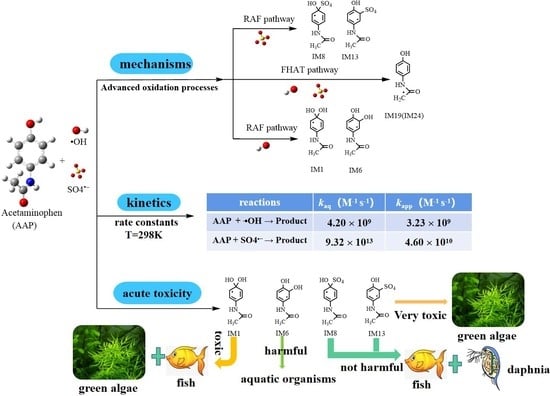
Theoretical Calculation on the Reaction Mechanisms, Kinetics and Toxicity of Acetaminophen Degradation Initiated by Hydroxyl and Sulfate Radicals in the Aqueous Phase
Abstract
:1. Introduction
2. Computational Methods
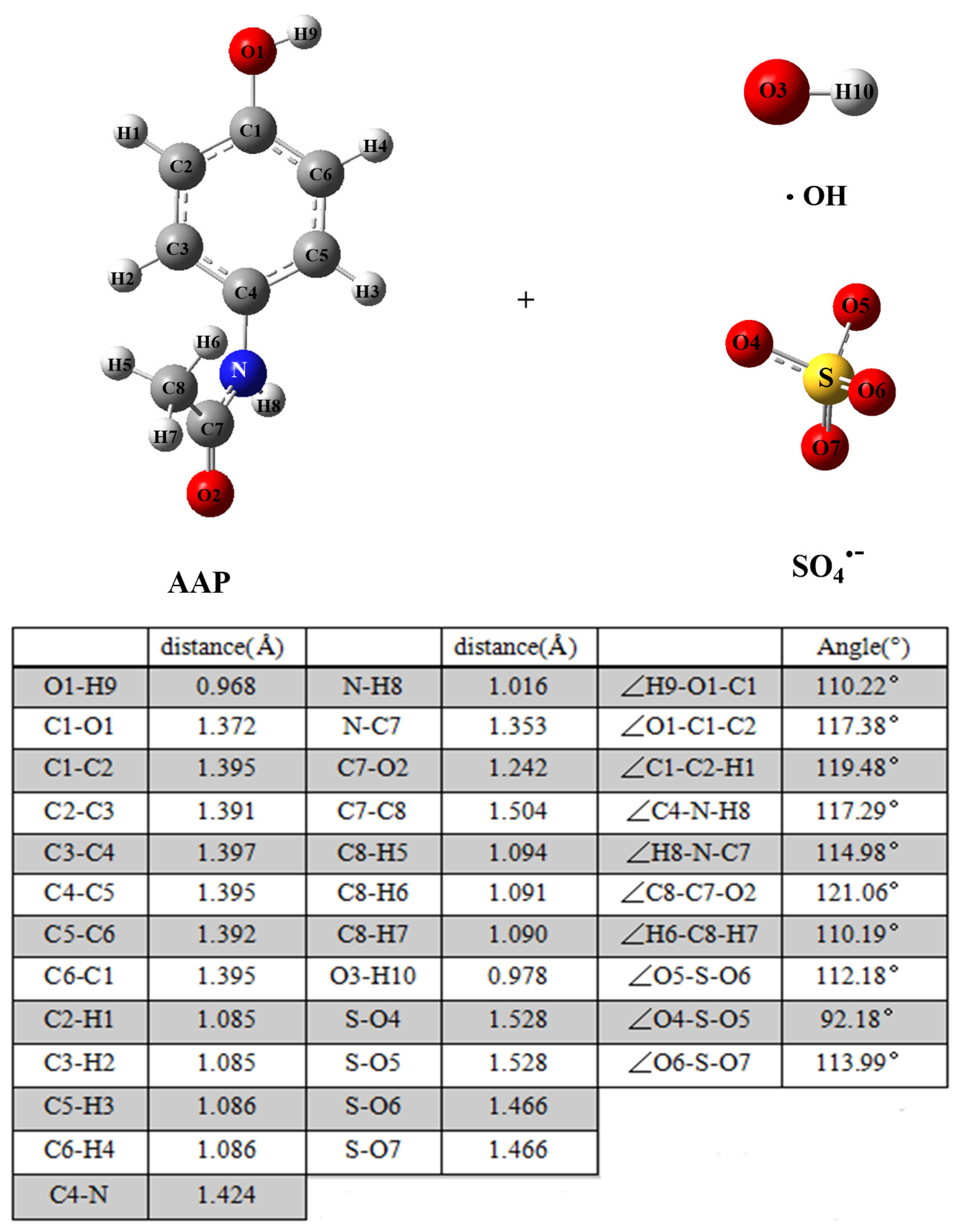
