Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Chemicals
2.3. Toxicity Assays
2.3.1. Zebrafish Embryonic Toxicity (ZET) Assay
2.3.2. General and Behavioral Toxicity (GBT) Assay
2.3.3. Dose Curve Fitting/Behavioral Analysis
3. Results
3.1. Toxicity Assays
3.2. Comparison of Toxicity Ranking Profiles
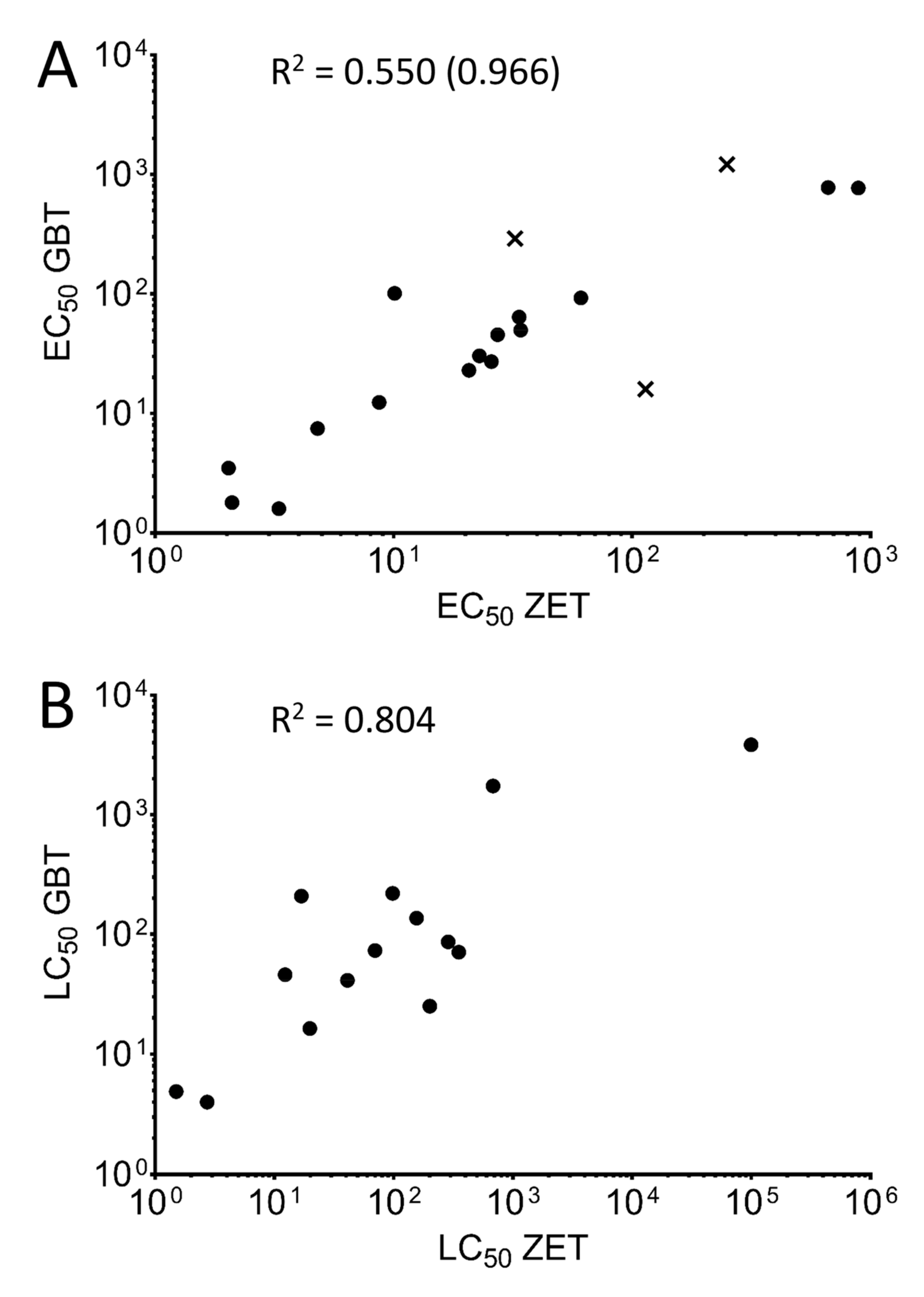
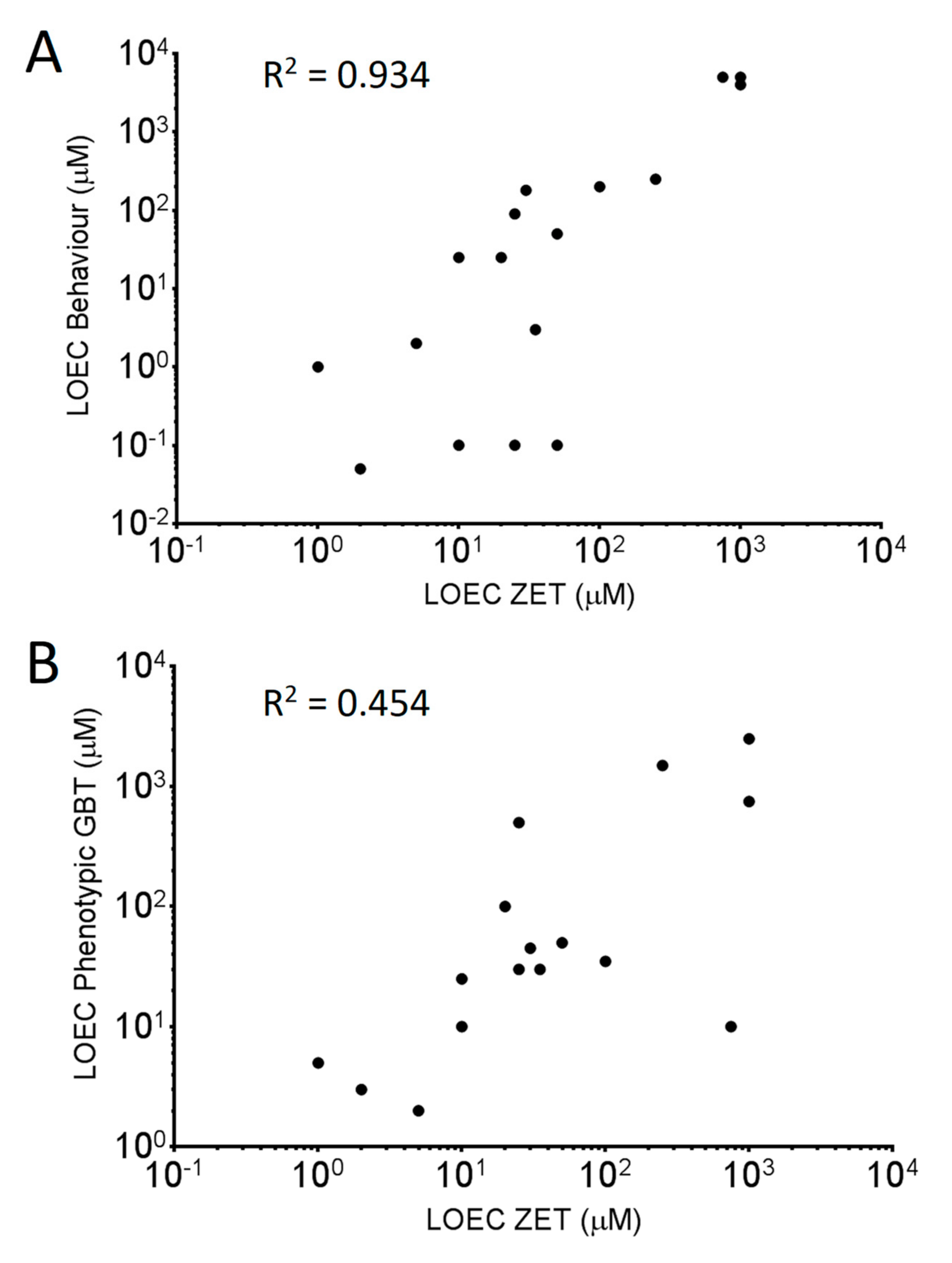
3.3. Comparing Sensitivities of the Two Toxicity Assays
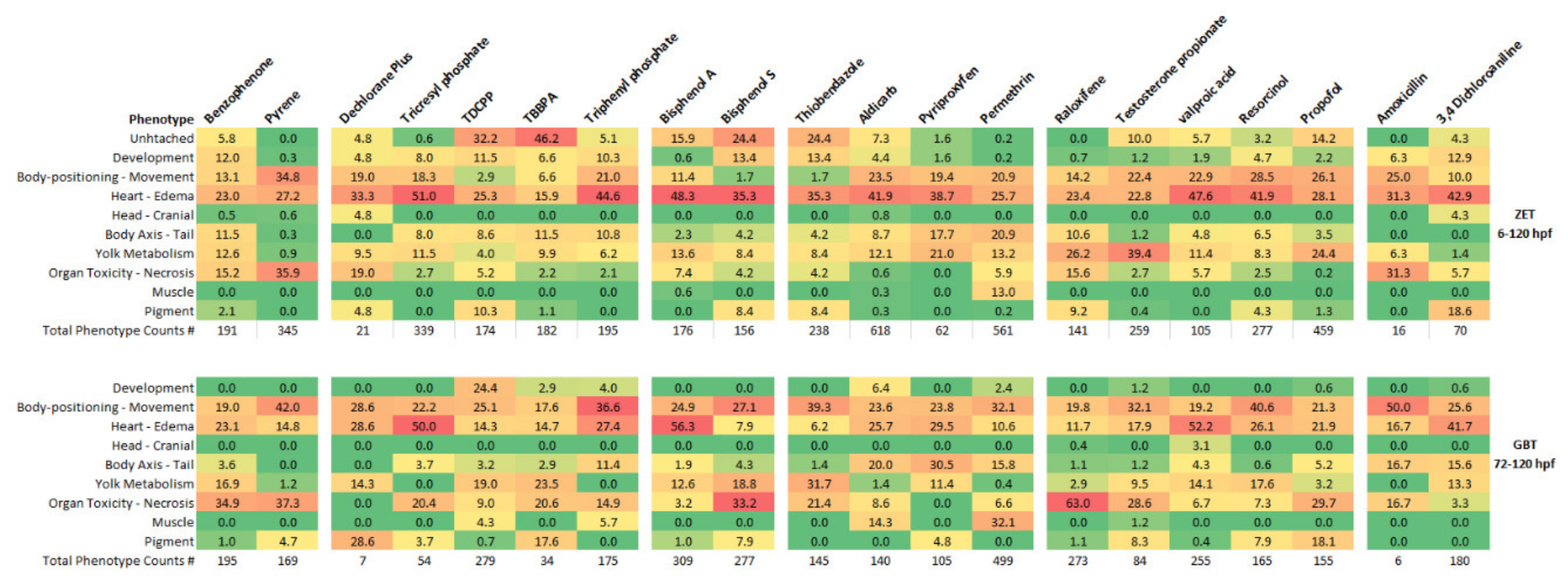
3.4. Observed Phenotypes and Phenotypic Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caballero, M.V.; Candiracci, M. Zebrafish as Toxicological model for screening and recapitulate human diseases. J. Unexplored Med. Data 2018, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Luna, J.; Al-Jubouri, Q.; Al-Nuaimy, W.; Sneddon, L.U. Impact of stress, fear and anxiety on the nociceptive responses of larval zebrafish. PLoS ONE 2017, 12, e0181010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenbergen, P.J.; Bardine, N. Antinociceptive effects of buprenorphine in zebrafish larvae: An alternative for rodent models to study pain and nociception? Appl. Anim. Behav. Sci. 2014, 152, 92–99. [Google Scholar] [CrossRef]
- Truong, L.; Gonnerman, G.; Simonich, M.T.; Tanguay, R.L. Assessment of the developmental and neurotoxicity of the mosquito control larvicide, pyriproxyfen, using embryonic zebrafish. Environ. Pollut. 2016, 218, 1089–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, R.; Legler, J.; Legradi, J. Alterations in locomotor activity of feeding zebrafish larvae as a consequence of exposure to different environmental factors. Environ. Sci. Pollut. Res. Int 2018, 25, 4085–4093. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.D.; Berrue, F.; Morash, M.; Achenbach, J.C.; Hill, J.; McDougall, J.J. Comparison of cannabinoids with known analgesics using a novel high throughput zebrafish larval model of nociception. Behav. Brain Res. 2018, 337, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Alzualde, A.; Behl, M.; Sipes, N.S.; Hsieh, J.H.; Alday, A.; Tice, R.R.; Paules, R.S.; Muriana, A.; Quevedo, C. Toxicity profiling of flame retardants in zebrafish embryos using a battery of assays for developmental toxicity, neurotoxicity, cardiotoxicity and hepatotoxicity toward human relevance. Neurotoxicol. Teratol. 2018, 70, 40–50. [Google Scholar] [CrossRef]
- Quevedo, C.; Behl, M.; Ryan, K.; Paules, R.S.; Alday, A.; Muriana, A.; Alzualde, A. Detection and Prioritization of Developmentally Neurotoxic and/or Neurotoxic Compounds Using Zebrafish. Toxicol. Sci. 2019, 168, 225–240. [Google Scholar] [CrossRef]
- Malafoglia, V.; Colasanti, M.; Raffaeli, W.; Balciunas, D.; Giordano, A.; Bellipanni, G. Extreme Thermal Noxious Stimuli Induce Pain Responses in Zebrafish Larvae. J. Cell. Physiol. 2014, 229, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Organisation for Economic Co-Operation and Development. Test. No. 236: Fish. Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- Saverino, C.; Gerlai, R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008, 191, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Gilmour, G.; Pioli, E.Y.; Dix, S.L.; Smith, J.W.; Conway, M.W.; Jones, W.T.; Loomis, S.; Mason, R.; Shahabi, S.; Tricklebank, M.D. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: Implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology 2009, 205, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Zebrafish (Danio rerio) embryos as a model for testing proteratogens. Toxicology 2011, 281, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Brannen, K.C.; Panzica-Kelly, J.M.; Danberry, T.L.; Augustine-Rauch, K.A. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, A.L.; Stedman, D.B.; Ball, J.; Hillegass, J.M.; Flood, A.; Zhang, C.X.; Panzica-Kelly, J.; Cao, J.; Coburn, A.; Enright, B.P.; et al. Inter-laboratory assessment of a harmonized zebrafish developmental toxicology assay-progress report on phase I. Reprod. Toxicol. 2012, 33, 155–164. [Google Scholar] [CrossRef]
