Potential Environmental Risk Characteristics of PCB Transformation Products in the Environmental Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
- (1)
- The data sources of the PCB environmental characteristics
- (2)
- The data sources of transformation pathways and transformation products of PCBs in the environmental media
2.2. 3D-QSAR Model Construction of PCB Toxicity (Phytotoxicity and Estrogen Toxicity)
3. Results
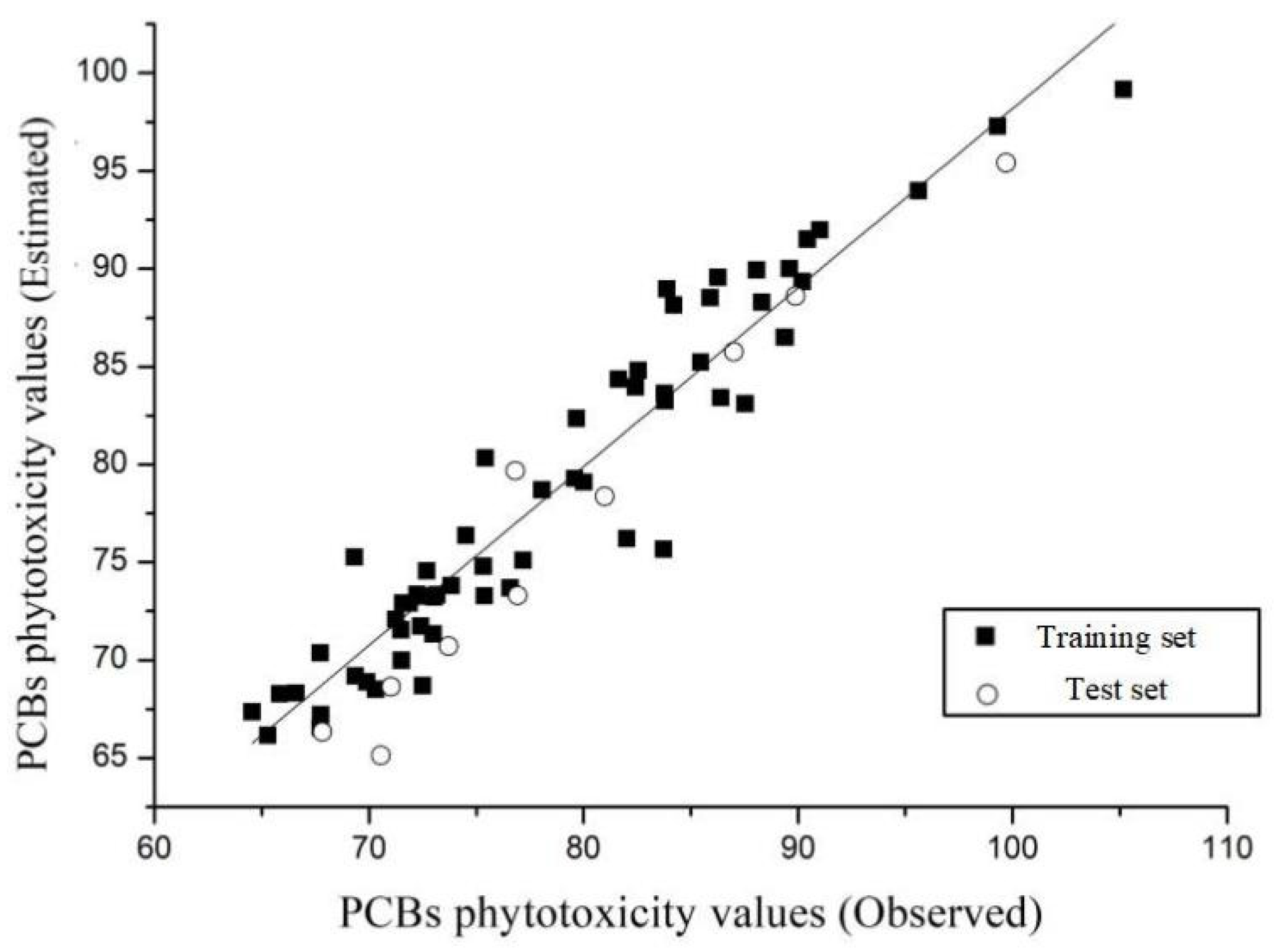
3D-QSAR Model Construction and Evaluation of PCB Toxicity (Phytotoxicity and Estrogen Toxicity)
4. Discussion
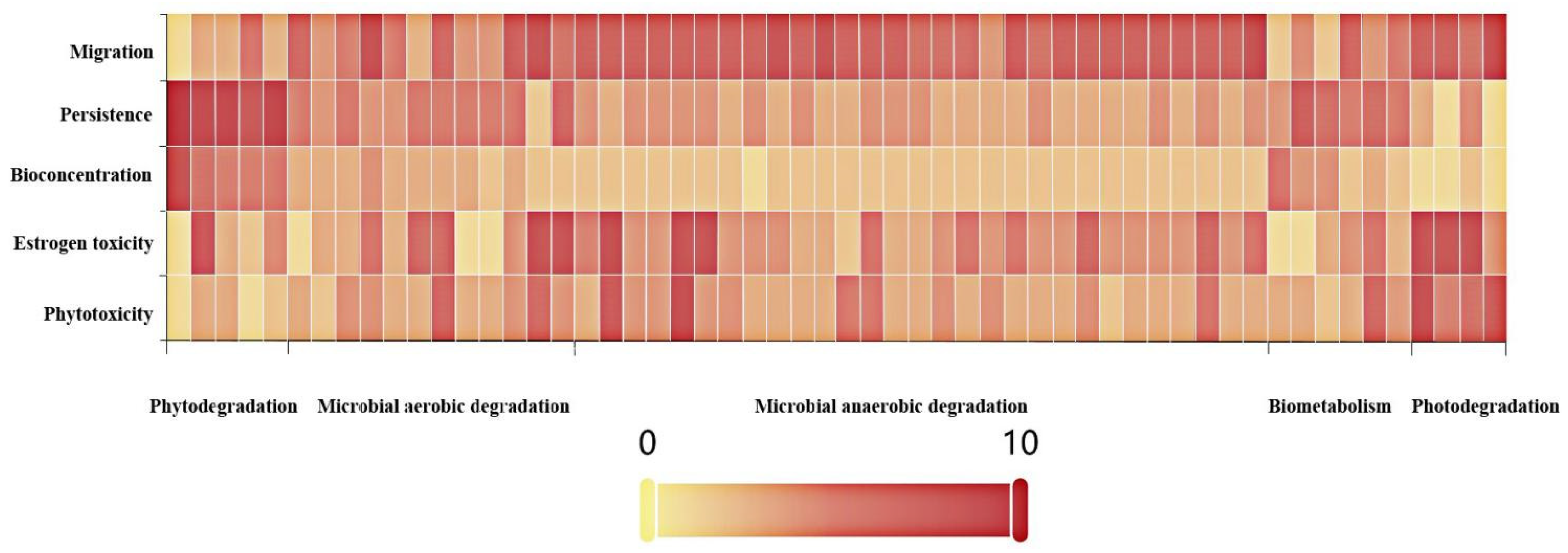
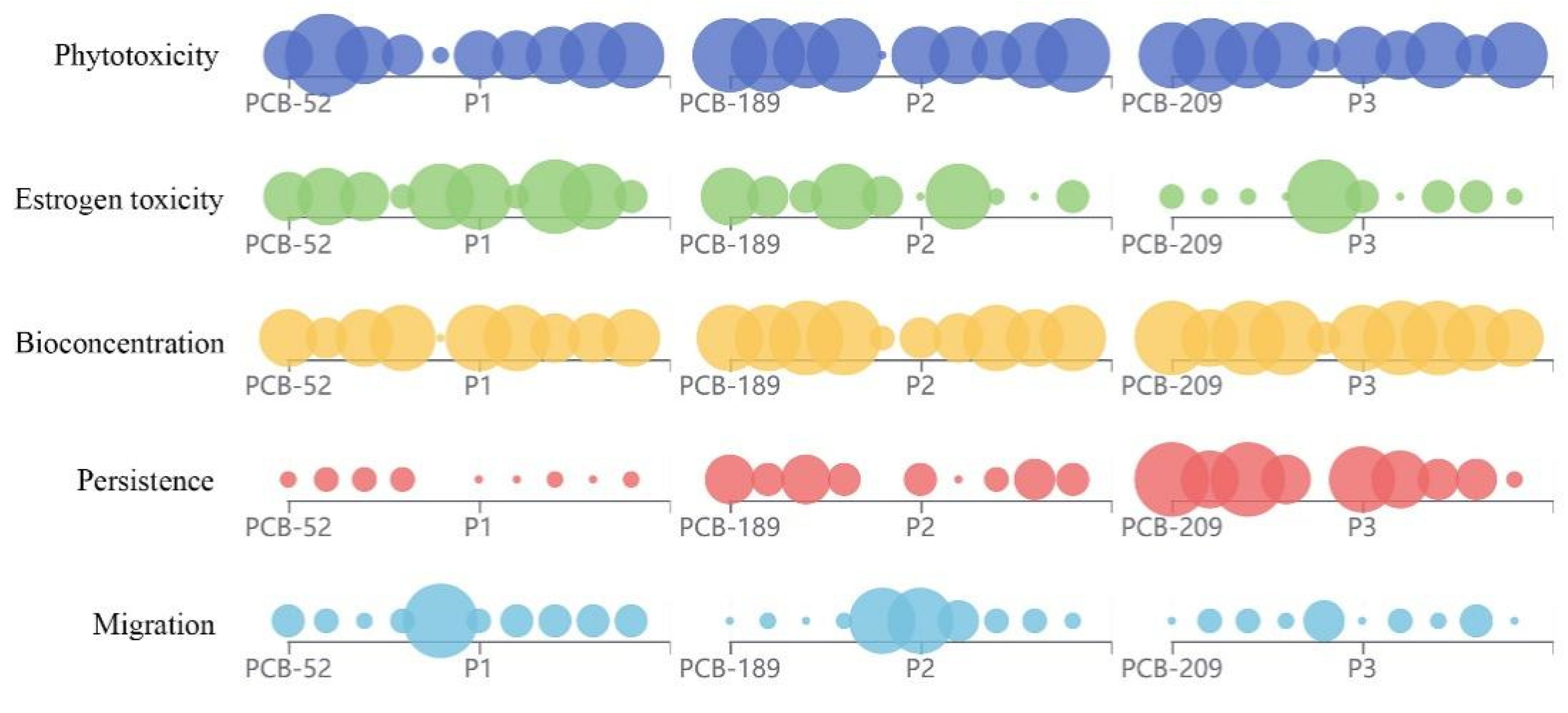
4.1. The Estimation of the Environmental Risk Characteristics of PCB Transformation Products in Environmental Media
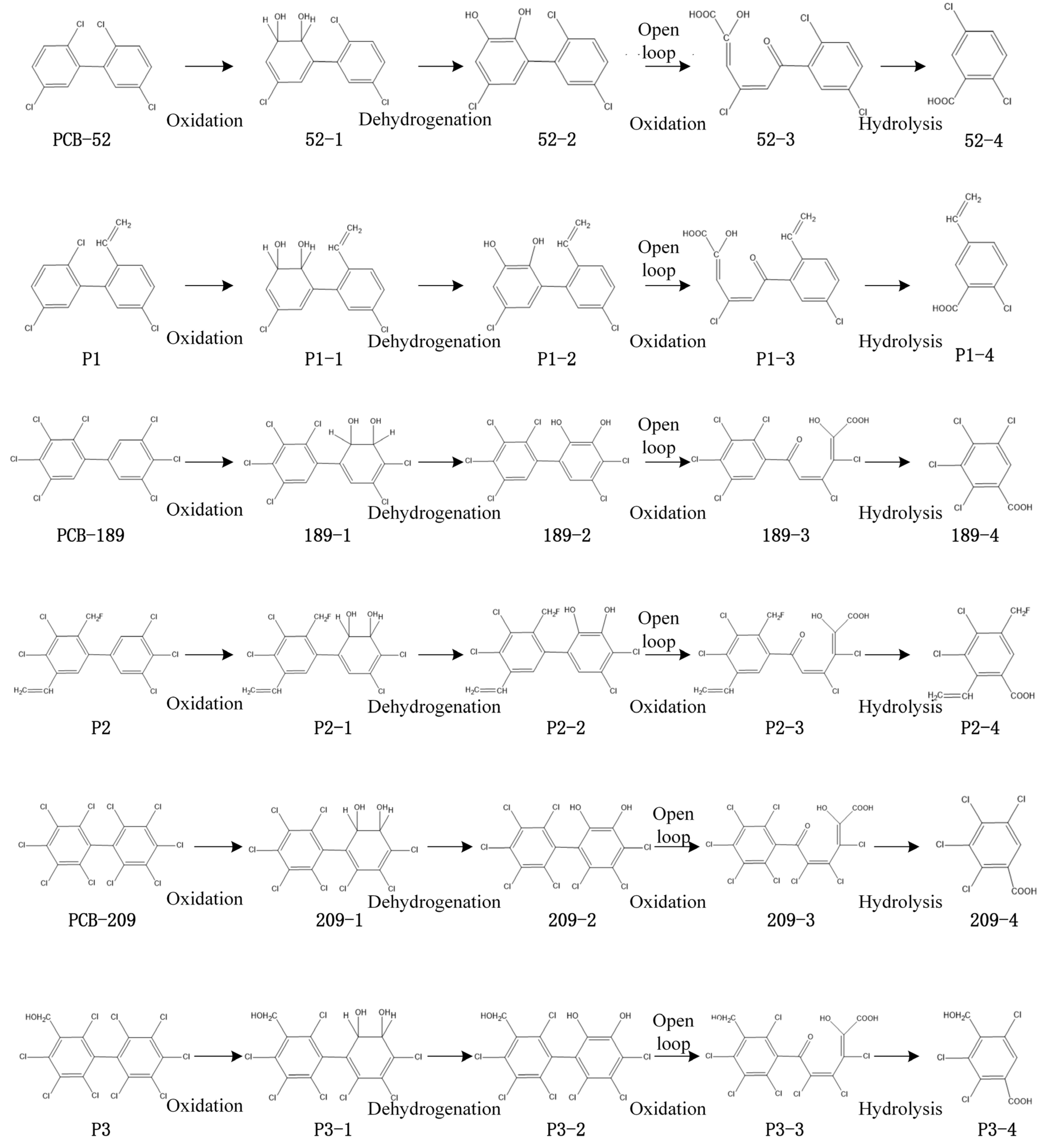
4.2. The Estimation of the Environmental Risk Characteristics of Environmentally Friendly PCB Transformation Products in Plants
4.3. The Validation of the Total Score and Its Estimated Value of PCBs and Their Products Containing Different Chemical Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tanabe, S. PCB problems in the future: Foresight from current knowledge. Environ. Pollut. 1988, 50, 5–28. [Google Scholar] [CrossRef]
- Novakova, M.; Mackova, M.; Chrastilova, Z.; Viktorova, J.; Macek, T. Cloning the bacterial bphC gene into Nicotiana tabacum to improve the efficiency of PCB phytoremediation. Biotechnol. Bioeng. 2009, 102, 29–37. [Google Scholar] [CrossRef]
- Uchida, E.; Ouchi, T.; Suzuki, Y.; Yoshida, T.; Habe, H.; Yamaguchi, I.; Omori, T.; Nojiri, H. Secretion of bacterial xenobioticdegrading enzymes from transgenic plants by an apoplastic expressional system: An applicability for phytoremediation. Environ. Sci. Technol. 2005, 39, 7671–7677. [Google Scholar] [CrossRef]
- Sylvestre, M. Genetically modified organisms to remediate polychlorinated biphenyls. Where do we stand? Int. Biodeter. Biodegr. 2004, 54, 153–162. [Google Scholar] [CrossRef]
- Abramowicz, D.A.; Brannan, M.J.; Dort, H.M.V.; Gallagher, E.L. Factors influencing the rale of polychlorinated biphenyls dechlorlnation in Hudson River sediments. Environ. Sci. Technol. 1993, 27, 1125–1131. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium burds. Science 1983, 221, 661–663. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, H.M.; Letcher, R.J.; Baker, J.E. Metabolism of PCBs by the deep-water sculpin (Myoxocephalus thompsoni). Environ. Sci. Technol. 2001, 35, 4747–4752. [Google Scholar] [CrossRef]
- Ghosh, J.P.; Achari, G.; Langford, C.H. Reductive Dechlorination of PCBs Using Photocatalyzed UV Light. Acta Hydrochim. Hydrobiol. 2012, 40, 455–460. [Google Scholar] [CrossRef]
- Grimm, F.A.; Hu, D.; Kania-Korwel, I.; Lehmler, H.J.; Ludewig, G.; Hornbuckle, K.C.; Duffel, M.W.; Bergman, Å.; Robertson, L.W. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 2015, 45, 245–272. [Google Scholar] [CrossRef]
- Specker, J.L.; Sullivan, C.V. Vitellogenesis in fishes: Status and perspectives. In Perspectives in Comparative Endocrinology; Davey, K.G., Peter, R.E., Tobe, S.S., Eds.; National Research Council Canada: Ottawa, ON, Canada, 1994; pp. 304–315. [Google Scholar]
- Sumpter, J.P.; Jobling, S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ. Health Persp. 1995, 103, 173–178. [Google Scholar]