2.1. Mechanism Calculation
2.2. Kinetic Calculation
2.3. Ecotoxicity Calculation
3. Results and Discussion
3.1. Degradation Mechanisms
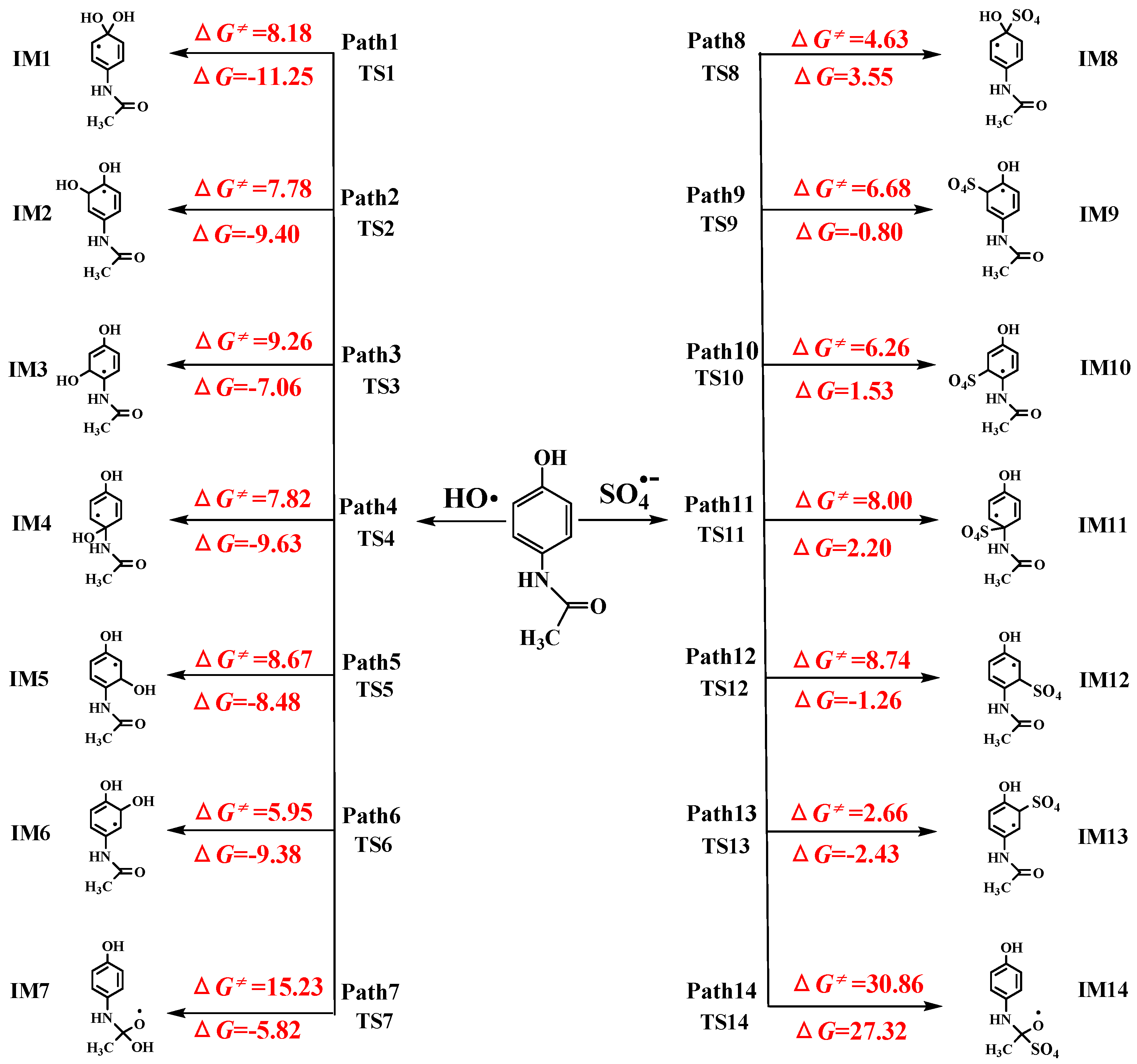
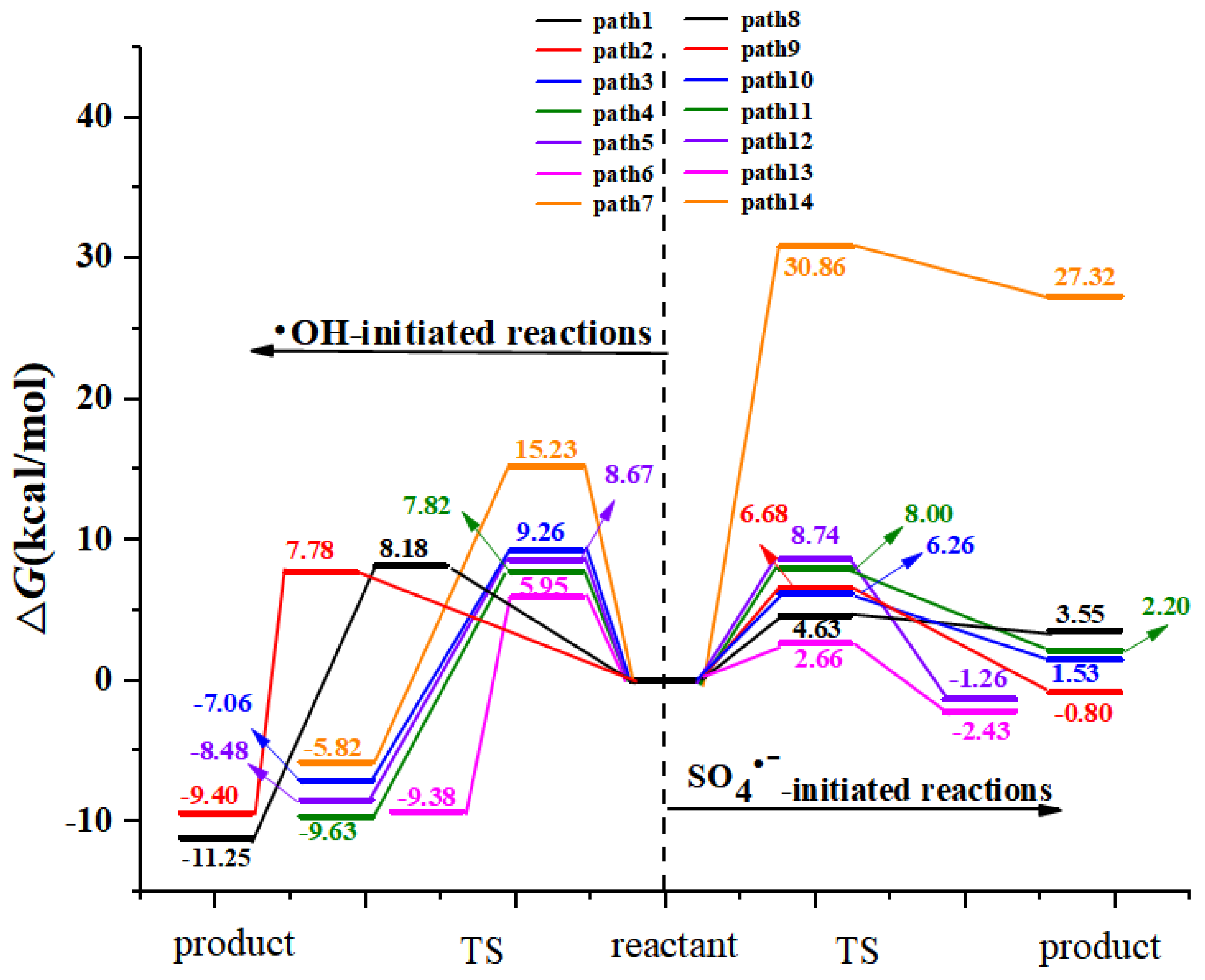
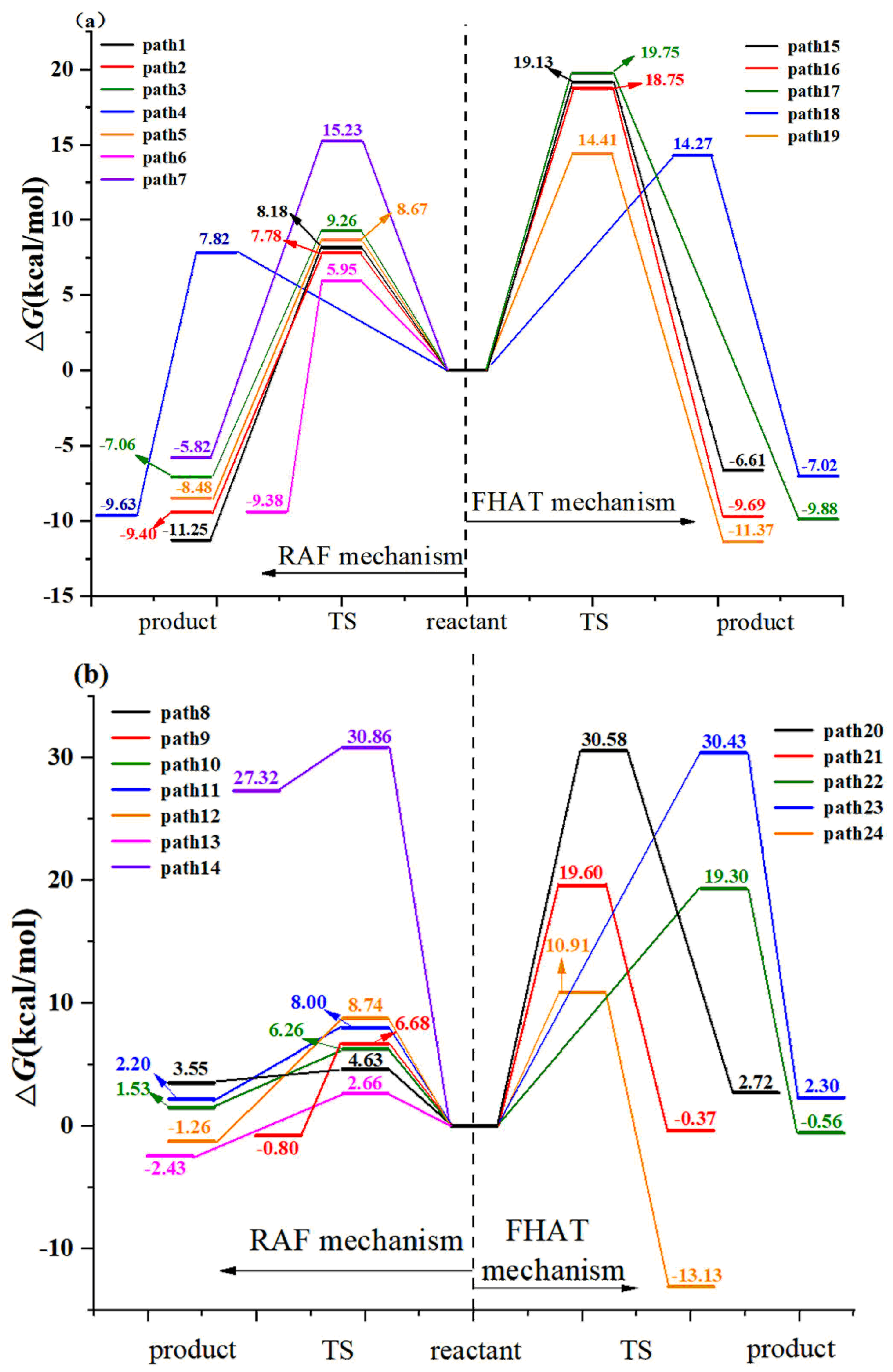
