Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Solvents, Reagents, and Chemicals
2.1.2. Gac Seeds
2.2. Methods
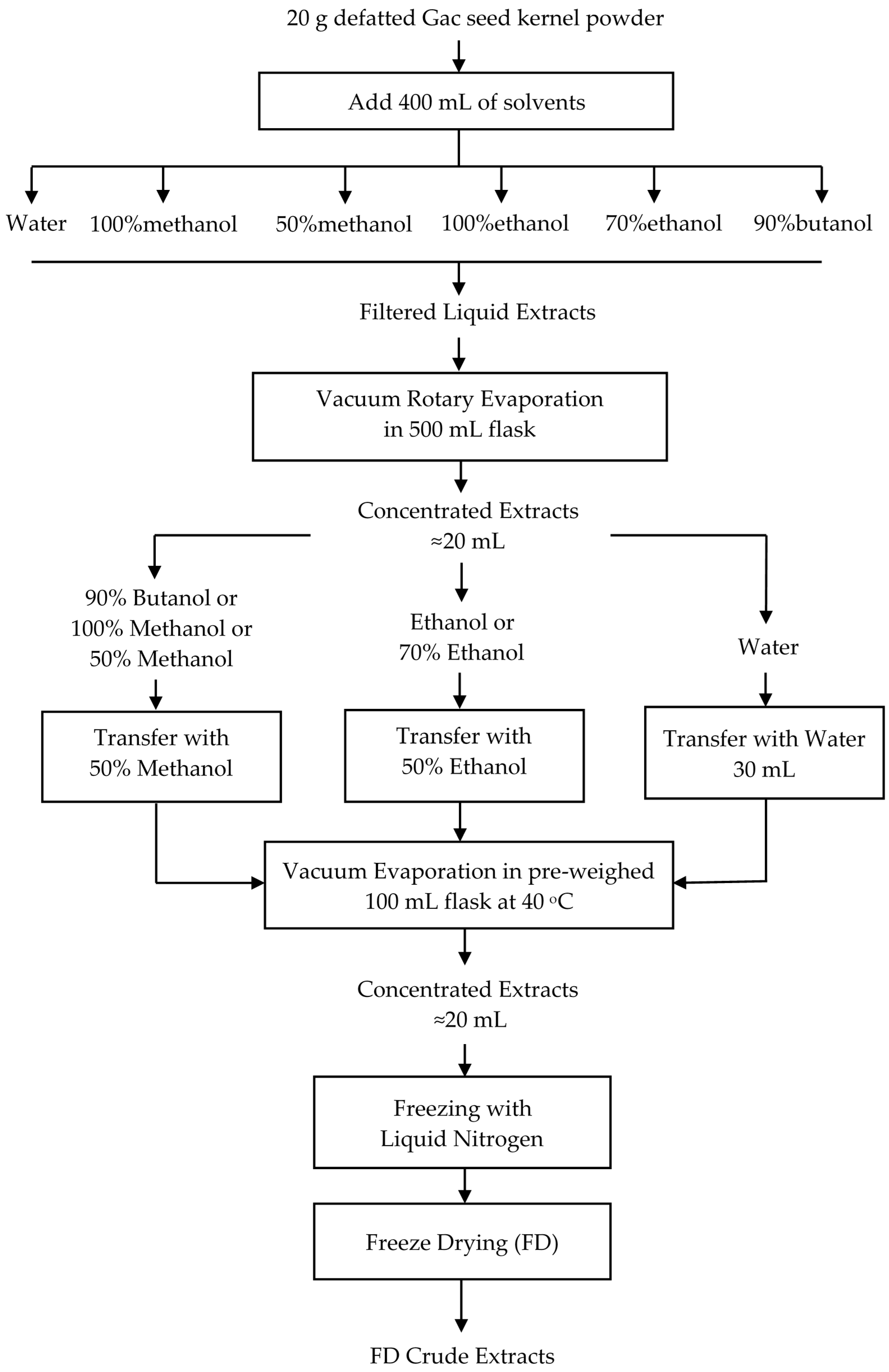
2.2.1. Extraction
2.2.2. Freeze Drying Extracts
2.2.3. Determination of Extractable Yield
2.2.4. Determination of Dry Mass Yield
2.2.5. Determination of Trypsin Inhibitor Activity (TIA)
Reagent Preparation
Determination of TIA
Calculation
- AI: Change in absorbance due to inhibition per 1 mL of extract;
- AI = (Ab – Aa) – (Ad – Ac), subscripts as per Table 1;
- S: Weight (mg) of the FD crude extract dissolved in 1 mL;
- m%: Moisture content of the FD crude extract powder.
