Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines
2.3. Standard Methods
2.4. Reversal of Anti-Parasitic Activity
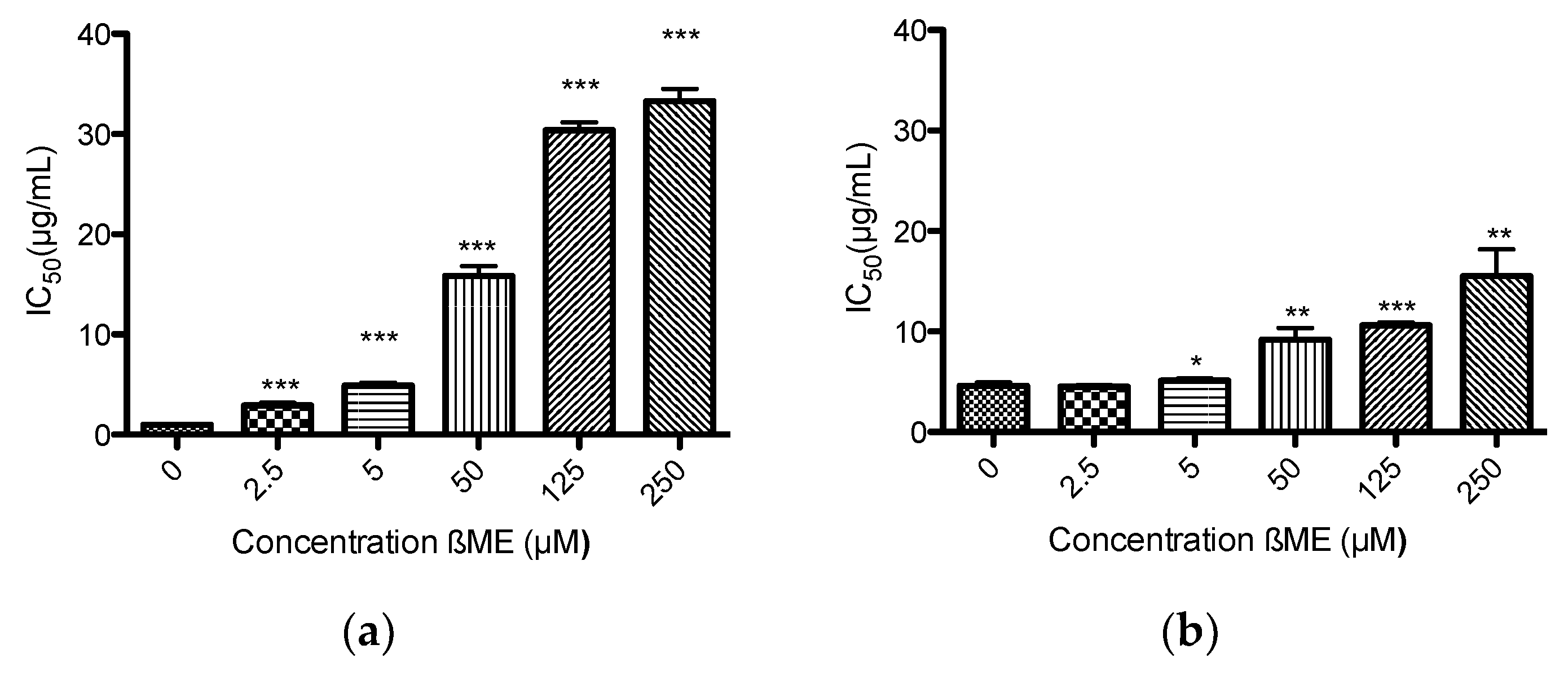
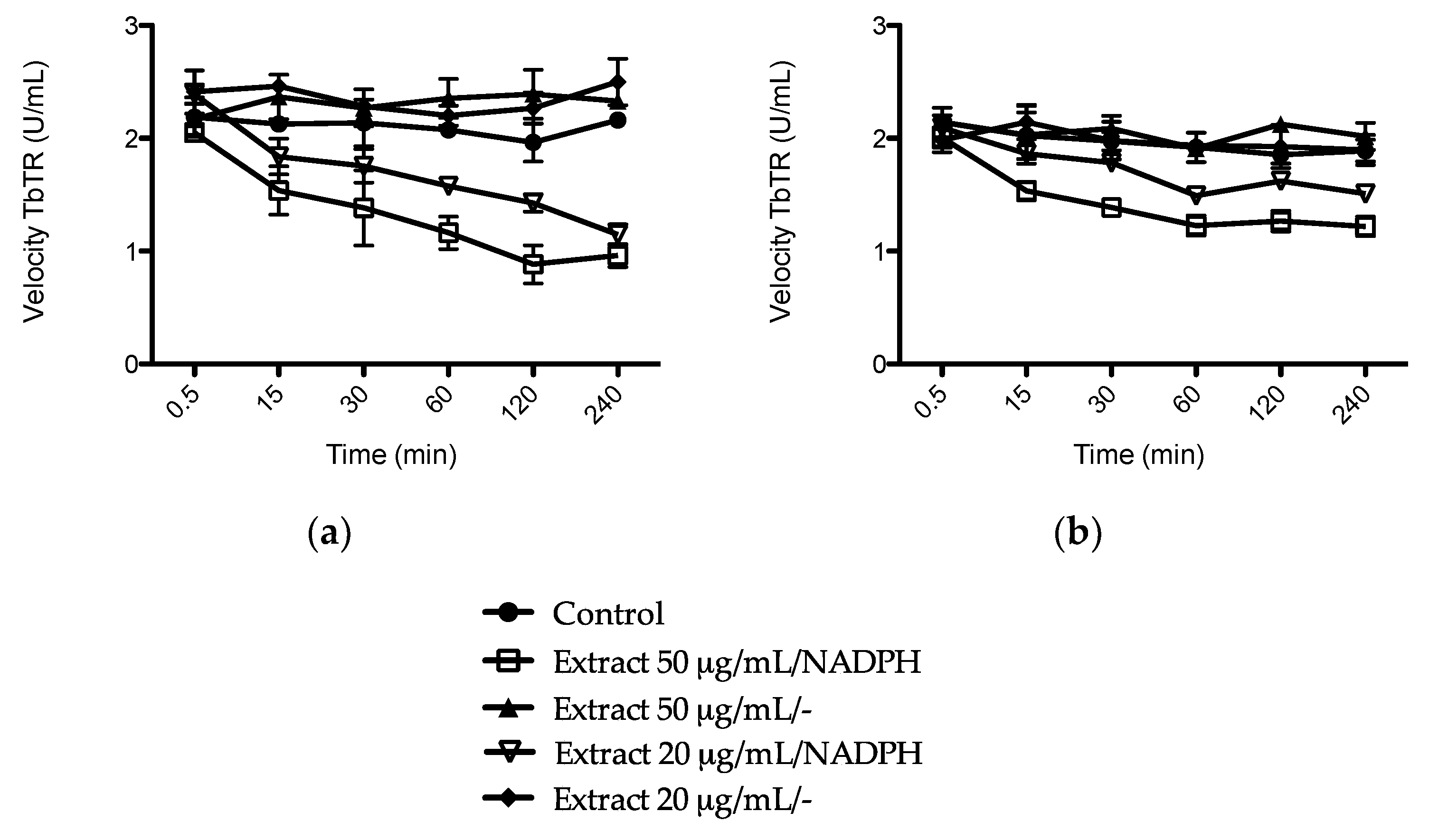
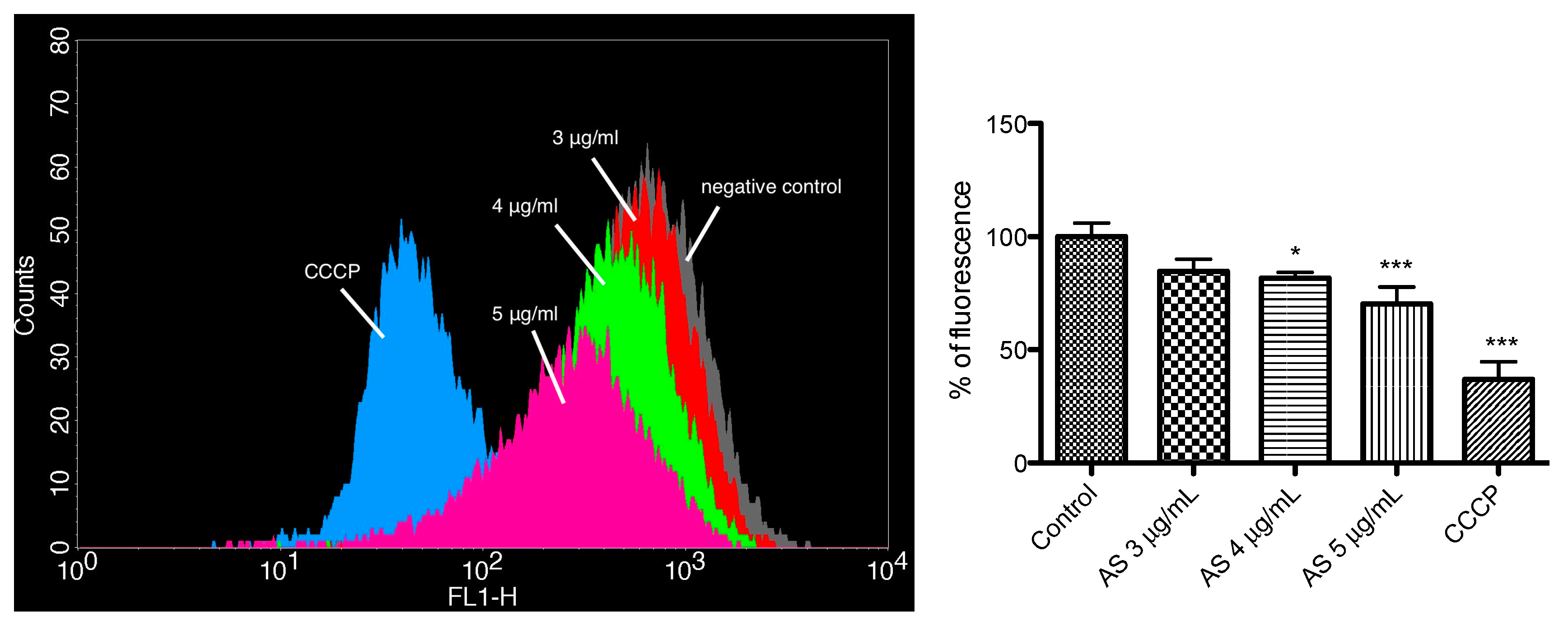
2.5. Mitochondrial Membrane Potential Assay
2.6. Drug Combinations
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs and Poisons; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Mnayer, D.; Fabiano-Tixier, A.S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Munday, C.M. Relative activities of organosulfur compounds derived from onions and garlic in increasing tissue activities of quinone reductase and glutathione transferase in rat tissues. Nutr. Cancer 2001, 40, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Sendl, A. Allium sativum and Allium ursinum: Part 1 Chemistry, analysis, history, botany. Phytomedicine 1995, 1, 323–339. [Google Scholar] [CrossRef]
- Suleria, H.A.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Nejad, B.; Saki, J. Effect of aqueous Allium cepa and Ixora brachiata root extract on Leishmania major promastigotes. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e15442. [Google Scholar] [CrossRef] [PubMed]
- Saleheen, D.; Ali, S.A.; Yasinzai, M.M. Antileishmanial activity of aqueous onion extract In Vitro. Fitoterapia 2004, 75, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wabwoba, B.W.; Anjili, C.O.; Ngeiywa, M.M.; Ngure, P.K.; Kigondu, E.M.; Ingonga, J.; Makwali, J. Experimental