3.1.1. Radical Adduct Formation
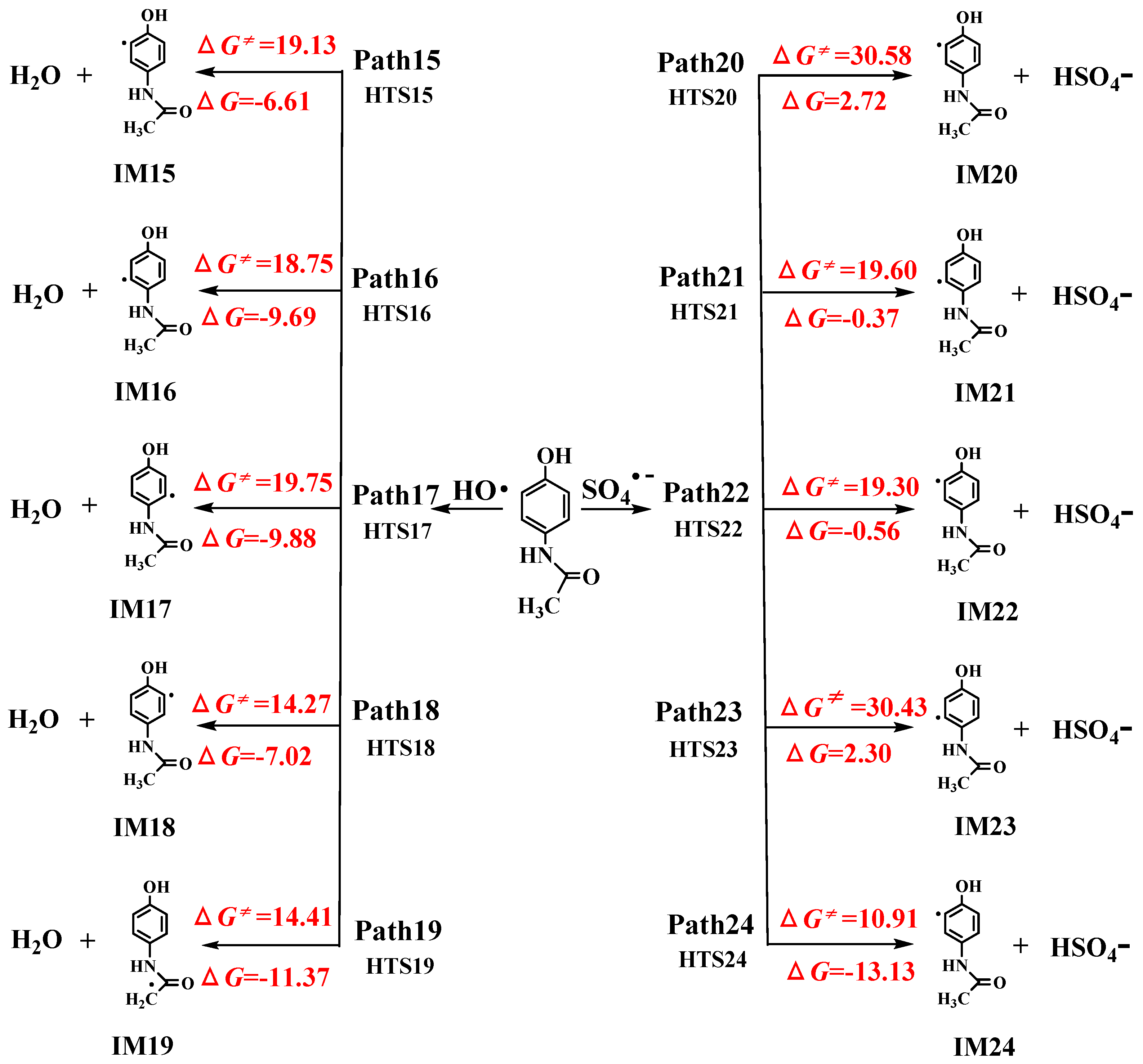
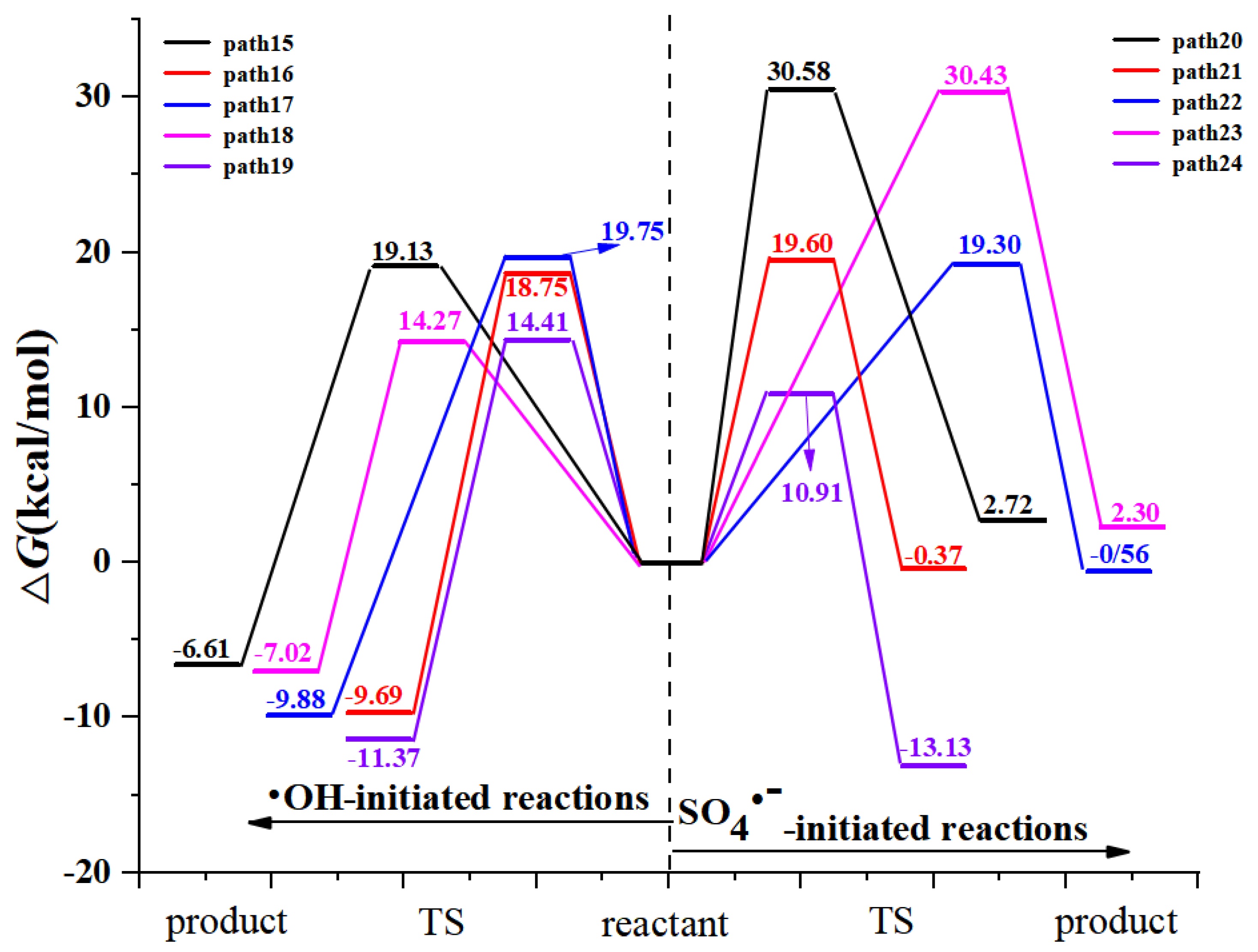
3.1.2. Formal Hydrogen Atom Transfer
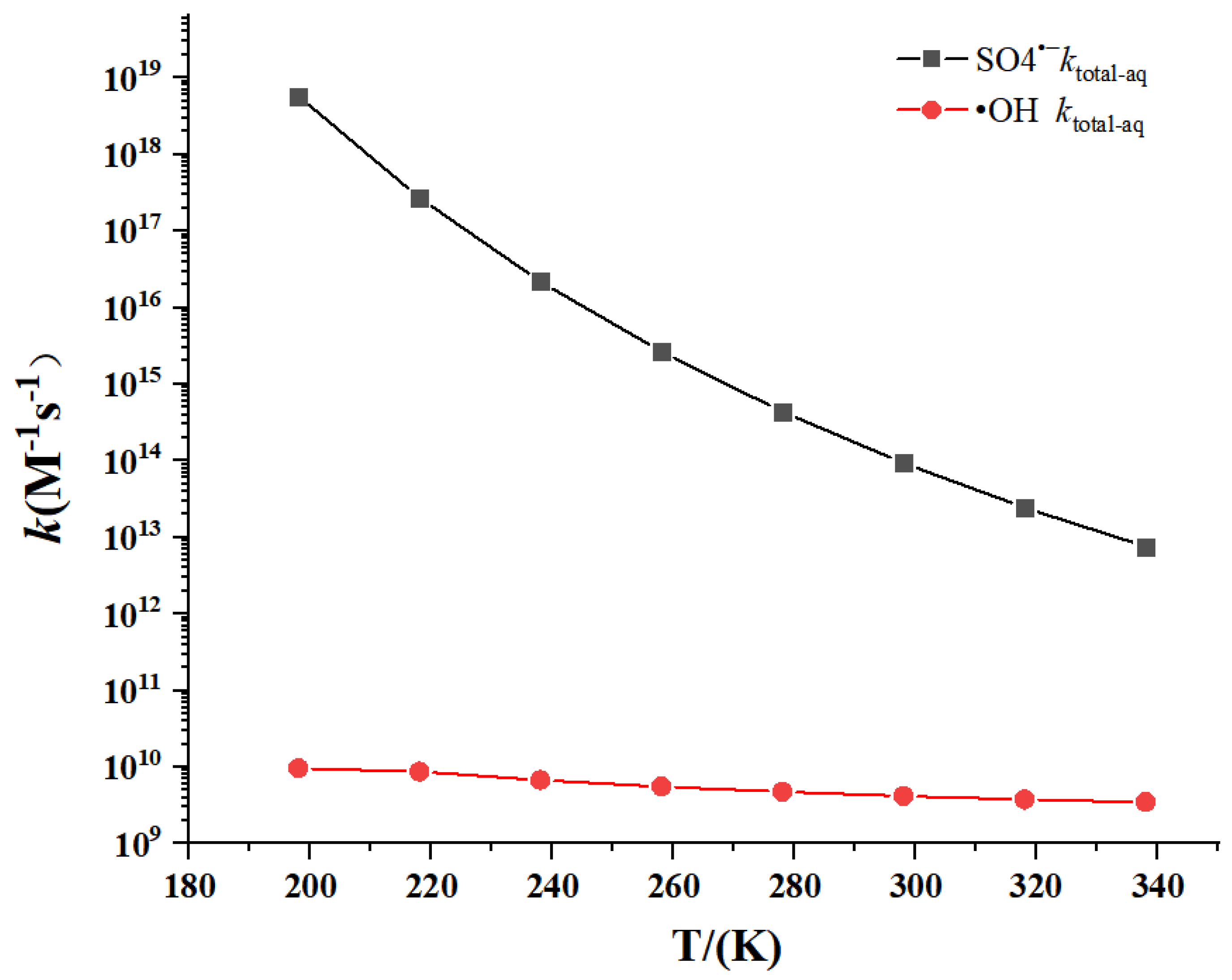
3.2. Kinetics
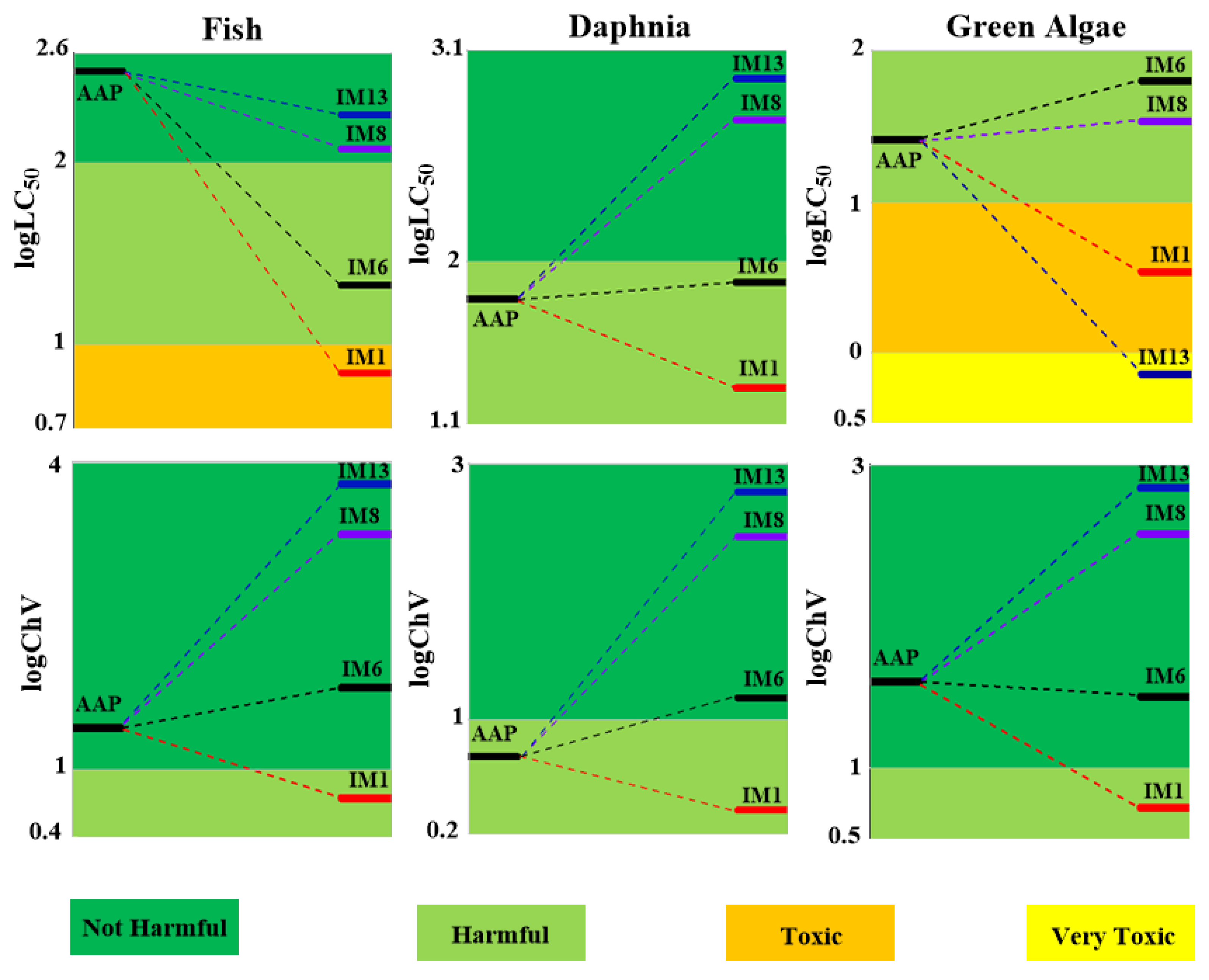
3.3. The Aquatic Toxicities of AAP and Its Degradation Intermediates
3.3.1. Toxicity of AAP
3.3.2. Toxicities of the Degradation Products
4. Conclusions
- (1)
- M06-2X/6-311+G (3df, 2p)//M06-2X/6-31+G (d, p) has been used to study the •OH-initiated and SO4•−-initiated transformation mechanism of AAP. •OH and SO4•− with AAP reactions have the same reaction sites, even reaction mechanisms. The results implied that the C6 addition is prominent pathway in RAF mechanisms and hydrogen abstraction of methyl group is dominant pathway for both reactions in FHAT mechanism. RAF takes precedence over FHAT.
