Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Extraction
2.3. Cell Culture and Viability by MTT Assay
2.4. Effect of Two- and Three-Drug Combinations on Cell Viability
2.5. ABC Transporter Assay with the Substrate Calcein-AM in CCRF-CEM and CEM/ADR5000 Cells
2.6. ABC Transporter Assay with the Substrate Rhodamine 123 in CCRF-CEM, CEM/ADR 5000 Cells, and Caco-2 Cells
2.7. Gene Expression Analysis by by Real-Time PCR
2.8. Reversal Ratio
2.9. Combination Index (CI)
- Cdox,50 is the IC50 of dox in combination.
- IC50,dox is the IC50 of dox alone in a cell line.
- CSM is the constant concentration of a single substance in combination.
- IC50,SM is the IC50 of a single substance (a secondary metabolite or a plant extract) in the cell line.
- Cdig is the concentration of digitonin used in combination. It is 0.5 μM in this study.
- IC50,dig is the IC50 of digitonin in the cell line.
2.10. Statistical Analysis
3. Results
3.1. Cytotoxicity of Single Substances against Drug Sensitive and Resistant Cells
3.2. Combinations Enhance the Cytotoxicity of Doxorubicin
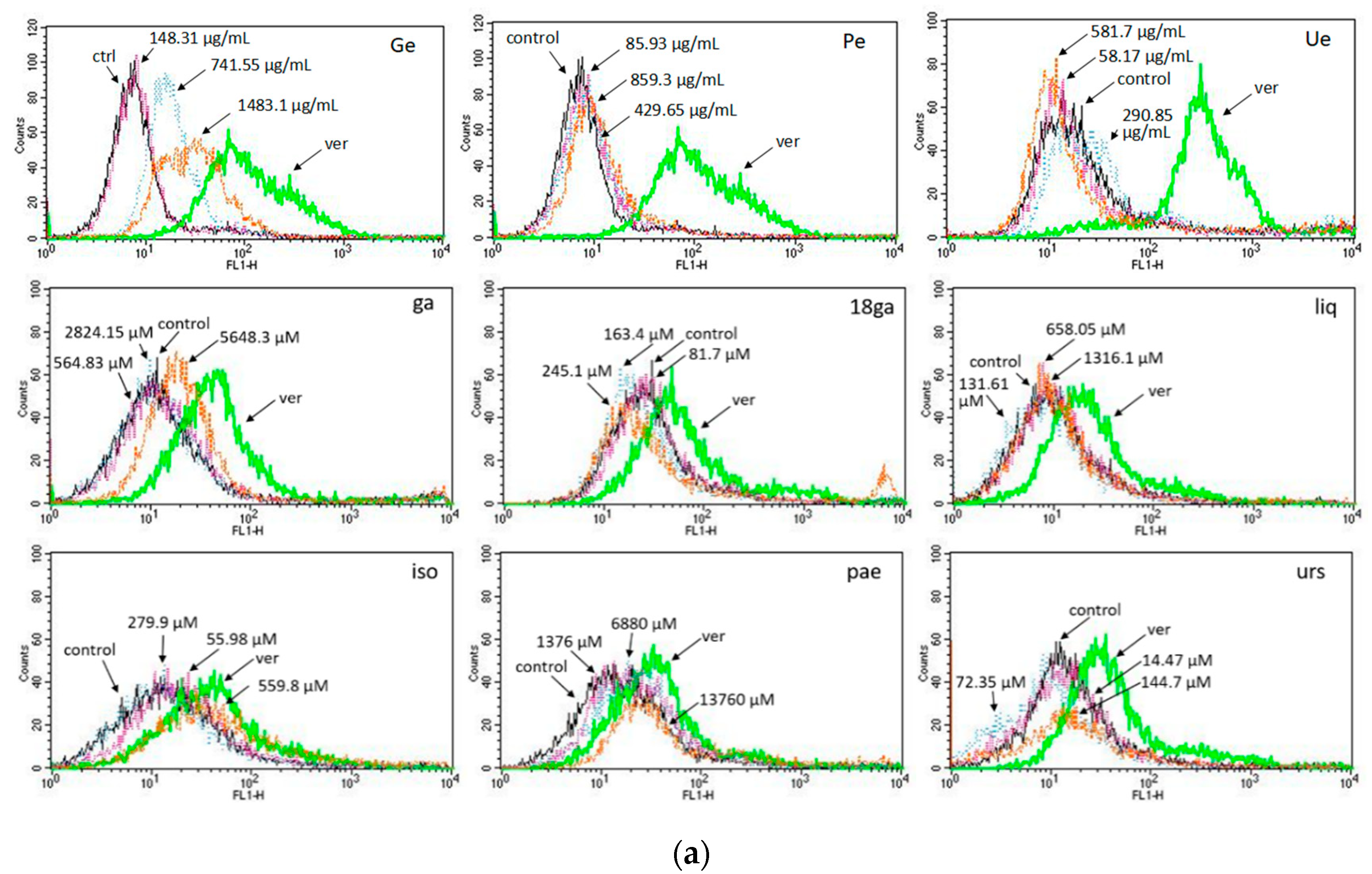
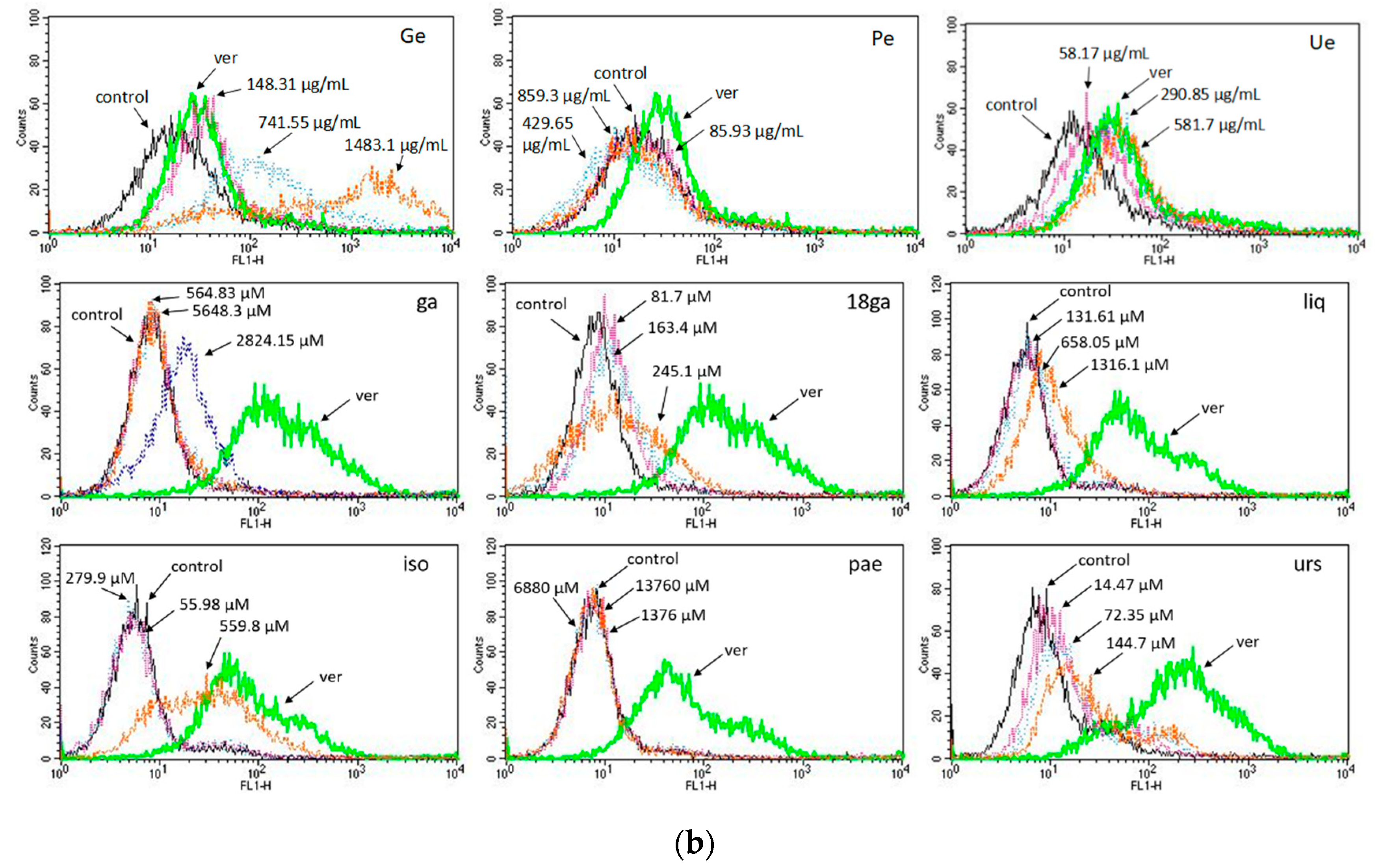
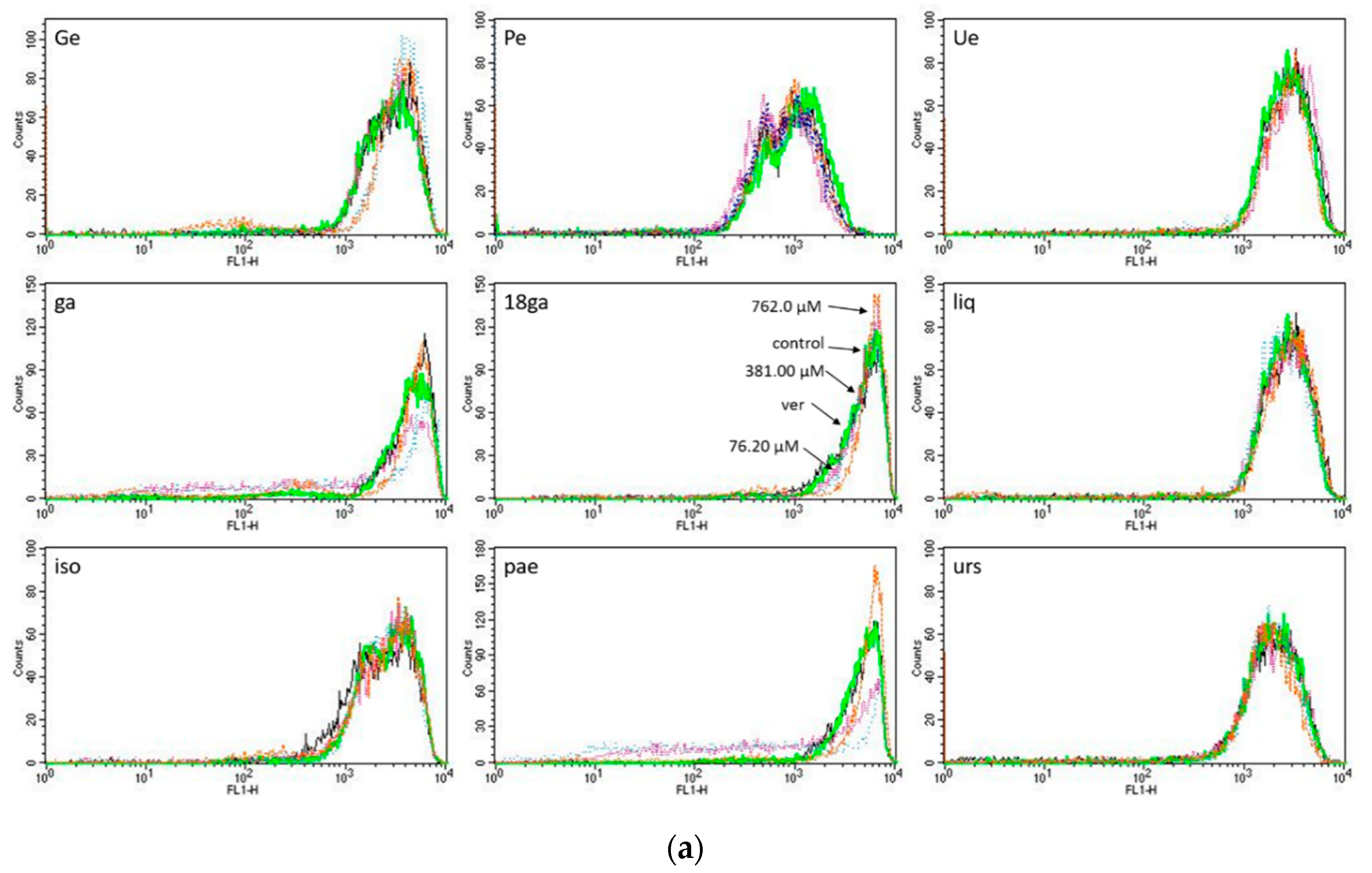
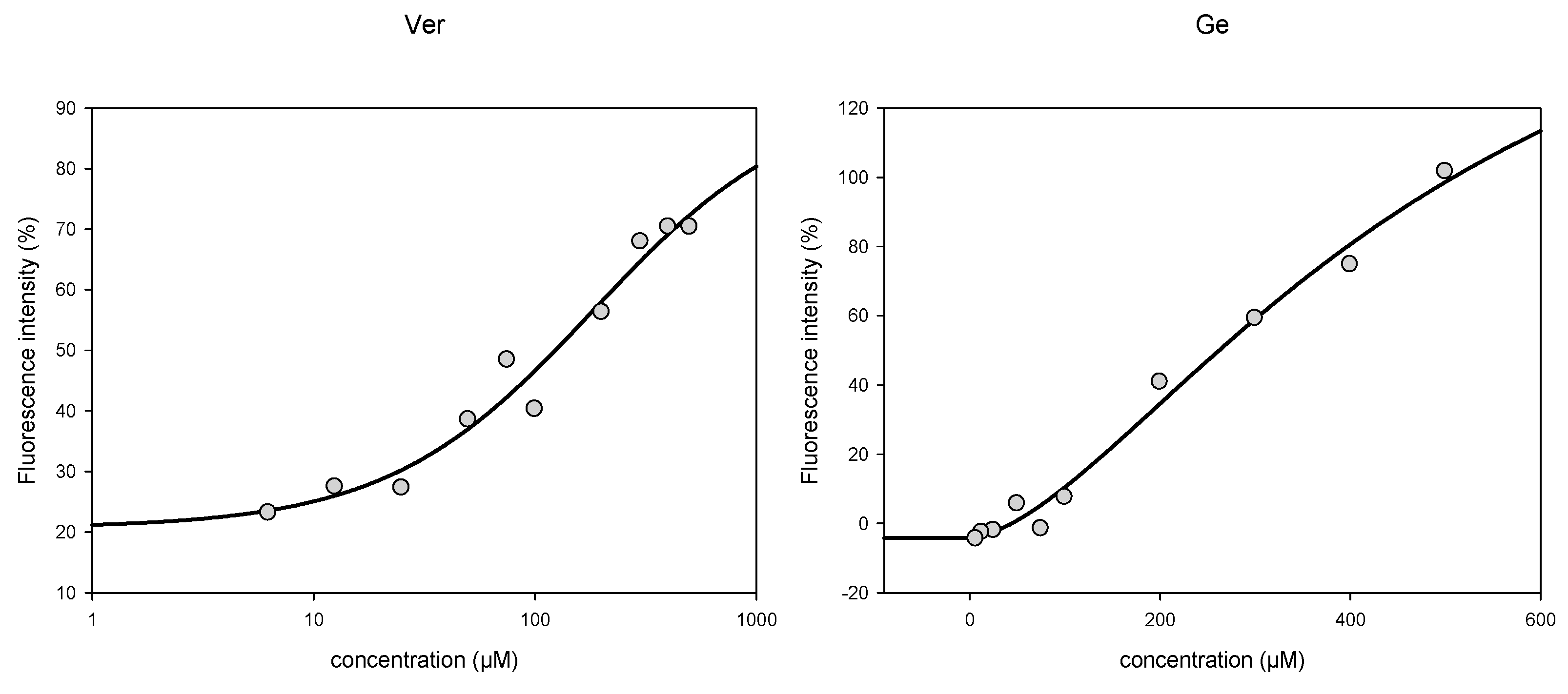
3.3. Activity towards ABC Transporters in CEM/ADR 5000 Cells
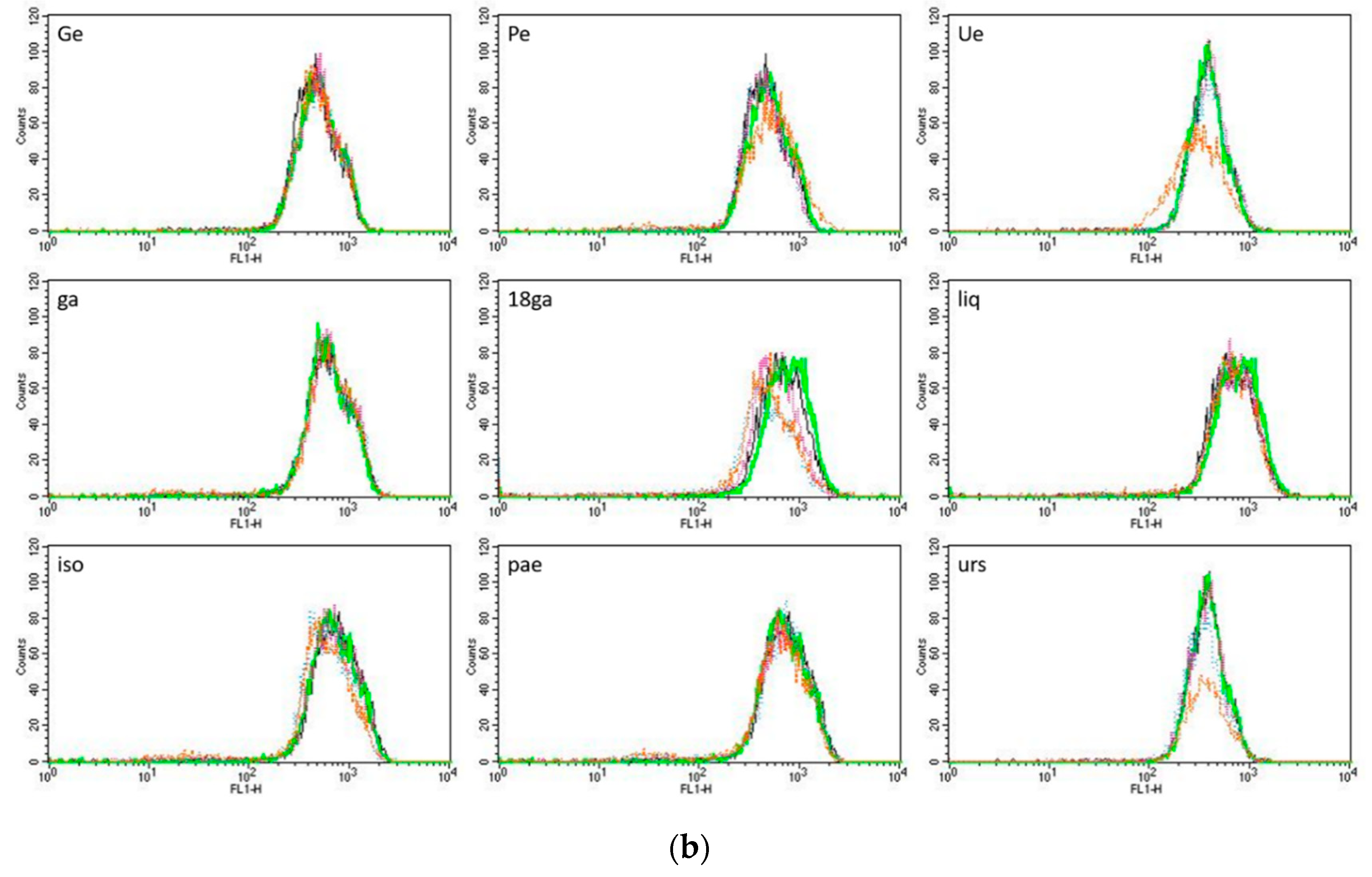
3.4. Rhodamine 123 Retention in Caco-2 Cells
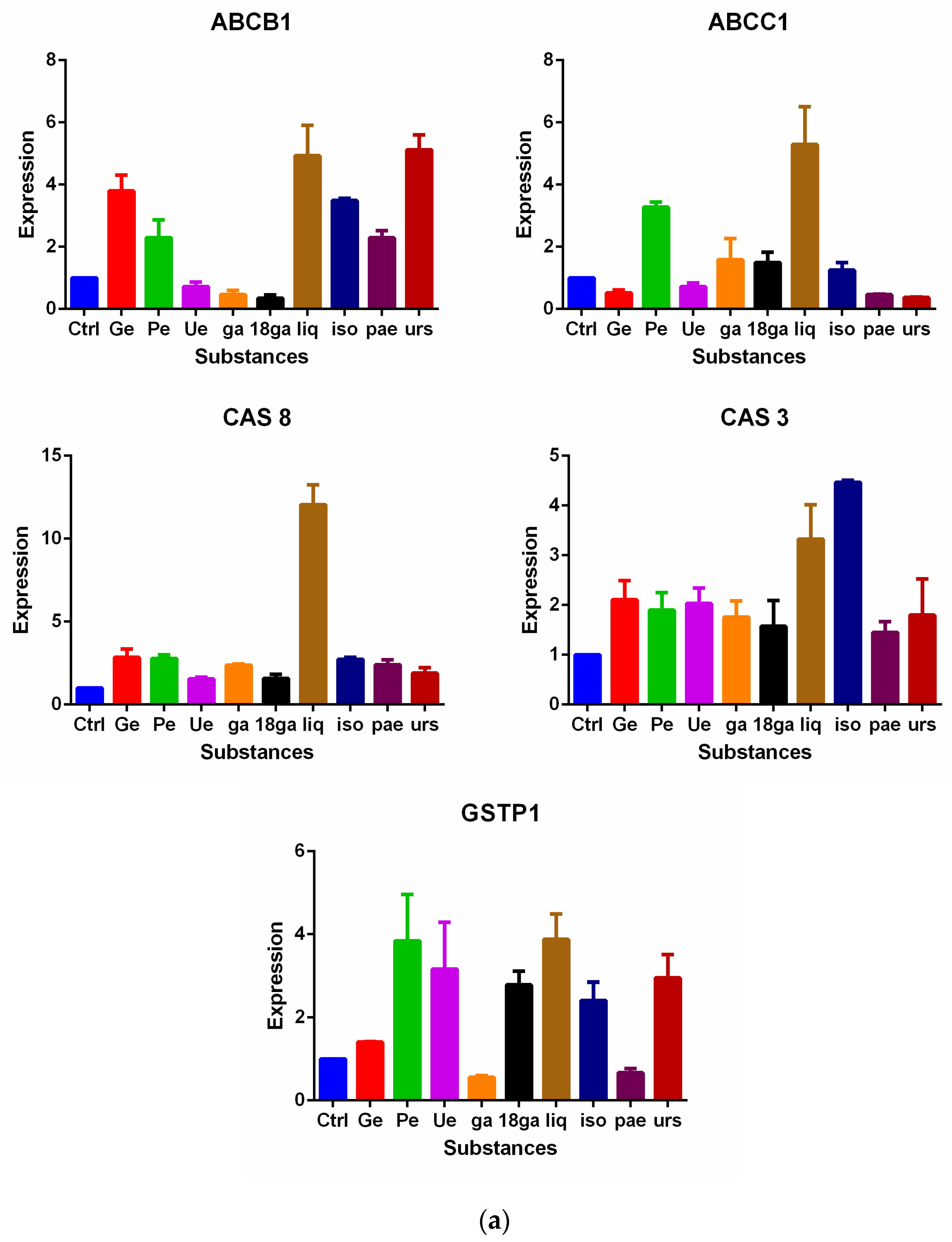
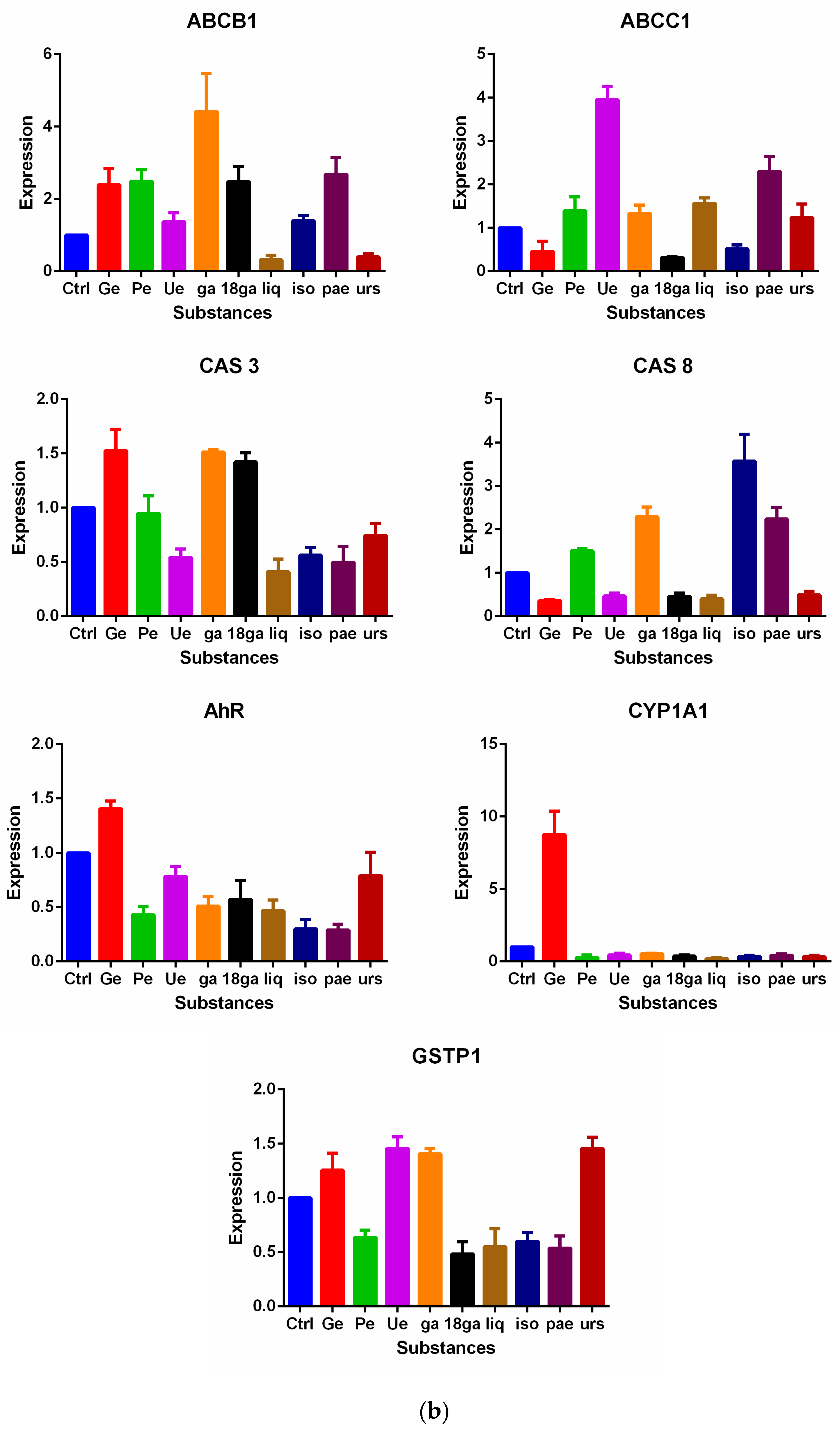
3.5. Influence on Gene Expression of MDR Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C. Multiple drug resistance mechanisms in cancer. Mol. Biotechnol. 2010, 46, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, A.A.; Rybalkina, E.Y. Recent advances in the studies of molecular mechanisms regulating multidrug resistance in cancer cells. Biochemistry 2018, 83, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist. Updates 