chemotherapy with Allium sativum (Liliaceae) methanolic extract in rodents infected with Leishmania major and Leishmania donovani. J. Vector Borne Dis. 2010, 47, 160–167. [Google Scholar] [PubMed]
- Gallwitz, H.; Bonse, S.; Martinez-Cruz, A.; Schlichting, I.; Schumacher, K.; Krauth-Siegel, R.L. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: Crystallographic, kinetic, and spectroscopic studies. J. Med. Chem. 1999, 42, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Sendl, A.; Elbl, G.; Steinke, B.; Redl, K.; Breu, W.; Wagner, H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med 1992, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.; Shin, I.; Shimon, L.J.; Miron, T.; Wilchek, M.; Mirelman, D.; Frolow, F.; Rabinkov, A. Thiol-disulfide organization in alliin lyase (alliinase) from garlic (Allium sativum). Protein Sci. 2009, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Munchberg, U.; Anwar, A.; Mecklenburg, S.; Jacob, C. Polysulfides as biologically active ingredients of garlic. Org. Biomol. Chem. 2007, 5, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Benkeblia, N.; Lanzotti, V. Allium thiosulfinates: Chemistry, biological properties and their potential utilization in food preservation. Food 2007, 1, 193–201. [Google Scholar]
- Neglected Tropical Diseases, World Health Organization (WHO). Available online: http://www.who.int/neglected_diseases/diseases/en/ (accessed on 6 June 2016).