2.2.6. Determination of Total Saponin Content (TSC)
2.2.7. Determination of Total Phenolic Content (TPC)
2.2.8. Determination of Antioxidant Capacity
DPPH
ABTS
FRAP
2.2.9. Determination of Cytotoxicity
Cell Lines and Culture
In Vitro Cytotoxicity Assay
2.2.10. Statistical Analyses
3. Results
3.1. Effect of Solvent on the Extractable Yield and the Dry Mass Yield
3.2. Effect of Solvents on the Content of Bioactive Compounds
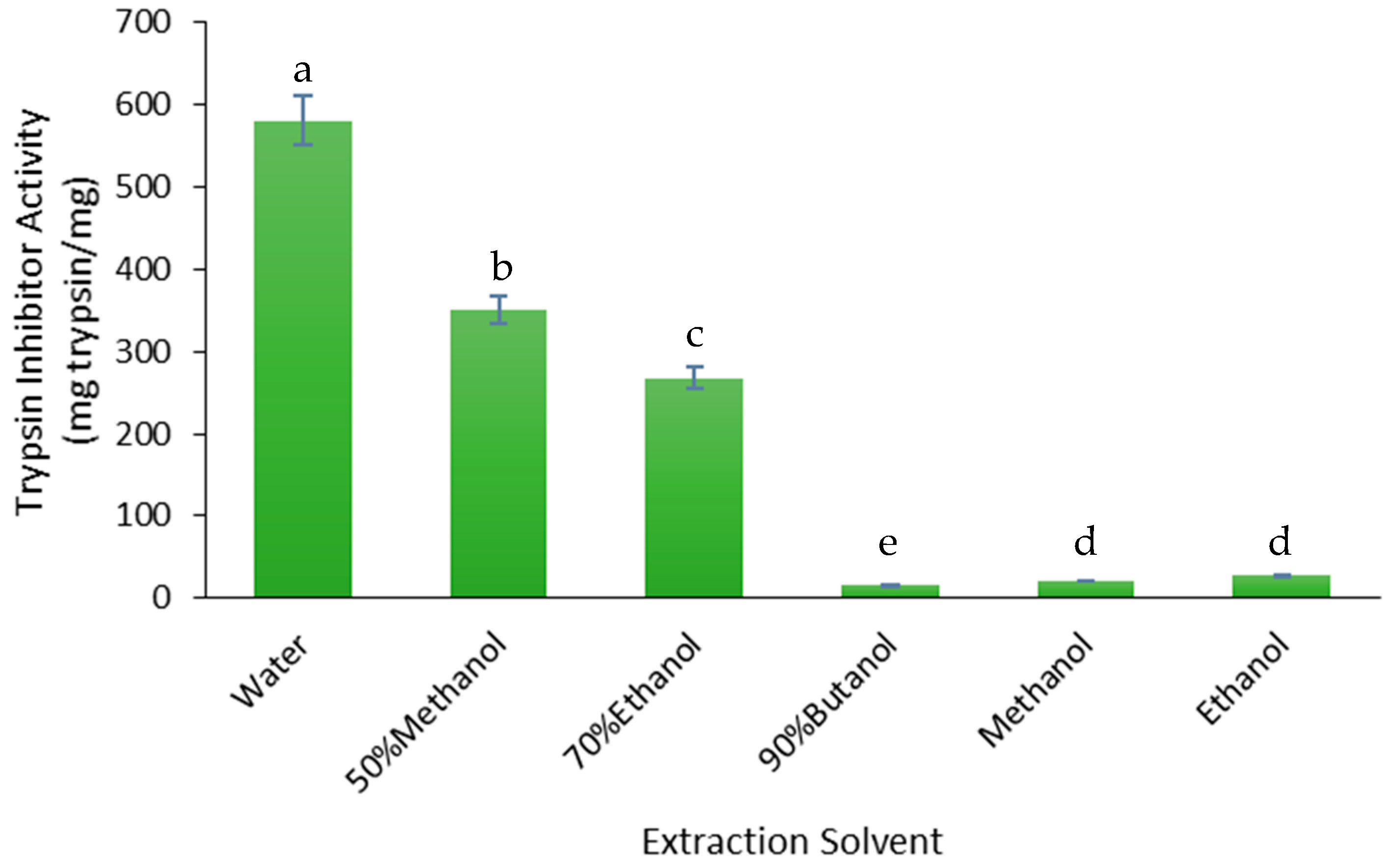
3.2.1. Trypsin Inhibitors
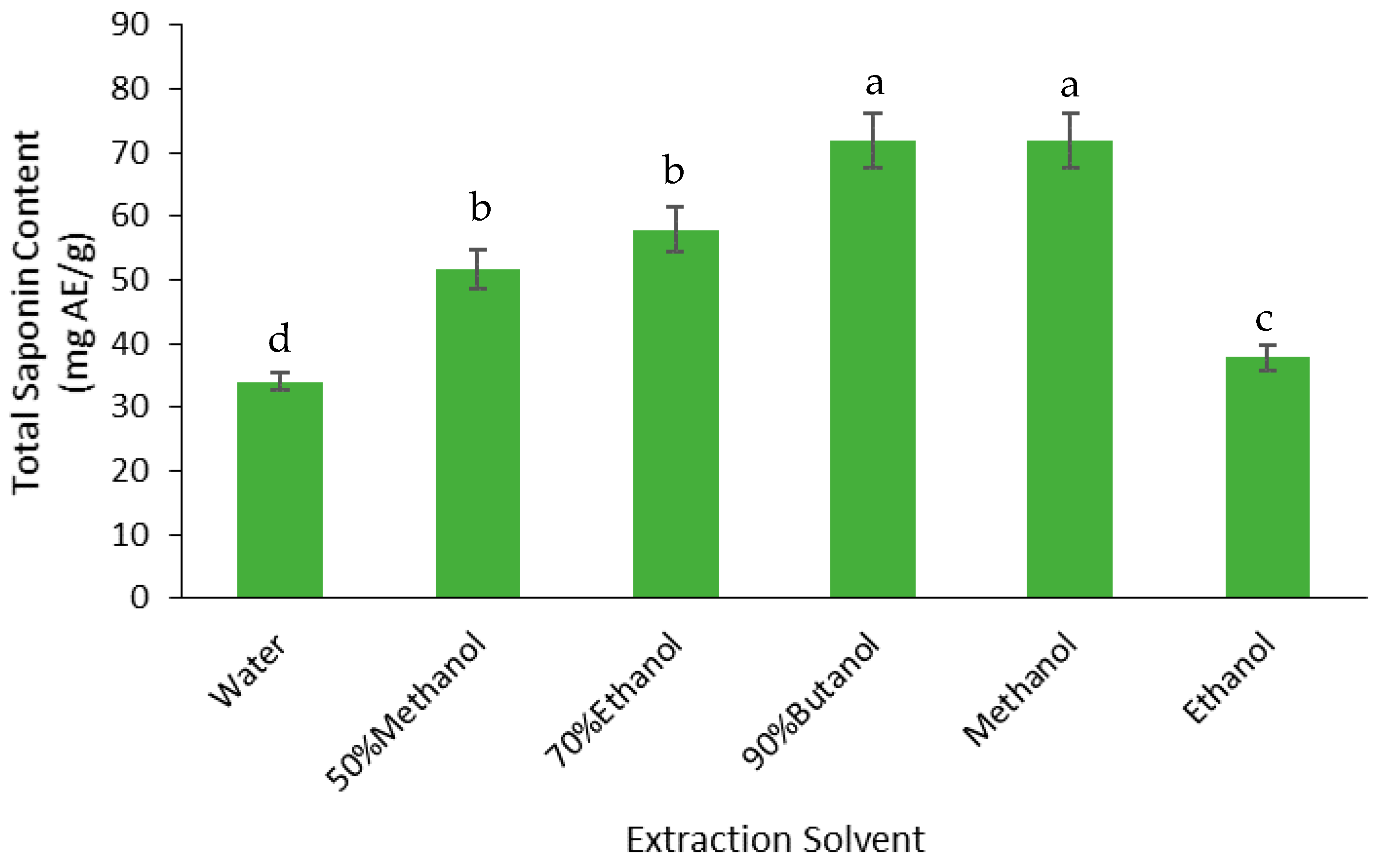
3.2.2. Saponins
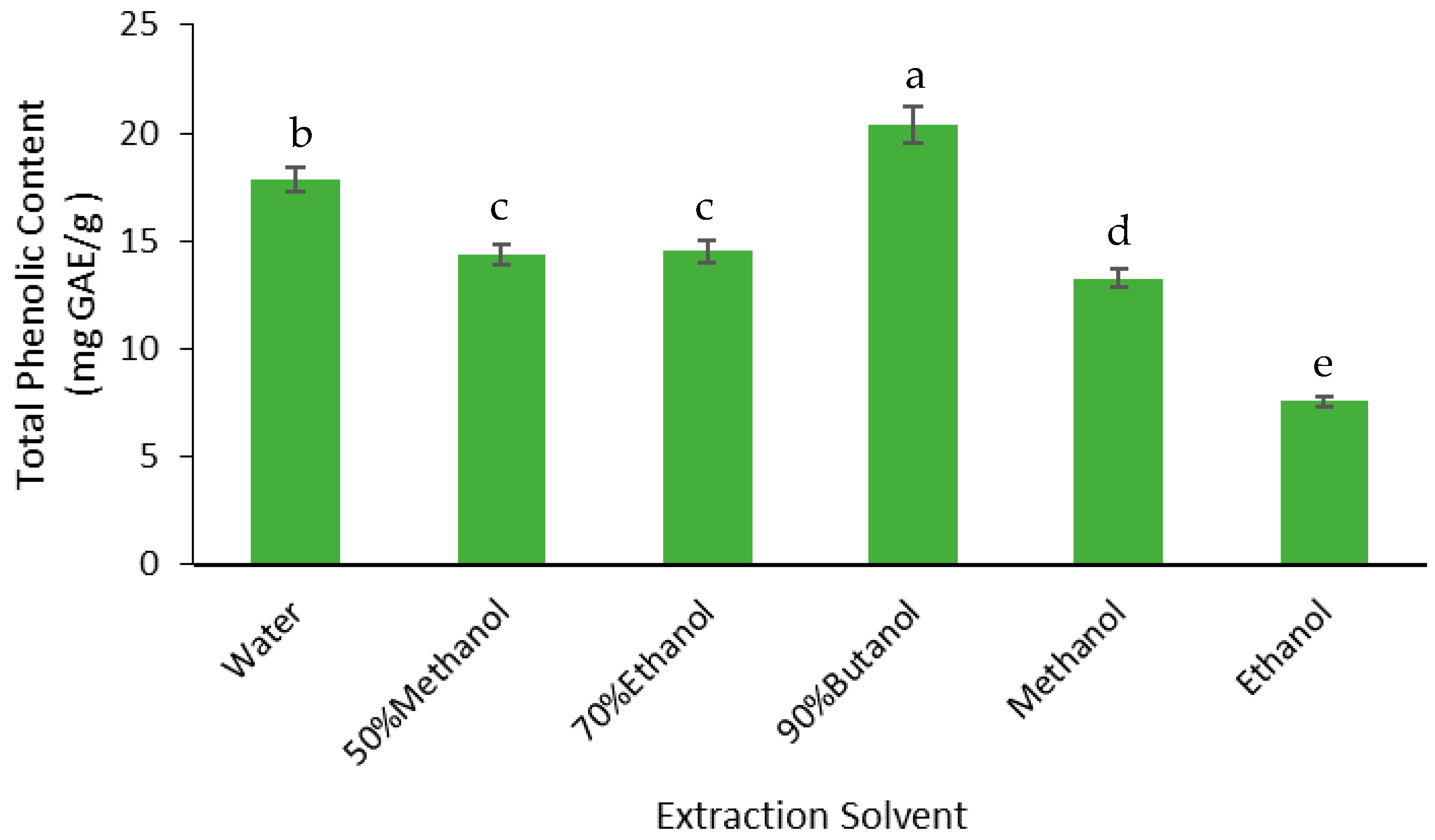
3.2.3. Phenolics
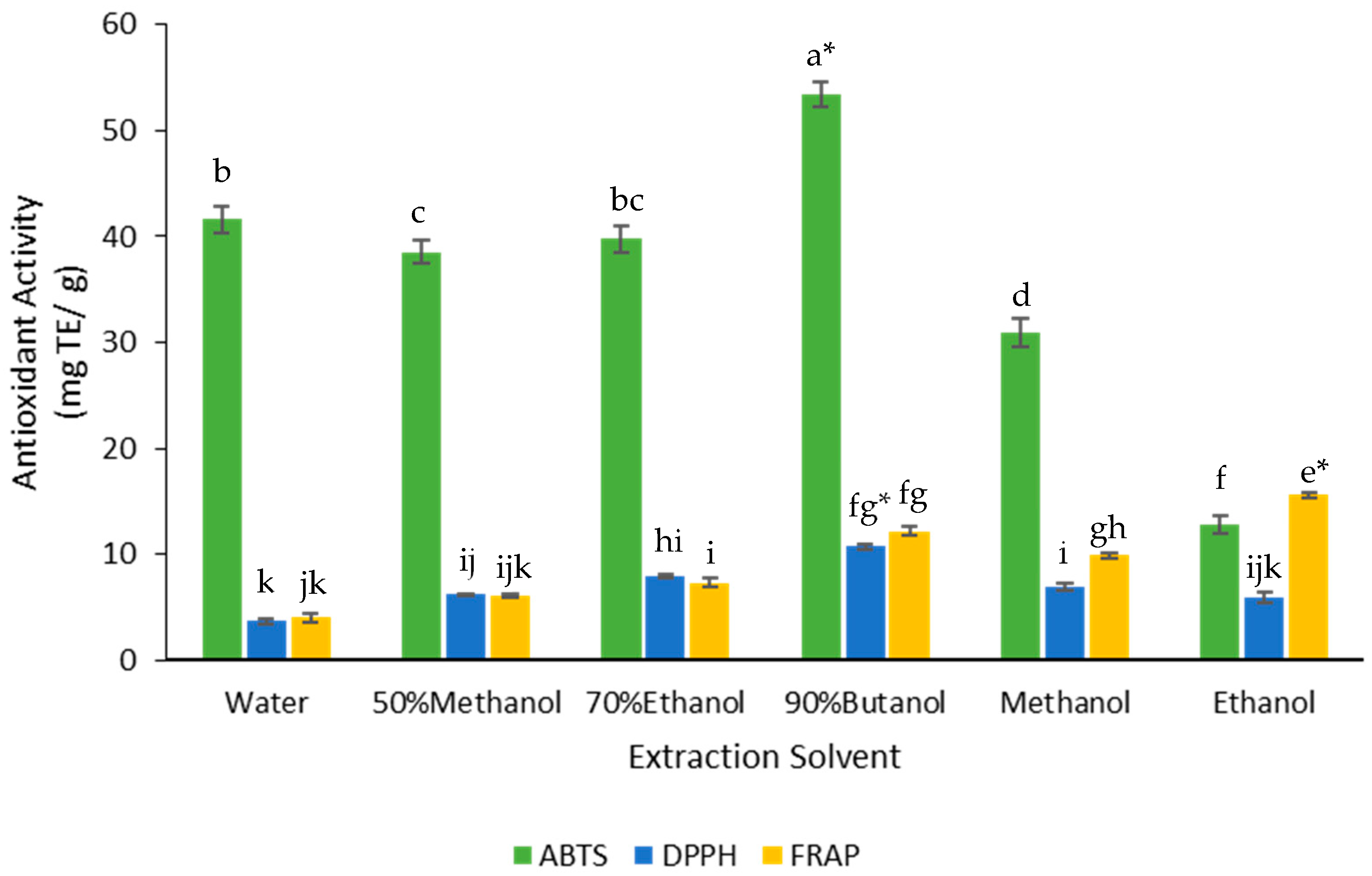
3.3. Effect of Solvents on Antioxidant Activity
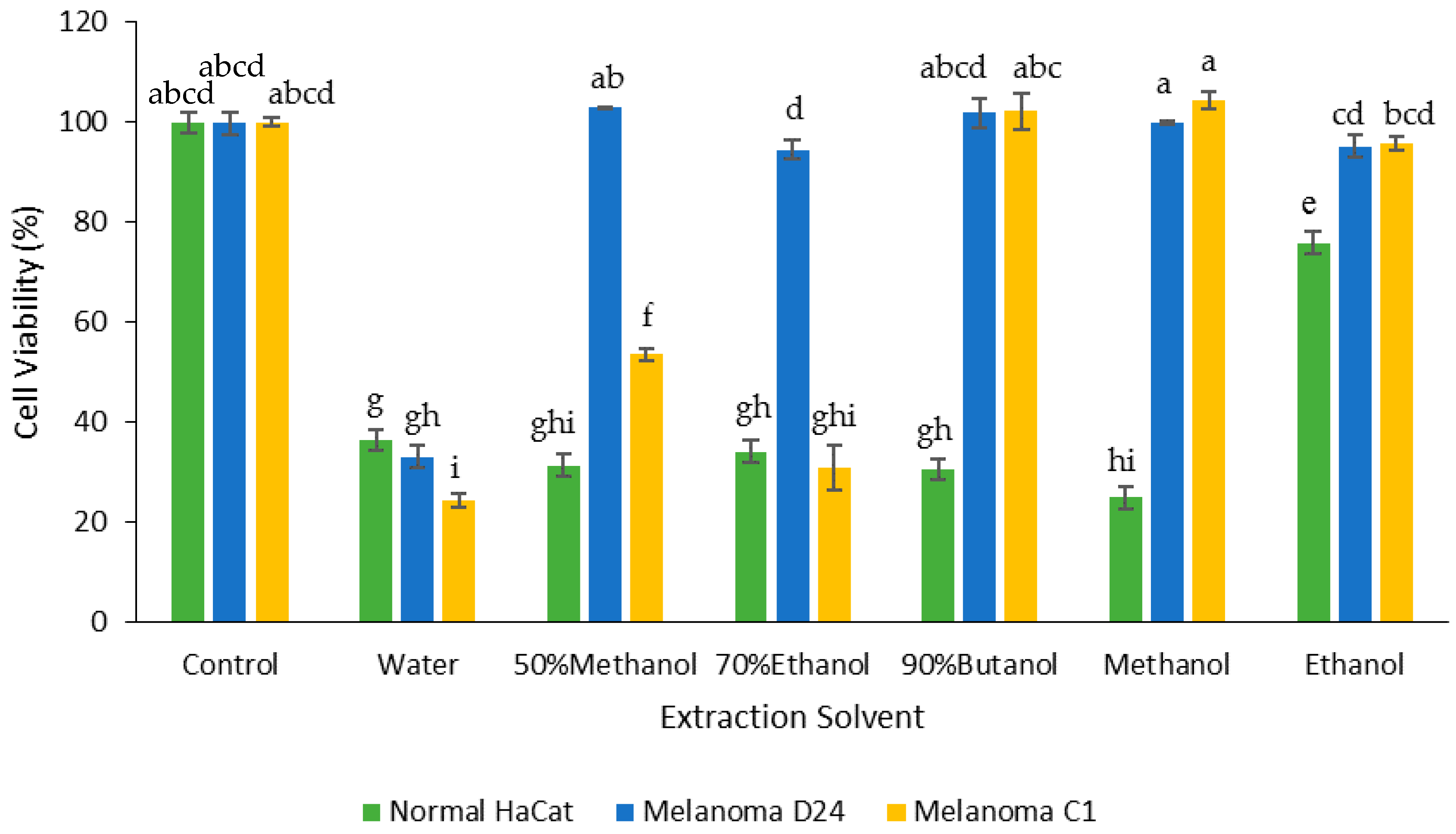
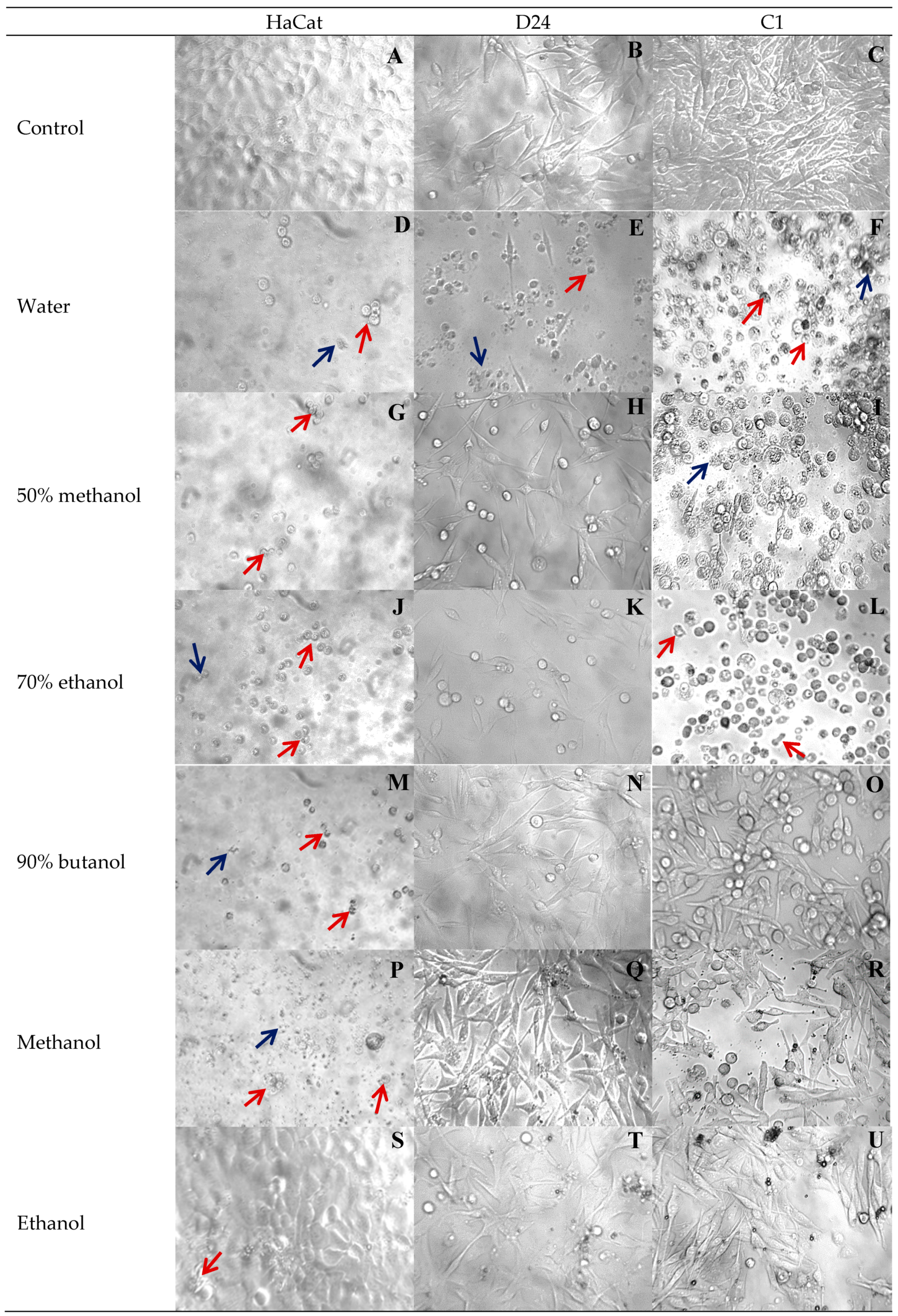
3.4. Effect of Extraction Solvent on Cancer Cell Viability
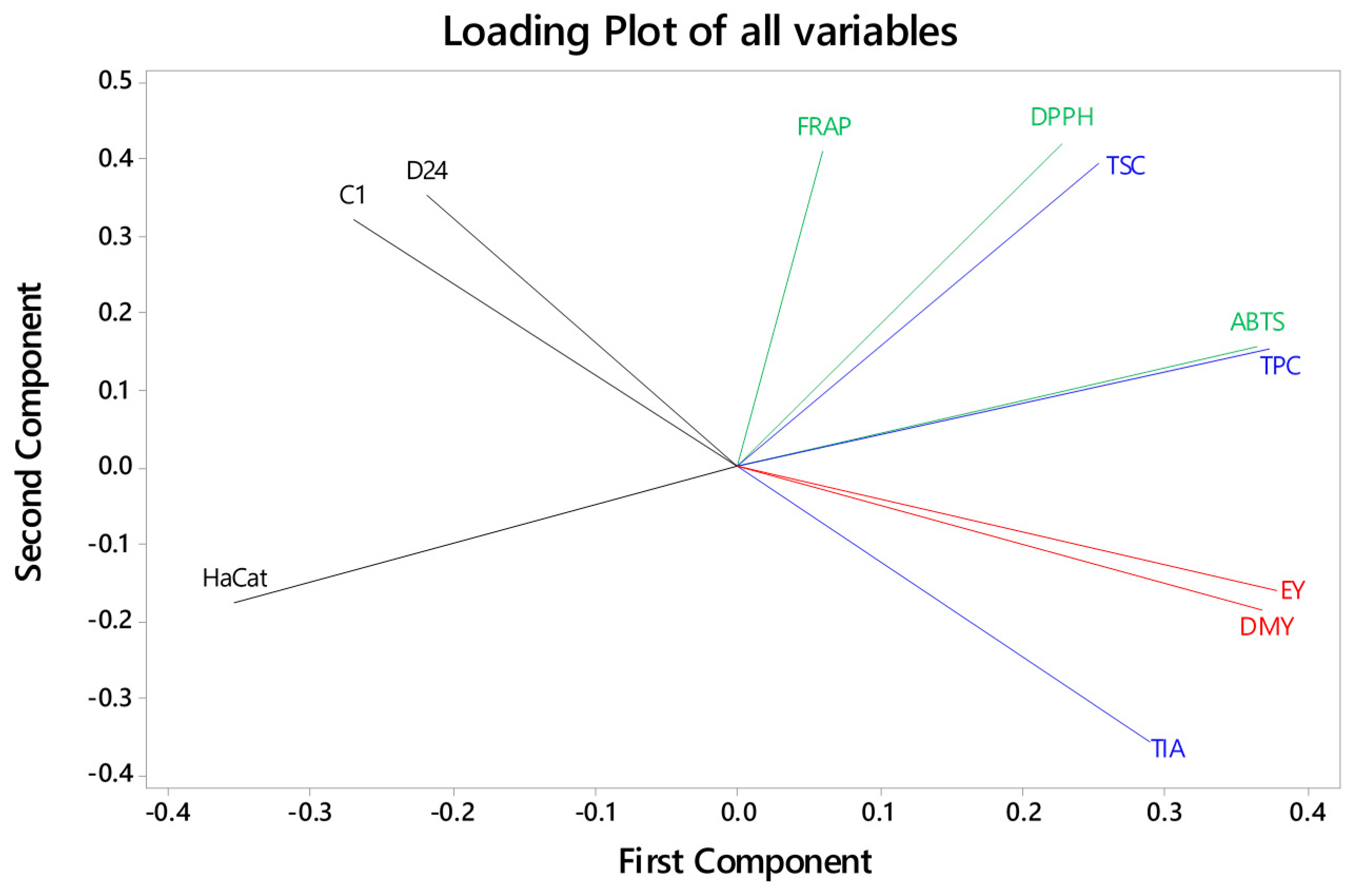
3.5. Correlations between Extract Yields, Bioactive Compounds, Antioxidant Activity, and Cancer Cell Viability across the FD Crude Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wimalasiri, D.; Piva, T.; Urban, S.; Huynh, T. Morphological and genetic diversity of Momordica cochinchinenesis (Cucurbitaceae) in Vietnam and Thailand. Genet. Resour. Crop Evol. 2016, 63, 19–33. [Google Scholar] [CrossRef]
- Behera, T.; John, K.J.; Bharathi, L.; Karuppaiyan, R. Momordica. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Heidelberg, Germany; New York, NY, USA, 2011; pp. 217–246. [Google Scholar]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2015, 50, 567–577. [Google Scholar] [CrossRef]
- Huynh, T.; Nguyen, M.H.; Dao, N. Biomedical importance of Momordica cochinchinensis (GAC) fruit and future applications. BJSTR 2018, 8. in press. [Google Scholar]