2016, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Piwnica-Worms, D.; Ratner, L. Multidrug resistance transporters and modulation. Curr. Opin. Oncol. 2000, 12, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Cole, S.P.C.; Bhardwaj, G.; Gerlach, J.H.; Mackie, J.E.; Grant, C.E.; Almquist, K.C.; Stewart, A.J.; Kurz, E.U.; Duncan, A.M.V.; Deeley, R.G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992, 258, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Bakos, É.; Hegedüs, T.; Holló, Z.; Welker, E.; Tusnády, G.E.; Zaman, G.J.R.; Flens, M.J.; Váradi, A.; Sarkadi, B. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J. Biol. Chem. 1996, 271, 12322–12326. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.J.; Tchou-Wong, K.M.; Greenberg, A.K.; Pass, H.; Rom, W.N. Aryl hydrocarbon receptor and lung cancer. Anticancer Res. 2013, 33, 1247–1256. [Google Scholar] [PubMed]
- Hao, N.; Whitelaw, M.L. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 2013, 86, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Antona, C.; Ingelman-Sundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Hu, X.; Jia, W.; Gong, C.; Song, E.; Hamar, P. Glutathion S transferase Pi indicates chemotherapy resistance in breast cancer. J. Surg. Res. 2003, 113, 102–108. [Google Scholar] [CrossRef]
- Duvoix, A.; Schmitz, M.; Schnekenburger, M.; Dicato, M.; Morceau, F.; Galteau, M.-M.; Diederich, M. Transcriptional regulation of glutathione S-transferase Pi-1 in human leukemia. BioFactors 2003, 17, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Colvin, O.M.; Conney, A.H.; Ernster, V.L.; Garber, J.E.; Greenwald, P.; Gudas, L.J.; Waun, K.H.; Kelloff, G.J.; Kramer, R.A.; et al. Prevention of cancer in the next millennium: Report of the chemoprevention working group to the American association for cancer research. Cancer Res. 1999, 59, 4743–4758. [Google Scholar]
- Fiskum, G. Intracellular levels and distribution of Ca2+ in digitonin permeabilized cells. Cell Calcium 1985, 6, 25–37. [Google Scholar] [CrossRef]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.E.; Wink, M. Medicinal Plants of the World, 2nd ed.; CABI: Wallingford, UK, 2017. [Google Scholar]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, B.; Fan, J.; Qiao, X.; Ye, M. Antitussive and expectorant activities of licorice and its major compounds. Bioorg. Med. Chem. 2017, 26, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, S. Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int. Immunopharmacol. 2012, 14, 27–31. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. Assessment Report on Paeonia lactiflora Pallas, Radix (Paeoniae Radix Alba). Available online: https://www.ema.europa.eu/documents/herbal-report/draft-assessment-report-paeonia-lactiflora-pallas-radix-paeoniae-radix-alba_en.pdf (accessed on 31 January 2017).
