1. Introduction
The entire world desires an atmosphere that provides clean air and has reduced levels of carbon dioxide, nitrous oxide, methane, ozone, and fluorocarbons, which contribute to global disturbances. These gases are called greenhouse effect gases. The atmosphere now faces two problems that contribute to the increased greenhouse effect gases. The first is the rapid deforestation caused by forest fires and land requirements for developmental purposes. The other is the use of fossil fuels for a variety of purposes. Fossil fuels generate gases that contribute to the greenhouse gas effect. The real concern is in the state of the global climate for the generations ahead. The United Nations (UN) has proclaimed several steps to mitigate greenhouse gas emissions. By this effort, it has reduced the burning of 64,000 kilotons of wood and reduced the release of 118,000 tons of CO
2 into the environment (
http://www.undp.org/content/undp/en/home/ourwork/climate-and-disaster-resilience/climate-change/mitigating-greenhouse-gas-emissions.html). By encouraging the use of renewable sources of energy, several wind farms and solar panels have been developed to reduce greenhouse effect gases.
Among the greenhouse gases involved in environmental disturbances, carbon dioxide, nitrogen oxides, and methane are given utmost importance, as the concentrations of these gases change more than the others, thus requiring immediate attention. Hence, this review is devoted to these gases. The climatic conditions are influenced by greenhouse gases [
1,
2]. This study is not aimed at correlating the gas levels to climate changes, although indirect relationships have been established, and hopefully this article will stimulate an interest in evolving such correlations.
Weather and climatic conditions are often considered important in our day-to-day life and, as a result, we hear/learn of these environmental conditions through the radio and TV. These channels often give us information such the temperature, wind speed, rain, snow, ice conditions, UV index, cloud cover, humidity, dew point, and barometric pressure, as shown in
Figure 1. The weather channel seldom gives the CO
2, CH
4, N
2O, or O
3 levels. This may be because of the non-availability of data and the methodology needed to be adapted to measure the concentrations of greenhouse gases. In 2012, a network of three tall tower stations was set up to obtain the long-term background levels of CO
2, CH
4, and NO
X in the United Kingdom. The results obtained from the network showed that the greenhouse gases were increasing in mole fractions. The CO
2 and CH
4 concentrations showed nighttime maxima and daytime minima [
3].
1.1. Status of Greenhouse Gases
The concentrations of greenhouse effect gases in the environment have been periodically monitored by several agencies, and in this section we review the concentrations of these gases in the environment with a view to developing sensors.
1.1.1. Status of Carbon Dioxide Gas
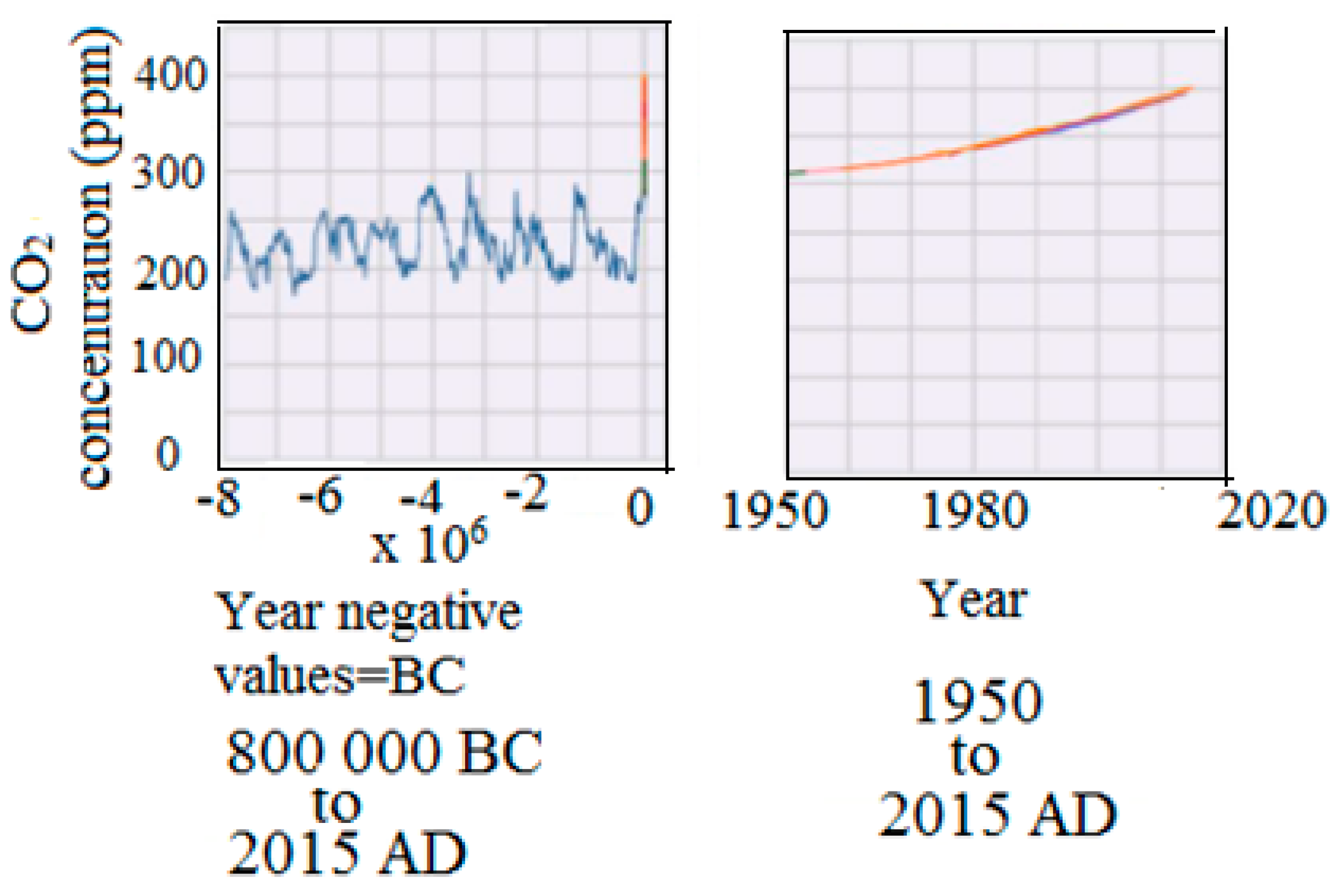
The carbon dioxide level in the atmosphere is currently reported to be at about 400 ppm, compared to a low value of about 200 ppm in 800,000 BC (
Figure 2). This reveals a rapid growth of the carbon dioxide levels in the atmosphere. This increase is attributed to fuel combustion, forest fires, volcanic eruption, and volatile organic compounds. The obvious choice to overcome this problem is to reduce the use of fossil fuels, increase the use of nonpolluting fuels, begin forest conservation efforts, and stop volcanoes from erupting [
1,
2]. The effectiveness of the measures to be taken requires sensors to monitor the carbon dioxide levels in the atmosphere. This has resulted in the evolution of gravimetric sensors. These sensors fall into the category of micro-to-nano electromechanical systems that are based on chemical, electrical conductivity, optical, magnetic, acoustic, capacitive, or ultrasonic properties. The active material response to the exposed gas is revealed by the sensor’s physicochemical interactions.
1.1.2. Status of Methane
Methane is another important gas contributing to the greenhouse gas effect. Its increase is greatly changing the climatic condition. It absorbs the sun’s heat and warms the atmosphere. It is 84 times more potent than carbon dioxide in this activity. It is produced by the natural decomposition of rice paddies, marshes, the guts of animals, the rotting of rubbish and the distribution of fossil fuels like coal, oil, or gas. The use of alternatives to natural gas such as hydrogen in a variety of living conditions [
4] could certainly reduce the contribution of this gas towards the greenhouse gas effect. Methane gas in the atmosphere has not been a serious concern, as it was increasing by 0.5 parts per billion per year until the year 2000. The current statistics show that it increased by 12.5 parts per billion in 2014. The methane mole fraction (ppb) is expected to reach about 1850 by 2018, and was approximately 1780 in the year 2000 (
https://www.washingtonpost.com/news/energy-environment/wp/2016/12/11/atmospheric-levels-of-methane-a-powerful-greenhouse-gas-are-spiking-scientists-report/?utm_term=.4a77e6472f2c). A recently published review [
5] demonstrated that a sensor developed for carbon dioxide should consider its response to methane in the atmosphere.
Figure 3 shows the simulated effect of methane interference in the measurement of carbon dioxide gas. The error in measurement of carbon dioxide concentration is indicated in the Figure for different concentration ratios of carbon dioxide to methane.
The effect of methane and carbon dioxide gases on the environment was recently analyzed by Charnay et al. [
6] using a 3D climate carbon model, and their results showed a change in the global albedo from 0.40 to 0.23 depending on the relative proportions of carbon dioxide and methane levels. The global temperature was estimated by using the albedo value to increase from −11.5 °C to 65 °C. This model predicts that a carbon dioxide level of 1 bar could produce hot climates at a low land fraction and cloud feedback. For the Earth to reach the high temperature requires carbon dioxide in the atmosphere to be 1 bar at 3.8 Ga.
1.1.4. Status of Fluorocarbons
The fluorocarbons are innumerable, and they can be divided into two classes: one class acting on the ozone layer that reduces the ultraviolet rays reaching the Earth, and another class having environmental effects. The first-class compounds are methyl chloroform, halon-1211, CFC-12, HCFC-22, and HCFC-141b. The other class includes sulfur hexafluoride, HFC-23, HFC-134a, PFC-14, PFC-16, and nitrogen trifluoride. The ozone-depleting fluorocarbons, except for methyl chloroform, are either increasing or reaching a constant value. Their levels range from about 5 to 400 parts per thousand (ppt) in the atmosphere (
https://www.epa.gov/climate-indicators/climate-change-indicators-atmospheric-concentrations-greenhouse-gases). Since fluorocarbons are not at the concentration levels of CO
2 or CH
4, they will not be considered here.
1.2. Sensors for Greenhouse Gases
Since the importance of the influence of greenhouse gases on the environment was understood, several different sensors have been developed and are well-reviewed in the literature [
7,
8,
9,
10,
11,
12].
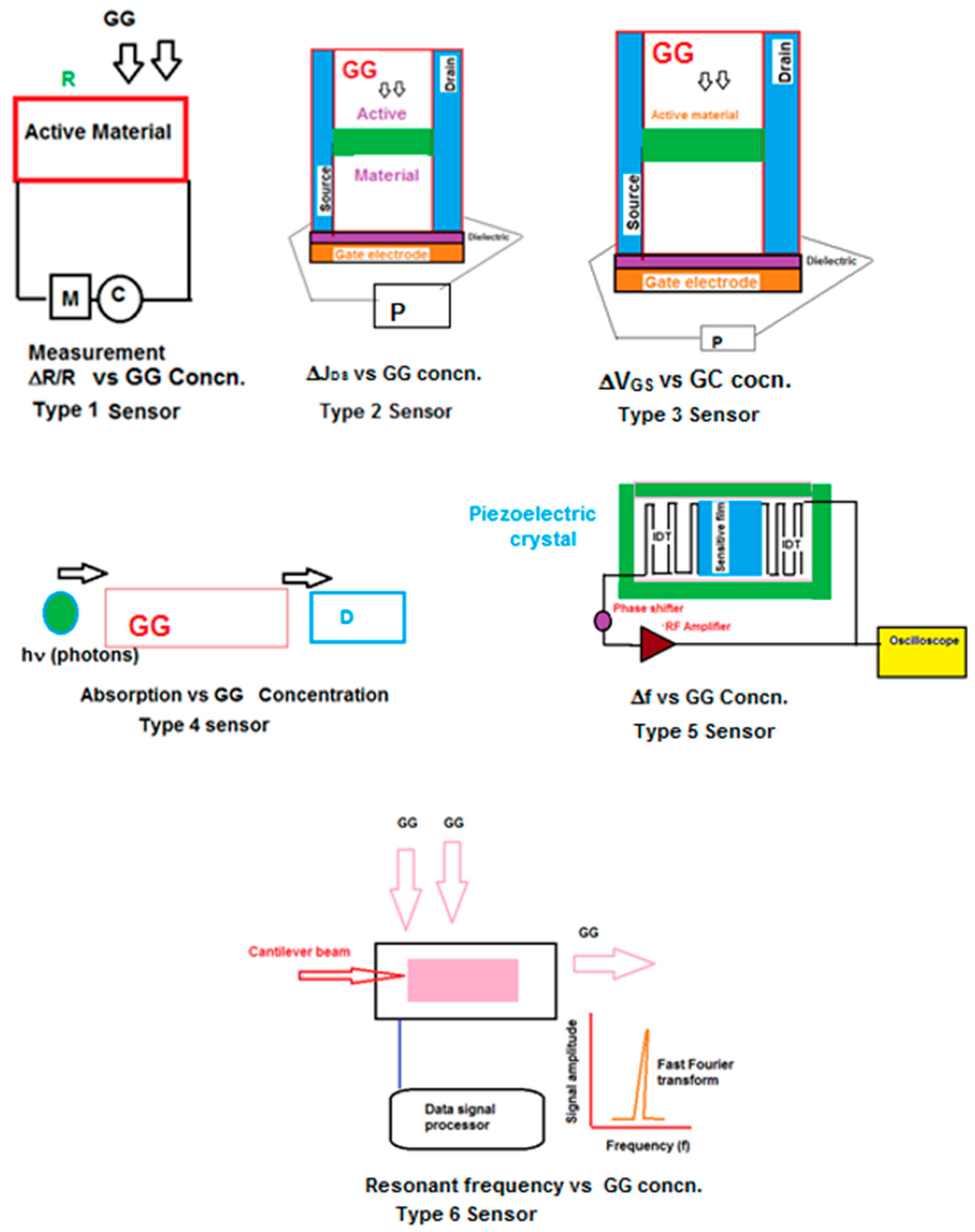
Figure 4 describes the six different types of sensors used in the measurement of greenhouse gases.