- Masayo, I.; Hikaru, O.; Tatsuo, Y.; Masako, T.; Yoshie, R.; Shuji, H.; Kunihide, M.; Ryuichi, H. Studies on the constituents of Momordica cochinchinensis Spreng. I. Isolation and characterization of the seed saponins, Momordica saponins I and II. Chem. Pharm. Bull. 1985, 33, 464–478. [Google Scholar] [CrossRef]
- Chan, L.Y.; Wang, C.K.L.; Major, J.M.; Greenwood, K.P.; Lewis, R.J.; Craik, D.J.; Daly, N.L. Isolation and characterization of peptides from Momordica cochinchinensis seeds. J. Nat. Prod. 2009, 72, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.; Fong, W.; Ng, T. Multiple trypsin inhibitors from Momordica cochinchinensis seeds, the Chinese drug mubiezhi. Peptides 2004, 25, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.Y.; He, W.; Tan, N.; Zeng, G.; Craik, D.J.; Daly, N.L. A new family of cystine knot peptides from the seeds of Momordica cochinchinensis. Peptides 2013, 39, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M. (Ed.) Mu Bie Zi (Semen momordicae). In Chinese Materia Medicia Beijing; Traditional Chinese Materia Medica Press: Beijing, China, 2005; Volume 2, pp. 601–602. [Google Scholar]
- Lin, Z.Y.; Liu, X.; Yang, F.; Yu, Y.Q. Structural characterization and identification of five triterpenoid saponins isolated from Momordica cochinchinensis extracts by liquid chromatography/tandem mass spectrometry. Int. J. Mass Spectrom. 2012, 328, 43–66. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Kim, N.; Kim, B.; Kim, J.-H.; Lee, B.-Y.; Park, J.H.; Lee, M.K.; Lee, H.S.; Jang, I.-J.; Kim, J.S.; et al. Gastroprotective action of cochinchina momordica seed extract is mediated by activation of CGRP and inhibition of cPLA2/5-LOX pathway. Dig. Dis. Sci. 2009, 54, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Chin, Y.