- Connor, K.; Ramamoorthy, K.; Moore, M.; Mustain, M.; Chen, I.; Safe, S.; Zacharewski, T.; Gillesby, B.; Joyeux, A.; Balaguer, P. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: Structure activity relationships. Toxicol. Appl. Pharm. 1997, 145, 111–123. [Google Scholar] [CrossRef]
- Al-Anati, L.; Viluksela, M.; Strid, A.; Bergman, Å.; Andersson, P.L.; Stenius, U.; Högberg, J. Hydroxyl metabolite of PCB 180 induces DNA damage signaling and enhances the DNA damaging effect of benzo[a]pyrene. Chem.-Biol. Interact. 2015, 239, 164–173. [Google Scholar] [CrossRef]
- Machala, M.; Bláha, L.; Lehmler, H.J. Toxicity of hydroxylated and quinoid PCB metabolites: Inhibition of gap junctional intercellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chem. Res. Toxicol. 2004, 17, 340–347. [Google Scholar] [CrossRef]
- Letcher, R.J.; Klasson-Wehler, E.; Bergman, A. The Handbook of Environmental Chemistry, Volume 3K: Anthropogenic Compounds; Springer: Heidelberg, Germany, 2000; pp. 315–359. [Google Scholar]
- Li, M.H.; Wang, X.L.; Chu, Z.H.; Li, Y. Multiple-Site Molecular Modification of Dioxin-like PCB-189 to Eliminate Bioconcentration. Pol. J. Environ. Stud. 2021, 30, 1655–1675. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, X.; Jiang, L.; Li, Y. Prediction of octanol-air partition coefficients for polychlorinated biphenyls (PCBs) using 3D-QSAR models. Ecotoxicol. Environ. Safe 2016, 124, 202–212. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Qiu, Y.; Gu, W.; Li, Y. Prediction of Stability for Polychlorinated Biphenyls in Transformer Insulation Oil Through Three-dimensional Quantitative Structure-activity Relationship Pharmacophore Model and Full Factor Experimental Design. Chem. Res. Chin. Univ. 2016, 32, 348–356. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Wan, K.; Yin, X.; Yang, Y. Altitude distributions and source analysis of OCPs and PCBs in surface soils of Changbai Mountain, Northeast China. Bull. Environ. Contam. Toxicol. 2017, 98, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, F.; Liu, Y.W.; Chang, H.Q.; Li, Z.J.; Xue, J.M. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- Sylvestre, M.; Macek, T.; Mackova, M. Transgenic plants to improve rhizoremediation of polychlorinated biphenyls (PCBs). Curr. Opin. Biotechnol. 2009, 20, 242–247. [Google Scholar] [CrossRef]
- Gibbs, B.F. Enhanced Degradation of Polychlorinated Biphenyls by Surfactants and a Dioxygenase Enzyme Complex; Concordia University: Montreal, QC, Canada, 2007. [Google Scholar]
- Seah, S.Y.; Labbé, G.; Nerdinger, S.; Johnson, M.R.; Snieckus, V.; Eltis, L.D. Identification of a serine hydrolase as a key determinant in the microbial degradation of polychlorinated biphenyls. J. Biol. Chem. 2000, 275, 15701–15708. [Google Scholar] [CrossRef] [Green Version]
- Gervold, S.K.F.; May, H.D.; Sowers, K.R. Microbial reductive dechlorination of aroclor 1260 in baltimore harbor sediment microcosms is catalyzed by three phylotypes within the phylum chloroflexi. Appl. Environ. Microb. 2007, 73, 3009–3018. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.; Chiu, T.; Yen, J.; Wang, Y. Dechlorination pathways of ortho-substituted PCBs by UV irradiation in n-hexane and their correlation to the charge distribution on carbon atom. Chemosphere 2003, 51, 775–784. [Google Scholar] [CrossRef]