- Leishmaniasis, World Health Organization (WHO). Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 8 June 2016).
- Leroux, A.E.; Krauth-Siegel, R.L. Thiol redox biology of trypanosomatids and potential targets for chemotherapy. Mol. Biochem. Parasitol. 2015, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [PubMed]
- Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Tulbaghia violacea and Allium ursinum extracts exhibit anti-parasitic and antimicrobial activities. Molecules 2018, 23, 313. [Google Scholar] [CrossRef] [PubMed]
- Divo, A.A.; Patton, C.L.; Sartorelli, A.C. Evaluation of rhodamine 123 as a probe for monitoring mitochondrial function in Trypanosoma brucei spp. J. Eukaryot. Microbiol. 1993, 40, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Krstin, S.; Mohamed, T.; Wang, X.; Wink, M. How do the alkaloids emetine and homoharringtonine kill trypanosomes? An insight into their molecular modes of action. Phytomedicine 2016, 23, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wientjes, M.G.; Au, J.L. Evaluation of combination chemotherapy: Integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin. Cancer Res. 2004, 10, 7994–8004. [Google Scholar] [CrossRef] [PubMed]
- Ferary, S.; Auger, J. What is the true odour of cut Allium? Complementarity of various hyphenated methods: Gas chromatography-mass spectrometry and high-performance liquid chromatography-mass spectrometry with particle beam and atmospheric pressure ionization interfaces in sulphenic acids rearrangement components discrimination. J. Chromatogr. A 1996, 750, 63–74. [Google Scholar]