-W.; Chung, Y.H.; Park, Y.H.; Yoo, H.; Min, D.S.; Lee, B.; Kim, J. Anti-gastritis and wound healing effects of Momordicae Semen extract and its active component. Immunopharmacol. Immunotoxicol. 2013, 35, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Kim, N.; Kim, B.; Kim, J.-H.; Lee, B.-Y.; Park, J.H.; Lee, M.K.; Lee, H.S.; Kim, J.S.; Jung, H.C.; et al. Enhancement of gastric ulcer healing and angiogenesis by cochinchina Momordica seed extract in rats. J. Korean Med. Sci. 2010, 25, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Kim, J.H.; Lee, S.; Jung, K.; Kim, K.H.; Cho, J.Y. Src/Syk-targeted anti-inflammatory actions of triterpenoidal saponins from Gac (Momordica cochinchinensis) Seeds. Am. J. Chin. Med. 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Chin, Y.-W.; Yoon, K.D.; Chae, H.-S.; Kim, C.Y.; Yoo, H.; Kim, J. Anti-inflammatory properties of a triterpenoidal glycoside from Momordica cochinchinensis in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2013, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tien, P.G.; Kayama, F.; Konishi, F.; Tamemoto, H.; Kasono, K.; Hung, N.T.; Kuroki, M.; Ishikawa, S.E.; Van Nguyen, C.; Kawakami, M. Inhibition of tumor growth and angiogenesis by water extract of Gac fruit (Momordica cochinchinensis Spreng). Int. J. Oncol. 2005, 26, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Y.-M.; Zhan, Y.-Z.; Liu, C.-X. Momordica cochinchinensis seed extracts suppress migration and invasion of human breast cancer ZR-75-30 cells via down-regulating MMP-2 and MMP-9. Asian Pac. J. Cancer Prev. 2014, 15, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Ichiishi, E.; Mukainaka, T.; Toriumi, M.; Ukiya, M.; Yasukawa, K.; Nishino, H. Anti-tumor promoting effects of multiflorane-type triterpenoids and cytotoxic activity of karounidiol against human cancer cell lines. Cancer Lett. 2001, 173, 9–14. [Google Scholar] [CrossRef]