- Ellis, L.D.; Soo, E.C.; Achenbach, J.C.; Morash, M.G.; Soanes, K.H. Use of the zebrafish larvae as a model to study cigarette smoke condensate toxicity. PLoS ONE 2014, 9, e115305. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- D’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [Green Version]
- Incardona, J.P.; Scholz, N.L. The influence of heart developmental anatomy on cardiotoxicity-based adverse outcome pathways in fish. Aquat. Toxicol. 2016, 177, 515–525. [Google Scholar] [CrossRef]
- Hao, R.; Bondesson, M.; Singh, A.V.; Riu, A.; McCollum, C.W.; Knudsen, T.B.; Gorelick, D.A.; Gustafsson, J.A. Identification of estrogen target genes during zebrafish embryonic development through transcriptomic analysis. PLoS ONE 2013, 8, e79020. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.; Herrero-Nogareda, L.; Van Antro, M.; Campos, M.P.; Casado, M.; Barata, C.; Pina, B.; Navarro-Martin, L. Morphometric signatures of exposure to endocrine disrupting chemicals in zebrafish eleutheroembryos. Aquat. Toxicol. 2019, 214, 105232. [Google Scholar] [CrossRef]
- Sant, K.E.; Timme-Laragy, A.R. Zebrafish as a Model for Toxicological Perturbation of Yolk and Nutrition in the Early Embryo. Curr. Environ. Health Rep. 2018, 5, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Collier, T.K.; Scholz, N.L. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004, 196, 191–205. [Google Scholar] [CrossRef]