- Arnault, I.; Christides, J.P.; Mandon, N.; Haffner, T.; Kahane, R.; Auger, J. High-performance ion-pair chromatography method for simultaneous analysis of alliin, deoxyalliin, allicin and dipeptide precursors in garlic products using multiple mass spectrometry and UV detection. J. Chromatogr. A 2003, 991, 69–75. [Google Scholar] [CrossRef]
- Mondy, N.; Naudin, A.; Christides, J.P.; Mandon, N.; Auger, J. Comparison of GC-MS and HPLC for the analysis of Allium volatiles. Chromatographia 2001, 53, 356–360. [Google Scholar] [CrossRef]
- Calvey, E.M.; White, K.D.; Matusik, J.E.; Sha, D.; Block, E. Allium chemistry: Identification of organosulfur compounds in ramp (Allium tricoccum) homogenates. Phytochemistry 1998, 49, 359–364. [Google Scholar] [CrossRef]
- Calvey, E.M.; Matusik, J.E.; White, K.D.; DeOrazio, R.; Sha, D.; Block, E. Allium chemistry: Supercritical fluid extraction and LC-APCI-MS of thiosulfinates and related compounds from homogenates of garlic, onion, and ramp. Identification in garlic and ramp and synthesis of 1-propanesulfinothioic acid S-allyl ester. J. Agric. Food Chem. 1997, 45, 4406–4413. [Google Scholar] [CrossRef]
- Aoyagi, M.; Kamoi, T.; Kato, M.; Sasako, H.; Tsuge, N.; Imai, S. Structure and bioactivity of thiosulfinates resulting from suppression of lachrymatory factor synthase in onion. J. Agric. Food Chem. 2011, 59, 10893–10900. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Cerami, A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992, 46, 695–729. [Google Scholar] [CrossRef] [PubMed]