- Borja, J.; Taleon, D.M.; Auresenia, J.; Gallardo, S. Polychlorinated biphenyls and their biodegradation. Process. Biochem. 2005, 40, 1999–2013. [Google Scholar] [CrossRef]
- Pan, L.L. Contamination of Polychlorinated Biphenyls in Agricultural Soils from the Yangtze River Delta and Their Metabolic Pathway in Rice. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2018. [Google Scholar]
- Wekhof, A. Treatment of contaminated water, air and soil with UV flashlamps. Environ. Prog. Sustain. 1991, 10, 241–247. [Google Scholar] [CrossRef]
- Huang, Y.; Su, X.O.; Wang, R.G.; Zhang, W. Advances on hydroxylated polychlorinated biphenyls metabolites and the estrogenic effects. Asian J. Ecotoxicol. 2018, 13, 58–68. [Google Scholar]
- Guo, L. Study on the Degradation Characteristics of Polychlorinated Biphenyls by Rhodococcus Biphenylivorans TG9. Master’s Thesis, Zhejiang University, Hangzhou, China, 2018. [Google Scholar]
- Wang, Z.H. Photodegradation of PCBs in Anionic Surfactant (SDS) Solution. Master’s Thesis, Hunan University, Changsha, China, 2002. [Google Scholar]
- Li, M.H.; Zhang, W.H.; Hou, Y.L.; Sun, R.H.; Li, Y. A Novel 3D-QSA2R Model Assisted with a Log-Normalized Method and Its Application in Molecule Modification. Pol. J. Environ. Stud. 2020, 29, 3675–3682. [Google Scholar] [CrossRef]
- Li, M.H.; Li, Y. A Vector Normalized Method-assisted 3D-QSA2R Model and Its Application in the Molecular Modification of PCBs with Higher Flame Retardancy and Lower Toxicity. Sci. Adv. Mater. 2021, 13, 80–87. [Google Scholar] [CrossRef]
- He, J.; Zhou, T.; Young, J.C.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food. Sci. Technol. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Winneke, G.; Walkowiak, J.; Lilienthal, H. PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology 2002, 181, 161–165. [Google Scholar] [CrossRef]
- Shen, H.; Ding, G.; Wu, Y.; Pan, G.; Zhou, X.; Han, J.; Li, J.; Wen, S. Polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs), polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) in breast milk from Zhejiang, China. Environ. Int. 2012, 42, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Megson, D.; Sullivan, O.G.; Comber, S.; Worsfold, P.J.; Lohan, M.C.; Edwards, M.R.; Shields, W.J.; Sandau, C.D.; Patterson, J.D.G. Elucidating the structural properties that influence the persistence of PCBs in humans using the National Health and Nutrition Examination Survey (NHANES) dataset. Sci. Total Environ. 2013, 461–462, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hegseth, M.N.; Camus, L.; Helgason, L.B.; Bocchetti, R.; Gabrielsen, G.W.; Regoli, F. Hepatic antioxidant responses related to levels of PCBs and metals in chicks of three Arctic seabird species. Comp. Biochem. Physiol. Part C 2011, 154, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, V.; Stefano, Z.; Elena, A.; Carlo, B.; Andrea, G.; Rossano, P. Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in Antarctic ice-free areas: Influence of local sources on lakesand soils. Microchem. J. 2015, 120, 26–33. [Google Scholar]