- Guo, J.; Pang, J.; Wang, X.; Shen, Z.; Jin, M.; Li, J. In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J. Gastroenterol. 2006, 12, 4078–4081. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.; Wu, J.; Ren, B.; Zhang, H. Euscaphic acid, a new hypoglycemic natural product from Folium Eriobotryae. Pharmazie 2008, 63, 765–767. [Google Scholar] [PubMed]
- Su, X.; Wu, L.; Hu, M.; Dong, W.; Xu, M.; Zhang, P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomed. Pharmacother. 2017, 95, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Yamaki, T.; Ueno, K.; Kitagawa, S. Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals. Cancer Chemother. Pharmacol. 2008, 62, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ha, J.Y.; Kim, K.; Jung, Y.; Jung, J.; Oh, S. Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef] [PubMed]
- Kalani, K.; Agarwal, J.; Alam, S.; Khan, F.; Pal, A.; Srivastava, S.K. In silico and in vivo anti-malarial studies of 18β glycyrrhetinic acid acid from Glycyrrhiza glabra. PLoS ONE 2013, 8, e74761. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Wang, B.; Sheng, L.; Liu, Z.; Yang, S.; Li, Y. Inhibitory effects of herbal constituents on P-glycoprotein in vitro and in vivo: Herb–drug interactions mediated via P-gp. Toxicol. Appl. Pharmacol. 2014, 275, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, Y.J.; Lee, M.S.; Han, E.S.; Lee, S.J. 18β glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wong, H.; Feng, Y.; Zhang, Z. Liquiritigenin exhibits antitumour action in pituitary adenoma cells via Ras/ERKs and ROS-dependent mitochondrial signalling pathways. J. Pharm. Pharmacol. 2013, 66, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Park, G.H.; Song, K. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology 2013, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, C.; Shieh, T.; Chen, H.; Huang, T.; Hsia, S. The liquorice dietary component isoliquiritigenin chemosensitizes human uterine sarcoma cells to doxorubicin and inhibits cell growth by inducing apoptosis and autophagy via inhibition of m-TOR signaling. J. Funct. Foods 2017, 33, 332–344. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Wang, Y.; Zheng, H.; Yu, W.; Chai, H.; Zhang, J.; Falck, J.R.; Guo, A.M.; Yue, J.; et al. Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer. Toxicol. Appl. Pharmacol. 2013, 272, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Kuo, P.; Lin, L.; Lin, C. Isoliquiritigenin inhibits cell proliferation and induces apoptosis in human hepatoma cells. Planta Med. 2005, 71, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, X.; Zhu, H.; Gao, Y.; Tian, J.; Fu, F. Paeoniflorin inhibits systemic inflammation and improves survival in experimental sepsis. Basic Clin. Pharmacol. Toxicol. 2009, 105, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Z.; Yang, S.; Du, J.; Wang, S. Immunoregulatory effects of paeoniflorin exerts anti-asthmatic effects via modulation of the Th1/Th2 equilibrium. Inflammation 2015, 38, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Song, W.; Xu, Y.; Ye, Y.; Feng, L. Paeoniflorin ameliorates ischemic neuronal damage in vitro via adenosine A1 receptor-mediated transactivation of epidermal growth factor receptor. Acta Pharmacol. Sin. 2015, 36, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhu, W.; Zhang, Y.; Shu, Y.; Liu, P. Paeoniflorin modulates multidrug resistance of a human gastric cancer cell line via the inhibition of NF-κB activation. Mol. Med. Rep. 2012, 5, 351–356. [Google Scholar] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, P.M.; Chan, J.Y.; Lam, H.; Au, S.W.; Kong, S.; Tsui, S.K.; Waye, M.M.; Mak, T.C.; Fung, K. Anti-proliferative effect of ursolic acid on multidrug resistant hepatoma cells R-HepG2 by apoptosis induction. Cancer Biol. Ther. 2007, 6, 1377–1385. [Google Scholar] [CrossRef]