- Wilson, L.B.; Truong, L.; Simonich, M.T.; Tanguay, R.L. Systematic Assessment of Exposure Variations on Observed Bioactivity in Zebrafish Chemical Screening. Toxics 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Leuthold, D.; Kluver, N.; Altenburger, R.; Busch, W. Can Environmentally Relevant Neuroactive Chemicals Specifically Be Detected with the Locomotor Response Test in Zebrafish Embryos? Environ. Sci. Technol. 2019, 53, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Jarema, K.A.; Hunter, D.L.; Shaffer, R.M.; Behl, M.; Padilla, S. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 2015, 52, 194–209. [Google Scholar] [CrossRef] [Green Version]
- Kluver, N.; Konig, M.; Ortmann, J.; Massei, R.; Paschke, A.; Kuhne, R.; Scholz, S. Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ. Sci. Technol. 2015, 49, 7002–7011. [Google Scholar] [CrossRef]
- Hotston, R.D. Outbreak of polyneuritis due to orthotricresyl phosphate poisoning. Lancet 1946, 9, 207. [Google Scholar] [CrossRef]
- Parens, R.; Nijhout, H.F.; Morales, A.; Xavier Costa, F.; Bar-Yam, Y. A Possible Link between Pyriproxyfen and Microcephaly. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Faria, M.; Bedrossiantz, J.; Prats, E.; Rovira Garcia, X.; Gomez-Canela, C.; Pina, B.; Raldua, D. Deciphering the mode of action of pollutants impairing the fish larvae escape response with the vibrational startle response assay. Sci. Total Environ. 2019, 672, 121–128. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Novoa-Luna, K.A.; Bedrossiantz, J.; Gomez-Canela, C.; Gomez-Olivan, L.M.; Raldua, D. Development of a vibrational startle response assay for screening environmental pollutants and drugs impairing predator avoidance. Sci. Total Environ. 2019, 650, 87–96. [Google Scholar] [CrossRef]
| Type | Compound Name | CAS RN | Purity | MW (g/mol) | Use | Log p | DMSO Vehicle % (v/v) |
|---|---|---|---|---|---|---|---|
| Chemical precursors | Pyrene | 129-00-0 | 99% | 202.25 | precursor for dyes, plastics, and pesticides | 4.9 | 1.0% |
| Benzophenone | 119-61-9 | ≥99% | 182.22 | UV blocker, flavour ingredient, fragrance enhancer | 3.4 | 0.5% (ZET)/1.0% (GBT) | |