- Kan, L.; Hu, Q.; Chao, Z.; Song, X.; Cao, X. Chemical constituents of unsaponifiable matter from seed oil of Momordica cochinchinensis. China J. Chin. Mater. Med. 2006, 31, 1441–1444. [Google Scholar]
- Hernandez, J.F.; Gagnon, J.; Chiche, L.; Nguyen, T.M.; Andrieu, J.P.; Heitz, A.; Trinh Hong, T.; Pham, T.T.; Le Nguyen, D. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry 2000, 39, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, J.; Craik, D.J. Discovery, structure, function, and applications of cyclotides: Circular proteins from plants. J. Exp. Bot. 2016, 67, 4801–4812. [Google Scholar] [CrossRef] [PubMed]
- Sever, N.; Filipic, M.; Brzin, J.; Lah, T.T. Effect of cysteine proteinase inhibitors on murine B16 melanoma cell invasion in vitro. Biol. Chem. 2002, 383, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Heidtmann, H.-H.; Salge, U.; Abrahamson, M.; Bencina, M.; Kastelic, L.; Kopitar-Jerala, N.; Turk, V.; Lah, T.T. Cathepsin B and cysteine proteinase inhibitors in human lung cancer cell lines. Clin. Exp. Metastasis 1997, 15, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A. Protease inhibitors and carcinogenesis: A review. Cancer Investig. 1996, 14, 597–608. [Google Scholar] [CrossRef]
- Hocman, G. Chemoprevention of cancer: Protease inhibitors. Int. J. Biochem. 1992, 24, 1365–1375. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Parveen, R.; Afreen, S.; Azam, M.; Sen, P.; Sharma, Y.; Haque, Q.M.R.; Fatma, T.; Manzoor, N.; Fatima, S. Trypsin inhibitors from Cajanus cajan and Phaseolus limensis possess antioxidant, anti-Inflammatory, and antibacterial activity. J. Diet. Suppl. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, G.; Kyerematen, G.; Farah, M.H. Preliminary chemical characterization of pharmacologically active compounds in aqueous plant extracts. J. Ethnopharmacol. 