Estimated Dietary Bisphenol-A Exposure and Adiposity in Samoan Mothers and Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Sample
2.2. BPA-Relevant Dietary Data
2.3. Estimated Dietary BPA Exposure
2.4. Participant Characteristic and Household Data
2.5. Measures of Adiposity
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. BPA Indices and Participant Characteristics
3.3. BPA Indices and Adiposity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Djalalinia, S.; Qorbani, M.; Peykari, N.; Kelishadi, R. Health impacts of obesity. Pak. J. Med. Sci. 2015, 31, 239–242. [Google Scholar] [PubMed]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharm. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s second Scientific Statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Faure, S. Is bisphenol A an environmental obesogen? Fundam. Clin. Pharmacol. 2017, 31, 594–609. [Google Scholar] [CrossRef]
- Metz, C.M. Bisphenol A: Understanding the Controversy. Workplace Health Saf. 2016, 64, 28–36. [Google Scholar] [CrossRef]
- NHANES National Health and Nutrition Examination Survey 2011–2012 Data Documentation, Codebook, and Frequencies, Environmental Phenols & Parabens. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/EPH_G.htm (accessed on 20 July 2020).
- Vom Saal, F.S.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef]
- Somm, E.; Schwitzgebel, V.M.; Toulotte, A.; Cederroth, C.R.; Combescure, C.; Nef, S.; Aubert, M.L.; Hüppi, P.S. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect. 2009, 117, 1549–1555. [Google Scholar] [CrossRef]
- Ribeiro, E.; Ladeira, C.; Viegas, S. Occupational exposure to Bisphenol A (BPA): A reality that still needs to be unveiled. Toxics 2017, 13, 22. [Google Scholar] [CrossRef]
- Lin, S.; Naseri, T.; Linhart, C.; Morrell, S.; Taylor, R.; McGarvey, S.T.; Magliano, D.J.; Zimmet, P. Trends in diabetes and obesity in Samoa over 35 years, 1978–2013. Diabet. Med. 2017, 34, 654–661. [Google Scholar] [CrossRef]
- Hawley, N.L.; Minster, R.L.; Weeks, D.E.; Viali, S.; Reupena, M.S.; Sun, G.; Cheng, H.; Deka, R.; Mcgarvey, S.T. Prevalence of adiposity and associated cardiometabolic risk factors in the samoan genome-wide association study. Am. J. Hum. Biol. 2014, 26, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Specter, S.E. Poverty and obesity: The role of energy density and energy costs. Am. J. Clin. Nutr. 2004, 79, 6–16. [Google Scholar] [CrossRef]
- Hartle, J.C.; Navas-Acien, A.; Lawrence, R.S. The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ. Res. 2016, 150, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Salamanca-Fernández, E.; Rodríguez-Barranco, M.; Arrebola, J.P.; Vela, F.; Díaz, C.; Chirlaque, M.D.; Colorado-Yohar, S.; Jiménez-Zabala, A.; Irizar, A.; Guevara, M.; et al. Bisphenol-A in the European Prospective Investigation into Cancer and Nutrition cohort in Spain: Levels at recruitment and associated dietary factors. Environ. Res. 2020, 182, 109012. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef]