- Nabekura, T.; Yamaki, T.; Hiroi, T.; Ueno, K.; Kitagawa, S. Inhibition of anticancer drug efflux transporter P-glycoprotein by rosemary phytochemicals. Pharmacol. Res. 2010, 61, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ludescher, C.; Thalerd, J.; Drach, D.; Spitaler, M.; Gattringer, C.; Huber, H.; Hofmann, J. Detection of activity of P-glycoprotein in human tumor samples using rhodamine 123. Br. J. Haematol. 1992, 82, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Holló, Z.; Homolya, L.; Davis, C.W.; Sarkadi, B. Calcein accumulation as a fluorometric functional assay of the multidrug tranaporter. Biochim. Biophys. Acta 1994, 1191, 384–388. [Google Scholar] [CrossRef]
- Twentyman, P.R.; Rhodes, T.; Rayner, S. A comparison of rhodamine 123 accumulation and efflux in cells with P-glycoprotein-mediated and MRP-associated multidrug resistance phenotypes. Eur. J. Cancer 1994, 30, 1360–1369. [Google Scholar] [CrossRef]
- Olson, D.P.; Taylor, B.J.; Ivy, S.P. Detection of MRP functional activity: Calcein AM but not BCECF AM as a multidrug resistance-related protein (MRP1) substrate. Cytometry 2001, 46, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Glavinas, H.; von Richter, O.; Vojnits, K.; Mehn, D.; Wilhelm, I.; Nagy, T.; Janossy, J.; Krizbai, I.; Couraud, P.; Krajcsi, P. Calcein assay: A high-throughput method to assess P-gp inhibition. Xenobiotica 2011, 41, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.M.; Hait, W.N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol. Rev. 1990, 42, 155–199. [Google Scholar] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N., Jr. Statistical analysis of real-time PCR data. BMC Bioinformat. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wientjes, M.G.; Au, J.L.-S. Evaluation of combination chemotherapy: Integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin. Cancer Res. 2004, 10, 7994–8004. [Google Scholar] [CrossRef] [PubMed]
- Chou, T. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, T.; Tong, Y.; Chen, X.; Chen, X.; Wang, Y.; Lu, J. A systematic review of the anticancer properties of compounds isolated from liquorice (Gancao). Planta Med. 2015, 81, 1670–1687. [Google Scholar] [PubMed]
- You, M.; Kim, M.; Jeong, K.; Kim, E.; Kim, Y.; Kim, H. Loquat (Eriobotrya japonica) leaf extract inhibits the growth of MDA-MB-231 tumors in nude mouse xenografts and invasion of MDA-MB-231 cells. Nutr. Res. Pract. 2016, 10, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Digitonin synergistically enhances the cytotoxicity of plant secondary metabolites in cancer cells. Phytomedicine 2012, 19, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Ji, S.; Yu, S.; Lin, X.; Jin, H.; Duan, Y.; Zhang, L.; Guo, D.; Ye, M. Identification of key licorice constituents which interact with cytochrome p450: Evaluation by LC/MS/MS cocktail assay and metabolic profiling. AAPS J. 2014, 16, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Wink, M. Synergistic interactions of saponins and monoterpenes in HeLa cells, Cos7 cells and in erythrocytes. Phytomedicine 2011, 18, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
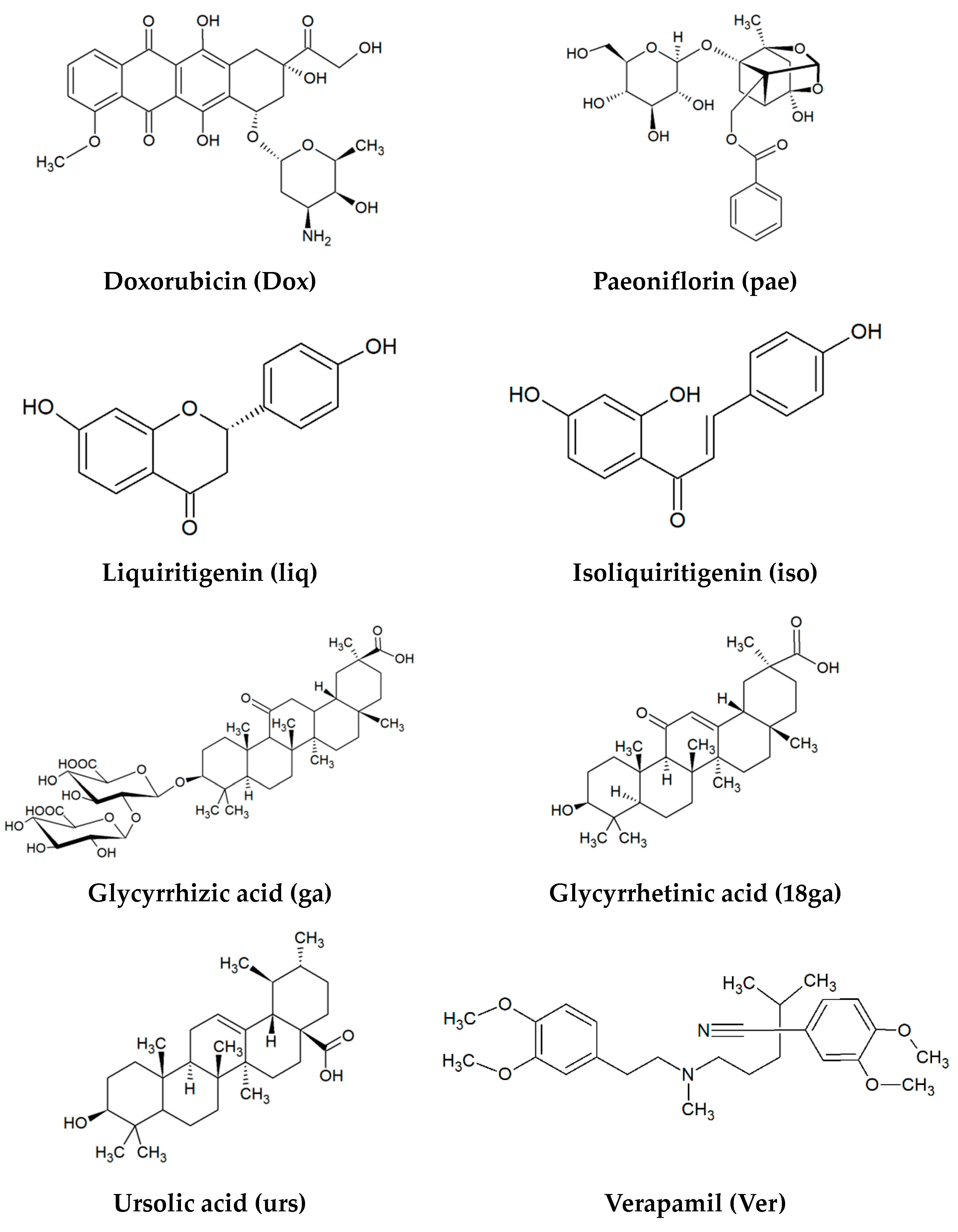

| Gene | Sequences | Amplicon |
|---|---|---|
| RPL13 | Forward: GAGGCGGAACAAGTCCAC | 75 nt |
| Reverse: AGGATGAGTTTGGAGCGGTA | ||
| ABCB1 | Forward: TTACATTCAGGTTTCATTTTGGTG | 90 nt |
| Reverse: TCCTGTCGCATTATAGCATGA | ||
| ABCC1 | Forward: TTGGGATTTTTGCTGTGGA | 73 nt |
| Reverse: ATATGCCCCGACTTCTTTCC | ||
| GSTP1 | Forward: AATGGCAACGGGAAACAGT | 77 nt |
| Reverse: TTGGTCCTGGAGAAAGGAAG | ||
| CAS 3 | Forward: TCTCATGCTGCAGAGGGTACT | 77 nt |
| Reverse: TGGAAGTTTGAGGTAGCTTCATAG | ||
| CAS 8 | Forward: GGAAAGCAATCTGTCCTTCCT | 61 nt |
| Reverse: CAGCAAATCCAGTCTATTAATTCG | ||
| AhR | Forward: AGAGTTGGACCGTTTGGCTA | 86 nt |
| Reverse: CGCTGAGCCTAAGAACTGAAA | ||
| CYP1A1 | Forward: CCACCAAGAACTGCTTAGCC | 74 nt |
| Reverse: CAGCTGGGTTTCCAGAGC |
| Sub-stances | CCRF-CEM | CEM/ADR 5000 | Caco-2 | HCT-116 |
|---|---|---|---|---|
| Dox | 0.17 ± 0.06 | 93.24 ± 19.02 | 5.40 ± 1.27 | 0.73 ± 0.04 |
| Dig | 10.65 ± 1.07 | 13.06 ± 2.38 | 11.28 ± 0.74 | 0.70 ± 0.10 |
| Ge | 96.63 ± 4.21 | 148.31 ± 9.57 | 352.97 ± 8.10 | 305.04 ± 4.78 |