Type 1 sensors generally have a nonmetallic substrate on which the active material is deposited and kept in either ambient or selected experimental conditions (e.g., inert atmosphere) in the measuring chamber. The change in resistance of the active material is measured as a function of concentration of the greenhouse gas. The successive measurement requires flushing the measuring chamber. The type 2 sensor is a field effect transistor having active material placed between the source and drain. The source, drain, and gate electrodes are connected to a potentiostat. The drain current is measured as a function of greenhouse gas concentration. The type 3 sensor is a modification of the type 2 sensor, in that the voltage is plotted as a function of greenhouse gas concentration. The type 4 sensor depicts the general scheme for electromagnetic interaction with the greenhouse gas. The photons used in these measurements are generally in the energy range of 104–10−2 kcal. Type 5 sensors utilize a piezo electric crystal carrying interdigitized electrodes. The active material is placed between digitized electrodes. The radio frequency (Rf) shift is measured with different greenhouse gas concentrations. This type of sensor is also called a SAW (surface acoustic wave) sensor. The type 6 sensor is a micromechanical sensor using a cantilever beam which has a coating of an active material. The resonant frequency of the cantilever beam is measured as a function of the greenhouse gas concentration. This review contains examples of all six different sensor types.
Conventional instrumental analysis such as optical spectroscopy, Fourier transform infrared spectroscopy (FTIR), semiconducting devices, mass spectroscopy, and Raman spectroscopy were used to analyze the greenhouse gases. These techniques will continue to be used, as they provide the advantages of selectivity and sensitivity. With the discovery of new nanomaterials, several less-expensive methods such as using resistance measurement (Type 1 sensor), field effect transistor (Types 2 and 3 sensors), optoacoustic (Type 4 sensor), wireless measurements (Type 5 sensor; surface acoustic wave (SAW)) and micromechanical measurements (Type 6) have recently been developed. These new methods and new research oriented towards new materials have provided speed and accuracy in the measurement of greenhouse gases.
2. Carbon Dioxide Sensors
Carbon dioxide gas is safe for humans up to 5000 ppm, and is dangerous when it reaches concentrations of 40,000 ppm. A short-term exposure of 30,000 ppm is bearable (
http://www.cdc.gov/niosh/idlh/intridl4.html) [
13,
14]. The level of carbon dioxide in the atmosphere is changing every year. The present level of carbon dioxide in the atmosphere is over 400 ppm. The presence of it in the atmosphere affects the albedo value of the Earth in reflecting the solar radiation and is currently estimated at 0.39. While the planet Mercury has an albedo value of 0.1 (receives the maximum amount of sunlight), the planet Venus has a value 0.84 [
4]. The temperature of the Venus is estimated at 462 °C, with carbon dioxide levels in the atmosphere amounting to 96.5%. Based on these facts, the need for limiting carbon dioxide in the Earth’s atmosphere is of the utmost importance. Hence, a good and reliable sensor for carbon dioxide is required to monitor the atmospheric carbon dioxide. A variety of sensors have been developed based on optical absorption, change in semiconducting property, electrical resistance, amperometry, and field effect transistors. The semiconducting materials used in these sensors operate at high temperatures (>200 °C). The optical detectors use either fiber optics methods with sophisticated instrumentation or conventional infrared detectors. The electrical methods have been successfully used for the detection of carbon dioxide in biomedical applications.
The main thrust in this decade has been to find new materials that enable easy detection and determination. Gravimetric sensors have been of great interest in this category. The infrared sensors are developed using a combination of a wavelength filter and a detector. This technology can reach a detection limit of 10 ppm of CO
2, with an upper limit of 10,000 ppm [
15,
16,
17,
18]. Mayrwögera et al. [
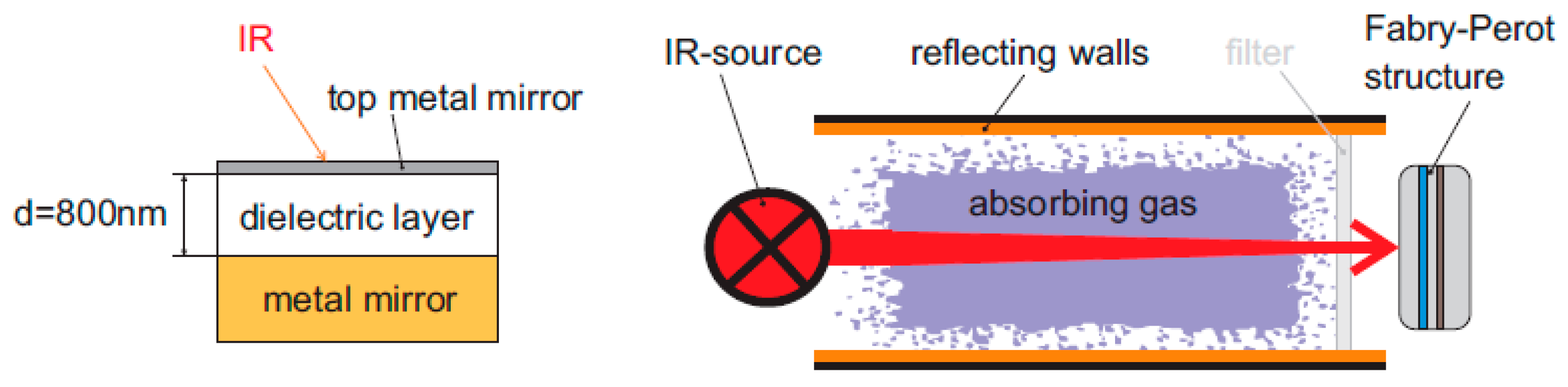
15] proposed a Fabry–Perot-based bolometer using a glass plate as a simple infrared filter for carbon dioxide determination (Type 4 sensor).
Figure 5 shows the analyte that is mixed with nitrogen for analysis.
The interference coming from water vapor in the measurement of carbon dioxide concentration was removed by using a glass filter, as shown in
Figure 6.
Several conducting polymer-based resistive sensors have been developed for the detection of carbon dioxide. Doping polyaniline (PANI) has been shown to play a role in the detection of CO
2 [
18,
19,
20]. The working range of the CO
2 sensor has been reported as 102–104 ppm.
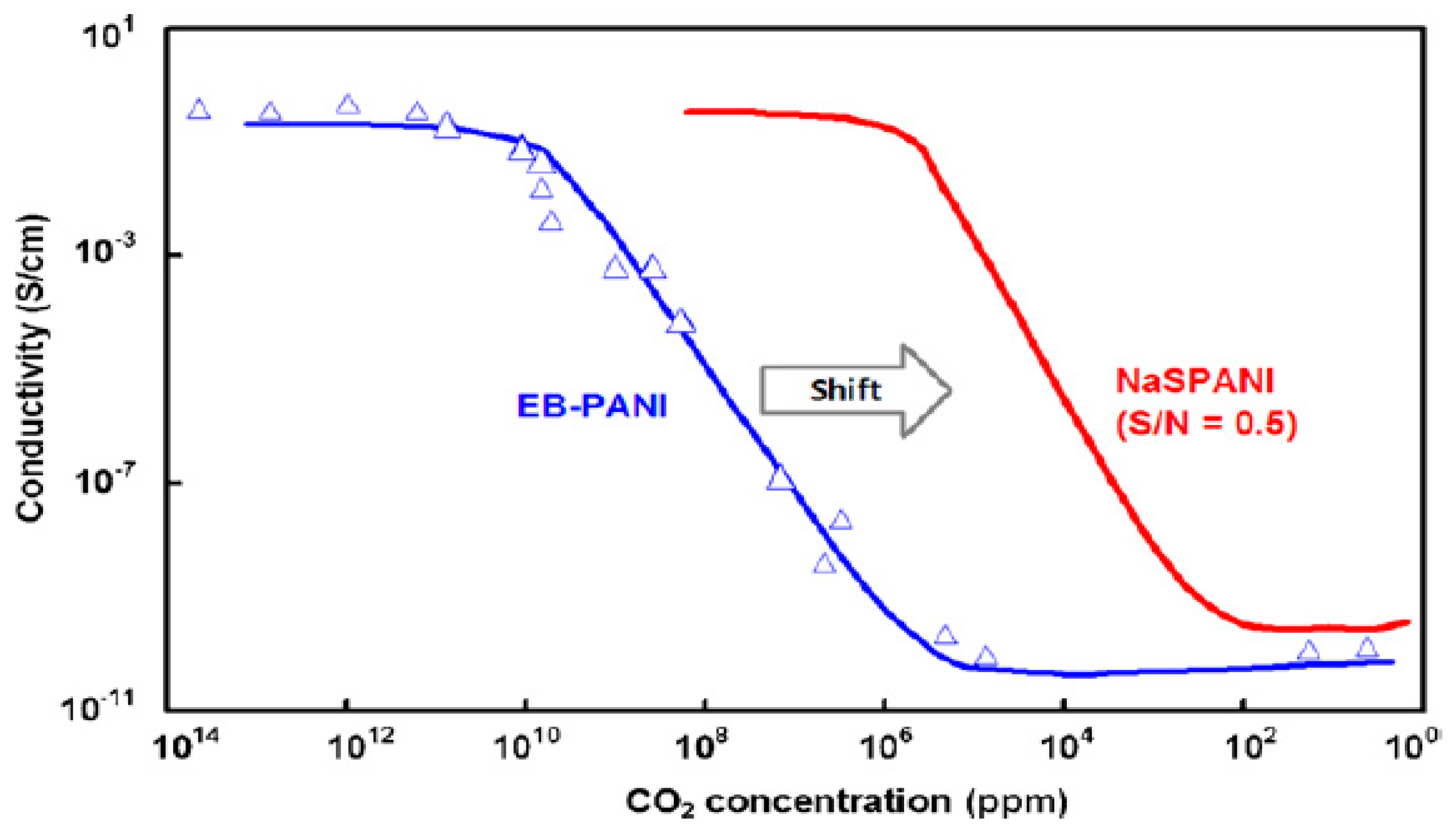
Figure 7 shows the conductivity change with the concentration of CO
2. The conductivity decreases when carbon dioxide is adsorbed on the sensor material (PANI; Type 1 sensor). The sensor performance has been shown to depend on whether it has an emeraldine base or sulfonated polymer as its active material. Both of them respond to carbon dioxide, but the emeraldine base’s response to lower ppm levels has been reported to be negligible.
The carbon dioxide concentrations in the atmosphere and the corresponding pH are controlled by the humidity in the atmosphere. The dissolved carbon dioxide is in equilibrium with other species [
19,
20], and
Table 1 shows the calculated concentrations of the equilibrating species.
The true concentration of CO
2 in the atmosphere can be evaluated by taking the concentrations in
Table 1 into consideration, as there is equilibrium between CO
2 and the protonated species as
An infrared fiber optic optical spark plug sensor was used for measuring CO
2 and water. As both the molecules are infrared-active with strong overlap, the spark plug was kept close to the electrodes for the in-cylinder measurement of CO
2 and gaseous water (Type 4 sensor). A tungsten halide lamp with two infrared detectors having different optical band pass filter was used. The test was carried out using a spark-ignited engine [
21]. Air quality monitoring sensors using a cluster of metal oxide (MO
X;MiCS-5525) [
22] or electrochemical sensors were used for the analysis of a mixture of nitrogen monoxide and carbon dioxide. The measurements were used to assess the practicality of the carbon dioxide sensor for a data quality objective with 25% uncertainty [
23].
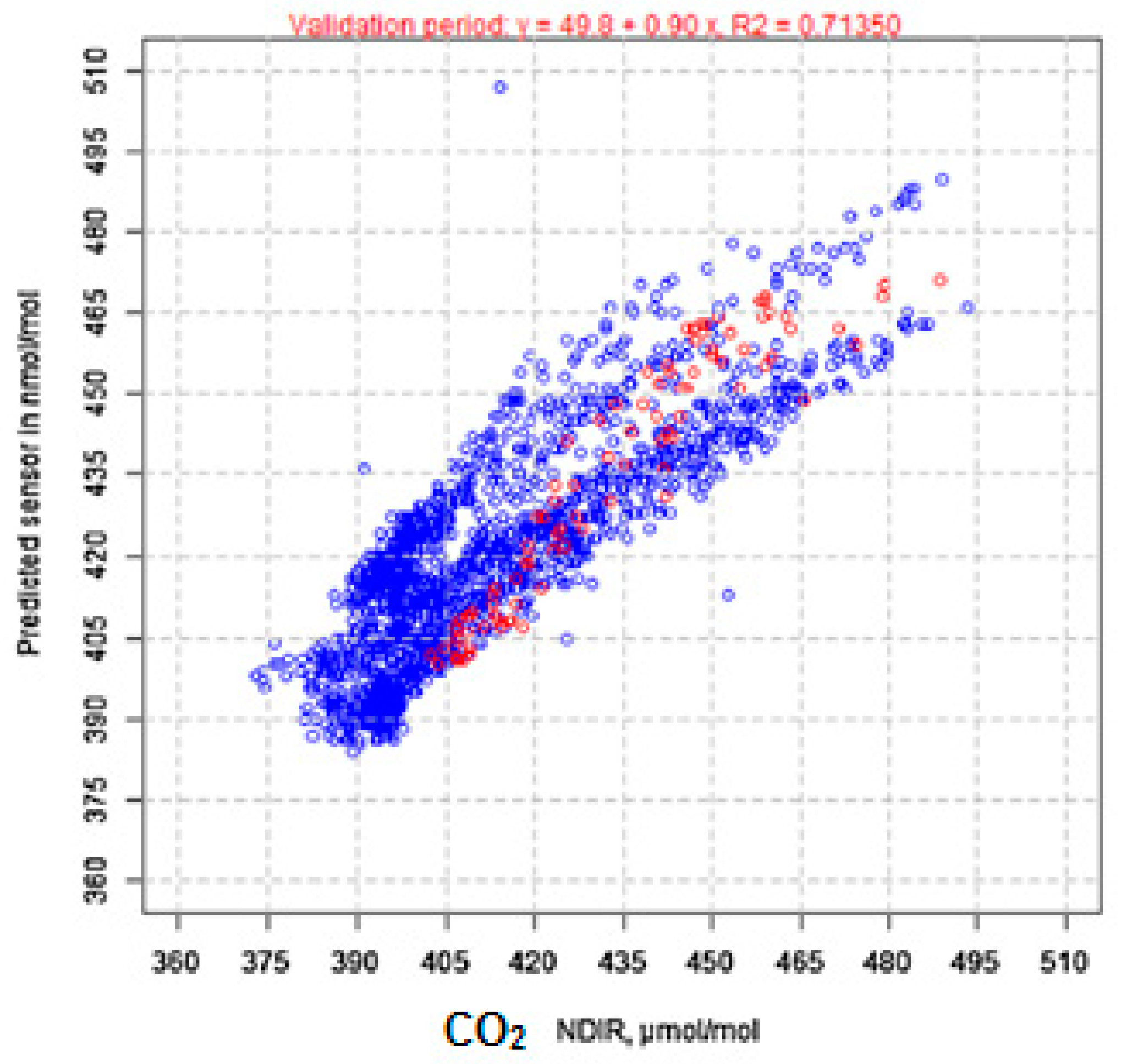
Table 2 shows the sensors used for data acquisition.