| Flame retardants | Dechlorane Plus | 13560-89-9 | 98% | 653.7 | flame retardant | 8 | 1.0% |
| Tricresyl phosphate (TCrP) | 1330-78-5 | 90% | 368.4 | flame retardant | 5.1 | 1.0% | |
| Tris(dichloro-isopropyl) phosphate (TDCPP) | 13674-87-8 | ≥95% | 430.9 | flame retardant | 3.3 | 0.5% | |
| 3,3′,5,5′-tetrabromobisphenol A (TBBPA) | 79-94-7 | 97% | 543.9 | brominated flame retardant | 6.8 | 0.5% | |
| Triphenyl phosphate (TPhP) | 115-86-6 | ≥99% | 326.28 | plasticizer and flame retardant | 4.6 | 0.5% | |
| Plasticizers | Bisphenol A | 80-05-7 | ≥99% | 228.29 | plasticizer | 3.3 | 0.5% |
| Bisphenol S | 80-09-1 | >98% | 250.27 | plasticizer | 1.9 | 1.0% | |
| Pesticides/Fungicides | Thiabendazole | 148-79-8 | >99% | 201.25 | veterinary fungicide | 2.5 | 0.5% |
| Aldicarb | 116-06-3 | ≥98% | 190.27 | pesticide | 1.1 | 1.0% (ZET)/0.5% (GBT) | |
| Pyriproxyfen | 95737-68-1 | ≥98% | 321.4 | veterinary drug for flea control | 4.8 | 1.0% | |
| Permethrin | 52645-53-1 | ≥90% | 391.3 | human and veterinary insecticide (head lice and scabies) | 6.5 | 0.5% | |
| Pharmaceuticals | Raloxifene HCl | 82640-04-8 | >99% | 510 | treatment of osteoporosis and breast cancer | 6.1 (non-ionic version) | 0.5% |
| Testosterone propionate | 57-85-2 | 98% | 344.5 | anabolic steroid, treatment of breast cancer | 4.4 | 0.5% (ZET)/1.0% (GBT) | |
| Valproic acid | 99-66-1 | >97.5% | 144.21 | anticonvulsant | 2.8 | 0.5% | |
| Resorcinol | 108-46-3 | ≥99% | 110.11 | topical treatment of skin disorders | 0.8 | 1.0% | |
| Propofol | 2078-54-8 | 97% | 178.27 | sedative | 3.8 | 0.5% | |
| Controls | Amoxicillin | 26787-78-0 | potency: ≥900 μg per mg | 365.4 | antibiotic, negative control | −2 | 0.5% (ZET)/1.0% (GBT) |
| 3,4-Dichloroaniline | 95-76-1 | 98% | 162.01 | positive control in OECD FET | 2.7 | 0.5% |
| Phenotypic Group | Phenotypes |