1985, 14, 193–201. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Mahatmanto, T.; Poth, A.G.; Mylne, J.S.; Craik, D.J. A comparative study of extraction methods reveals preferred solvents for cystine knot peptide isolation from Momordica cochinchinensis seeds. Fitoterapia 2014, 95, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Abascal, K.; Ganora, L.; Yarnell, E. The effect of freeze-drying and its implications for botanical medicine: A review. Phytother. Res. 2005, 19, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Klomklao, S.; Benjakul, S.; Kishimura, H.; Chaijan, M. Extraction, purification and properties of trypsin inhibitor from Thai mung bean (Vigna radiata (L.) R. Wilczek). Food Chem. 2011, 129, 1348–1354. [Google Scholar] [CrossRef]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Effect of solvents and extraction methods on recovery of bioactive compounds from defatted Gac (Momordica cochinchinensis Spreng.) seeds. Separations 2018, 5, 39. [Google Scholar] [CrossRef]
- Makkar, H.P.; Siddhuraju, P.; Becker, K. Trypsin Inhibitor. In Plant Secondary Metabolites; Humana Press: New York, NY, USA, 2007; pp. 1–6. [Google Scholar]
- Stauffer, C.E. Measuring trypsin inhibitor in soy meal: Suggested improvements in the standard method. Cereal Chem. 1990, 67, 296–302. [Google Scholar]
- Tan, S.P.; Tuyen, K.C.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]
- Tan, S.P.; Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J. Food Sci. 2014, 79, E1372–E1381. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A combined assay of cell vability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm Bull. 1996, 19, 1518–1520. [Google Scholar] [CrossRef] [PubMed]