Figure 8 shows the carbon dioxide levels obtained from the sensor with linear regression analysis.
It was shown that the predicted values were lower compared to calibration. When an electrochemical sensor was used for the measurement of CO
2, the interference of ozone was encountered and was removed by linear/multi linear regression [
23,
24].
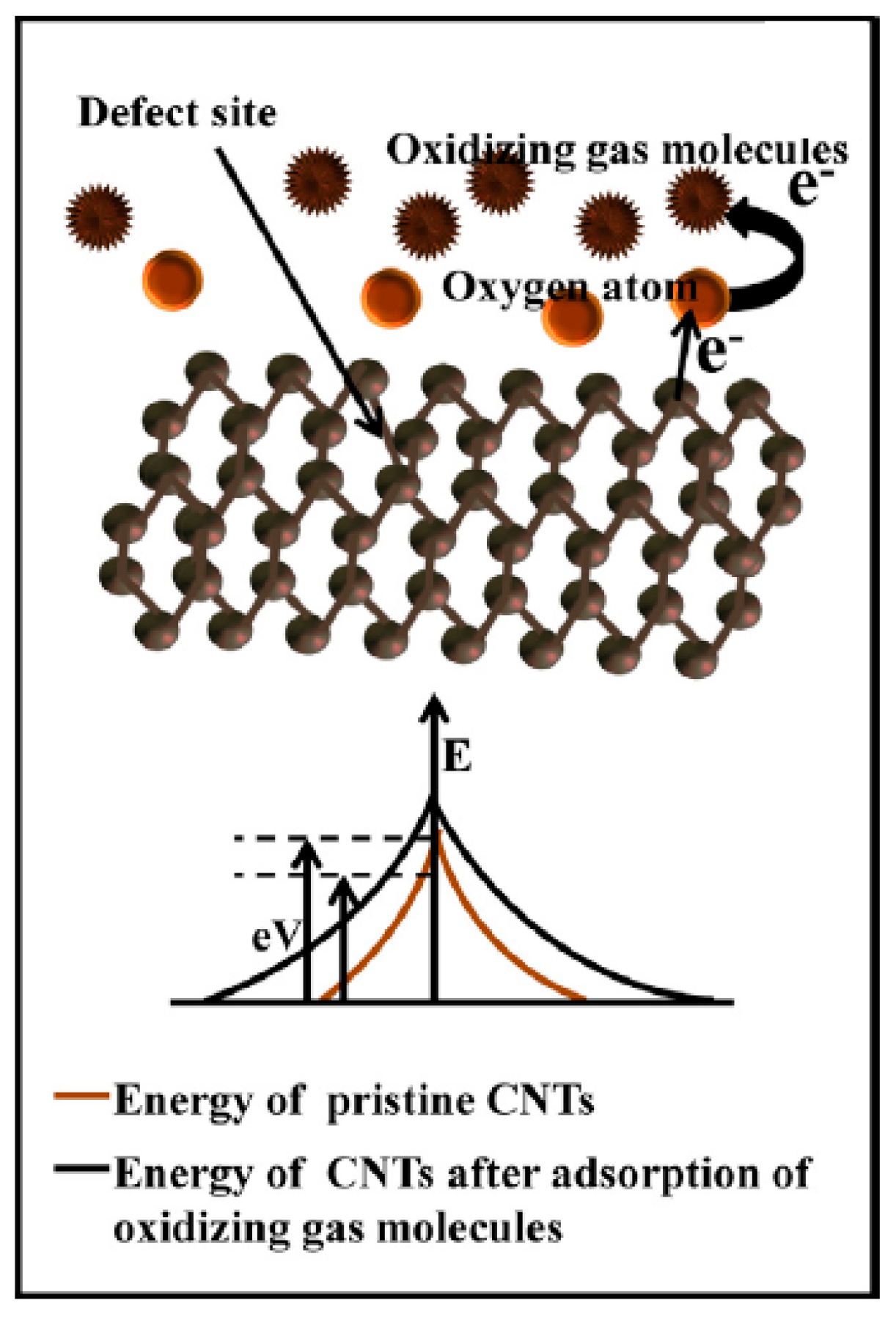
Carbon nanotube (CNT)-based sensors have been developed for carbon dioxide gas utilizing the principle of physicochemical adsorption of the gas by the carbon nanotubes. Two types of sensors have been developed based on this principle. One type is based on resistance change upon adsorption of the gas on the active material, and the other is dependent on the adsorption of the gas on the active material having effects on transistor properties such as the voltage or current in the field effect transistor [
13] (Types 2 and 3 sensors). The adsorption of carbon dioxide gas produces an increase in resistance that is attributed to an increase in the energy barrier for electron movement, as shown in
Figure 9.
A number of reports directed towards measuring CO
2 using a chemoresistive method with CNTs have been reported in the literature [
13,
15]. Trans et al. [
25] have fabricated a field effect transistor using carbon nanotubes (NTFET) whose sensitivity was examined by Star et al. [
26,
27,
28]. A prototype sensor chip was packaged for measuring the carbon dioxide level using a computer.
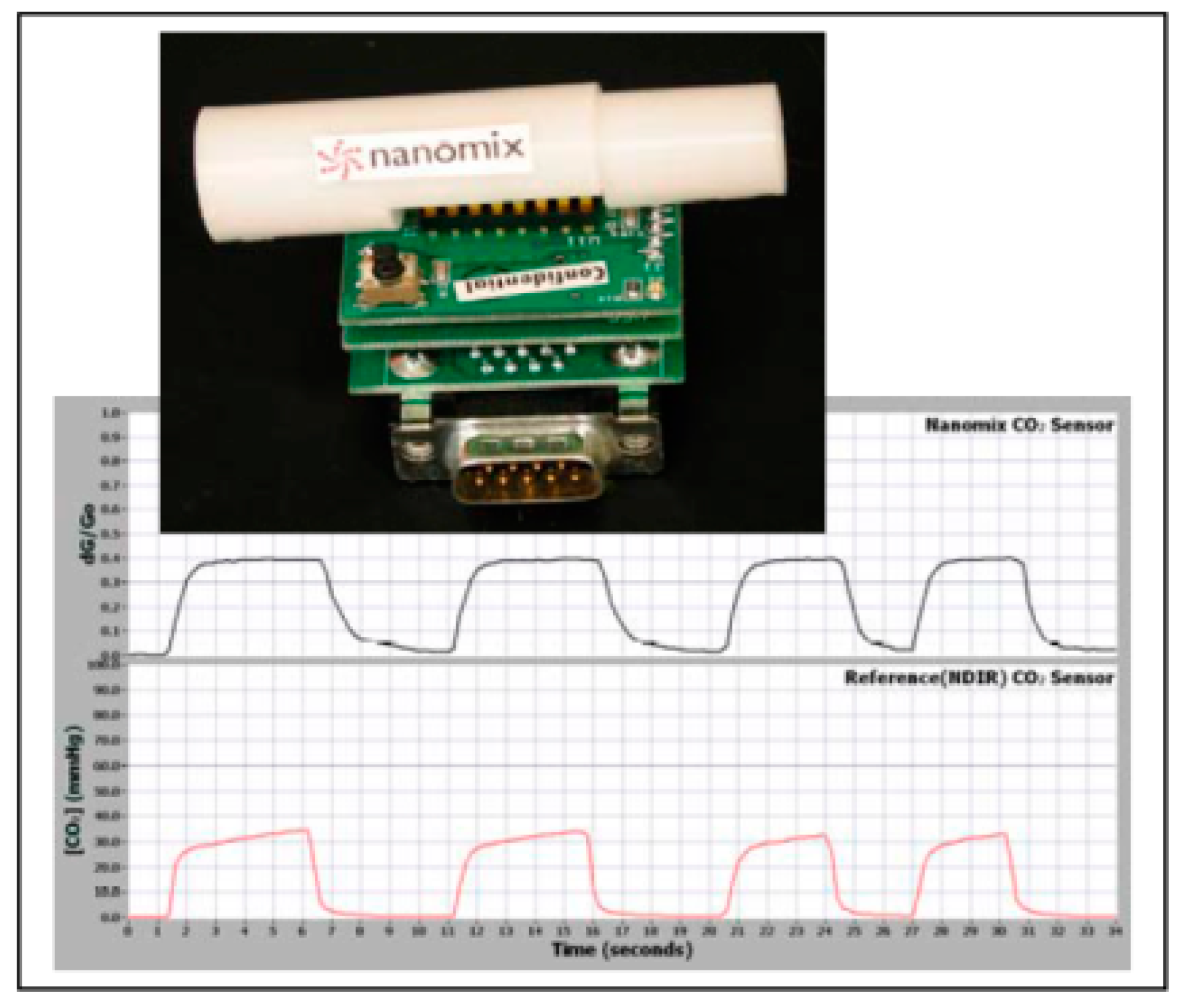
Figure 10 shows the response of the sensor for carbon dioxide in breath analysis. The reproducibility in the response to successive injections of the gas was established by these measurements. The NTFET board containing the field effect transistor [
29,
30] is shown in
Figure 11. The reproducibility of the pulses shows the feasibility of its usage for successive measurements.
The sensor monitors the %CO
2 that can be detected by the NTFET, which can be used conveniently in several locations, such as in hospitals and on-site by paramedics. Being a noninvasive and disposable device, it can be used in the monitoring of greenhouse gas for about 6 h. The performance of the NTFET in measuring the concentration of CO
2 in the presence of moisture has also been carried out. It had a tolerance up to 80% RH (relative humidity). Sensors made with CNTs for gases generally have the following advantages: (a) room-temperature operation; (b) facile property adjustment; (c) high sensitivity and response time; (d) easy device fabrication; (e) low selectivity; and (f) long time instability [
10,
31,
32,
33,
34,
35,
36,
37,
38,
39].
The low selectivity and long time instability have been an active area of investigation in the past decade, and several new composites of carbon nanotubes have been recommended for gas detection. Chen et al. [
19] have reported that cleaning the carbon nanotubes with ultraviolet light resulted in a dramatically enhanced performance. The mechanism by which ultra violet light enhances the performance has not been explored in this work. Presumably it makes the surface active by the oxidation of amorphous carbon or defect centers in the nanotubes. A quantum mechanical investigation of carbon dioxide adsorption on S-functionalized boron nitride and aluminum nitride (AlN) nanotubes has shown it to be exothermic, opening the prospects of developing a thermal sensor for carbon dioxide [
40]. The adsorption of carbon dioxide on AlN nanocages and nanosheets has also been investigated using density functional calculations [
41,
42], with emphasis on chirality. Amine-functionalized TiO
2 has been used to capture the carbon dioxide from flue gases, and is amenable for the development of a gas sensor. The presence of moisture has been demonstrated to have a significant effect on adsorption/desorption processes [
43].