- Covaci, A.; Voorspoels, S.; Roosens, L.; Jacobs, W.; Blust, R.; Neels, H. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere 2008, 73, 170–175. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.S.; Zhang, L.; Li, J.G.; Yao, L.; Self, S.G.; Sun, X.; Tang, N.J. Levels of PCDDs, PCDFs and dl-PCBs in the blood of childbearing-aged women living in the vicinity of a chemical plant in Tianjin: A primary study. Chemosphere 2015, 118, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gauger, K.J.; Kato, Y.; Haraguchi, K.; Lehmler, H.J.; Robertson, L.W.; Bansal, R.; Zoeller, R.T. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ. Health Persp. 2004, 112, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Hauser, R. Exposure to Polychlorinated Biphenyls (PCBs) and Male Reproduction. Syst. Biol. Reprod. Med. 2010, 56, 122–131. [Google Scholar] [CrossRef] [Green Version]
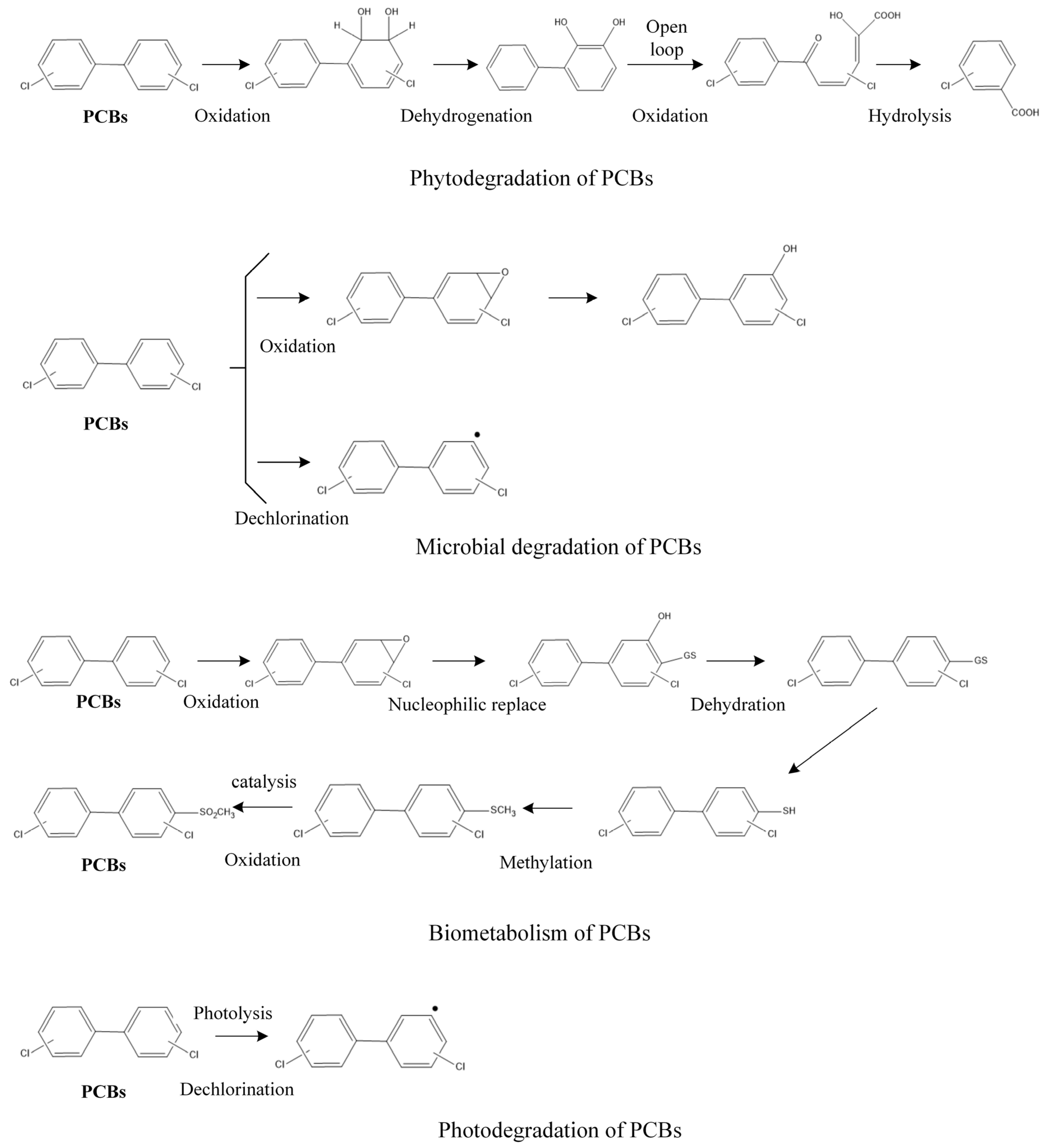

| NO. | Degradation Pathway | Parent Molecule | Degradation or Metabolites |
|---|---|---|---|
| 1 | Plant degradation | PCB-3 | 4-CBA |
| 2 | PCB-4 | 2-CBA | |
| 3 | PCB-5 | 2,3-CBA | |
| 4 | PCB-11 | 3-CBA | |
| 5 | PCB-31 | 2,5-CBA | |
| 6 | Microbial aerobic degradation | PCB-97 | 4′-OH-CB97 |
| 7 | PCB-101 | 4′-OH-CB101 | |
| 8 | PCB-107 | 4-OH-CB107 | |
| 9 | PCB-109 | 4-OH-CB109 | |
| 10 | PCB-118 | 3-OH-CB118 | |
| 11 | PCB-148 | 4-OH-CB148 | |
| 12 | PCB-153 | 3-OH-CB153 | |
| 13 | PCB-162 | 4-OH-CB162 | |
| 14 | PCB-172 | 4′-OH-CB172 | |
| 15 | PCB-187 | 4-OH-CB187 | |
| 16 | PCB-199 | 4′-OH-CB199 | |
| 17 | PCB-202 | 4-OH-CB202 | |
| 18 | Microbial anaerobic degradation | PCB-90 | PCB-49 PCB-68 |
| 19 | PCB-91 | PCB-51 | |
| 20 | PCB-92 | PCB-52 PCB-72 | |
| 21 | PCB-95 | PCB-53 | |
| 22 | PCB-99 | PCB-47 | |
| 23 | PCB-101 | PCB-49 | |
| 24 | PCB-102 | PCB-51 | |
| 25 | PCB-130 | PCB-90 | |
| 26 | PCB-132 | PCB-91 | |
| 27 | PCB-135 | PCB-94 | |
| 28 | PCB-137 | PCB-90 PCB-99 | |
| 29 | PCB-138 | PCB-99 | |
| 30 | PCB-146 | PCB-90 | |
| 31 | PCB-147 | PCB-91 | |
| 32 | PCB-149 | PCB-102 | |
| 33 | PCB-151 | PCB-95 | |
| 34 | PCB-153 | PCB-99 | |
| 35 | PCB-154 | PCB-100 | |
| 36 | PCB-170 | PCB-130 PCB-137 PCB-138 | |
| 37 | PCB-174 | PCB-149 | |
| 38 | PCB-180 | PCB-153 PCB-146 | |
| 39 | PCB-183 | PCB-154 | |
| 40 | PCB-187 | PCB-149 | |
| 41 | Biometabolism | PCB-49 | 3′-MeSO2-CB49 |
| 42 | PCB-64 | 4-MeSO2-CB64 | |
| 43 | PCB-70 | 3-MeSO2-CB70 | |
| 44 | PCB-110 | 3-MeSO2-CB110 | |
| 45 | PCB-149 | 4-MeSO2-CB149 | |
| 46 | PCB-174 | 4-MeSO2-CB174 | |
| 47 | Photodegradation | PCB-47 | PCB-15 |
| 48 | PCB-40 | PCB-11 | |
| 49 | PCB-101 | PCB-70 | |
| 50 | PCB-171 | PCB-35 |
| PCBs (IUPAC) | Phytotoxicity | Estrogen Toxicity | PCBs (IUPAC) | Phytotoxicity | Estrogen Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estd. | Obs. | Relative Error (%) | Estd. | Obs. | Relative Error (%) | Estd. | Obs. | Relative Error (%) | Estd. | Obs. | Relative Error (%) | ||
| 0 | 80.694 | 71.424 | 105 | 91.091 | 65.655 | ||||||||
| 1 | 80.325 | 75.426 | −6.50 | 70.958 | 106 | 81.145 | 69.353 | ||||||
| 2 | 84.363 | 81.624 | −3.36 | 70.674 | 69.396 | −1.84 | 107 | 84.562 | 70.567 | ||||
| 3 | 83.949 | 82.426 | −1.85 | 67.889 | 65.720 a | −3.30 | 108 | 89.361 | 69.117 | ||||
| 4 | 73.802 | 73.822 | 0.03 | 63.977 | 63.512 | −0.73 | 109 | 67.583 | 57.749 | ||||
| 5 | 79.090 | 80.007 | 1.15 | 71.672 | 110 | 68.633 | 71.019 a | 3.36 | 61.736 | 60.469 | −2.10 | ||