- Dekant, W.; Völkel, W. Human exposure to bisphenol A by biomonitoring: Methods, results and assessment of environmental exposures. Toxicol. Appl. Pharmacol. 2008, 228, 114–134. [Google Scholar] [CrossRef]
- Stacy, S.L.; Eliot, M.; Calafat, A.M.; Chen, A.; Lanphear, B.P.; Hauser, R.; Papandonatos, G.D.; Sathyanarayana, S.; Ye, X.; Yolton, K.; et al. Patterns, Variability, and Predictors of Urinary Bisphenol A Concentrations during Childhood. Environ. Sci. Technol. 2016, 50, 5981–5990. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Jia, L.T.; Needham, L.L.; Calafat, A.M. Stability of the conjugated species of environmental phenols and parabens in human serum. Environ. Int. 2009, 35, 1160–1163. [Google Scholar] [CrossRef]
- Lorber, M.; Schecter, A.; Paepke, O.; Shropshire, W.; Christensen, K.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 2015, 77, 55–62. [Google Scholar] [CrossRef]
- Choy, C.C.; Desai, M.M.; Park, J.J.; Frame, E.A.; Thompson, A.A.; Naseri, T.; Reupena, M.S.; Duckham, R.L.; Deziel, N.C.; Hawley, N.L. Child, maternal and household-level correlates of nutritional status: A cross-sectional study among young Samoan children. Public Health Nutr. 2017, 20, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.C.; Wang, D.; Baylin, A.; Soti-Ulberg, C.; Naseri, T.; Reupena, M.S.; Thompson, A.A.; Duckham, R.L.; Hawley, N.L. Dietary patterns are associated with child, maternal and household-level characteristics and overweight/obesity among young Samoan children. Public Health Nutr. 2018, 21, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Tse, L.A.; Lee, P.M.Y.; Ho, W.M.; Lam, A.T.; Lee, M.K.; Ng, S.S.M.; He, Y.; Leung, K.-S.; Hartle, J.C.; Hu, H.; et al. Bisphenol A and other environmental risk factors for prostate cancer in Hong Kong. Environ. Int. 2017, 107, 1–7. [Google Scholar] [CrossRef]
- Deziel, N.C.; Freeman, N.C.G.; Hartle, J.C. Exposure science: Ingestion. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 823–832. ISBN 9780444639523. [Google Scholar]
- Choy, C.C.; Hawley, N.L.; Naseri, T.; Reupena, M.S.; McGarvey, S.T. Associations between socioeconomic resources and adiposity traits in adults: Evidence from Samoa. SSM Popul. Health 2020, 10, 100556. [Google Scholar] [CrossRef] [PubMed]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the Global Physical Activity Questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef]
- MacCallum, R.C.; Zhang, S.; Preacher, K.J.; Rucker, D.D. On the practice of dichotomization of quantitative variables. Psychol. Methods 2002, 7, 19–40. [Google Scholar] [CrossRef]
- Janz, K.F.; Broffitt, B.; Levy, S.M. Validation evidence for the netherlands physical activity questionnaire for young children: The iowa bone development study. Res. Q. Exerc. Sport 2005, 76, 363–369. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 7 June 2020).
- Gochfeld, M.; Burger, J. Disproportionate exposures in environmental justice and other populations: The importance of outliers. Am. J. Public Health 2011, 101, 53. [Google Scholar] [CrossRef]