- (2)
- At 298 K, the total apparent rate constant of AAP with SO4•− is larger than that of •OH. The calculated rate constants basically matched with experimental values. Theoretical calculations predicted the kinetic data at 198 K–338 K.
- (3)
- Toxic assessment shows that some representative degradation intermediates present an acute threat to the target organisms. Thus, subsequent degradation should be implemented until they are degraded into non-toxic substances.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2015, 57, 12842–12860. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Govindan, K.; Raja, M.; Noel, M.; James, E.J. Degradation of pentachlorophenol by hydroxyl radicals and sulfate radicals using electrochemical activation of peroxomonosulfate, peroxodisulfate and hydrogen peroxide. J. Hazard. Mater. 2014, 272, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khan, J.A.; Shah, N.S.; Sayed, M.; Khan, H.M. Carbamazepine degradation by UV and UV-assisted AOPs: Kinetics, mechanism and toxicity investigations. Process. Saf. Environ. 2018, 117, 307–314. [Google Scholar] [CrossRef]
- Khodadadi, T.; Solgi, E.; Mortazavi, S.; Nourmoradi, H. Comparison of advanced oxidation methods of Fenton, UV/Fenton, and O3/Fenton in treatment of municipal wastewater. Desalin. Water Treat. 2020, 206, 108–115. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Miino, M.C.; Arab, H.; Bestetti, M.; Franz, S. Efficiency and energy demand in polishing treatment of wastewater treatment plants effluents: Photoelectrocatalysis vs. Photocatalysis and photolysis. Water 2021, 13, 821. [Google Scholar] [CrossRef]
- Moussavi, G.; Pourakbar, M.; Aghayani, E.; Mahdavianpour, M. Investigating the aerated VUV/PS process simultaneously generating hydroxyl and sulfate radicals for the oxidation of cyanide in aqueous solution and industrial wastewater. Chem. Eng. J. 2018, 350, 673–680. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, Q.; Hong, J.M. Sulfate radical degradation of acetaminophen by novel iron–copper bimetallic oxidation catalyzed by persulfate: Mechanism and degradation pathways. Appl. Surf. Sci. 2017, 422, 443–451. [Google Scholar] [CrossRef]
- Li, M.X.; Sun, J.F.; Han, D.D.; Wei, B.; Mei, Q.; An, Z.X.; Wang, X.Y.; Cao, H.J.; Xie, J.; He, M.X. Theoretical investigation on the contribution of HO•, SO4•− and CO3•− radicals to the degradation of phenacetin in water: Mechanisms, kinetics, and toxicity evaluation. Ecotoxicol. Environ. Saf. 2020, 204, 110977. [Google Scholar] [CrossRef]
- Xiao, R.Y.; He, L.; Luo, Z.H.; Spinney, R.; Wei, Z.S.; Dionysiou, D.D.; Zhao, F.P. An experimental and theoretical study on the degradation of clonidine by hydroxyl and sulfate radicals. Sci. Total Environ. 2020, 710, 136333. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.N.; Wang, L.M. Kinetics and mechanism of syringic acid degradation initiated by hydroxyl radical and sulphate radical in the aqueous phase. Chemosphere 2020, 256, 126997. [Google Scholar] [CrossRef]
- Gao, L.W.; Mao, Q.M.; Luo, S.; Cao, L.Y.; Xie, X.D.; Yang, Y.; Deng, Y.F.; Wei, Z.S. Experimental and theoretical insights into kinetics and mechanisms of hydroxyl and sulfate radicals-mediated degradation of sulfamethoxazole: Similarities and differences. Environ. Pollut. 2020, 259, 113795. [Google Scholar] [CrossRef]
- Wang, S.L.; Wu, J.F.; Lu, X.Q.; Xu, W.X.; Gong, Q.; Ding, J.Q.; Dan, B.S.; Xie, P.C. Removal of acetaminophen in the Fe2+/persulfate system: Kinetic model and degradation pathways. Chem. Eng. J. 2019, 358, 1091–1100. [Google Scholar] [CrossRef]
- Li, B.Q.; Ma, X.Y.; Deng, J.; Li, Q.S.; Chen, W.Z.; Li, G.X.; Chen, G.Y.; Wang, J.P. Comparison of acetaminophen degradation in UV-LED-based advance oxidation processes: Reaction kinetics, radicals contribution, degradation pathways and acute toxicity assessment. Sci. Total Environ. 2020, 723, 137993. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Miao, X.R.; Zhang, J.; Du, J.; Xu, S.D.; Tang, J.H.; Zhang, Y.Y. DFT studies on the reaction mechanism and kinetics of dibutyl phthalate initiated by hydroxyl and sulfate radicals: Prediction of the most reactive sites. Chem. Eng. J. 2020, 381, 122680. [Google Scholar] [CrossRef]
- Liu, W.; Lv, G.C.; Sun, X.M.; He, L.; Zhang, C.X.; Li, Z.Q. Theoretical study on the reaction of anthracene with sulfate radical and hydroxyl radical in aqueous solution. Ecotoxicol. Environ. Saf. 2019, 183, 109551. [Google Scholar] [CrossRef]
- Mei, Q.; Sun, J.F.; Han, D.D.; Wei, B.; An, Z.X.; Wang, X.Y.; Xie, J.; Zhan, J.H.; He, M.X. Sulfate and hydroxyl radicals-initiated degradation reaction on phenolic contaminants in the aqueous phase: Mechanisms, kinetics and toxicity assessment. Chem. Eng. J. 2019, 373, 668–676. [Google Scholar] [CrossRef]