| 6 | 83.632 | 83.766 | 0.16 | 71.588 | 111 | 82.886 | 73.235 | 68.404 | −7.06 | ||||
| 7 | 83.259 | 83.786 | 0.63 | 67.399 | 112 | 63.621 | 62.042 | 62.049 a | 0.01 | ||||
| 8 | 85.749 | 87.006 a | 1.44 | 68.852 | 113 | 70.030 | 64.786 | ||||||
| 9 | 78.359 | 80.980 a | 3.24 | 72.131 | 114 | 83.185 | 66.746 | ||||||
| 10 | 71.729 | 72.398 | 0.92 | 64.205 | 115 | 58.217 | 55.372 | ||||||
| 11 | 83.118 | 87.533 | 5.04 | 73.814 | 73.504 | −0.42 | 116 | 61.924 | 58.568 | ||||
| 12 | 88.281 | 88.323 | 0.05 | 67.752 | 117 | 67.104 | 57.672 | 57.287 | −0.67 | ||||
| 13 | 89.938 | 88.073 | −2.12 | 67.219 | 67.002 | −0.32 | 118 | 88.524 | 85.897 | −3.06 | 65.011 | ||
| 14 | 81.876 | 72.630 | 119 | 67.740 | 57.761 | ||||||||
| 15 | 89.550 | 86.282 | −3.79 | 65.043 | 120 | 86.663 | 68.555 | ||||||
| 16 | 75.274 | 69.318 | −8.59 | 64.841 | 121 | 71.060 | 55.813 | ||||||
| 17 | 72.967 | 60.820 | 122 | 90.103 | 68.870 | 68.399 | −0.69 | ||||||
| 18 | 72.089 | 71.252 | −1.17 | 63.389 | 123 | 93.991 | 95.620 | 1.70 | 64.795 | ||||
| 19 | 69.084 | 56.327 | 124 | 88.268 | 69.449 | ||||||||
| 20 | 82.354 | 79.668 | −3.37 | 72.228 | 70.152 | −2.96 | 125 | 75.464 | 60.670 | ||||
| 21 | 82.154 | 68.549 | 126 | 99.156 | 105.165 | 5.71 | 66.748 | ||||||
| 22 | 84.490 | 69.539 | 127 | 92.611 | 71.565 | ||||||||
| 23 | 76.212 | 82.007 | 7.07 | 72.676 | 128 | 68.328 | 66.586 | −2.62 | 58.372 | 59.488 | 1.88 | ||
| 24 | 63.846 | 64.834 | 69.621 | 6.88 | 129 | 71.759 | 62.271 | ||||||
| 25 | 86.493 | 89.403 | 3.25 | 67.989 | 130 | 74.548 | 72.689 | −2.56 | 62.574 | 59.408 a | −5.33 | ||
| 26 | 81.368 | 72.709 | 131 | 62.701 | 52.006 | ||||||||
| 27 | 74.870 | 64.884 | 132 | 66.789 | 54.858 | ||||||||
| 28 | 88.612 | 89.893 a | 1.42 | 65.302 | 133 | 73.554 | 65.404 | ||||||
| 29 | 80.237 | 67.876 | 134 | 62.993 | 56.028 | ||||||||
| 30 | 70.770 | 60.778 | 56.932 | −6.76 | 135 | 64.861 | 59.012 | ||||||
| 31 | 83.420 | 86.392 | 3.44 | 70.012 | 136 | 64.381 | 50.250 | ||||||
| 32 | 65.574 | 61.050 | 137 | 67.115 | 58.501 | ||||||||
| 33 | 89.357 | 90.196 | 0.93 | 68.142 | 67.742 | −0.59 | 138 | 68.889 | 69.900 | 1.45 | 57.865 | ||
| 34 | 87.497 | 71.727 | 139 | 68.238 | 50.422 | ||||||||
| 35 | 86.138 | 69.104 | 140 | 60.491 | 48.887 | ||||||||
| 36 | 91.986 | 91.012 | −1.07 | 73.519 | 141 | 74.464 | 61.583 | ||||||
| 37 | 91.435 | 64.331 | 142 | 70.237 | 52.663 | ||||||||
| 38 | 92.285 | 69.477 | 66.772 | −4.05 | 143 | 66.244 | 54.364 | ||||||
| 39 | 91.511 | 90.431 | −1.19 | 69.204 | 144 | 64.648 | 55.168 | ||||||
| 40 | 73.327 | 73.154 | −0.24 | 65.302 | 145 | 74.108 | 61.477 | 63.476 a | 3.15 | ||||
| 41 | 68.109 | 61.187 | 146 | 75.031 | 60.764 | ||||||||
| 42 | 74.542 | 61.114 | 147 | 65.471 | 52.282 | ||||||||
| 43 | 74.076 | 64.081 | 148 | 70.797 | 52.261 | ||||||||
| 44 | 66.061 | 65.927 | 149 | 68.510 | 54.156 | 54.240 | 0.16 | ||||||
| 45 | 60.125 | 56.469 | 150 | 79.539 | 46.136 | ||||||||
| 46 | 66.153 | 65.282 | −1.33 | 54.948 | 151 | 67.629 | 57.030 | 56.600 | −0.76 | ||||
| 47 | 65.818 | 57.198 | 60.010 | 4.69 | 152 | 77.851 | 63.524 | ||||||
| 48 | 65.849 | 60.330 | 153 | 66.585 | 67.731 | 1.69 | 57.174 | ||||||
| 49 | 71.324 | 61.956 | 65.967 a | 6.08 | 154 | 67.694 | 50.534 | ||||||
| 50 | 68.467 | 53.531 | 51.652 | −3.64 | 155 | 74.516 | 64.116 | ||||||
| 51 | 63.434 | 52.778 | 156 | 86.505 | 89.360 | 3.19 | 66.754 | ||||||