A nano thin film functionalized chemiresistor sensor operating at room temperature (Type 1 sensor) has been reported for the detection of carbon dioxide in the range of 50−500 ppm [
44]. The sensor had negligible interference from ammonia, carbon monoxide, methane, and nitrogen dioxide. The sensing of carbon dioxide using a wireless network [
13,
45,
46,
47] (Type 5 sensor) uses analyte-induced changes such as in mass, elasticity, or complex permittivity [
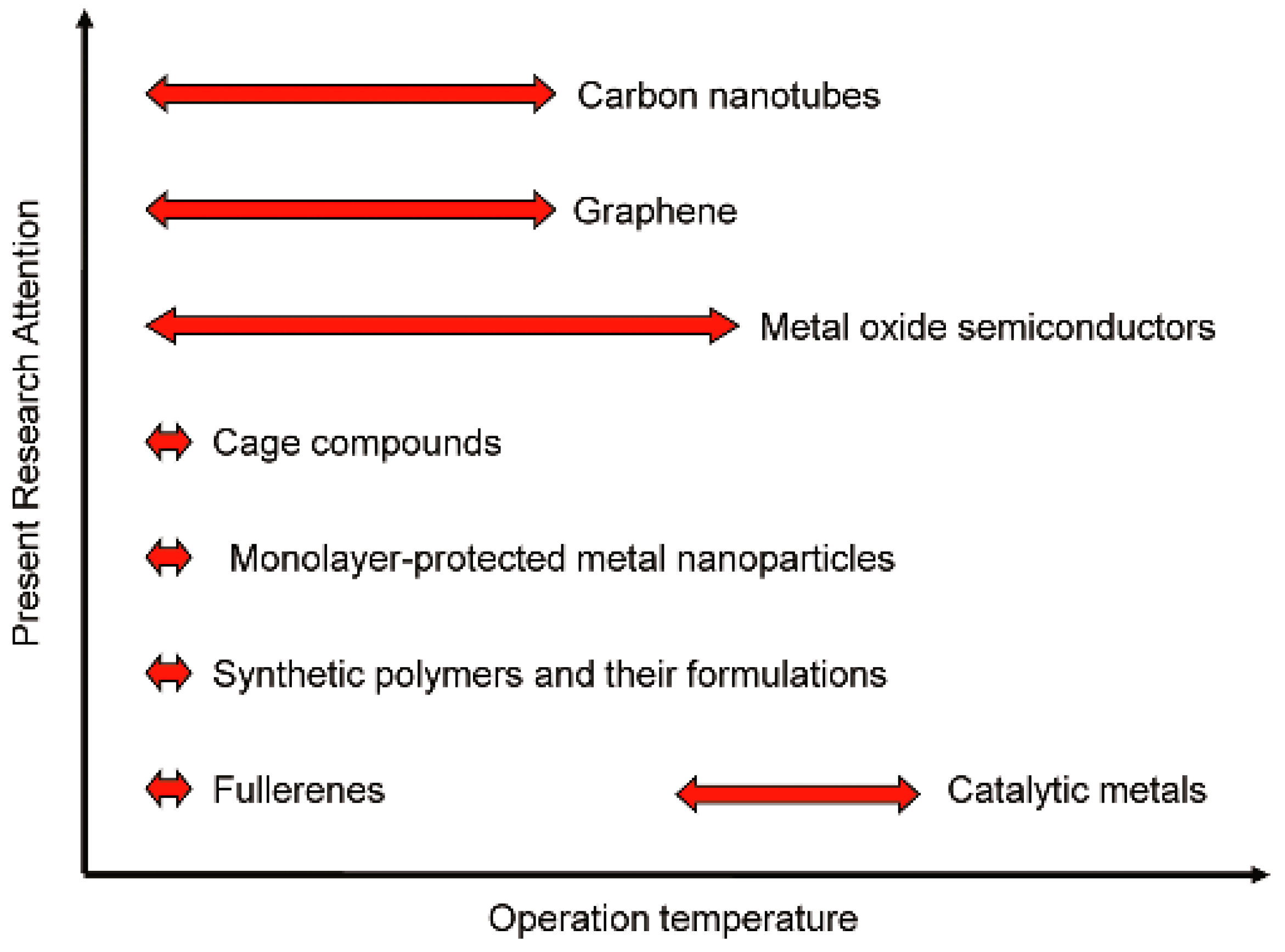
48]. The diverse temperature requirements in this class of carbon network are shown in
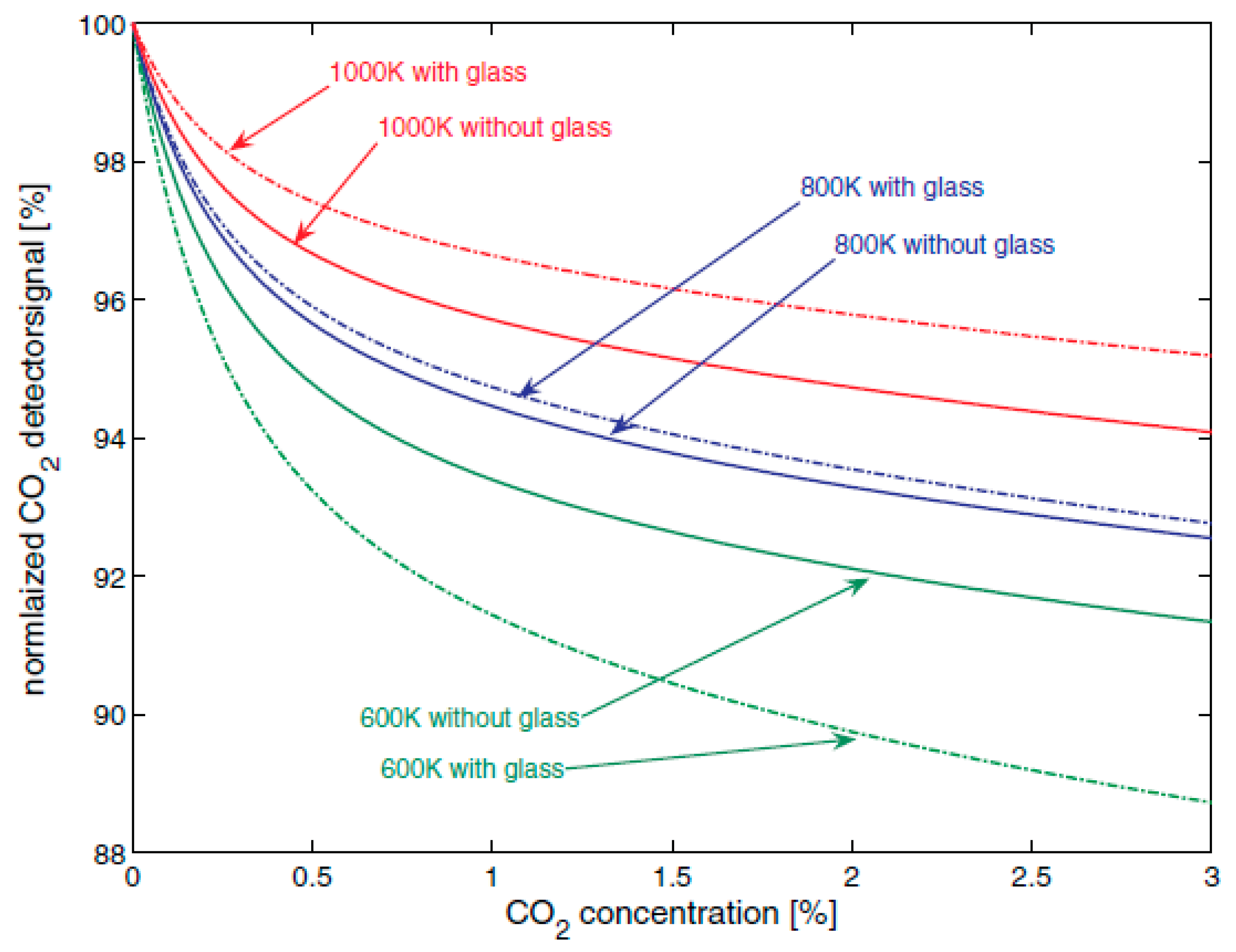
Figure 12.
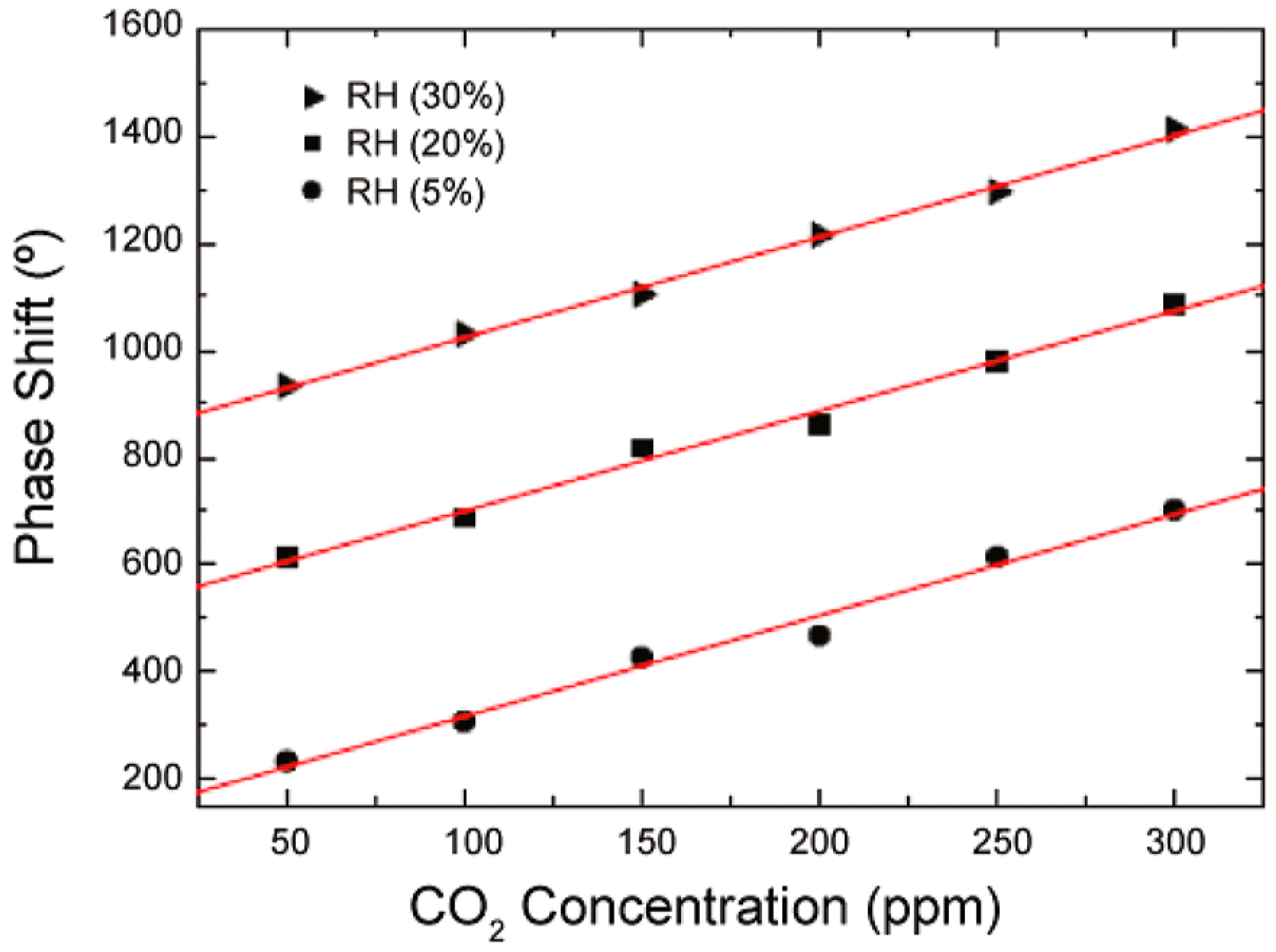
A SAW (surface acoustic wave) sensor using random copolymer Teflon AF2400 (
Figure 13) prepared from tetrafluoroethylene and 2,2-bis(trifluoromethyl)-4,5-difluoro-1,3-dioxole showed variations in carbon dioxide detection depending on the amount of water present. The phase shift changes with carbon dioxide level are shown in
Figure 14, showing a phase shift of about 1.14°/ppm of CO
2.
The permittivity and conductivity of multiwalled carbon nanotubes has been used for the detection of carbon dioxide [
48]. The incorporation of a SiO
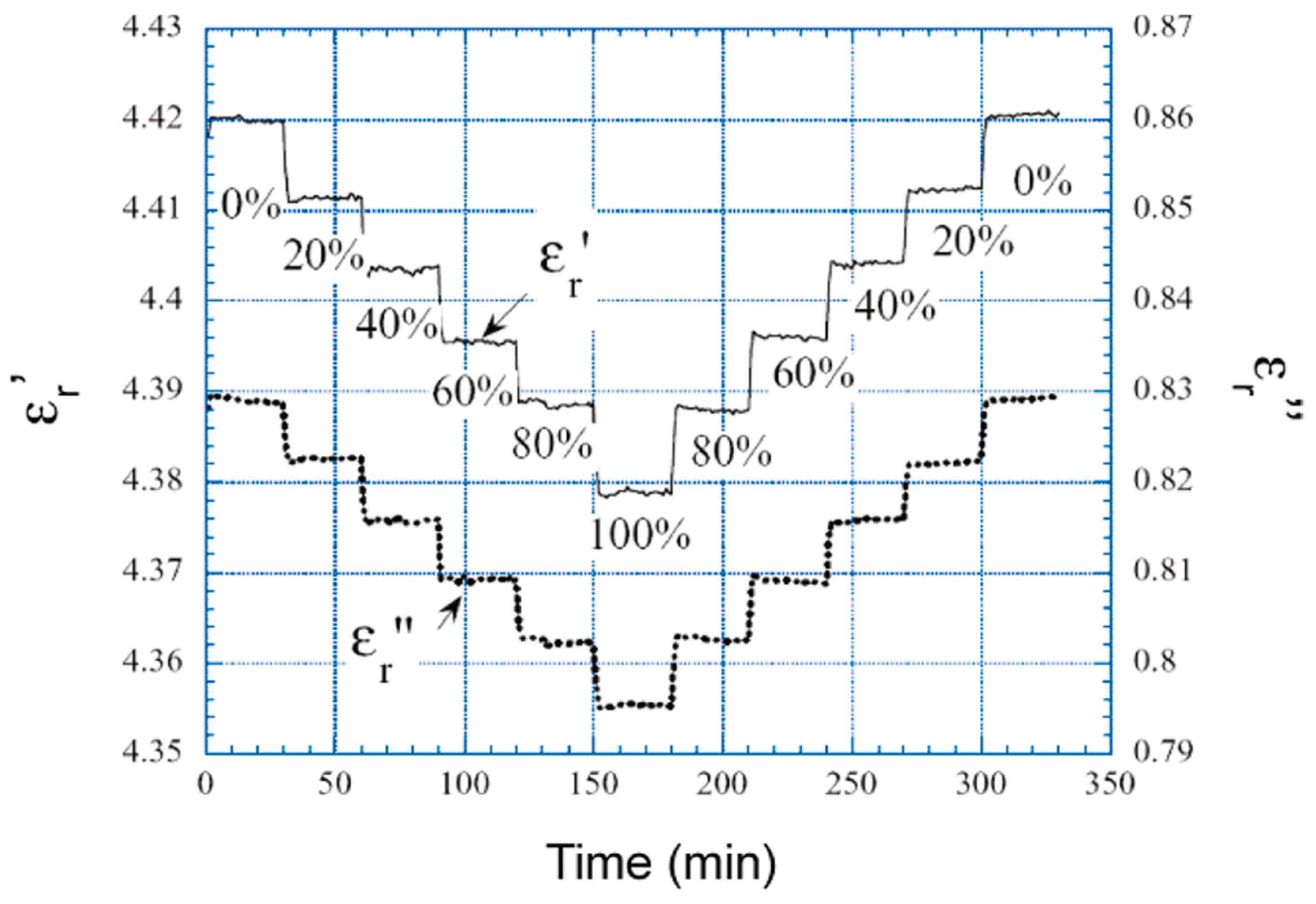
2 matrix along with the incorporation of a wireless inductor-capacitor resonator in the sensor showed a decrease in the effective permittivity (see
Figure 15). The analyte induced changes in complex permittivity (εr’ − j εr’’), where εr’ and εr’’ are the real and imaginary parts of the complex permittivity are measured in this approach. The imaginary permittivity of the sensing material is directly proportional to its conductivity. A hysteresis-free operation of the sensor is remarkable and advantageous for fast measurements.