- Wimalasiri, D. Genetic Diversity, Nutritional and Biological Activity of Momordica cochinchinensis (Cucurbitaceae). Ph.D. Thesis, RMIT University, Melbourne, Australia, 2015. [Google Scholar]
- Arimatsu, P.; Sawangsook, O. Purification and characterization of Trypsin inhibitor from Momordica cochinchinensis Spreng. seed and its effects to Spodoptera litura. Asian J. Plant Sci. 2012, 11, 195–199. [Google Scholar] [CrossRef]
- Cascales, L.; Henriques, S.T.; Kerr, M.C.; Huang, Y.-H.; Sweet, M.J.; Daly, N.L.; Craik, D.J. Identification and characterization of a new family of cell-penetrating peptides cyclic cell-penetrating peptides. J. Biol. Chem. 2011, 286, 36932–36943. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Wolf, W.J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125, 581S. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y. Plant Protease Inhibitors as Cancer Chemopreventive Agents; Springer: Berlin, Germany, 2003; ISBN 978-3-642-05514-0. [Google Scholar]
- Troll, W.; Kennedy, A.R. Protease Inhibitors as Cancer Chemopreventive Agents; Plenum Pub Corp: New York, NY, USA, 1993; ISBN 0306443902. [Google Scholar]
- Kennedy, A.R. Chemopreventive agents: Protease inhibitors. Pharmacol. Ther. 1998, 78, 167–209. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Iqbal, S.; Khong, N.M.; Ooi, D.-J.; Ismail, M. Antioxidant activity of phenolics–saponins rich fraction prepared from defatted kenaf seed meal. LWT-Food Sci. Technol. 2014, 56, 181–186. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Chemistry and Pharmacology of Natural Products, Saponin; Cambridge University Press: Cambridge, NY, USA, 1995; ISBN 0-521-32970-1. [Google Scholar]
- US Food and Drug Administration (FDA). Guidance for Industry; USHaH Services, Ed.; US Food and Drug Administration (FDA): Silver Spring, MD, USA, 2012.