- Yang, J.X.; Lv, G.C.; Wang, Z.H.; Sun, X.M.; Gao, J. Mechanisms, kinetics and eco-toxicity assessment of singlet oxygen, sulfate and hydroxyl radicals-initiated degradation of fenpiclonil in aquatic environments. J. Hazard. Mater. 2021, 409, 124505. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.H.; Su, R.H.; Luo, S.; Spinney, R.; Cai, M.Q.; Xiao, R.Y.; Wei, Z.S. Comparison of the reactivity of ibuprofen with sulfate and hydroxyl radicals: An experimental and theoretical study. Sci. Total. Environ. 2017, 590, 751–760. [Google Scholar] [CrossRef]
- He, L.; Sun, X.M.; Zhu, F.P.; Ren, S.J.; Wang, S.G. OH-initiated transformation and hydrolysis of aspirin in AOPs system: DFT and experimental studies. Sci. Total Environ. 2017, 592, 33–40. [Google Scholar] [CrossRef]
- Yao, J.F.; Tang, Y.Z.; Zhang, Y.J.; Ruan, M.; Chen, F.; Wu, W.Z.; Sun, J.Y. Quantum chemical study of the mechanisms, kinetics, and ecotoxicity assessment of OH radical-initiated reactions of 2, 2′, 4, 4′, 5, 5′-hexabrominated diphenyl ether (BDE-153) in atmosphere and wastewater. Chem. Eng. J. 2021, 422, 129916. [Google Scholar] [CrossRef]
- Zou, M.T.; Qi, Y.M.; Qu, R.J.; Al-Basher, G.; Pan, X.X.; Wang, Z.Y.; Huo, Z.L.; Zhu, F. Effective degradation of 2,4-dihydroxybenzophenone by zero-valent iron powder (Fe0)-activated persulfate in aqueous solution: Kinetic study, product identification and theoretical calculations. Sci. Total Environ. 2021, 771, 144743. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, V.; Barone, G.A.; Petersson, H.; Nakatsuji, X.; et al. Gaussian 16 (Revision A.03); Gaussian, Inc.: Pittsburgh, PA, USA, 2016. [Google Scholar]
- Maeda, S.; Harabuchi, Y.; Ono, Y.; Taketsugu, T.; Morokuma, K. Intrinsic reaction coordinate: Calculation, bifurcation, and automated search. Int. J. Quant. Chem. 2015, 115, 258–269. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Canneaux, S.; Bohr, F.; Henon, E. KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results. J. Comput. Chem. 2014, 35, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z. Formation and chlorination of carbazole, phenoxazine, and phenazine. Environ. Sci. Technol. 2015, 49, 2215–2221. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Sun, J.F.; Han, D.D.; Bao, L.; Mei, Q.; Wei, B.; An, Z.X.; He, M.X.; Yuan, S.L.; Xie, J.; et al. Gaseous and heterogeneous reactions of low-molecular-weight (LMW) unsaturated ketones with O3: Mechanisms, kinetics, and effects of mineral dust in tropospheric chemical processes. Chem. Eng. J. 2020, 395, 125083. [Google Scholar] [CrossRef]
- Yao, J.F.; Sun, Y.N.; Tang, Y.Z.; Zhang, Y.J.; Wu, W.Z.; Sun, J.Y. Atmospheric oxidation of 4-(2-methoxyethyl) phenol initiated by OH radical in the presence of O2 and NOx: A mechanistic and kinetic study. Int. J. Quantum. Chem. 2021, 121, e26650. [Google Scholar] [CrossRef]
- An, Z.X.; Sun, J.F.; Han, D.D.; Mei, Q.; Wei, B.; Wang, X.Y.; He, M.X. Theoretical study on the mechanisms, kinetics and ecotoxicity assessment of OH-initiated reactions of guaiacol in atmosphere and wastewater. Sci. Total Environ. 2019, 685, 729–740. [Google Scholar] [CrossRef]
- Ji, Y.M.; Zhao, J.; Terazono, H.; Misawa, K.; Levitt, N.P.; Li, Y.X.; Lin, Y.; Peng, J.F.; Wang, Y.; Duan, L.; et al. Reassessing the atmospheric oxidation mechanism of toluene. Proc. Natl. Acad. Sci. USA 2017, 114, 8169–8174. [Google Scholar] [CrossRef] [Green Version]
- Collins, F.C.; Kimball, G.E. Diffusion-controlled reaction rates. J. Colloid Sci. 1949, 4, 425–437. [Google Scholar] [CrossRef]
- Steubing, W. Ueber die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in Flüssigkeiten suspendierten Teilchen. Z. Chem. Ind. Kolloide 1908, 3, 230. [Google Scholar] [CrossRef]
- ECOSAR v2.0. Available online: https://www.epa.gov/oppt/newchems/tools/21ecosar.htm (accessed on 1 January 2017).
- Bai, F.Y.; Ni, S.; Tang, Y.Z.; Pan, X.M.; Zhao, Z. Ciprofloxacin transformation in aqueous environments: Mechanism, kinetics, and toxicity assessment during OH-mediated oxidation. Sci. Total. Environ. 2020, 699, 134190. [Google Scholar] [CrossRef]
- Shada, A.; Chen, J.; Qu, R.J.; Dar, A.A.; Bin-Jumah, M.; Allam, A.A.; Wang, Z.Y. Degradation of sulfadimethoxine in phosphate buffer solution by UV alone, UV/PMS and UV/H2O2: Kinetics, degradation products, and reaction pathways. Chem. Eng. J. 2020, 398, 125357. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.X.; Zeng, X.L.; Feng, M.B.; Qu, R.J.; Wang, Z.Y.; Nesnas, N.; Sharma, V.K. Ferrate(VI) oxidation of polychlorinated diphenyl sulfides: Kinetics, degradation, and oxidized products. Water Res. 2018, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shiroudi, A.; Deleuze, M.S.; Canneaux, S. Theoretical study of the oxidation mechanisms of naphthalene initiated by hydroxyl radicals: The O2 addition reaction pathways. Phys. Chem. Chem. Phys. 2015, 17, 13719–13732. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Sun, J.F.; Mei, Q.; Wei, B.; An, Z.X.; Cao, H.J.; Zhang, C.; Xie, J.; Zhan, J.H.; Wang, W.X.; et al. Acetaminophen degradation by hydroxyl and organic radicals in the peracetic acid-based advanced oxidation processes: Theoretical calculation and toxicity assessment. J. Hazard. Mater. 2021, 416, 126250. [Google Scholar] [CrossRef] [PubMed]
 .
.
 .