| Pe | 56.15 ± 1.99 | 85.93 ± 3.58 | 134.64 ± 14.90 | 202.14 ± 12.09 |
| Ue | 50.88 ± 4.51 | 58.17 ± 3.05 | 98.95 ± 11.57 | 67.61 ± 2.89 |
| ga | 406.71 ± 24.77 | 564.83 ± 19.06 | 602.65 ± 31.39 | 1415.46 ± 113.43 |
| 18ga | 76.20 ± 0.93 | 81.72 ± 0.43 | 126.18 ± 26.86 | 113.17 ± 1.77 |
| liq | 88.41 ± 4.86 | 131.61 ± 6.82 | 746.43 ± 92.93 | 349.27 ± 16.83 |
| iso | 26.09 ± 0.83 | 55.98 ± 1.60 | 98.79 ± 11.27 | 28.26 ± 0.89 |
| pae | 802.11 ± 28.93 | 1376.96 ± 195.12 | 822.27 ± 297.99 | 1146.11 ± 120.48 |
| urs | 10.28 ± 0.39 | 14.47 ± 0.65 | 22.37 ± 1.22 | 13.10 ± 0.19 |
| Ver | - | 130.08 ± 32.49 | 35.29 ± 3.32 | - |
| Substances | Two-Drug Combinations (PSM + Dox) | Three-Drug Combinations (PSM + Dox + 0.5 μM Digitonin) | ||||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM of Doxorubicin) | Reversal Ratio | CI | Interpretation | IC50 (μM of Dox) | Reversal Ratio | CI | Interpretation | |
| Dox alone | 93.24 ± 19.02 | 1.00 | NR | NR | 93.24 ± 19.02 | 1.00 | NR | NR |
| Ge (80 µg/mL—IC26) | 31.84 ± 5.45 | 2.93 | 0.88 | + (slight syn) | 23.54 ± 10.76 | 3.96 | 0.83 | ++ (mod syn) |
| Pe (50 µg/mL—IC3) | 74.02 ± 9.17 | 1.26 | 1.37 | -- (mod ant) | 59.35 ± 22.22 | 1.57 | 1.25 | -- (mod ant) |
| Ue (30 µg/mL—IC15) | 49.93 ± 13.28 | 1.87 | 1.06 | ± (nearly add) | 37.44 ± 7.89 | 2.49 | 0.96 | ± (nearly add) |
| ga (300 µM—IC10) | 32.34 ± 2.55 | 2.88 | 0.88 | + (slight syn) | 26.08 ± 2.18 | 3.76 | 0.85 | ++ (mod syn) |
| 18ga (50 µM—IC1) | 38.11 ± 6.91 | 2.45 | 1.02 | ± (nearly add) | 26.38 ± 10.49 | 3.53 | 0.93 | ± (nearly add) |
| liq (40 µM—IC18) | 57.50 ± 8.17 | 1.62 | 0.92 | ± (nearly add) | 47.56 ± 7.46 | 1.96 | 0.85 | ++ (mod syn) |
| iso (20 µM—IC12) | 31.17 ± 8.60 | 2.99 | 0.69 | +++ (syn) | 25.21 ± 6.10 | 3.70 | 0.66 | +++ (syn) |
| pae (500 µM—IC11) | 40.27 ± 5.13 | 2.32 | 0.79 | ++ (mod syn) | 34.17 ± 9.04 | 2.73 | 0.76 | ++ (mod syn) |
| urs (6 µM—IC17) | 30.13 ± 10.49 | 3.10 | 0.74 | ++ (mod syn) | 25.96 ± 7.67 | 3.59 | 0.73 | ++ (mod syn) |
| Substances | Two-Drug Combinations (PSM + Dox) | Three-Drug Combinations (PSM + Dox + 0.5 μM Digitonin) | ||||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM of Dox) | Reversal Ratio | CI | Interpretation | IC50 (μM of Dox) | Reversal Ratio | CI | Interpretation | |
| Dox. alone | 5.40 ± 1.27 | 1.00 | NR | NR | 5.40 ± 1.27 | 1.00 | NR | NR |
| Ge (200 µg/mL—IC5) | 3.21 ± 0.72 | 1.68 | 1.16 | - (slight ant) | 2.14 ± 1.08 | 2.52 | 1.01 | ± (nearly add) |
| Pe (25 µg/mL—IC10) | 3.44 ± 0.97 | 1.32 | 0.82 | ++ (mod syn) | 0.42 ± 0.20 | 2.52 | 0.31 | +++ (syn) |
| Ue (40 µg/mL—IC6) | 1.10 ± 0.08 | 4.91 | 0.60 | +++ (syn) | 0.52 ± 0.18 | 10.38 | 0.53 | +++ (syn) |
| ga (350 µM—IC10) | 1.74 ± 0.56 | 3.10 | 0.90 | ± (nearly add) | 0.68 ± 0.27 | 7.94 | 0.75 | ++ (mod syn) |
| 18ga (25 µM—IC20) | 2.08 ± 0.97 | 2.60 | 0.58 | +++ (syn) | 0.96 ± 0.45 | 5.63 | 0.42 | +++ (syn) |
| liq (200 µM—IC20) | 11.45 ± 3.78 | 0.47 | 2.39 | --- (ant) | 7.83 ± 3.65 | 0.69 | 1.76 | --- (ant) |
| iso (30 µM—IC23) | 6.36 ± 1.78 | 0.85 | 1.48 | --- (ant) | 4.61 ± 1.29 | 1.17 | 1.20 | -- (mod ant) |
| pae (200 µM—IC27) | 3.59 ± 0.79 | 1.50 | 0.90 | ± (nearly add) | 3.02 ± 0.89 | 1.79 | 0.84 | ++ (mod syn) |
| urs (8 µM—IC12) | 1.45 ± 0.55 | 3.72 | 0.63 | +++ (syn) | 0.32 ± 0.07 | 16.88 | 0.46 | +++ (syn) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.-X.; Wink, M. Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites. Medicines 2018, 5, 123. https://doi.org/10.3390/medicines5040123
Zhou J-X, Wink M. Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites. Medicines. 2018; 5(4):123. https://doi.org/10.3390/medicines5040123
Chicago/Turabian StyleZhou, Jun-Xian, and Michael Wink. 2018. "Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites" Medicines 5, no. 4: 123. https://doi.org/10.3390/medicines5040123
APA StyleZhou, J.-X., & Wink, M. (2018). Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites. Medicines, 5(4), 123. https://doi.org/10.3390/medicines5040123