|---|---|
| Development | Gross morphological abnormality |
| Overall short body | |
| Body positioning—Movement | Loss of lateral recumbency |
| Constant movement | |
| Heart—Edema | Slow heart rate |
| Pericardial edema | |
| Blood pooling | |
| Yolk syncitial layer edema | |
| Head edema | |
| Large gut lumen | |
| Head—Cranial | Small head |
| Gray/cloudy head | |
| Body axis—tail | Curved tail—dorsal/ventral axis |
| Curved tail | |
| Yolk metabolism | Large yolk (for developmental time point) |
| Gray/cloudy yolk | |
| Organ toxicity—necrotic | Dark coloured liver |
| Dark coloured nose/mandible | |
| Muscle | Ruffled tail fin |
| Pigment | Lighter pigment |
| Overall darker colour | |
| Melanocyte aggregation |
| EC50 (µM) | LC50 (µM) | Maximum Exposure Concentration (µM) Tested | ||||
|---|---|---|---|---|---|---|
| ZET | GBT | ZET | GBT | |||
| 6–120 hpf | 72–120 hpf | 6–120 hpf | 72–120 hpf | ZET | GBT | |
| Benzophenone | 114 | 16 | - | 787.9 | 5000 | 5000 |
| Pyrene | 33.6 * | 64.1 * | 156 * | 136.8 * | 2000 | 2000 |
| Dechlorane Plus | - | - | - | - | 62.5 | 62.5 |
| Tricresyl Phosphate | 20.7 * | 23 * | 201.2 * | 25.1 * | 2000 | 500 |
| TBBPA | 2.03 | 3.5 | 2.7 | 4.0 | 50 | 10 |
| TDCPP | 8.7 * | 12.4 * | 12.3 | 45.9 | 500 | 500 |
| Triphenyl phosphate | 4.8 * | 7.5 * | 19.8 | 16.4 | 50 | 50 |
| Bisphenol A | 34.1 * | 49.8 * | 69.8 * | 73.2 * | 100 | 250 |
| Bisphenol S | 664 * | 778 * | 1722 | - | 4000 | 2500 |
| Thiobendazole | 32.3 | 291 | 59.0 | - | 90 | 1000 |
| Aldicarb | 2.1 | 1.8 | 1.5 | 4.9 | 2000 | 500 |
| Pyriproxyfen | 60.99 | 92.8 | 98.2 | 220.1 | 500 | 500 |
| Permethrin | 3.3 | 1.6 | - | - | 50 | 10 |
| Raloxifene HCl | 25.7 * | 27.2 * | 41.0 * | 41.2 * | 90 | 75 |
| Testosterone Propionate | 27.3 | 45.7 | 286.7 | 86.4 | 900 | 450 |
| Valproic Acid | 250 | 1215 | 682.2 | 1743 | 2500 | 2500 |
