The Confounder-Mediator Dilemma: Should We Control for Obesity to Estimate the Effect of Perfluoroalkyl Substances on Health Outcomes?
Abstract
1. Introduction
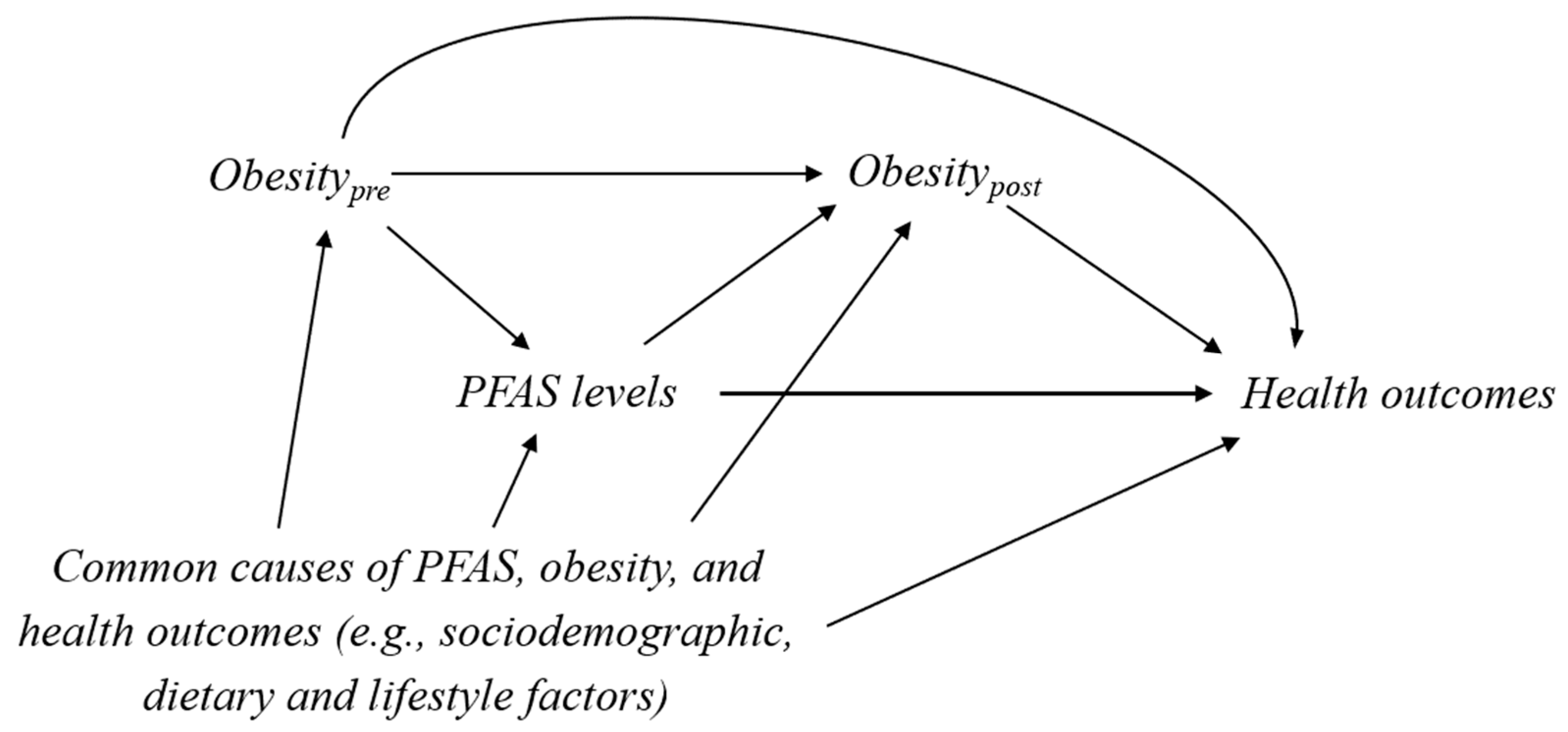
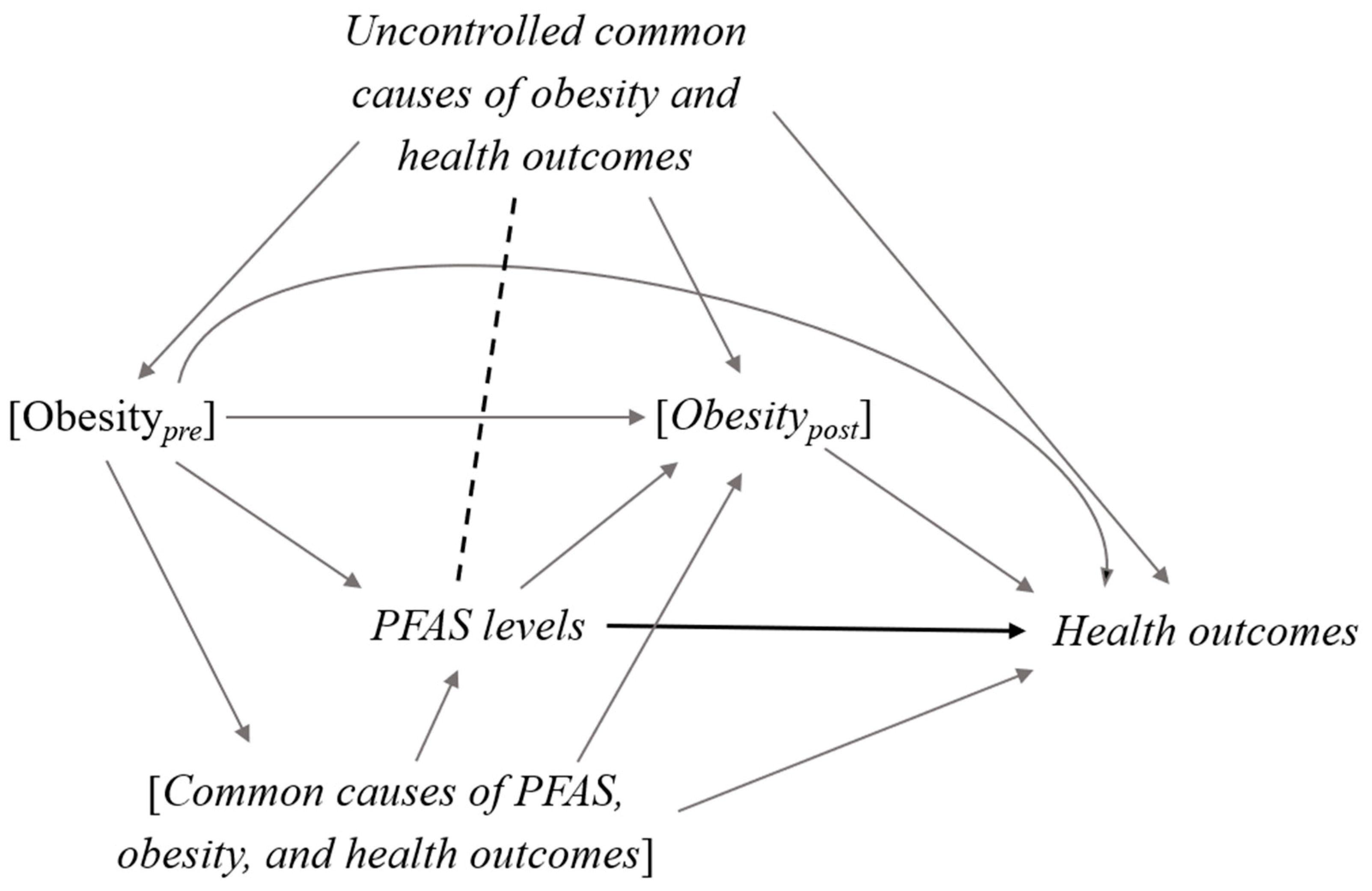
2. Confounder-Mediator Dilemma
3. Example Illustration I: The Association between PFAS and Thyroid Hormones during Pregnancy in the Danish National Birth Cohort
4. Example Illustration II: The Association between PFAS and Cardiovascular Disease Using the US National Health and Nutrition Examination Survey
5. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothman, K.; Grenland, S.; Lash, T.L. Modern Epidemiology; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Schisterman, E.F.; Cole, S.R.; Platt, R.W. Overadjustment Bias and Unnecessary Adjustment in Epidemiologic Studies. Epidemiology 2009, 20, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.R.; Platt, R.W.; Schisterman, E.F.; Chu, H.; Westreich, D.; Richardson, D.; Poole, C. Illustrating bias due to conditioning on a collider. Int. J. Epidemiol. 2010, 39, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díaz, S.; Schisterman, E.F.; Hernán, M.A. The Birth Weight “Paradox” Uncovered? Am. J. Epidemiol. 2006, 164, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.H.; Stokes, A. Obesity Paradox. Epidemiology 2014, 25, 454–461. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Per- and Polyfluoroalkyl Substances (PFAS). Available online: https://www.epa.gov/pfas (accessed on 24 July 2020).