| 52 | 67.203 | 67.751 | 0.81 | 64.519 | 157 | 93.025 | 65.739 | ||||||
| 53 | 68.356 | 66.008 | 158 | 69.996 | 60.483 | 55.534 a | −8.91 | ||||||
| 54 | 66.777 | 60.121 | 159 | 84.798 | 82.536 | −2.74 | 69.399 | ||||||
| 55 | 85.426 | 69.076 | 160 | 65.245 | 58.509 | ||||||||
| 56 | 88.107 | 68.780 | 161 | 71.927 | 56.126 | 57.228 | 1.93 | ||||||
| 57 | 79.168 | 73.179 | 75.837 | 3.51 | 162 | 86.262 | 69.840 | ||||||
| 58 | 86.389 | 72.286 | 163 | 66.042 | 64.367 | ||||||||
| 59 | 66.140 | 65.444 | 164 | 73.207 | 73.038 | −0.23 | 59.661 | ||||||
| 60 | 87.500 | 66.423 | 67.218 | 1.18 | 165 | 66.114 | 62.462 | ||||||
| 61 | 78.246 | 68.885 | 166 | 60.991 | 54.165 | ||||||||
| 62 | 65.854 | 60.866 | 167 | 90.001 | 89.597 | −0.45 | 65.217 | ||||||
| 63 | 81.240 | 70.529 | 168 | 77.170 | 57.249 | ||||||||
| 64 | 64.290 | 57.249 | 169 | 95.088 | 67.929 | 69.672 | 2.50 | ||||||
| 65 | 60.271 | 62.106 | 64.739 | 4.07 | 170 | 73.365 | 72.234 | −1.57 | 58.643 | ||||
| 66 | 92.187 | 64.582 | 70.824 a | 8.81 | 171 | 70.282 | 49.261 | ||||||
| 67 | 83.173 | 68.420 | 172 | 69.300 | 61.413 | ||||||||
| 68 | 90.326 | 68.134 | 173 | 70.549 | 53.322 | 55.317 | 3.61 | ||||||
| 69 | 74.005 | 61.429 | 174 | 70.357 | 67.737 | −3.87 | 55.179 | ||||||
| 70 | 86.766 | 69.266 | 68.144 | −1.65 | 175 | 82.557 | 56.668 | ||||||
| 71 | 68.635 | 61.179 | 58.979 | −3.73 | 176 | 71.336 | 72.986 | 2.26 | 63.146 | ||||
| 72 | 84.899 | 72.836 | 77.695 | 6.25 | 177 | 66.333 | 67.823 a | 2.20 | 52.939 | 50.357 | −5.13 | ||
| 73 | 73.877 | 64.293 | 178 | 67.363 | 57.564 | ||||||||
| 74 | 85.224 | 85.467 | 0.28 | 65.771 | 179 | 66.274 | 48.973 | ||||||
| 75 | 64.592 | 57.676 | 180 | 68.687 | 72.489 | 5.24 | 57.952 | 55.123 | −5.13 | ||||
| 76 | 91.184 | 68.336 | 181 | 73.058 | 49.108 | ||||||||
| 77 | 97.273 | 99.318 | 2.06 | 64.961 | 182 | 65.665 | 51.597 | ||||||
| 78 | 95.861 | 70.370 | 183 | 71.546 | 71.487 | −0.08 | 50.906 | 52.807 a | 3.60 | ||||
| 79 | 88.812 | 69.859 | 184 | 73.752 | 43.131 | ||||||||
| 80 | 88.972 | 83.873 | −6.08 | 74.719 | 185 | 75.222 | 53.835 | ||||||
| 81 | 95.415 | 99.705 a | 4.30 | 66.087 | 70.222 | 5.89 | 186 | 73.291 | 75.368 | 2.76 | 58.614 | ||
| 82 | 75.659 | 83.729 | 9.64 | 61.660 | 64.109 | 3.82 | 187 | 71.067 | 52.759 | ||||
| 83 | 75.123 | 64.490 | 188 | 77.819 | 65.671 | ||||||||
| 84 | 60.484 | 58.008 | 189 | 88.147 | 84.203 | −4.68 | 66.052 | ||||||
| 85 | 67.346 | 64.546 | −4.34 | 57.494 | 190 | 67.586 | 59.613 | 58.962 | −1.10 | ||||
| 86 | 67.811 | 60.494 | 191 | 75.088 | 77.172 | 2.70 | 50.567 | 51.685 | 2.16 | ||||
| 87 | 68.117 | 62.251 | 192 | 67.662 | 58.945 | ||||||||
| 88 | 60.852 | 53.986 | 193 | 69.188 | 69.360 | 0.25 | 58.711 | ||||||
| 89 | 61.130 | 52.143 | 194 | 73.294 | 76.926 a | 4.72 | 57.909 | ||||||
| 90 | 73.421 | 60.341 | 195 | 69.996 | 71.502 | 2.11 | 50.303 | ||||||
| 91 | 62.968 | 53.689 | 196 | 73.869 | 51.909 | ||||||||
| 92 | 64.058 | 65.180 | 197 | 76.361 | 74.519 | −2.47 | 61.428 | ||||||
| 93 | 62.641 | 55.883 | 198 | 74.917 | 54.322 | ||||||||
| 94 | 71.336 | 55.614 | 199 | 73.260 | 72.533 | −1.00 | 53.729 | ||||||
| 95 | 65.125 | 70.561 a | 7.70 | 58.455 | 200 | 73.676 | 76.580 | 3.79 | 62.805 | 61.690 | −1.81 | ||
| 96 | 79.861 | 66.412 | 201 | 68.497 | 70.293 | 2.56 | 58.049 | ||||||