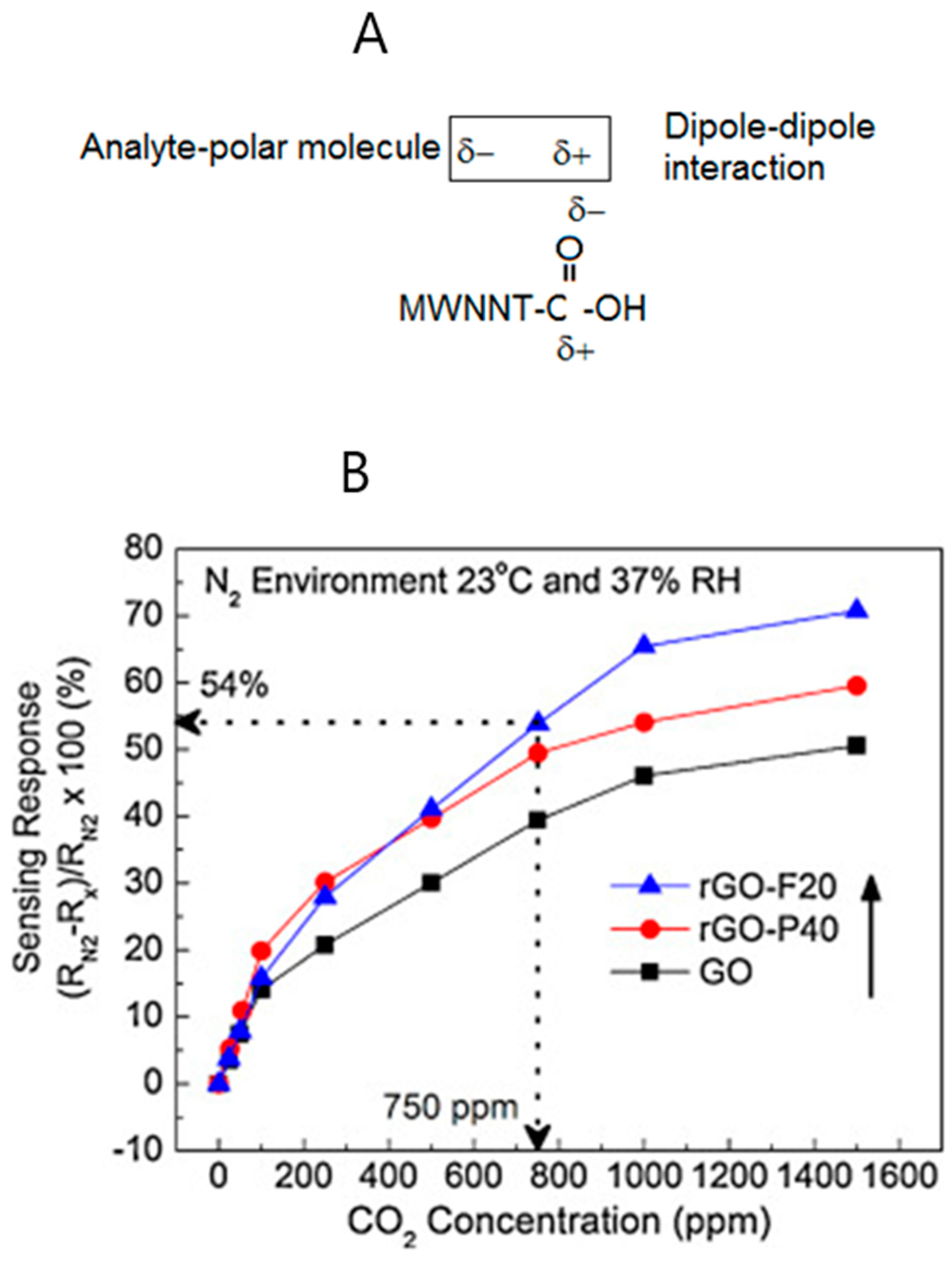
The sensor operates through a dipole–dipole interaction mechanism as shown below (
Figure 16A). The functionalized multiwalled carbon nanotubes (MWCNTs) have a partial negative charge to which the analyte is bridged through its partial positive charge end, as shown in the above illustration [
10]. The optical sensing of carbon dioxide has been reported using different types of membranes [
38]. As an offshoot of carbon nanotubes, the discovery of graphene by Novoselov and Geim in 2004 has opened up new sensors based on carbon [
39]. In one approach, 3 μL of a 1 mg/mL graphene oxide solution was spin-coated on silicon fingers of 3 μm width. The graphene resistive sensor’s response in the concentration range of 0–1500 ppm was measured [
49,
50,
51,
52].
Figure 16B provides the resistance response with carbon dioxide concentration at ppm levels.
The initial resistance measurement of the sensor was carried out in nitrogen atmosphere (RN2). The change in resistance [
46] of the sensor when carbon dioxide gas was injected (Rx) was used in the construction of the graph in
Figure 16. Recently, a miniature resistive carbon dioxide sensor has been reported that operates in the concentration range of 50–50,000 ppm. A photoacoustic spectroscopic method has been developed for the simultaneous determination of carbon dioxide and methane gases [
52] with high precision and a large dynamic range.
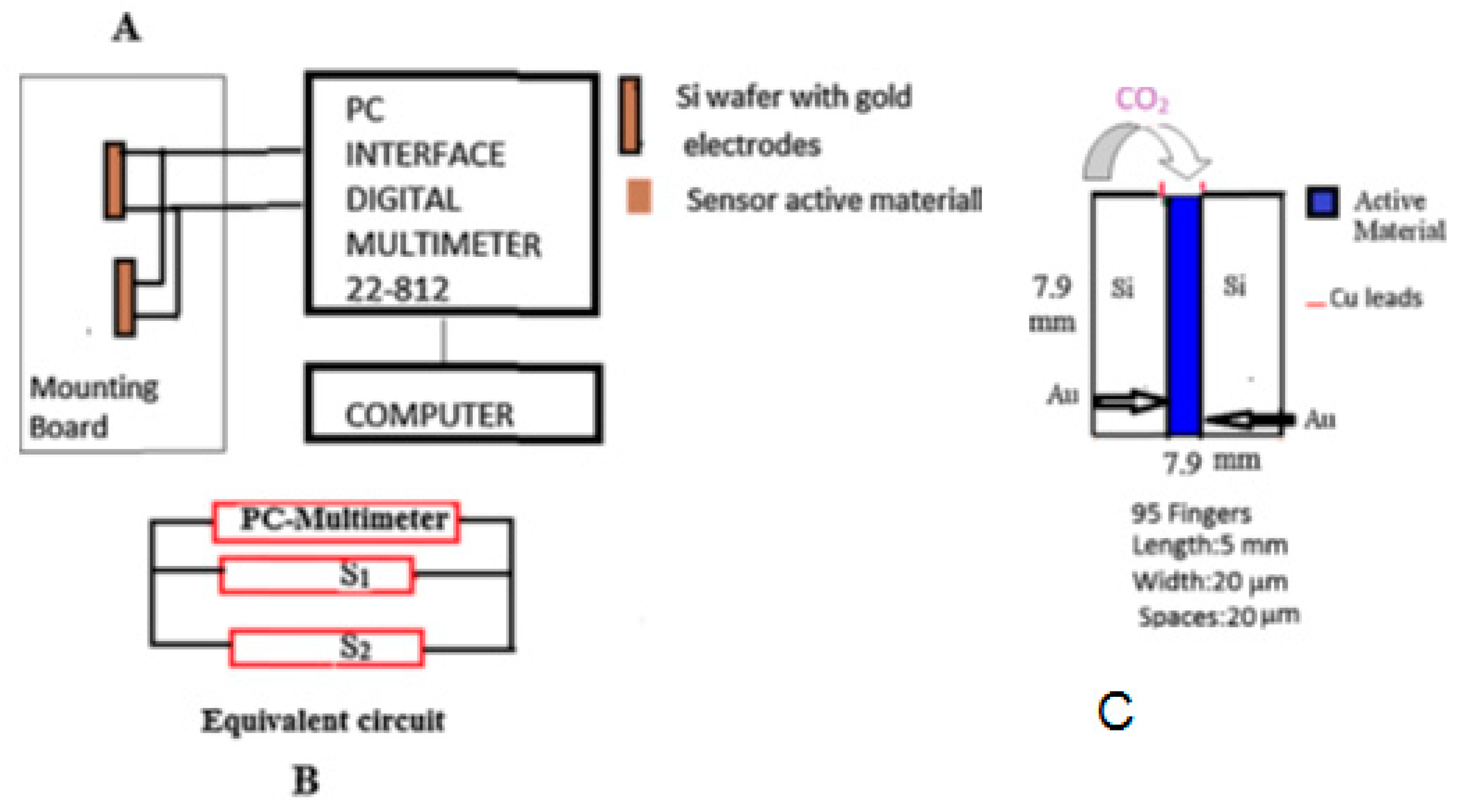
A new silicon substrate micro sensor has been developed [
5] using a composite made of carbon nanotube and Baytron-P that senses carbon dioxide at 22 °C. The sensor was constructed with a Si chip by depositing the composite between two gold electrodes. Two identical Si chips were connected in a parallel configuration (
Figure 17) to reduce the initial resistance of the sensor. The resistance of the sensor decreased proportionally upon exposure to the concentration of carbon dioxide.
The sensor showed a semiconducting behavior with a negative temperature coefficient (Type 1 sensor). The response time of the sensor was about 40 s. Fourier transform infrared spectroscopy showed peaks for the nanocomposite at 1056 cm−1, 1195 cm−1, 1296 cm−1, 1635 cm−1, 2083 cm−1, 2345 cm−1, and 3278 cm−1. The carbon dioxide adsorption on the composite resulted in the polystyrene sulfonate absorption band shifting from 1195 cm−1 to 1176 cm−1, suggesting a phase separation occurring in the nanocomposite that resulted in the increased conductivity.
Metal oxide semiconductor (MOS) films, nanowires, nanocage, powder, and microspheres have been investigated [
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69] for carbon dioxide sensing. The sensors developed using this approach operate at a temperature range of 200–700 °C and can detect concentrations in the range of 100–10,000 ppm with a response time falling in the range of 3 s to 9.5 h. The recovery times are also in the range 4–700 s. The performance of an MOS sensor depends on its morphology and composition. The results obtained here have shown that the grain size significantly influences the performance sensitivity (Types 2 and 3 sensors).