- Elnima, E.I.; Ahmed, S.A.; Mekkawi, A.G.; Mossa, J.S. The antimicrobial activity of garlic and onion extracts. Pharmazie 1983, 38, 747–748. [Google Scholar] [PubMed]
- Benmalek, Y.; Yahia, O.A.; Belkebir, A.; Fardeau, M.L. Anti-microbial and anti-oxidant activities of Illicium verum, Crataegus oxyacantha ssp monogyna and Allium cepa red and white varieties. Bioengineered 2013, 4, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, X.; Deng, L.; Zhao, X.; Wei, Y.; Gao, Z.; Jia, J.; Xu, J.; Sun, C. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J. Microbiol. 2015, 8, e14814. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; An update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, V.; Wink, M. Alkaloids induce programmed cell death in bloodstream forms of trypanosomes (Trypanosoma b. brucei). Molecules 2008, 13, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
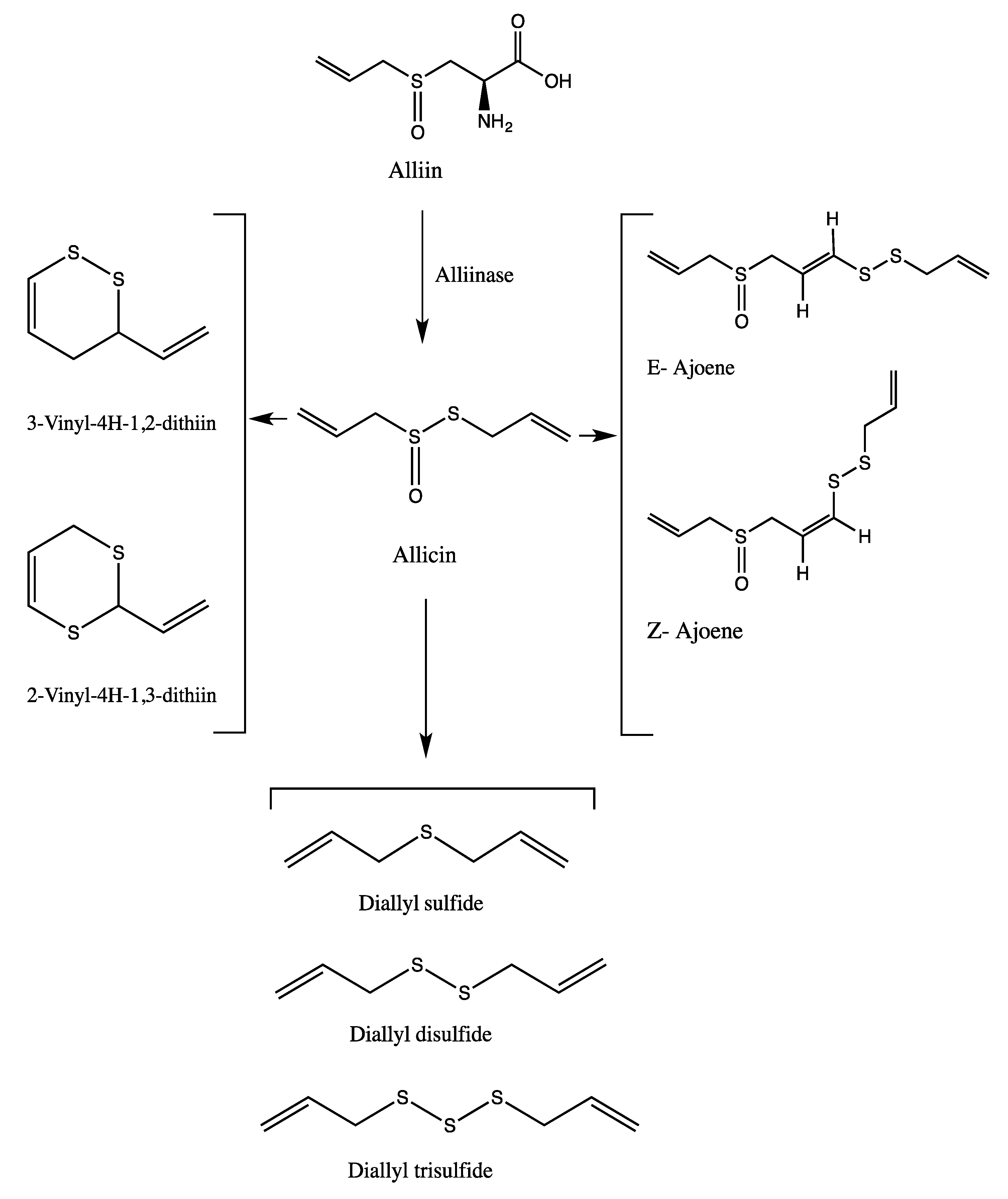
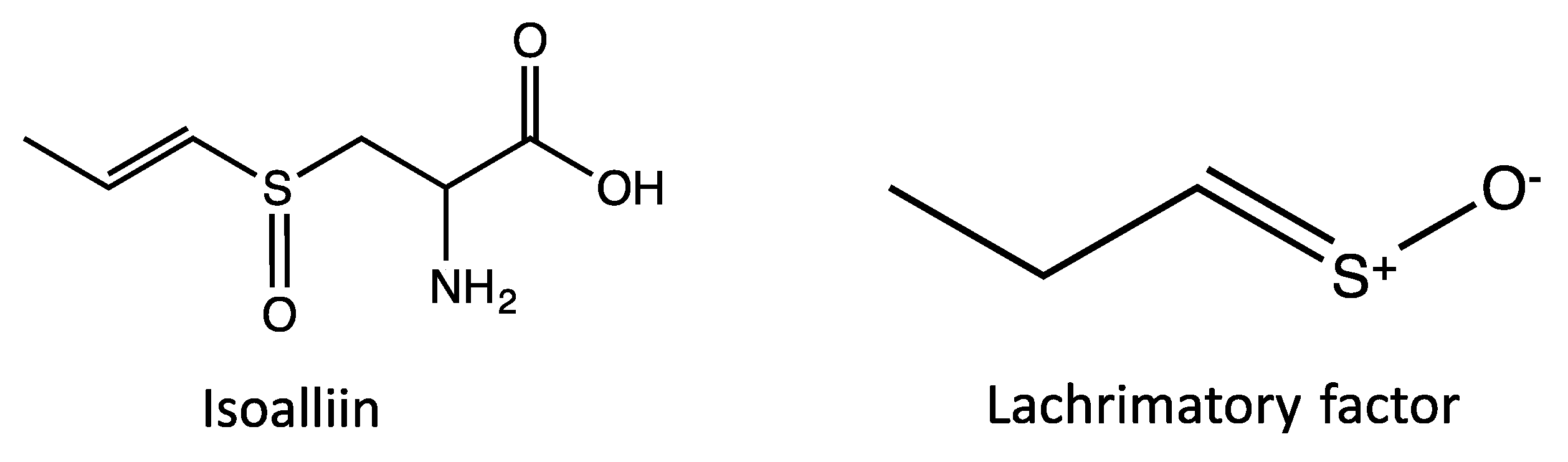
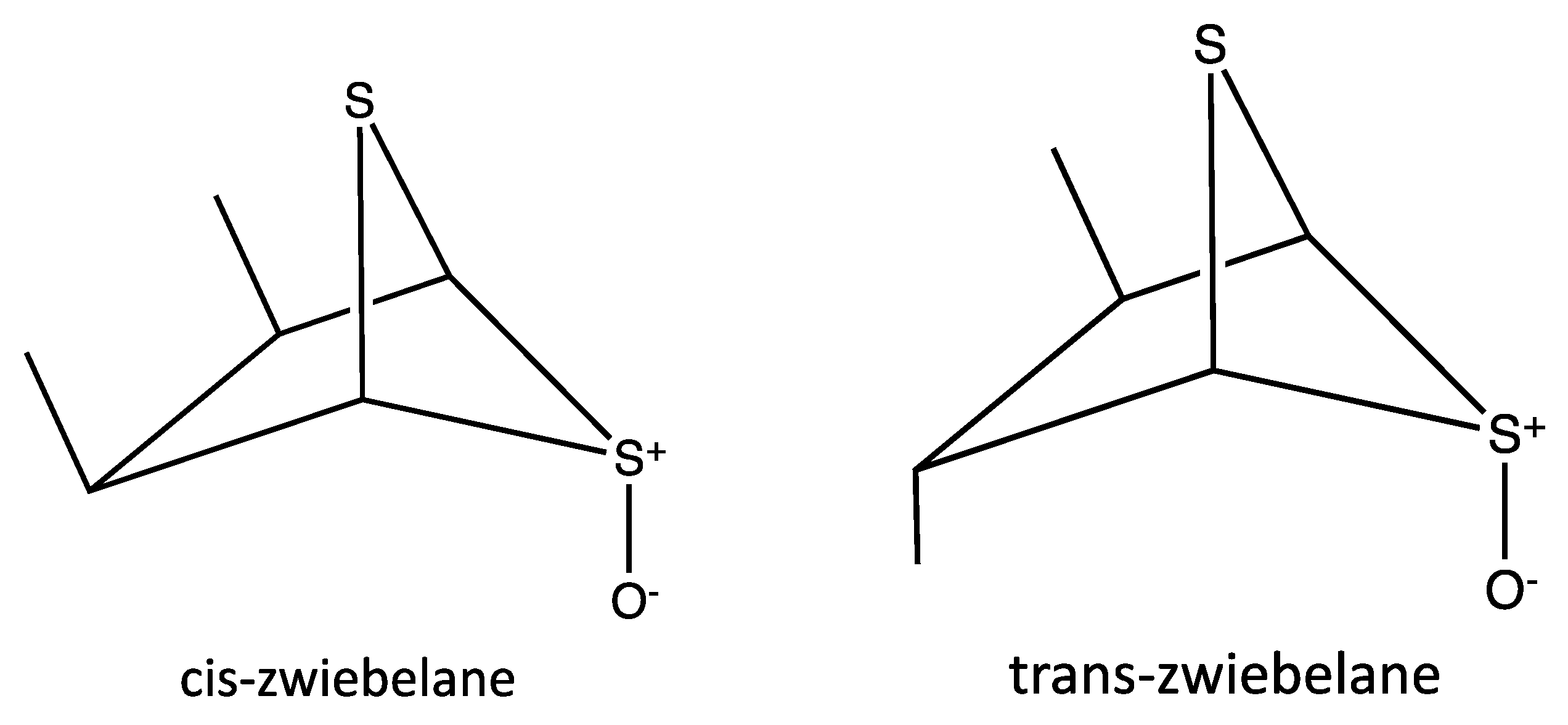
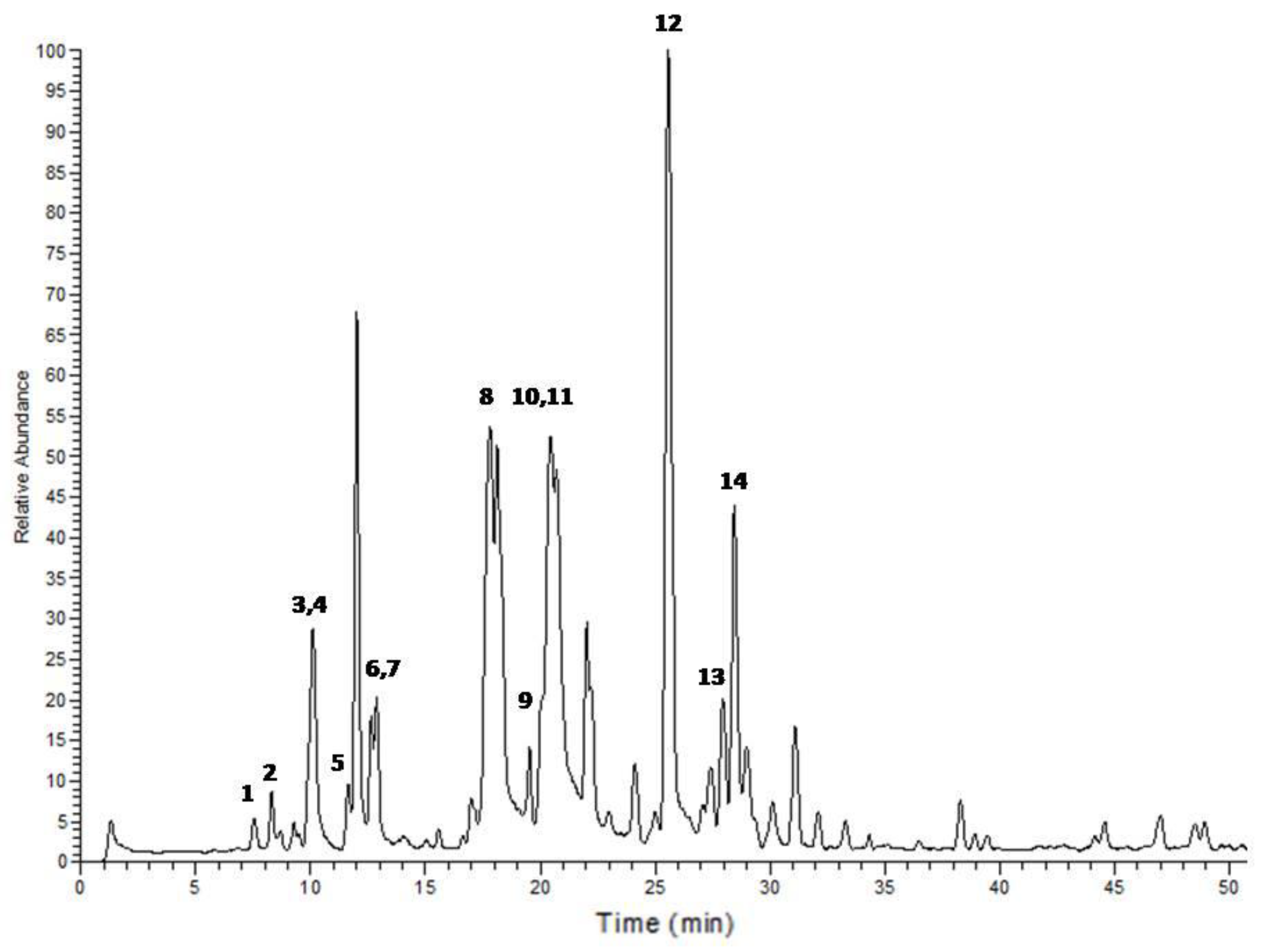
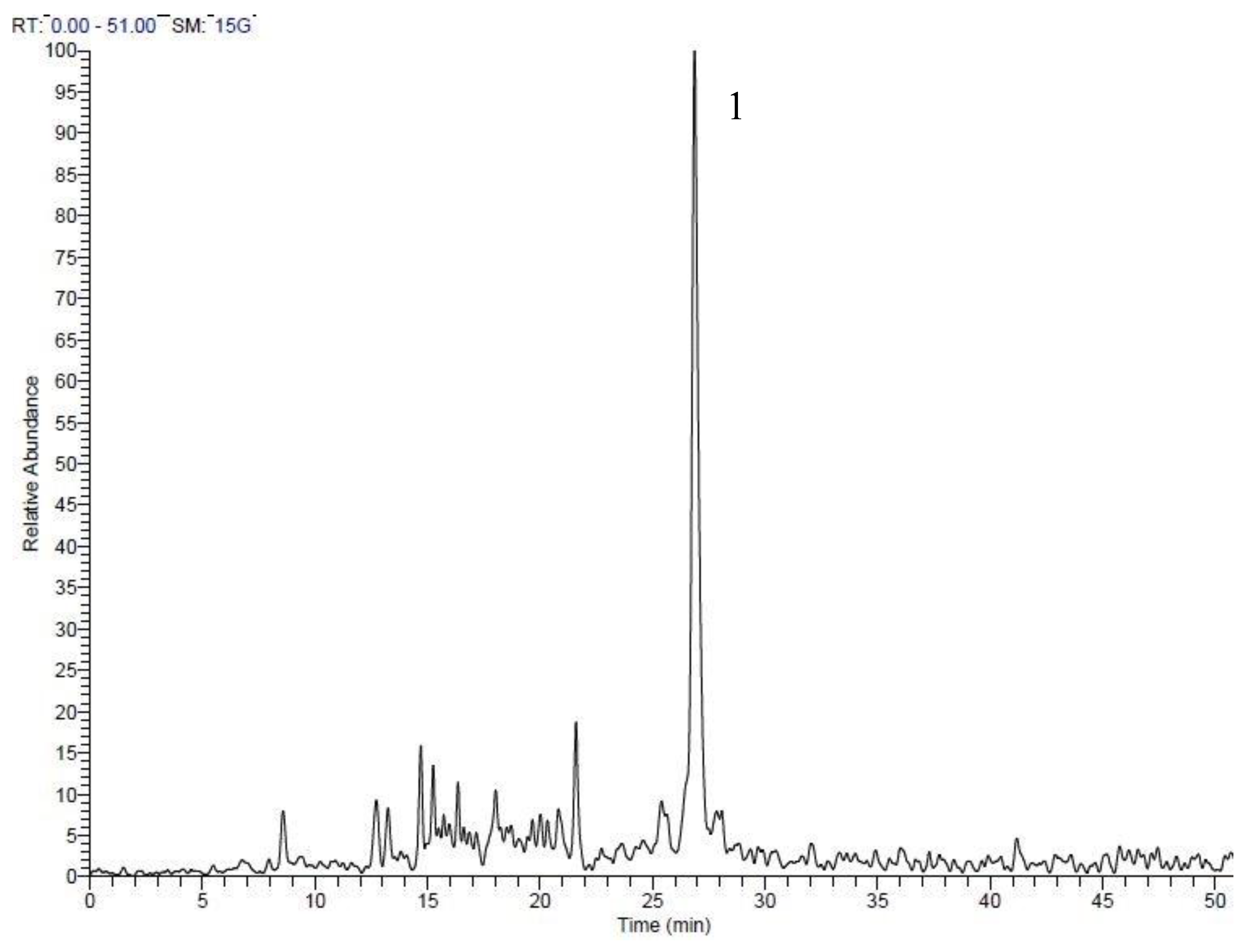
| Peak No. | tR | Area % | [M + H]+ | Tentative identification | Reference |
|---|---|---|---|---|---|
| 1 | 7.54 | 0.69 | 227 | 1-Ethyl-2-(3-(propylsulfinyl)propyl)disulfane | Tentative |
| 2 | 8.31 | 1.12 | 249 | 1-Allyl-2-((1E,3E)-4-(vinyldisulfanyl)buta-1,3-dien-1-yl)disulfane | Tentative |
| 3 | 9.89 | 0.64 | 137 | Methanesulfinothioic acid S-(E)-1-propenyl ester | [24] |
| 4 | 10.08 | 6.34 | 137 | Methanesulfinothioic acid S-(Z)-1-propenyl ester | [24] |