- Moon, M.K. Concern about the safety of bisphenol a substitutes. Diabetes Metab. J. 2019, 43, 46–48. [Google Scholar] [CrossRef]
- Robertson, T.J.; Farrelly, T.A. Bisphenol A (BPA) exposure in New Zealand: A basis for discussion. J. R. Soc. N. Z. 2015, 45, 184–196. [Google Scholar] [CrossRef]
- Administration, R. of the P.D. of H.F. and D. Ban of Bisphenol A (BPA) from Infant Feeding Bottles and Sippy Cups as Child Care Article Products. 2019. Available online: https://www.fda.gov.ph/fda-circular-no-2019-004-ban-of-bisphenol-a-bpa-from-infant-feeding-bottles-and-sippy-cups-as-child-care-article-products/ (accessed on 20 July 2020).
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 993, 559–566. [Google Scholar] [CrossRef]
- Resnik, D.B.; Elliott, K.C. Bisphenol a and risk management ethics. Bioethics 2015, 29, 182–189. [Google Scholar] [CrossRef]
- Nelson, J.W.; Scammell, M.K.; Hatch, E.E.; Webster, T.F. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003–2006. Environ. Health A Glob. Access Sci. Source 2012, 11, 10. [Google Scholar] [CrossRef]
- Covaci, A.; Den Hond, E.; Geens, T.; Govarts, E.; Koppen, G.; Frederiksen, H.; Knudsen, L.E.; Mørck, T.A.; Gutleb, A.C.; Guignard, C.; et al. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environ. Res. 2015, 141, 77–85. [Google Scholar] [CrossRef]
- Kim, T.J.; Von Dem Knesebeck, O. Income and obesity: What is the direction of the relationship? A systematic review and meta-analysis. BMJ Open 2018, 8, 019862. [Google Scholar]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Wang, D.; Hawley, N.L.; Thompson, A.A.; Lameko, V.; Reupena, M.S.; McGarvey, S.T.; Baylin, A. Dietary Patterns Are Associated with Metabolic Outcomes among Adult Samoans in a Cross-Sectional Study. J. Nutr. 2017, 147, 628–635. [Google Scholar] [CrossRef]
- Bhandari, R.; Xiao, J.; Shankar, A. Urinary bisphenol a and obesity in US children. Am. J. Epidemiol. 2013, 177, 1263–1270. [Google Scholar] [CrossRef]
- Do, M.T.; Chang, V.C.; Mendez, M.A.; de Groh, M. Urinary bisphenol a and obesity in adults: Results from the canadian health measures survey. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 403–412. [Google Scholar] [CrossRef]
- DiBello, J.R.; McGarvey, S.T.; Kraft, P.; Goldberg, R.; Campos, H.; Quested, C.; Laumoli, T.S.; Baylin, A. Dietary patterns are associated with metabolic syndrome in adult Samoans. J. Nutr. 2009, 139, 1933–1943. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Gerona, R.R.; Kannan, K.; Taylor, J.A.; Van Breemen, R.B.; Dickenson, C.A.; Liao, C.; Yuan, Y.; Newbold, R.R.; Padmanabhan, V.; et al. A round robin approach to the analysis of bisphenol a (BPA) in human blood samples. Environ. Health A Glob. Access Sci. Source 2014, 13, 25. [Google Scholar] [CrossRef]
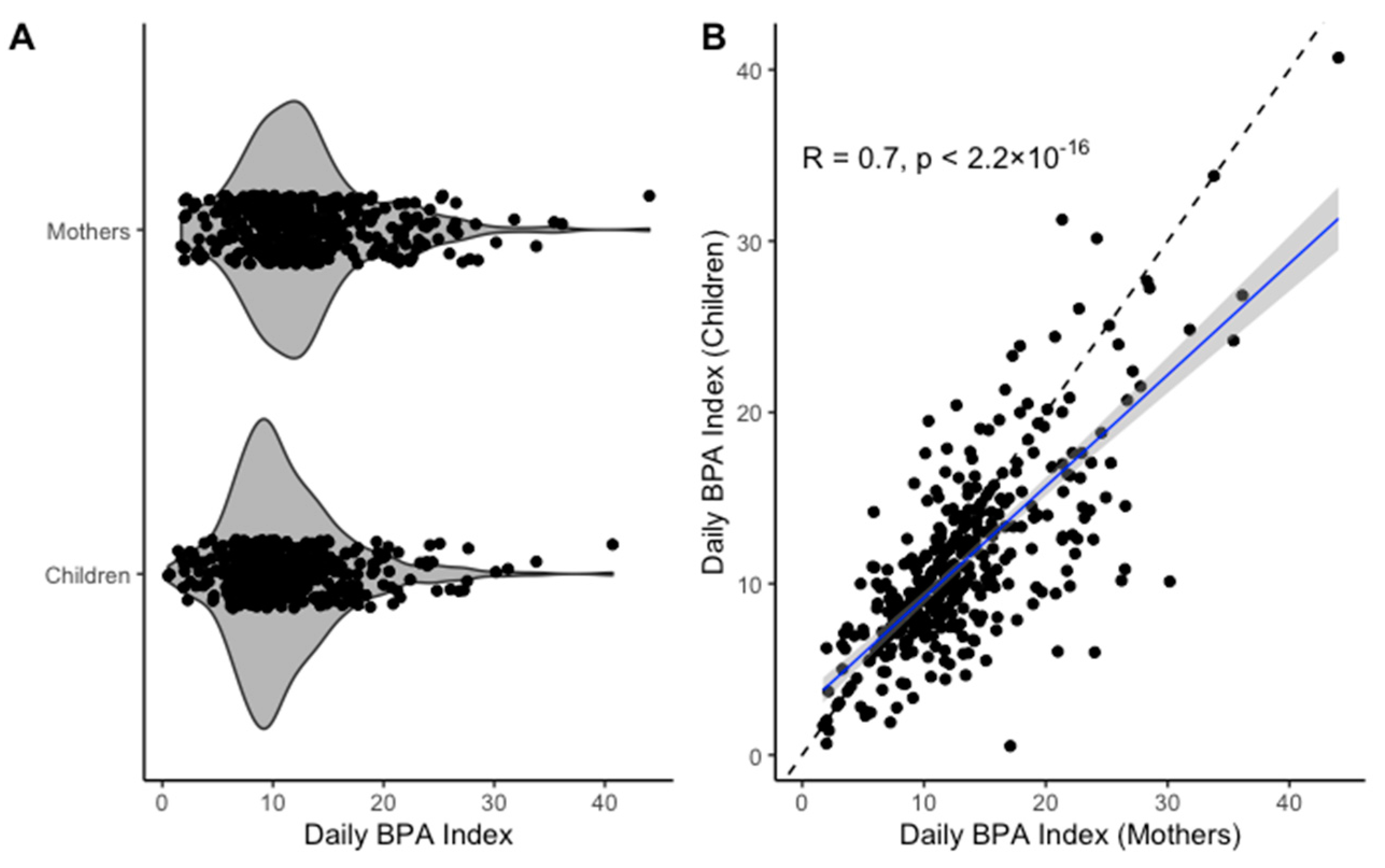
| Participant-Level Characteristics (Mothers) | ||
| n | Mean (SD) | |
| Age (years) | 399 | 34.9 (8.7) |
| Education (years) | 399 | 12.3 (1.7) |
| BMI (kg/m2) | 376 | 34.9 (6.7) |
| AC (cm) | 376 | 109.3 (14.6) |
| n | % | |