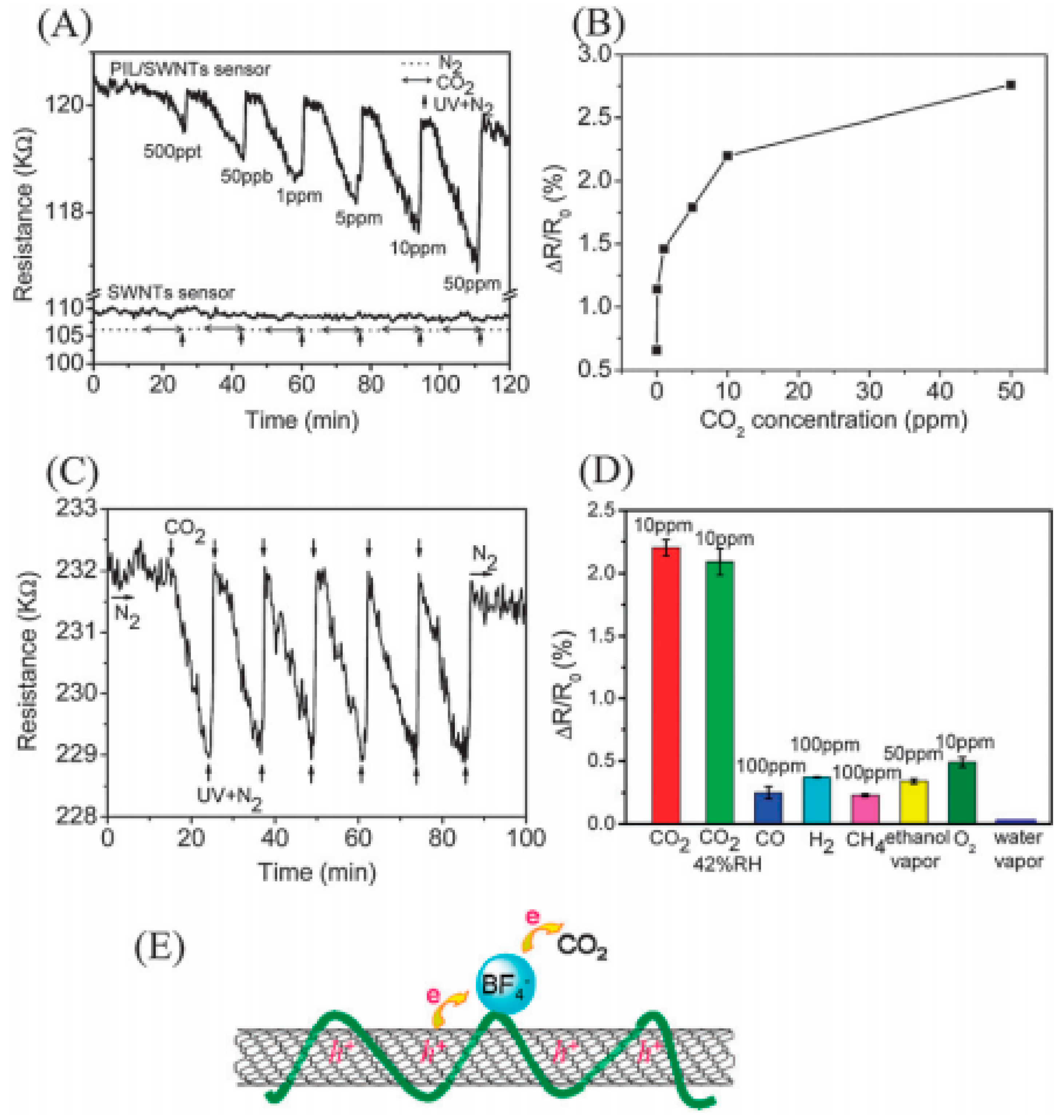
Poly (ionic liquid)-wrapped single-walled carbon nanotubes have been found to be sensitive for carbon dioxide detection at low concentrations of CO
2 (500 ppt) [
67,
68,
69,
70,
71,
72]. The chemiresistive dynamic response of the carbon nanotubes is shown in
Figure 18 (Type 1 sensor), where the mechanism for sensing is based on the interaction between BF
4 anion and CO
2. The charge transfer interaction between BF
4- and CO
2 is depicted in
Figure 18E.
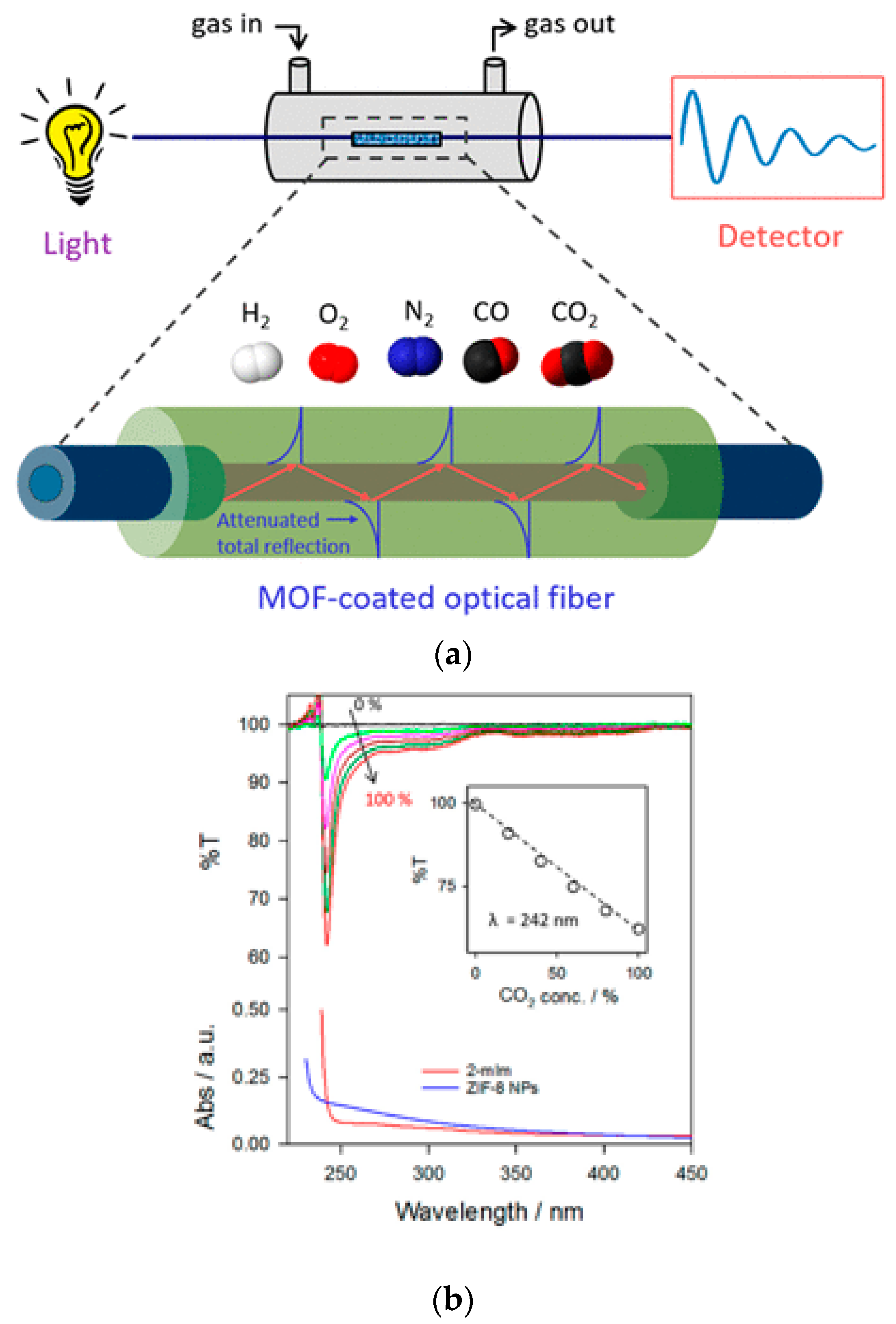
An impressive type 4 sensor for CO
2 has been reported using optical fiber coated with a metal oxide-zeolitic imidazole framework (ZIF)-8 MOF [
69].
A high sensitivity for CO
2 with negligible response for gases such as H
2, N
2, O
2, and CO has been reported. The percentage transmission of 242 nm radiation has been monitored as a function of CO
2 concentration, as shown in
Figure 19b. The linear change in the transmission of radiation with concentration with very little interference from other gases provides reliability for usage in the field.
Table 3 gives a list of sensor materials and their responses.
Solid-State Electrochemical Sensor
There have been attempts to develop a solid-sate electrochemical sensor for carbon dioxide [
71]. Mason et al. [
71] developed solid-state reference measuring electrodes and a beta alumina electrolyte substrate with a provision for heating, and made measurements at a temperature of 530 °C. At this temperature, the carbon dioxide sensing was found to be selective from other gases. A novel electrochemical sensor using a ceramic-type component of Na-β/β″ alumina and a reference electrode of glass sealed elemental Na has been developed for measuring CO
2 gas in the temperature range of 300 to 600 °C [
72] with a response time of seconds to minutes.
3. NOx Gas Sensors
Faraday rotation spectroscopy has been proposed for NO
2 determination (Type 4 sensor) [
67,
68,
69,
70,
71,
72,
73]. It uses a widely tunable external cavity quantum cascade laser (EC-QCL) and operates mode-hop free between 1600 cm
−1 to 1650 cm
−1 and allows Q-branch transition of NO
2 at 1613.2 cm
−1. A detection limit of 95 ppt has been reported. Meyyappan et al. [
73] used a simple casting of single-walled carbon nanotubes on an interdigitated electrode for the detection of NO
2 ranging from sub-ppm to 44 ppb. The response time of the sensor was on the order of seconds.
Among the sensors reported in the literature, there are a few sensors reaching the lowest detection limits of 0.01–0.5 ppb (
Table 4). The ZnO [
74,
75,
76], In
2O
3 [
77], and WO
3 [
78,
79] sensors showing variable response times fall into this category of low detection limits. A sensor developed with reduced graphene oxide–Cu
2O nanowire interestingly reached the lowest detection concentration of 0.064 ppm [
79,
80,
81,
82,
83,
84].
Nitrous oxide (N
2O) is released into the atmosphere from chemical plants producing nitric acid and polymers (
https://www.eia.gov/environment/emissions/ghg_report/ghg_nitrous.cfm). N
2O is a colorless toxic pollutant gas with a slightly sweet odor. It is widely used as an anesthetic and analgesic agent in the clinical field, and also as a propellant for pressurized containers in the food industry. It is neither flammable nor explosive. One molecule of N
2O has the same greenhouse warming power as 300 molecules of carbon dioxide. Two-thirds of anthropogenic N
2O emissions arise from agricultural soils [
86], where N
2O is formed as part of the bacterial denitrification pathway, in which soil and marine bacteria use oxidized nitrogen compounds as terminal electron acceptors for anaerobic respiration [
87]. Once that N
2O molecule reaches the upper atmosphere, it can stay there for more than 100 years before getting destroyed naturally. Even though nitrous oxide is a moderately undisruptive substance unlike pollutants known as NOx, it has recently been reported to participate in the depletion of the ozone layer in the stratosphere (
https://www.eia.gov/environment/emissions/ghg_report/ghg_nitrous.cfm). So, it is crucial to control and convert N
2O to a harmless gas by catalytic surface reactions.
Yoosefian [
88] performed density functional studies on the adsorption behavior of nitrous oxide (N
2O) onto intrinsic carbon nanotube (CNT) and Pd-doped (5,5) single-walled carbon nanotube (Pd-CNT). Pd dopant facilitates the adsorption of N
2O on the otherwise inert nanotube, as observed from the adsorption energies and global reactivity descriptor values. The adsorption energy of N
2O on CNT was investigated in three orientations: vertical (VC) and horizontal (HC) to the nanotube axis, and the nitrogen atom toward the C–C bond (NC). The full optimized structures are indicated in
Figure 20.
The adsorption of N
2O changes the electronic conductivity of Pd-CNT, which is attractive for developing the sensor. Recently, heterojunction sensors have been constructed with SnO
2/SnS
2, operating at 80 °C (Type 1), sensitive to NO
2 concentrations in the range of 1–8 ppm [
89,
90,
91,
92,
93,
94,
95,
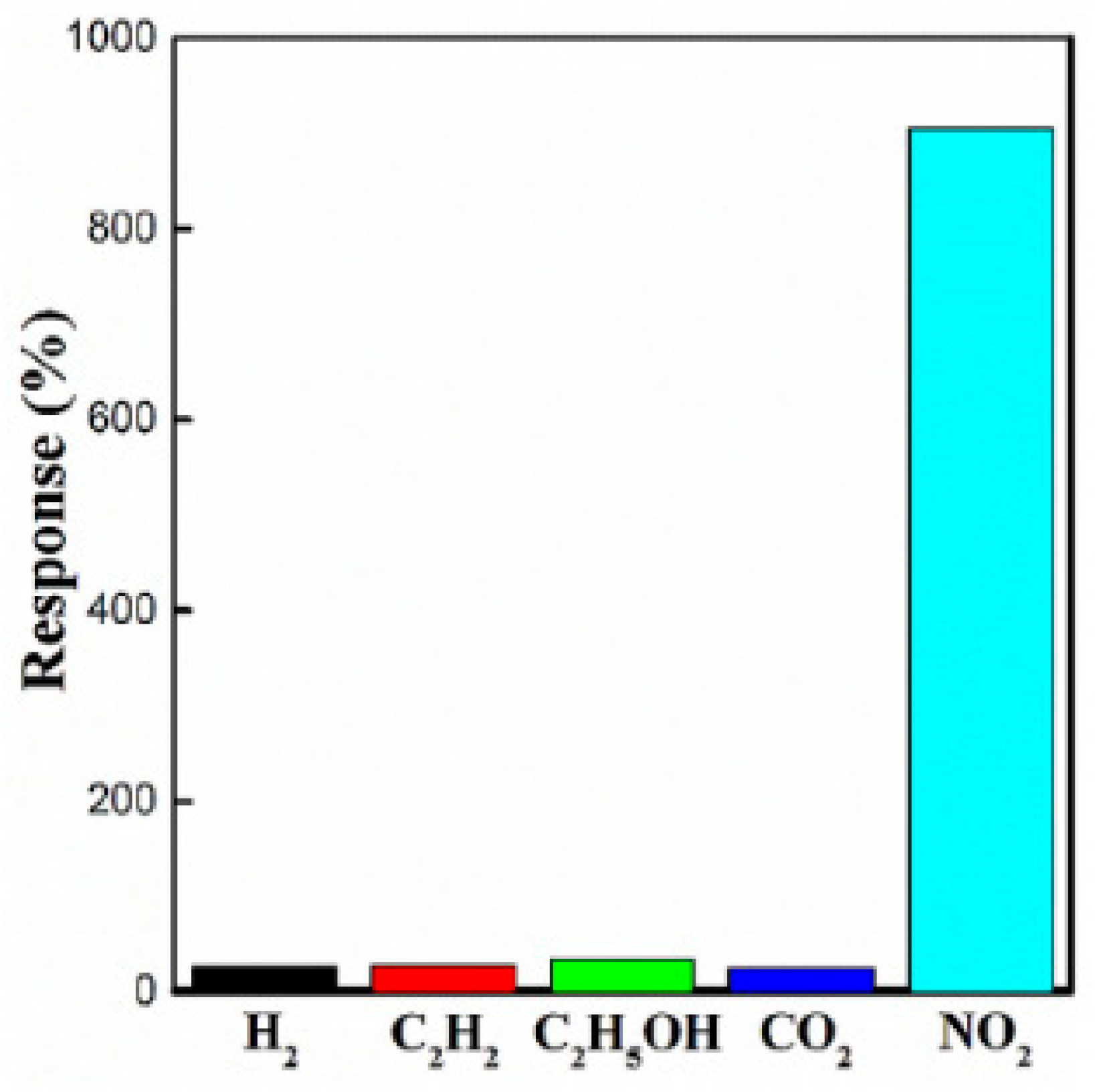
96]. The response of the sensor to other analytes is shown in
Figure 21.