| 97 | 76.133 | 61.494 | 59.222 | −3.84 | 202 | 68.262 | 65.828 | −3.70 | 49.883 | ||||
| 98 | 65.547 | 51.651 | 52.414 | 1.46 | 203 | 78.701 | 78.053 | −0.83 | 49.585 | ||||
| 99 | 65.061 | 56.699 | 204 | 72.909 | 71.865 a | −1.45 | 60.753 | ||||||
| 100 | 62.776 | 50.011 | 205 | 70.706 | 73.702 | 4.07 | 53.985 | ||||||
| 101 | 72.904 | 71.570 | −1.86 | 61.497 | 65.198 a | 5.68 | 206 | 76.912 | 55.815 | 59.438 a | 6.10 | ||
| 102 | 68.287 | 53.276 | 207 | 79.669 | 76.815 a | −3.72 | 60.496 | ||||||
| 103 | 73.465 | 54.758 | 208 | 74.789 | 75.332 | 0.72 | 55.552 | 55.405 | −0.27 | ||||
| 104 | 69.305 | 62.315 | 209 | 79.286 | 79.575 | 0.36 | 59.179 | 59.612 | 0.73 | ||||
| Molecular | Phytotoxicity | Change Rate (%) | Estrogen Toxicity | Change Rate (%) | Bioconcentration | Change Rate (%) | Persistence | Change Rate (%) | Migration | Change Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PCB-52 | 67.203 | 64.519 | 4.63 | 0.989 | 8.538 | |||||
| 52-1 | 90.708 | 34.98 | 65.576 | 1.64 | 3.879 | −16.22 | 1.011 | 2.22 | 9.061 | 6.13 |
| 52-2 | 75.438 | 12.25 | 64.884 | 0.57 | 5.034 | 8.73 | 1.095 | 10.72 | 10.111 | 18.42 |
| 52-3 | 58.492 | −12.96 | 55.954 | −13.28 | 5.372 | 16.03 | 1.102 | 11.43 | 9.431 | 10.46 |
| 52-4 | 39.965 | −40.53 | 69.928 | 8.38 | 1.159 | −74.97 | −0.831 | −184.02 | 3.449 | −59.60 |
| P1 | 65.259 | 69.296 | 5.477 | 0.811 | 9.686 | |||||
| P1-1 | 65.134 | −0.19 | 54.331 | −21.60 | 5.258 | −4.00 | 0.665 | −18.00 | 8.575 | −11.47 |
| P1-2 | 70.868 | 8.59 | 73.992 | 6.78 | 4.416 | −19.37 | 0.881 | 8.63 | 8.494 | −12.31 |
| P1-3 | 79.052 | 21.14 | 68.901 | −0.57 | 4.214 | −23.06 | 0.78 | −3.82 | 8.634 | −10.86 |
| P1-4 | 76.850 | 17.76 | 56.281 | −18.78 | 4.596 | −16.09 | 0.893 | 10.11 | 8.852 | −8.61 |
| PCB-189 | 88.147 | 66.052 | 5.440 | 1.567 | 11.517 | |||||
| 189-1 | 84.575 | −4.05 | 62.105 | −5.98 | 5.446 | 0.11 | 1.274 | −18.70 | 10.565 | −8.27 |
| 189-2 | 79.545 | −9.76 | 58.150 | −11.96 | 5.649 | 3.84 | 1.599 | 2.04 | 10.912 | −5.25 |
| 189-3 | 84.176 | −4.50 | 68.607 | 3.87 | 5.939 | 9.17 | 1.299 | −17.10 | 10.702 | −7.08 |
| 189-4 | 31.989 | −63.71 | 59.291 | −10.24 | 2.873 | −47.19 | −0.558 | −135.61 | 4.428 | −61.55 |
| P2 | 71.373 | 49.398 | 3.446 | 1.311 | 4.642 | |||||
| P2-1 | 75.601 | 5.92 | 69.555 | 40.81 | 4.439 | 28.82 | 0.694 | −47.06 | 7.406 | 59.54 |
| P2-2 | 67.157 | −5.91 | 52.344 | 5.96 | 5.459 | 58.42 | 1.038 | −20.82 | 9.928 | 113.87 |
| P2-3 | 80.525 | 12.82 | 50.002 | 1.22 | 4.725 | 37.12 | 1.49 | 13.65 | 9.046 | 94.87 |
| P2-4 | 86.936 | 21.81 | 58.514 | 18.45 | 5.536 | 60.65 | 1.212 | −7.55 | 10.088 | 117.32 |
| PCB-209 | 79.408 | 54.982 | 6.136 | 2.191 | 11.805 | |||||
| 209-1 | 85.844 | 8.10 | 50.643 | −7.89 | 4.726 | −22.98 | 1.85 | −15.56 | 10.003 | −15.26 |
| 209-2 | 77.534 | −2.36 | 51.355 | −6.60 | 6.204 | 1.11 | 2.079 | −5.11 | 9.816 | −16.85 |
| 209-3 | 61.850 | −22.11 | 47.372 | −13.84 | 6.079 | −0.93 | 1.606 | −26.70 | 10.169 | −13.86 |
| 209-4 | 50.929 | −35.86 | 71.124 | 29.36 | 3.190 | −48.01 | −0.487 | −122.23 | 7.964 | −32.54 |
| P3 | 75.056 | 57.487 | 5.500 | 1.863 | 11.102 | |||||
| P3-1 | 63.598 | −15.27 | 48.786 | −15.14 | 5.815 | 5.73 | 1.700 | −8.75 | 9.383 | −15.48 |
| P3-2 | 79.836 | 6.37 | 58.633 | 1.99 | 5.703 | 3.69 | 1.478 | −20.67 | 9.948 | −10.39 |