- WHO State of the Science of Endocrine Disrupting Chemicals—2012. Available online: http://apps.who.int/iris/bitstream/10665/78101/1/9789241505031_eng.pdf (accessed on 25 April 2018).
- Bjerregaard-Olesen, C.; Bach, C.C.; Long, M.; Ghisari, M.; Bossi, R.; Bech, B.H.; Nohr, E.A.; Henriksen, T.B.; Olsen, J.; Bonefeld-Jørgensen, E.C. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environ. Int. 2016, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Wong, L.-Y.; Jia, L.T.; Kuklenyik, Z.; Calafat, A.M. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ. Sci. Technol. 2011, 45, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, V.; Costa, O.; Iñiguez, C.; Fletcher, T.; Ballester, F.; Lopez-Espinosa, M.-J. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ. Int. 2017, 99, 15–28. [Google Scholar] [CrossRef]
- Nelson, J.W.; Hatch, E.E.; Webster, T.F. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect. 2010, 118, 197–202. [Google Scholar] [CrossRef]
- Geiger, S.D.; Xiao, J.; Ducatman, A.; Frisbee, S.; Innes, K.; Shankar, A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere 2014, 98, 78–83. [Google Scholar] [CrossRef]
- Huang, M.; Jiao, J.; Zhuang, P.; Chen, X.; Wang, J.; Zhang, Y. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national U.S. population. Environ. Int. 2018, 119, 37–46. [Google Scholar] [CrossRef]
- Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.-F.; Fleisch, A.F.; Lin, P.-I.D.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; et al. Association of Perfluoroalkyl and Polyfluoroalkyl Substances with Adiposity. JAMA Netw. Open 2018, 1, e181493. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.S.; Fei, C.; Gamborg, M.; Nohr, E.A.; Sørensen, T.I.A.; Olsen, J. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am. J. Epidemiol. 2013, 178, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J. Causal Diagrams for Empirical Research. Biometrika 1995, 82, 669–688. [Google Scholar] [CrossRef]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal Diagrams for Epidemiologic Research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef]
- Inoue, K.; Ritz, B.; Andersen, S.L.; Ramlau-Hansen, C.H.; Høyer, B.B.; Bech, B.H.; Henriksen, T.B.; Bonefeld-Jørgensen, E.C.; Olsen, J.; Liew, Z. Perfluoroalkyl Substances and Maternal Thyroid Hormones in Early Pregnancy; Findings in the Danish National Birth Cohort. Environ. Health Perspect. 2019, 127, 117002. [Google Scholar] [CrossRef]
- Greenland, S. Quantifying biases in causal models: Classical confounding vs collider-stratification bias. Epidemiology 2003, 14, 300–306. [Google Scholar] [CrossRef]
- Weiss, J.M.; Andersson, P.L.; Lamoree, M.H.; Leonards, P.E.G.; van Leeuwen, S.P.J.; Hamers, T. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol. Sci. 2009, 109, 206–216. [Google Scholar] [CrossRef]
- Yu, W.-G.; Liu, W.; Jin, Y.-H. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ. Toxicol. Chem. 2009, 28, 990–996. [Google Scholar] [CrossRef]
- Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ. Sci. Pollut. Res. Int. 2013, 20, 8045–8056. [Google Scholar] [CrossRef] [PubMed]
- Burrow, G.N.; Fisher, D.A.; Larsen, P.R. Maternal and fetal thyroid function. N. Engl. J. Med. 1994, 331, 1072–1078. [Google Scholar] [PubMed]
- Korevaar, T.I.M.; Medici, M.; Visser, T.J.; Peeters, R.P. Thyroid disease in pregnancy: New insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 2017, 13, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Preston, E.V.; Webster, T.F.; Oken, E.; Claus Henn, B.; McClean, M.D.; Rifas-Shiman, S.L.; Pearce, E.N.; Braverman, L.E.; Calafat, A.M.; Ye, X.; et al. Maternal Plasma per- and Polyfluoroalkyl Substance Concentrations in Early Pregnancy and Maternal and Neonatal Thyroid Function in a Prospective Birth Cohort: Project Viva (USA). Environ. Health Perspect. 2018, 126, 027013. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.; Smurthwaite, K.; Bräunig, J.; Trevenar, S.; D’Este, C.; Lucas, R.; Lal, A.; Korda, R.; Clements, A.; Mueller, J.; et al. The PFAS Health Study: Systematic Literature Review; The Australian National University: Canberra, ACT, Australia, 2018. [Google Scholar]
- Olsen, J.; Melbye, M.; Olsen, S.F.; Sørensen, T.I.; Aaby, P.; Andersen, A.M.; Taxbøl, D.; Hansen, K.D.; Juhl, M.; Schow, T.B.; et al. The Danish National Birth Cohort—Its background, structure and aim. Scand. J. Public Health 2001, 29, 300–307. [Google Scholar] [CrossRef]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C.G. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, P.-C.; Lin, Y.-C.; Lin, L.-Y. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 2009, 32, 702–707. [Google Scholar] [CrossRef]
- CDC Obesity Is a Common, Serious, and Costly Disease. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 7 November 2020).
- VanderWeele, T.J. Commentary: Resolutions of the birthweight paradox: Competing explanations and analytical insights. Int. J. Epidemiol. 2014, 43, 1368–1373. [Google Scholar] [CrossRef]
- Daniel, R.M.; Cousens, S.N.; Stavola, B.L.D.; Kenward, M.G.; Sterne, J.A.C. Methods for dealing with time-dependent confounding. Stat. Med. 2013, 32, 1584–1618. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Yan, Q.; Paul, K.; Walker, D.; Jones, D.; Ritz, B. Air pollution and adverse pregnancy and birth outcomes: Mediation analysis using metabolomic profiles. Curr. Environ. Health Rep. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.B.; Lee, J.S.; Ren, H.; Vallanat, B.; Liu, J.; Waalkes, M.P.; Abbott, B.D.; Lau, C.; Corton, J.C. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: Evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol. Sci. 2008, 103, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Heuvel, J.V.; Thompson, J.; Frame, S.; Gillies, P. Differential Activation of Nuclear Receptors by Perfluorinated Fatty Acid Analogs and Natural Fatty Acids: A Comparison of Human, Mouse, and Rat Peroxisome Proliferator-Activated Receptor-α, -β, and -γ, Liver X Receptor-β, and Retinoid X Receptor-α. Toxicol. Sci. 2006, 92, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Wei, S.; Li, M.; Yang, J.; Li, K.; Jin, L.; Xie, Y.; Giesy, J.P.; Zhang, X.; Yu, H. Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-throughput Targeted Metabolomics Approach. Sci. Rep. 2016, 6, 23963. [Google Scholar] [CrossRef]
- Kirkley, A.G.; Sargis, R.M. Environmental Endocrine Disruption of Energy Metabolism and Cardiovascular Risk. Curr. Diab. Rep. 2014, 14, 494. [Google Scholar] [CrossRef]
- Schaider, L.A.; Balan, S.A.; Blum, A.; Andrews, D.Q.; Strynar, M.J.; Dickinson, M.E.; Lunderberg, D.M.; Lang, J.R.; Peaslee, G.F. Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [Google Scholar] [CrossRef]
- Susmann, H.P.; Schaider, L.A.; Rodgers, K.M.; Rudel, R.A. Dietary Habits Related to Food Packaging and Population Exposure to PFASs. Environ. Health Perspect. 2019, 127, 107003. [Google Scholar] [CrossRef]
- Liew, Z.; Olsen, J.; Cui, X.; Ritz, B.; Arah, O.A. Bias from conditioning on live birth in pregnancy cohorts: An illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int. J. Epidemiol. 2015, 44, 345–354. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Mumford, S.L.; Schisterman, E.F. Conditioning on intermediates in perinatal epidemiology. Epidemiology 2012, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; VanderWeele, T.J. Compound Treatments and Transportability of Causal Inference. Epidemiology 2011, 22, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Taubman, S.L. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int. J. Obes. 2008, 32 (Suppl. 3), S8–S14. [Google Scholar] [CrossRef]
- Pearl, J. Does Obesity Shorten Life? Or is it the Soda? On Non-manipulable Causes. J. Causal Inference 2018, 6. [Google Scholar] [CrossRef]
| PFAS | Relative % Difference of TSH (95% CI) 1 | |
|---|---|---|
| Model 1 2 | Model 2 3 | |
| PFOS | ||
| Per IQR increase | 1.06 (0.96, 1.16) | 1.04 (0.96, 1.14) |
| Quartile 1 | Ref | Ref |
| Quartile 2 | 0.85 (0.68, 1.07) | 0.86 (0.69, 1.06) |
| Quartile 3 | 0.93 (0.75, 1.16) | 0.96 (0.78, 1.17) |
| Quartile 4 | 1.03 (0.84, 1.27) | 1.01 (0.83, 1.22) |
| PFOA | ||
| Per IQR increase | 1.02 (0.94, 1.11) | 1.01 (0.93, 1.10) |
| Quartile 1 | Ref | Ref |
| Quartile 2 | 0.95 (0.76, 1.19) | 0.96 (0.78, 1.19) |
| Quartile 3 | 1.01 (0.81, 1.25) | 1.02 (0.83, 1.25) |
| Quartile 4 | 1.09 (0.86, 1.39) | 1.08 (0.86, 1.36) |
| PFAS | Relative % Difference of TSH (95% CI) 1 | ||
|---|---|---|---|
| Non-Overweight, BMI < 25 (n = 1002) | Overweight, BMI ≥ 25 (n = 364) | P for Interaction2 | |
| PFOS | |||
| Per IQR increase | 1.08 (0.97, 1.21) | 0.94 (0.82, 1.09) | 0.19 |
| Quartile 1 | Ref | Ref | - |
| Quartile 2 | 0.87 (0.67, 1.13) | 0.79 (0.56, 1.12) | 0.83 |
| Quartile 3 | 1.05 (0.83, 1.33) | 0.64 (0.42, 0.98) | 0.15 |