| 5 | 11.68 | 1.29 | 137 | S-methyl 1-propenesulfinothioate/S-1-propenyl methanesulfinothioate | [24] |
| 6 | 12.64 | 2.08 | 251 | Gamma-l-glutamyl-l-cysteine | [25] |
| 7 | 12.92 | 3.06 | 251 | Gamma-l-glutamyl-l-cysteine | [25] |
| 8 | 17.88 | 23.66 | 163 | Allicin | [25] |
| 9 | 20.12 | 1.25 | 209 | (E)-1-allyl-2-(3-(methylesulfinyl)prop-1-en-1-yl)disulfane | Tentative |
| 10 | 20.37 | 11.78 | 163 | 2-Propene-1-sulfinothioic acid S-(E)-1-propenyl ester | [24] |
| 11 | 20.53 | 12.89 | 163 | Propene-1-sulfinothioic acid S-(Z)-1-propenyl ester | [24] |
| 12 | 25.59 | 23.39 | 235 | Ajoene | [26] |
| 13 | 27.95 | 3.31 | 237 | (E)-1-Propenyl 1-(1-propenylsulfinyl)propyl disulfide | [27] |
| 14 | 28.45 | 8.49 | 237 | 2-Propenyl 1-(2-propenylsulfinyl) propyl disulfide | [27] |
| Peak No. | tR | [M + H]+ | Proposed Compound | Reference |
|---|---|---|---|---|
| 1 | 26.87 | 163 | (cis/trans)-zwiebelane | [28] |
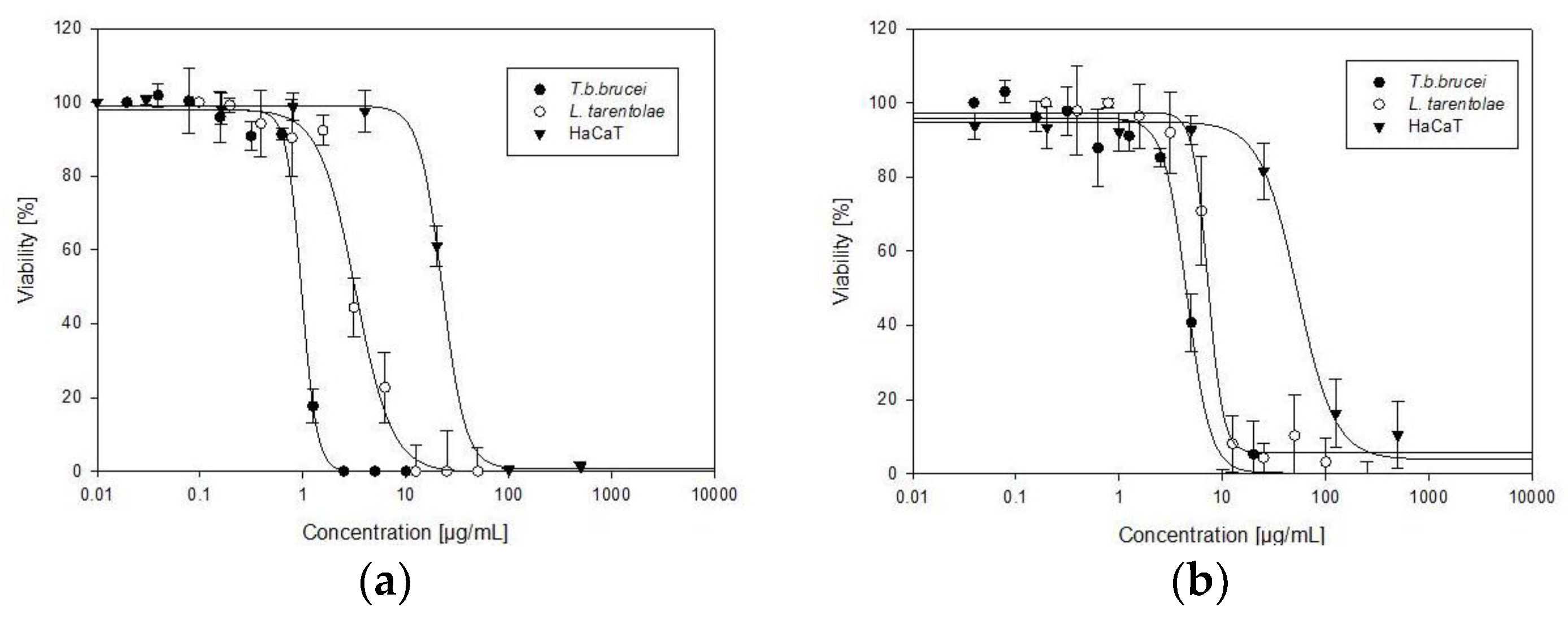
| Sample | IC50T. b. b. | IC50L. t. | IC50 HaCaT | Selectivity Index | |
|---|---|---|---|---|---|
| HaCaT/T. b. b. | HaCaT/L. t. | ||||
| Allium sativum | 0.95 ± 0.04 | 2.89 ± 0.4 | 22.27 ± 1.61 | 23 | 8 |
| Allium cepa | 4.59 ± 0.34 | 7.23 ± 0.78 | 44.56 ± 3.06 | 10 | 6 |
| Suramin | 0.13 ± 0.01 | NT | NT | / | / |
| Amphotericin B | NT | 0.13 ± 0.02 | NT | / | / |
| Doxorubicin | NT | NT | 1.04 ± 0.35 | / | / |
| Gram Type | Sample | A. sativum | A. cepa | Ciprofloxacin | Ampicillin | Nystatin | ||
|---|---|---|---|---|---|---|---|---|
| Indicator Strain | ||||||||
| MIC | MMC | MIC | MMC | MIC | MIC | MIC | ||
| + | Bacillus subtilis | 40 | 160 | 40 | >320 | ≤0.03 | ≤0.03 | NT |
