46.9 | 51.29 |
| 9 | 1 | 0 | −1 | 33 | 35.21 |
| 10 | 0 | −1 | 1 | 81 | 85.24 |
| 11 | 0 | −1 | −1 | 68.4 | 67.95 |
| 12 | −1 | −1 | 1 | 100 | 99.64 |
| 13 | −1 | 0 | −1 | 72.1 | 83.41 |
| 14 | −1 | 1 | −1 | 76 | 85.08 |
| 15 | 0 | 0 | −1 | 70.7 | 69.90 |
| 16 | 0 | 1 | 0 | 80 | 83.51 |
| 17 | 0 | 0 | 0 | 78 | 80.78 |
| 18 | −1 | 1 | 0 | 100 | 95.37 |
| 19 | −1 | −1 | −1 | 88.4 | 82.92 |
| 20 | 0 | 1 | −1 | 90 | 73.03 |
| 21 | 1 | 0 | 0 | 49.8 | 46.37 |
| 22 | −1 | 1 | 1 | 100 | 99.44 |
| 23 | 1 | 1 | 1 | 55 | 56.30 |
| 24 | 1 | 1 | −1 | 39 | 40.53 |
| 25 | 1 | 0 | 1 | 57.3 | 52.17 |
| 26 | −1 | 0 | 0 | 100 | 94.10 |
| 27 | 0 | 0 | 1 | 86 | 86.01 |
| Source | F-Value | p-Value | Standard Error |
|---|---|---|---|
| Model | 31.53 | <0.0001 | 3.65 |
| A—concentration | 231.66 | <0.0001 | 1.56 |
| B—temperature | 2.18 | 0.1577 | 1.57 |
| C—mass | 27.42 | <0.0001 | 1.55 |
| AB | 0.94 | 0.3458 | 1.88 |
| AC | 0.037 | 0.8506 | 1.85 |
| BC | 0.10 | 0.7542 | 1.86 |
| A2 | 4.58 | 0.0471 | 2.81 |
| B2 | 0.048 | 0.8293 | 2.68 |
| C2 | 4.07 | 0.0596 | 3.07 |
| Carbon | Modification Route | MetalIons | Adsorption Capacity (mg·g−1) | Ref. |
|---|---|---|---|---|
| AC from hazelnut husks | Zinc chloride activation | Cu2+ Pb2+ | 6.64 13.05 | [20] |
| AC from Ceiba pentandra hulls | Steam activation (SA) | Cu2+ Cd2+ | 20.8 19.5 | [21] |
| Commercial AC | Sodium diethyl dithiocarbamate (SDDC) | Cu2+ | 38.9 | [22] |
| AC from grape bagasse | Phosphoric acid | Cu2+ | 43.47 | [23] |
| CMK-3 | - | Cu2+ | 250 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y. Modeling of Copper Adsorption on Mesoporous Carbon CMK-3: Response Surface Design. ChemEngineering 2018, 2, 49. https://doi.org/10.3390/chemengineering2040049
Ezzeddine Z, Batonneau-Gener I, Pouilloux Y. Modeling of Copper Adsorption on Mesoporous Carbon CMK-3: Response Surface Design. ChemEngineering. 2018; 2(4):49. https://doi.org/10.3390/chemengineering2040049
Chicago/Turabian StyleEzzeddine, Zeinab, Isabelle Batonneau-Gener, and Yannick Pouilloux. 2018. "Modeling of Copper Adsorption on Mesoporous Carbon CMK-3: Response Surface Design" ChemEngineering 2, no. 4: 49. https://doi.org/10.3390/chemengineering2040049
APA StyleEzzeddine, Z., Batonneau-Gener, I., & Pouilloux, Y. (2018). Modeling of Copper Adsorption on Mesoporous Carbon CMK-3: Response Surface Design. ChemEngineering, 2(4), 49. https://doi.org/10.3390/chemengineering2040049