- Yasukawa, K.; Akihisa, T.; Tamura, T.; Takido, M. Inhibitory effect of karounidiol on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion. Biol. Pharm Bull. 1994, 17, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Optimisation of the microwave-assisted ethanol extraction of saponins from Gac (Momordica cochinchinensis Spreng) seeds. Medicines 2018, 5, 70. [Google Scholar] [CrossRef] [PubMed]
| Component | Reagent Blank (a) | Standard (b) | Sample Blank (c) | Sample (d) |
|---|---|---|---|---|
| Deionised water (mL) | 2 | 2 | 1 | 1 |
| Trypsin solution (mL) | - | 2 | - | 2 |
| Diluted extract (mL) | - | - | 1 | 1 |
| BAPNA (mL) | 5 | 5 | 5 | 5 |
| Acetic acid (mL) | 1 | 1 | 1 | 1 |
| Trypsin solution after reaction inactivation (mL) | 2 | - | 2 | - |
| Solvent | EY (g/100 g) | DM (g/100 g) | Original Volume (mL) | Collected Volume (mL) | Yield Loss (%) |
|---|---|---|---|---|---|
| Water | 15.5 ± 0.1 a | 13.1 ± 0.1 b | 400 ± 3 | 355 ± 0 | 15.3 ± 0.1 d |
| 50% Methanol | 10.2 ± 0.0 c | 9.1 ± 0.3 d | 400 ± 3 | 348 ± 3 | 10.9 ± 0.2 f |
| 70% Ethanol | 10.2 ± 0.0 c | 7.1 ± 0.4 e | 400 ± 3 | 340 ± 5 | 30.6 ± 0.3 a |
| 90% Butanol | 6.9 ± 0.1 e | 5.5 ± 0.1 f | 400 ± 3 | 350 ± 0 | 19.9 ± 0.1 c |
| Methanol | 6.7 ± 0.2 e | 4.8 ± 0.2 g | 400 ± 3 | 330 ± 0 | 27.8 ± 0.2 b |
| Ethanol | 4.4 ± 0.0 g | 3.7 ± 0.0 h | 400 ± 3 | 345 ± 0 | 14.9 ± 0.0 e |
| Yield | Bioactive Compound | Antioxidant Activity | Cell Viability | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EY | DMY | TIA | TSC | TPC | ABTS | DPPH | FRAP | HaCat | D24 | C1 | |
| EY | 1.00 | ||||||||||
| DMY | 0.98 † | 1.00 | |||||||||
| TIA | 0.96 ‡ | 0.97 ‡ | 1.00 | ||||||||
| TSC | −0.42 | −0.50 | −0.61 | 1.00 | |||||||
| TPC | 0.51 | 0.49 | 0.31 | 0.36 | 1.00 | ||||||
| ABTS | 0.48 | 0.43 | 0.29 | 0.44 | 0.97 † | 1.00 | |||||
| DPPH | −0.52 | −0.58 | −0.66 | 0.81 § | 0.37 | 0.46 | 1.00 | ||||
| FRAP | −0.93 ‡ | −0.89 § | −0.88 § | 0.19 | −0.48 | −0.50 | 0.44 | 1.00 | |||
| HaCat | −0.42 | −0.35 | −0.22 | −0.63 | −0.73 | −0.78 | −0.28 | 0.63 | 1.00 | ||
| D24 | −0.79 | −0.80 | −0.77 | 0.65 | −0.28 | −0.13 | 0.69 | 0.56 | −0.01 | 1.00 | |
| C1 | −0.88 § | −0.83 § | −0.92 § | 0.53 | −0.23 | −0.26 | 0.50 | 0.83 § | 0.18 | 0.61 | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, A.V.; Huynh, T.T.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds. Medicines 2018, 5, 104. https://doi.org/10.3390/medicines5030104
Le AV, Huynh TT, Parks SE, Nguyen MH, Roach PD. Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds. Medicines. 2018; 5(3):104. https://doi.org/10.3390/medicines5030104
Chicago/Turabian StyleLe, Anh V., Tien T. Huynh, Sophie E. Parks, Minh H. Nguyen, and Paul D. Roach. 2018. "Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds" Medicines 5, no. 3: 104. https://doi.org/10.3390/medicines5030104
APA StyleLe, A. V., Huynh, T. T., Parks, S. E., Nguyen, M. H., & Roach, P. D. (2018). Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds. Medicines, 5(3), 104. https://doi.org/10.3390/medicines5030104