| + | MRSA | 40 | >320 | 320 | >320 | 0.03 | 16 | NT |
| + | MRSA CI | 80 | >320 | 160 | >320 | 4 | 16 | NT |
| + | Staphylococcus epidermidis | 40 | >320 | 80 | >320 | 0.03 | 0.5 | NT |
| + | Enterococcus faecalis | 160 | >320 | >320 | >320 | 0.5 | 1 | NT |
| + | VRE | 320 | >320 | >320 | >320 | 0.5 | 1 | NT |
| + | Streptococcus pyogenes | 80 | 160 | 40 | 40 | 0.13 | <0.03 | NT |
| - | Escherichia coli | 40 | 160 | >320 | >320 | ≤0.03 | 4 | NT |
| - | Escherichia coli EHEC | 40 | >320 | >320 | >320 | ≤0.03 | 4 | NT |
| - | Klebsiella pneumoniae | 80 | >320 | >320 | >320 | 0.125 | >64 | NT |
| - | Klebsiella pneumoniae CI | 80 | >320 | >320 | >320 | <0.03 | 32 | NT |
| - | Pseudomonas aeruginosa | 40 | >320 | 160 | >320 | ≤0.03 | >64 | NT |
| F | Candida albicans | 5 | 5 | 160 | 160 | NT | NT | 10 |
| F | Candida parapsilosis | 5 | 5 | 160 | 160 | NT | NT | 10 |
| Extract | IC50 ± SD Extract Alone (μg/mL) | Drug | IC50 ± SD Drug Alone (μM) | Fixed Concentration of the Extract (μg/mL) | IC50 ± SD Drug in Combination (μM) | CI Value at IC50 | Interpretation |
|---|---|---|---|---|---|---|---|
| T. b. brucei | |||||||
| Allium cepa | 4.59 ± 0.34 | Diminazene | 0.24 ± 0.01 | 0.5 | 0.19 ± 0.09 | 0.90 | Additive |
| 1 | 0.24 ± 0.04 | 1.22 | No effect | ||||
| Pentamidine | 0.07 ± 0.01 | 0.5 | 0.11 ± 0.01 | 1.68 | No effect | ||
| 1 | 0.09 ± 0.01 | 1.50 | No effect | ||||
| Suramin | 0.09 ± 0.01 | 0.5 | 0.07 ± 0.01 | 0.89 | Synergism | ||
| 1 | 0.07 ± 0.01 | 1.00 | Additive | ||||
| L. tarentolae | |||||||