| P3-3 | 61.300 | −18.33 | 58.633 | 1.99 | 5.367 | −2.42 | 1.402 | −24.75 | 8.608 | −22.46 |
| P3-4 | 79.556 | 6.00 | 52.530 | −8.62 | 5.065 | −7.91 | 0.949 | −49.06 | 10.965 | −1.23 |
| NO. | Phytotoxicity | Estrogen Toxicity | |||||
|---|---|---|---|---|---|---|---|
| Total Cost | Estimated | Relative Error (%) | Total Cost | Estimated | Relative Error (%) | ||
| 1 | 4′-OH-CB97 | 75.468 | 71.34 | −5.47 | 66.793 | 55.05 | −17.58 |
| 2 | 4′-OH-CB101 | 74.627 | 62.36 | −16.44 | 65.252 | 59.60 | −8.66 |
| 3 | 4-OH-CB107 | 78.598 | 84.60 | 7.64 | 67.165 | 67.39 | 0.34 |
| 4 | 4-OH-CB109 | 75.046 | 67.77 | −9.70 | 65.372 | 59.92 | −8.34 |
| 5 | 3-OH-CB118 | 86.550 | 83.53 | −3.49 | 62.666 | 63.70 | 1.65 |
| 6 | 4-OH-CB148 | 68.485 | 66.70 | −2.61 | 71.272 | 55.92 | −21.54 |
| 7 | 3-OH-CB153 | 72.700 | 78.53 | 8.02 | 66.310 | 59.69 | −9.98 |
| 8 | 4-OH-CB162 | 97.896 | 85.20 | −12.97 | 61.408 | 64.24 | 4.61 |
| 9 | 4′-OH-CB172 | 80.308 | 66.42 | −17.29 | 65.140 | 55.88 | −14.22 |
| 10 | 4-OH-CB187 | 75.115 | 72.69 | −3.23 | 63.185 | 52.85 | −16.36 |
| 11 | 4′-OH-CB199 | 79.422 | 85.35 | 7.46 | 60.988 | 62.00 | 1.66 |
| 12 | 4-OH-CB202 | 77.024 | 69.99 | −9.13 | 55.911 | 56.65 | 1.32 |
| 13 | 3′-MeSO2-CB49 | 72.948 | 67.66 | −7.25 | 67.760 | 56.88 | −16.06 |
| 14 | 4-MeSO2-CB64 | 71.831 | 62.72 | −12.68 | 61.242 | 52.25 | −14.68 |
| 15 | 3-MeSO2-CB70 | 86.872 | 78.98 | −9.08 | 72.756 | 66.08 | −9.18 |
| 16 | 3-MeSO2-CB110 | 80.192 | 67.57 | −15.74 | 78.504 | 62.03 | −20.98 |
| 17 | 4-MeSO2-CB149 | 83.809 | 81.80 | −2.40 | 58.452 | 54.16 | −7.34 |
| 18 | 4-MeSO2-CB174 | 80.324 | 72.89 | −9.26 | 54.488 | 55.18 | 1.27 |
| 19 | P1 | 66.228 | 65.26 | −1.46 | 70.507 | 69.30 | −1.72 |
| 20 | P1-1 | 104.434 | 65.13 | −37.63 | 58.480 | 54.33 | −7.10 |
| 21 | P1-2 | 78.201 | 70.87 | −9.38 | 73.265 | 73.99 | 0.99 |
| 22 | P1-3 | 85.788 | 79.05 | −7.85 | 67.833 | 68.90 | 1.57 |
| 23 | P1-4 | 78.552 | 76.85 | −2.17 | 52.126 | 56.28 | 7.97 |
| 24 | P2 | 77.873 | 71.37 | −8.35 | 60.337 | 49.40 | −18.13 |
| 25 | P2-1 | 83.167 | 75.60 | −9.10 | 77.099 | 69.56 | −9.78 |
| 26 | P2-2 | 73.239 | 67.16 | −8.30 | 72.490 | 52.34 | −27.79 |
| 27 | P2-3 | 88.294 | 80.53 | −8.80 | 65.488 | 50.00 | −23.65 |
| 28 | P2-4 | 80.868 | 86.94 | 7.50 | 64.082 | 58.51 | −8.69 |
| 29 | P3 | 77.512 | 75.06 | −3.17 | 63.670 | 57.49 | −9.71 |
| 30 | P3-1 | 78.478 | 63.60 | −18.96 | 53.482 | 48.79 | −8.78 |
| 31 | P3-2 | 76.342 | 79.84 | 4.58 | 63.270 | 58.63 | −7.33 |
| 32 | P3-3 | 76.683 | 61.30 | −20.06 | 72.835 | 58.63 | −19.50 |
| 33 | P3-4 | 77.896 | 79.56 | 2.13 | 55.943 | 52.53 | −6.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; He, W.; Yang, H.; Sun, S.; Li, Y. Potential Environmental Risk Characteristics of PCB Transformation Products in the Environmental Medium. Toxics 2021, 9, 213. https://doi.org/10.3390/toxics9090213
Li M, He W, Yang H, Sun S, Li Y. Potential Environmental Risk Characteristics of PCB Transformation Products in the Environmental Medium. Toxics. 2021; 9(9):213. https://doi.org/10.3390/toxics9090213
Chicago/Turabian StyleLi, Minghao, Wei He, Hao Yang, Shimei Sun, and Yu Li. 2021. "Potential Environmental Risk Characteristics of PCB Transformation Products in the Environmental Medium" Toxics 9, no. 9: 213. https://doi.org/10.3390/toxics9090213