.
| Radicals | Redox Potential a (V) | The Range of Rate Constants b (M−1 s−1) | The Second-Order Rate Constants of Neutral Sulfamethoxazole c (M−1 s−1) | The Second-Order Rate Constants of Acetaminophen (M−1 s−1) | |
|---|---|---|---|---|---|
| Fe2+/PS d | UV-LED/H2O2 e | ||||
| •OH | 1.8–2.7 | 108–1010 | (7.27 ± 0.43) × 109 | (3.26 ± 0.41) × 109 | 5.15 × 109 |
| SO4•− | 2.5–3.1 | 107–1010 | (2.98 ± 0.32) × 109 | (1.80 ± 0.17) × 109 | 7.66 × 109 |
| Paths | kaq (M−1 s−1) | Raq (%) | kD (M−1 s−1) | kapp (M−1 s−1) |
|---|---|---|---|---|
| APP + •OH → IM1 (k1) | 8.04 × 107 | 1.9 | 9.80 × 109 | 7.97 × 107 |
| APP + •OH → IM2 (k2) | 1.87 × 108 | 4.5 | 9.80 × 109 | 1.83 × 108 |
| APP + •OH → IM3 (k3) | 1.51 × 107 | 0.4 | 9.80 × 109 | 1.51× 107 |
| APP + •OH → IM4 (k4) | 3.22 × 108 | 7.6 | 9.80 × 109 | 3.12 × 108 |
| APP + •OH → IM5 (k5) | 3.33 × 107 | 0.8 | 9.80 × 109 | 3.32 × 107 |
| APP + •OH → IM6 (k6) | 3.56 × 109 | 84.8 | 9.80 × 109 | 2.61 × 109 |
| APP + •OH → IM7 (k7) | 6.75 × 102 | 0 | 9.80 × 109 | 6.75 × 102 |
| APP + •OH → IM15 (k15) | 4.75 | 0 | 9.80 × 109 | 4.75 |
| APP + •OH → IM16 (k16) | 9.75 | 0 | 9.80 × 109 | 9.75 |
| APP + •OH → IM17 (k17) | 1.91 | 0 | 9.80 × 109 | 1.91 |
| APP + •OH → IM18 (k18) | 1.15 × 104 | 0 | 9.80 × 109 | 1.15 × 104 |
| APP + •OH → IM19 (k19) | 1.13 × 104 | 0 | 9.80 × 109 | 1.13 × 104 |
| APP + •OH → Product (ktotal) | 4.20 × 109 | 100 | 3.23 × 109 |
| Paths | k’aq (M−1 s−1) | R’aq (%) | k’D (M−1 s−1) | k’app (M−1 s−1) |
|---|---|---|---|---|
| APP + SO4•−→IM8 (k’8) | 6.00 × 1012 | 6.4 | 8.05 × 109 | 8.04 × 109 |
| APP + SO4•− → IM9 (k’9) | 1.61 × 1011 | 0.2 | 8.05 × 109 | 7.67 × 109 |
| APP + SO4•− → IM10 (k’10) | 2.60 × 1011 | 0.3 | 8.05 × 109 | 7.81 × 109 |
| APP + SO4•− → IM11 (k’11) | 3.28 × 1010 | 0.01 | 8.05 × 109 | 6.46 × 109 |
| APP + SO4•− → IM12 (k’12) | 2.52 × 1011 | 0.3 | 8.05 × 109 | 7.80 × 109 |
| APP + SO4•− → IM13 (k’13) | 8.65 × 1013 | 92.8 | 8.05 × 109 | 8.05 × 109 |
| APP + SO4•− → IM14 (k’14) | 1.77 × 10−6 | 0 | 8.05 × 109 | 1.77 × 10−6 |
| APP + SO4•− → IM20 (k’20) | 14.3 | 0 | 8.05 × 109 | 14.3 |
| APP + SO4•− → IM21 (k’21) | 1.11 × 102 | 0 | 8.05 × 109 | 1.11 × 102 |
| APP + SO4•− → IM22 (k’22) | 1.88 × 102 | 0 | 8.05 × 109 | 1.88 × 102 |
| APP + SO4•− → IM23 (k’23) | 5.55 | 0 | 8.05 × 109 | 5.55 |
| APP + SO4•− → IM24 (k’24) | 1.33 × 108 | 0 | 8.05 × 109 | 1.33 × 108 |
| APP + SO4•− → Product (k’total) | 9.32 × 1013 | 100 | 4.60 × 1010 |
| Paths | kaq (M−1 s−1) | Raq (%) | Paths | k’aq (M−1 s−1) | R’aq (%) |
|---|---|---|---|---|---|
| APP + •OH (FHAT) | 2.28 × 104 | 100 | APP + SO4•− (FHAT) | 1.33 × 108 | 100 |
| APP + •OH → IM15 (k15) | 4.75 | 0 | APP + SO4•− → IM20 (k’20) | 14.3 | 0 |
| APP + •OH → IM16 (k16) | 9.75 | 0 | APP + SO4•− → IM21 (k’21) | 1.11 × 102 | 0 |
| APP + •OH → IM17 (k17) | 1.91 | 0 | APP + SO4•− → IM22 (k’22) | 1.88 × 102 | 0 |
| APP + •OH → IM18 (k18) | 1.15 × 104 | 50.42 | APP + SO4•− → IM23 (k’23) | 5.55 | 0 |
| APP + •OH → IM19 (k19) | 1.13 × 104 | 49.58 | APP + SO4•− → IM24 (k’24) | 1.33 × 108 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Yao, J.; Sun, S.; Yan, S.; Sun, J. Theoretical Calculation on the Reaction Mechanisms, Kinetics and Toxicity of Acetaminophen Degradation Initiated by Hydroxyl and Sulfate Radicals in the Aqueous Phase. Toxics 2021, 9, 234. https://doi.org/10.3390/toxics9100234
Xu M, Yao J, Sun S, Yan S, Sun J. Theoretical Calculation on the Reaction Mechanisms, Kinetics and Toxicity of Acetaminophen Degradation Initiated by Hydroxyl and Sulfate Radicals in the Aqueous Phase. Toxics. 2021; 9(10):234. https://doi.org/10.3390/toxics9100234
Chicago/Turabian StyleXu, Mengmeng, Junfang Yao, Simei Sun, Suding Yan, and Jingyu Sun. 2021. "Theoretical Calculation on the Reaction Mechanisms, Kinetics and Toxicity of Acetaminophen Degradation Initiated by Hydroxyl and Sulfate Radicals in the Aqueous Phase" Toxics 9, no. 10: 234. https://doi.org/10.3390/toxics9100234
APA StyleXu, M., Yao, J., Sun, S., Yan, S., & Sun, J. (2021). Theoretical Calculation on the Reaction Mechanisms, Kinetics and Toxicity of Acetaminophen Degradation Initiated by Hydroxyl and Sulfate Radicals in the Aqueous Phase. Toxics, 9(10), 234. https://doi.org/10.3390/toxics9100234