| 7.23 ± 0.78 | Amphotericin B | 0.14 ± 0.02 | 0.75 | 0.15 ± 0.02 | 1.17 | No effect | |
| 1.5 | 0.15 ± 0.02 | 1.28 | No effect | ||||
| Pentamidine | 4.01 ± 0.86 | 0.75 | 3.82 ± 0.81 | 1.06 | Additive | ||
| 1.5 | 3.71 ± 0.94 | 1.13 | No effect | ||||
| T. b. brucei | |||||||
| Allium sativum | 0.95 ± 0.04 | Diminazene | 0.24 ± 0.01 | 0.1 | 0.20 ± 0.05 | 0.94 | Additive |
| 0.2 | 0.19 ± 0.03 | 1.00 | Additive | ||||
| Pentamidine | 0.07 ± 0.01 | 0.1 | 0.05 ± 0.02 | 0.82 | Synergism | ||
| 0.2 | 0.05 ± 0.02 | 0.92 | Additive | ||||
| Suramin | 0.09 ± 0.01 | 0.1 | 0.1 ± 0.01 | 1.22 | No effect | ||
| 0.2 | 0.1 ± 0.01 | 1.32 | No effect | ||||
| L. tarentolae | |||||||
| 2.89 ± 0.4 | Amphotericin B | 0.14 ± 0.02 | 0.32 | 0.14 ± 0.02 | 1.11 | No effect | |
| 0.64 | 0.13 ± 0.01 | 1.15 | No effect | ||||
| Pentamidine | 4.01 ± 0.86 | 0.32 | 3.5 ± 0.45 | 0.98 | Additive | ||
| 0.64 | 3.18 ± 0.46 | 1.01 | Additive | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae. Medicines 2018, 5, 37. https://doi.org/10.3390/medicines5020037
Krstin S, Sobeh M, Braun MS, Wink M. Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae. Medicines. 2018; 5(2):37. https://doi.org/10.3390/medicines5020037
Chicago/Turabian StyleKrstin, Sonja, Mansour Sobeh, Markus Santhosh Braun, and Michael Wink. 2018. "Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae" Medicines 5, no. 2: 37. https://doi.org/10.3390/medicines5020037
APA StyleKrstin, S., Sobeh, M., Braun, M. S., & Wink, M. (2018). Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae. Medicines, 5(2), 37. https://doi.org/10.3390/medicines5020037