| Quartile 4 | 1.03 (0.83, 1.28) | 0.95 (0.66, 1.35) | 0.84 |
| PFOA | |||
| Per IQR increase | 1.04 (0.94, 1.15) | 0.95 (0.82, 1.10) | 0.24 |
| Quartile 1 | Ref | Ref | - |
| Quartile 2 | 1.09 (0.84, 1.42) | 0.68 (0.50, 0.92) | 0.03 |
| Quartile 3 | 1.15 (0.90, 1.46) | 0.75 (0.53, 1.05) | 0.04 |
| Quartile 4 | 1.12 (0.86, 1.48) | 1.02 (0.72, 1.43) | 0.52 |
| PFAS | Prevalence Ratio of Cardiovascular Diseases (95% CI) | |
|---|---|---|
| Model 1 a | Model 2 b | |
| PFOS | ||
| Per IQR increase | 1.06 (1.03, 1.09) | 1.06 (1.03, 1.09) |
| Quartile 1 | Ref | Ref |
| Quartile 2 | 1.08 (0.76, 1.52) | 1.07 (0.76, 1.51) |
| Quartile 3 | 1.19 (0.84, 1.67) | 1.18 (0.84, 1.67) |
| Quartile 4 | 1.18 (0.85, 1.64) | 1.19 (0.86, 1.66) |
| PFOA | ||
| Per IQR increase | 1.04 (1.01, 1.08) | 1.04 (1.01, 1.08) |
| Quartile 1 | Ref | Ref |
| Quartile 2 | 1.01 (0.75, 1.36) | 1.00 (0.75, 1.34) |
| Quartile 3 | 1.14 (0.82, 1.58) | 1.14 (0.83, 1.56) |
| Quartile 4 | 1.30 (0.99, 1.70) | 1.31 (1.00, 1.71) |
| PFAS | Prevalence Ratio of Cardiovascular Diseases (95% CI) a | ||
|---|---|---|---|
| Obese, BMI < 30 (n = 4796) | Non-Obese, BMI ≥ 30 (n = 2615) | P for Interactionb | |
| PFOS | |||
| Per IQR increase | 1.09 (1.04, 1.14) | 1.04 (1.01, 1.09) | 0.18 |
| Quartile 1 | ref | ref | - |
| Quartile 2 | 0.89 (0.58, 1.35) | 1.34 (0.78, 2.29) | 0.28 |
| Quartile 3 | 1.01 (0.69, 1.50) | 1.51 (0.90, 2.54) | 0.29 |
| Quartile 4 | 1.08 (0.72, 1.60) | 1.39 (0.85, 2.27) | 0.64 |
| PFOA | |||
| Per IQR increase | 1.01 (0.95, 1.08) | 1.07 (1.04, 1.11) | 0.10 |
| Quartile 1 | ref | ref | - |
| Quartile 2 | 0.77 (0.53, 1.11) | 1.38 (0.85, 2.23) | 0.04 |
| Quartile 3 | 1.01 (0.65, 1.55) | 1.35 (0.87, 2.09) | 0.35 |
| Quartile 4 | 1.03 (0.73, 1.46) | 1.79 (1.15, 2.77) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, K.; Goto, A.; Sugiyama, T.; Ramlau-Hansen, C.H.; Liew, Z. The Confounder-Mediator Dilemma: Should We Control for Obesity to Estimate the Effect of Perfluoroalkyl Substances on Health Outcomes? Toxics 2020, 8, 125. https://doi.org/10.3390/toxics8040125
Inoue K, Goto A, Sugiyama T, Ramlau-Hansen CH, Liew Z. The Confounder-Mediator Dilemma: Should We Control for Obesity to Estimate the Effect of Perfluoroalkyl Substances on Health Outcomes? Toxics. 2020; 8(4):125. https://doi.org/10.3390/toxics8040125
Chicago/Turabian StyleInoue, Kosuke, Atsushi Goto, Takehiro Sugiyama, Cecilia Høst Ramlau-Hansen, and Zeyan Liew. 2020. "The Confounder-Mediator Dilemma: Should We Control for Obesity to Estimate the Effect of Perfluoroalkyl Substances on Health Outcomes?" Toxics 8, no. 4: 125. https://doi.org/10.3390/toxics8040125
APA StyleInoue, K., Goto, A., Sugiyama, T., Ramlau-Hansen, C. H., & Liew, Z. (2020). The Confounder-Mediator Dilemma: Should We Control for Obesity to Estimate the Effect of Perfluoroalkyl Substances on Health Outcomes? Toxics, 8(4), 125. https://doi.org/10.3390/toxics8040125