Table 5 gives the relative performances of different sensors for NO
2.
In comparison to these sensors, several N
2O gas sensors have been developed using metal oxides.
Table 6 gives the performance of N
2O sensors (Type 1). These oxide sensors operate at high temperatures in the region of 300–600 °C.
5. Modern Technology Using 3D and Ink Jet Printing of Gas Sensors
With rapid developments in finding active materials suitable for greenhouse gas sensors, there is need for consideration of the cost of making these devices. In this context, the 3D-printing of the active material would be of interest. High-quality 3D-printed desk-top devices have been produced at a low cost without conventional micromechanical systems. Taylor and Velásquez-García [
111] have reported a novel electrospray printed nanostructured graphene oxide for gas detection. A number of gases were examined in this work with a variety of detection limits. For CO
2, the detection limit was set at 1000 ppm. The preliminary reports opened up opportunities to modify the spray printing for extension to lower detection limits. Rieu et al. [
112] developed an ink jet-printed SnO
2 gas sensor on a plastic substrate. Both the gas-sensitive layer and the heating transducer were ink jet-printed. This method of making the sensor compares well with sol-gel tin oxide film. The following detection limits have been reported for CO and NO
2 (
Table 7) The limit of detection fell far above the other methods discussed in the earlier sections. Furthermore, the operating temperature of the sensor was in the range of 200–300 °C (
Table 7). Sulfonated graphene was used for detecting NO
2 at room temperature by Liua et al. [
113]. Sulfonated graphene and SnO
2 particles are combined to form the active material of the sensor. The process involves the direct deposition of SnO
2 nanoparticles on reduced graphene oxide. A high performance and a good sensitivity were achieved by this active material. A detection limit of 1 ppm of NO
2 was reached with this sensor. The room-temperature detection using sulfonated graphene opens up opportunities for developing other greenhouse gas sensors. A screen-printed piezoelectric microcantilever (Type 6 sensor) has been used to detect and determine NO
2 and CO gases using oxygen plasma-treated multiwalled carbon nanotubes as the sensitive layer [
114].
Figure 22 shows the response of the cantilever for the two gases, with both positive and negative shifts in resonant frequency, attributed to stress and mass effects at different analyte concentrations.
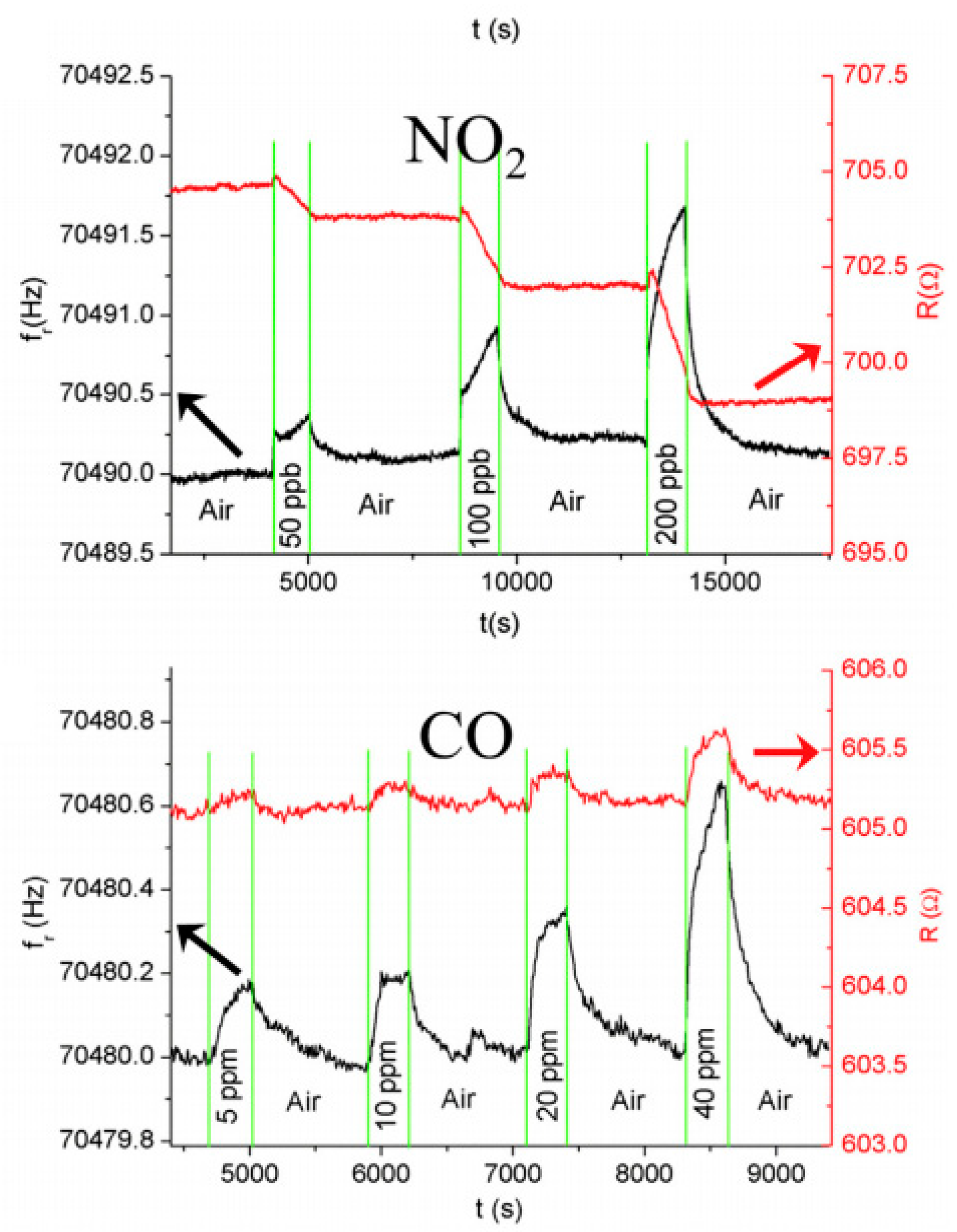
The sensor’s response to NO
2 was remarkable at ppb levels. A new generation of sensors for greenhouse gases utilizing 3D printing and ink jet printing is to be expected in the future, and are being researched in the literature [
114,
115,
116,
117,
118,
119]. Although some of the analytes described in this section do not fall into the class of greenhouse gases, they provide the pathway for the development of new greenhouse gas sensors through ink jet printing. With this technology, the sensors will have high reproducibility, sensitivity, and low cost. A 3D-printed CO
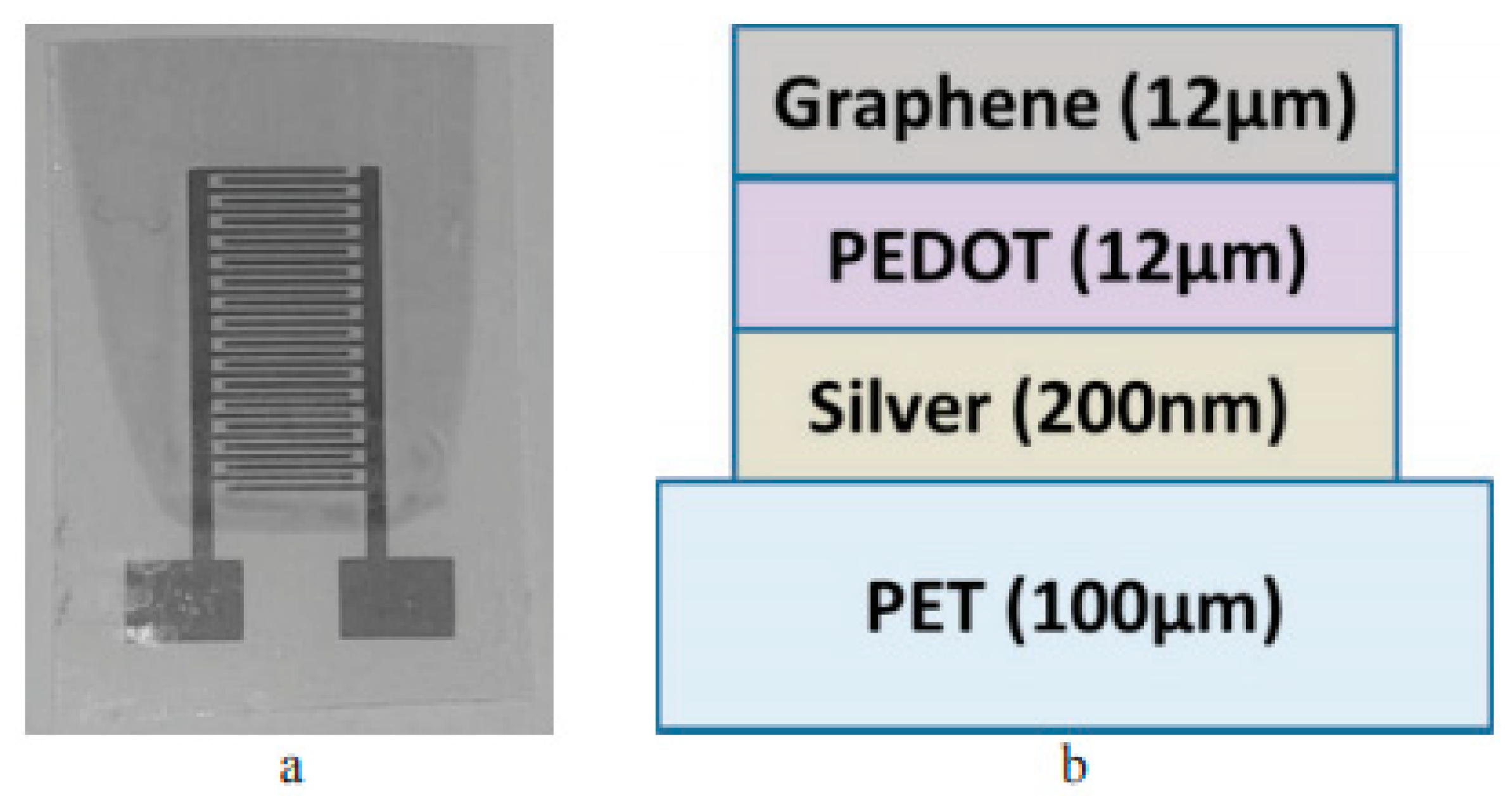
2 sensor was constructed with a double layer of PEDOT/PSS (poly(3,4-ethylenedioxythiophene)/polystyrene sulfonate) and graphene on interdigitated electrodes that was made by inkjet printing using conductive silver nanoparticles. The sensor works on the principle illustrated for type 1 and has sensitivity of 45 μohm/ppm [
117].
Figure 23 shows the different layers in the sensor and the interdigitated fingers.
The sensor’s responses at two different temperatures are given in
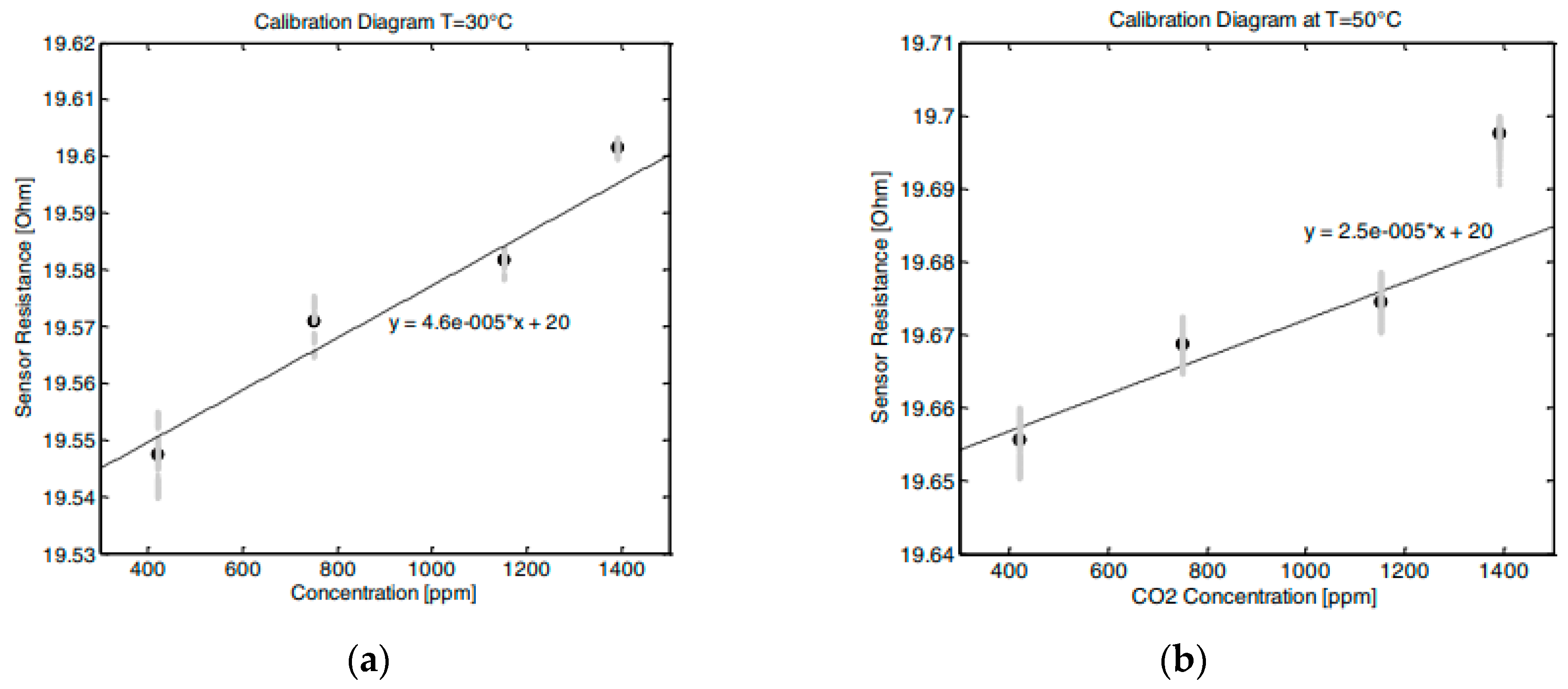
Figure 24.