| Resorcinol | 888.4 * | 771 * | - | 3849 | 5270 | 5000 |
| Propofol | 22.9 * | 30.4* | 351.6 | 70.9 | 1000 | 500 |
| Amoxicillin | - | - | - | - | 2500 | 5000 |
| 3,4-Dichloroaniline | 10.1 | 101.4 | 16.8 | 208.7 | 500 | 500 |
| Lowest Observed Effect Concentration (LOEC) (µM) | |||
|---|---|---|---|
| ZET | GBT | GBT | |
| (6–120 hpf) | (72–120 hpf) | Distance Travelled | |
| Benzophenone | 750 | 10 | 5000 |
| Pyrene | 50 | 50 | 50 |
| Dechlorane Plus | - | - | - |
| Tricresyl Phosphate | 25 | 30 | 0.1 * |
| TDCPP | 10 | 25 | 25 |
| TBBPA | 2 | 3 | 0.05 |
| Triphenyl phosphate | 10 | 10 | 0.1 * |
| Bisphenol A | 50 | 50 | 50 |
| Bisphenol S | 1000 | 750 | 5000 |
| Thiobendazole | 25 | 500 | 90 |
| Aldicarb | 1 | 5 | 1 |
| Pyriproxyfen | 50 | 50 | 0.1 |
| Permethrin | 5 | 2 | 2 * |
| Raloxifene HCl | 35 | 30 | 3 * |
| Testosterone Propionate | 30 | 45 | 180 |
| Valproic Acid | 250 | 1500 | 250 * |
| Resorcinol | 1000 | 2500 | 4000 |
| Propofol | 100 | 35 | 200 |
| Amoxicillin | - | - | - |
| 3,4-Dichloroaniline | 20 | 100 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achenbach, J.C.; Leggiadro, C.; Sperker, S.A.; Woodland, C.; Ellis, L.D. Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening. Toxics 2020, 8, 126. https://doi.org/10.3390/toxics8040126
Achenbach JC, Leggiadro C, Sperker SA, Woodland C, Ellis LD. Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening. Toxics. 2020; 8(4):126. https://doi.org/10.3390/toxics8040126
Chicago/Turabian StyleAchenbach, John C., Cindy Leggiadro, Sandra A. Sperker, Cindy Woodland, and Lee D. Ellis. 2020. "Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening" Toxics 8, no. 4: 126. https://doi.org/10.3390/toxics8040126
APA StyleAchenbach, J. C., Leggiadro, C., Sperker, S. A., Woodland, C., & Ellis, L. D. (2020). Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening. Toxics, 8(4), 126. https://doi.org/10.3390/toxics8040126