3D inkjet printing has provided an economical manufacturing technique for the CO
2 sensor [
118]. It uses a colloidal dispersion in the printing ink and is composed of La
0.8Sr
0.2MnO
3 (LSM)(Y
2O
3)
0.08(ZrO
2)
0.92(YSZ). It uses an electrochemical cell Ni-YSZ|YSZ|YSZ-LSM|LSM, with YSZ (yittria stabilized zirconia) as electrolyte sintered to a Ni-yittria stabilized zirconia (Ni-YSZ). The carbon dioxide reaction in the electrochemical fuel cell is
The inkjet 3D printing performance is dependent on the formation and ejection of droplets. Its propagation depends on acoustic pressure waves in the fluid held in the chamber behind the printing nozzle and in the YSZ-LSM LSM ((La
1−xSr
x)
1−yMnO
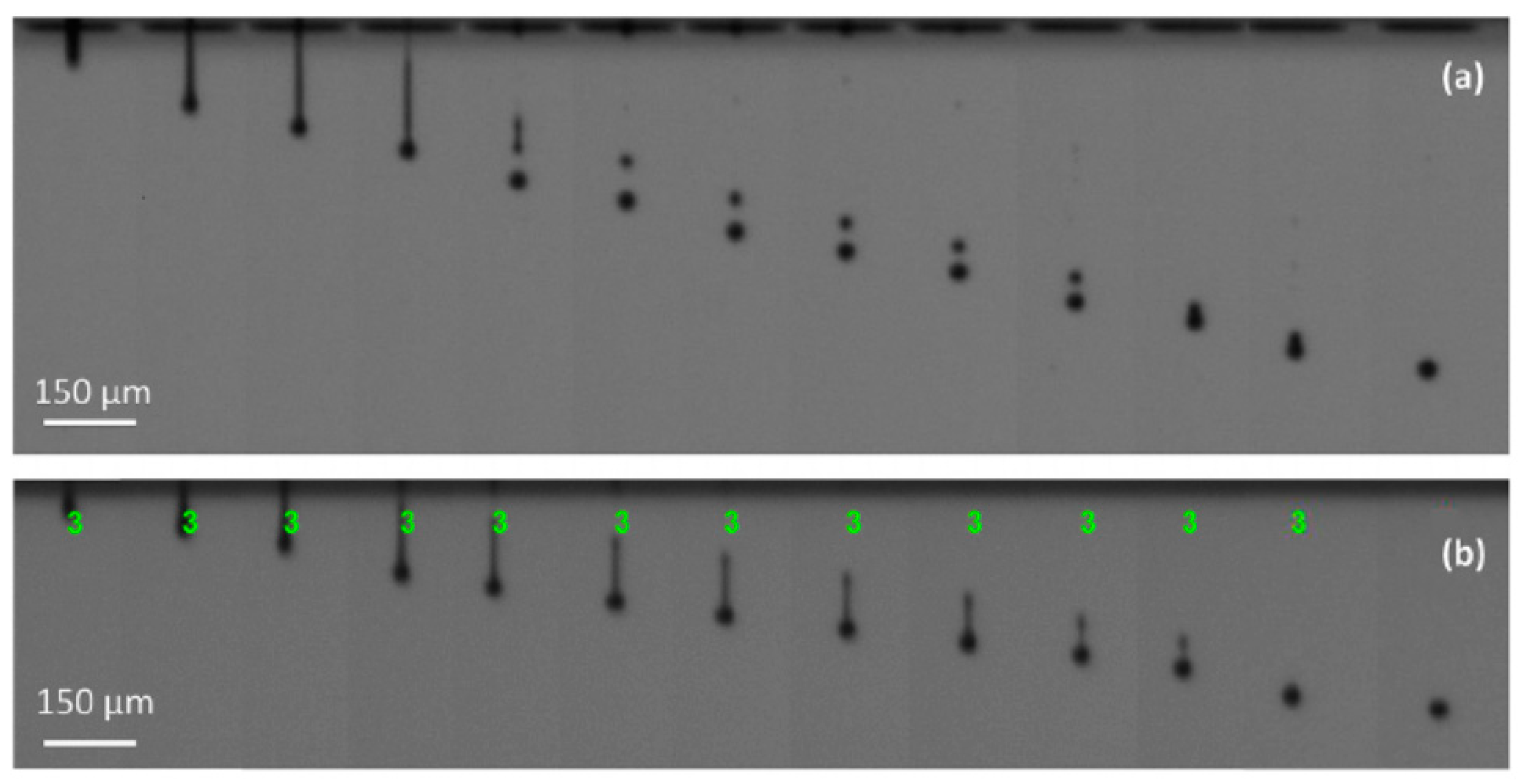
3) sensor. The droplets time interval is shown in
Figure 25. The colloidal LSM dispersions are stabilized using electrostatic dispersants which are commercially available from different manufacturers, as shown in
Table 8.
The performance of the fuel cell is linked to the concentration of CO
2, and it is in the initial stages of development. As discussed in the previous section on carbon dioxide, a pH electrode can be used as a CO
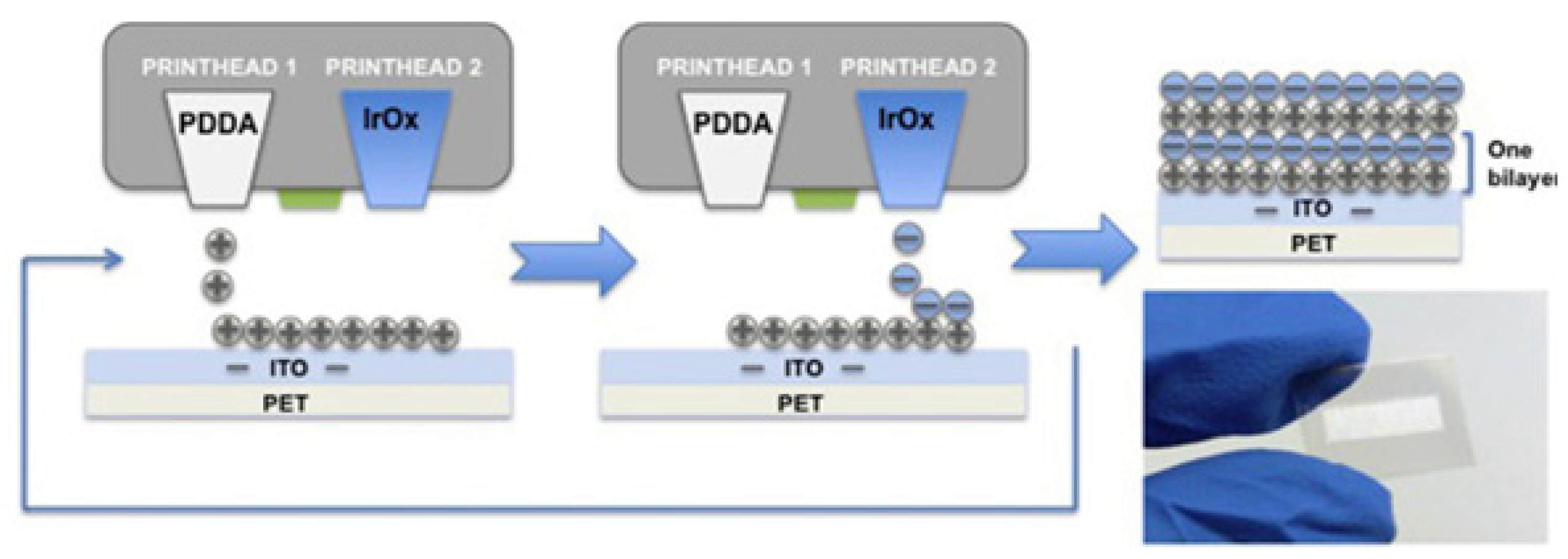
2 sensor. A large-scale layer-by-layer ink jet printing of flexible iridium oxide for a hydrogen ion sensor (
Figure 26) has been reported [
119]. The sensor’s active material is iridium oxide, which interacts with the analyte.
A flexible tin oxide gas sensor was developed by an inkjet printing process. Here the active material was tin oxide, printed on gold deposited in a digitated electrode spacing of 500 and 200 μm. Carbon monoxide was the analyte, and the measurements were done at 300 °C in the concentration range of 20–200 ppm [
120].
A type 3 sensor for CO
2 was recently been reported [
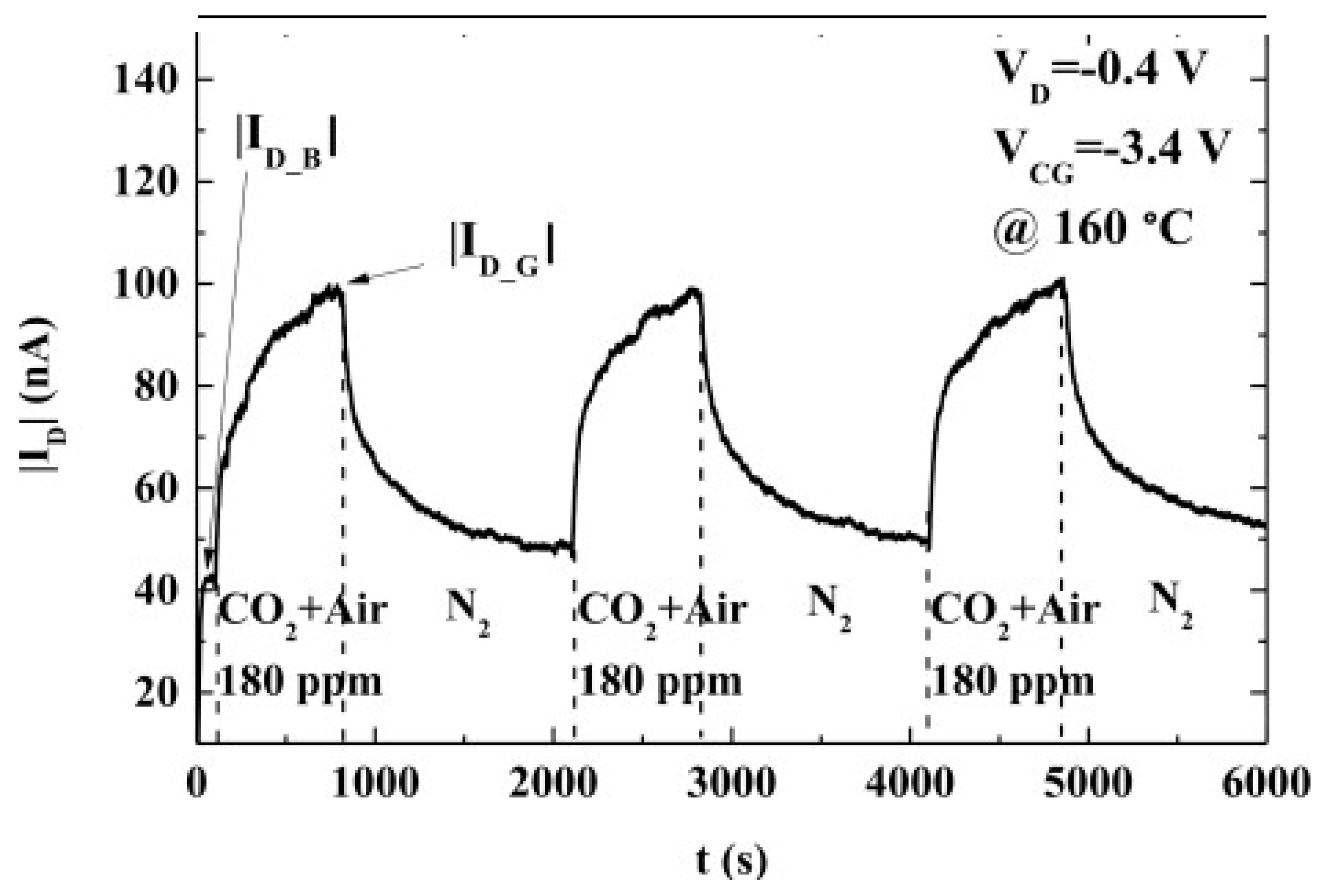
121] using a sodium ion conducting solid electrolyte having a floating gate passivated by an insulator stack and a control gate made of an interdigitated field gate (
Figure 27). The sensing material, (3-aminopropyl) triethoxysilane (APTES), was added by inkjet printing. The sensor operated at 160 °C in the concentration range of 125–325 ppm.
Inkjet printing technology has very high commercial value, and the developments have been rapidly pursued for long-term testing. Inkjet-printed graphene sensors (Type 1) for nitrogen oxide gas and inkjet printed platinum decorated tungsten oxide nanoparticles on silicon substrate for gas sensing (Type 1) have been reported [
122,
123]. The methodologies reported in these publications are interesting, and may be extendable to greenhouse gases in the future [
124,
125,
126].