| Physical Activity | ||
| 0 MVPA Minutes | 290 | 73.2% |
| >0 MVPA Minutes | 106 | 26.8% |
| Participant-Level Characteristics (Children) | ||
| n | Mean (SD) | |
| Age (years) | 399 | 5.3 (0.9) |
| BMI (kg/m2) | 392 | 16.6 (1.9) |
| AC (cm) | 392 | 55.5 (5.1) |
| n | % | |
| Physical Activity | ||
| Less than peers | 33 | 8.3% |
| About equal to peers | 172 | 43.1% |
| More than peers | 194 | 48.6% |
| Sex | ||
| Female | 205 | 51.4% |
| Male | 194 | 48.6% |
| Dietary Pattern | ||
| Less Modern | 199 | 49.9% |
| More Modern | 200 | 50.1% |
| Household-Level Characteristics | ||
| n | % | |
| Region | ||
| Apia Urban Area | 131 | 32.8% |
| Northwest Upolu | 140 | 35.1% |
| Rest of Upolu | 128 | 32.1% |
| Household Income | ||
| <30,000 talā | 233 | 59.4% |
| 30,000 to 90,000 talā | 90 | 23.0% |
| >90,000 talā | 69 | 17.6% |
| n | Mean (SD) | |
| Household Assets (total number) | 383 | 5.7 (4.0) |
| Food or Beverage Item | Exposure Score a | Mothers (n = 399) | Children (n = 399) | ||
|---|---|---|---|---|---|
| Daily Consumption b, Median (IQR) | Daily BPA Index c, Median (IQR) | Daily Consumption b, Median (IQR) | Daily BPA Index c, Median (IQR) | ||
| Item 1: Cold beverages from a hard plastic cup | 1.33 | 3.0 (2.0) | 3.99 (2.66) | 2.0 (2.0) | 2.66 (2.66) |
| Item 2: Hot beverages from a hard plastic cup | 2 | 2.0 (2.0) | 4.0 (4.0) | 2.0 (2.0) | 4.0 (4.0) |
| Item 3: Canned beverages | 1.67 | 0.29 (0.29) | 0.48 (0.48) | 0.29 (0.29) | 0.48 (0.48) |
| Item 4: Disposable, plastic bottled beverages | 1 | 0.14 (0.14) | 0.14 (0.14) | 0.14 (0.14) | 0.14 (0.14) |
| Item 5: Food packaged in a metal can | 3 | 0.43 (0.43) | 1.29 (1.29) | 0.43 (0.43) | 1.29 (1.29) |
| Item 6: Unheated/uncooked food packaged in plastic/film wrap | 0 | 0.14 (0.29) | 0 | 0.14 (0.29) | 0 |
| Item 7: Hot food stored in plastic film/packaging | 0 | 0.14 (0.29) | 0 | 0.14 (0.14) | 0 |
| Item 8: Food heated in the microwave/oven in contact with plastic | 2.67 | 0.14 (0.22) | 0.38 (0.58) | 0.14 (0.24) | 0.38 (0.63) |
| Item 9: Food microwaved in plastic containers | 2.33 | 0.14 (0.14) | 0.33 (0.33) | 0.14 (0.14) | 0.33 (0.33) |
| Daily BPA index c | 11.92 (6.17) | 9.97 (5.74) | |||
| Daily BPA Index a | |||||
|---|---|---|---|---|---|
| 95% CI ( ) | Percent Change b | 95% CI (Percent Change) | p | ||
| Age (years) | −0.001 | −0.01 to 0.01 | −0.10 | −1.00 to 1.01 | 0.72 |
| Maternal Education (Years) | −0.01 | −0.04 to 0.02 | −1.00 | −3.92 to 2.02 | 0.50 |
| Physical Activity | |||||
| >0 MVPA Minutes | Reference | ||||
| 0 MVPA Minutes | −0.21 | −0.33 to −0.10 | −18.94 | −28.11 to −9.52 | 0.0004 |
| Annual Household Income | |||||
| <5000 talā | Reference | ||||
| 5000 to 9999 talā | 0.01 | −0.11 to 0.14 | 1.01 | −10.42 to 15.03 | 0.81 |
| ≥10,000 talā | −0.33 | −0.47 to −0.19 | −28.11 | −37.50 to −17.30 | 0.00001 |
| Household Assets (total number) | 0.003 | −0.01 to 0.02 | 0.30 | −1.00 to 2.02 | 0.64 |
| Region | |||||
| Apia Urban Area | Reference | ||||
| Northwest Upolu | 0.003 | −0.12 to 0.13 | 0.30 | −11.31 to 13.88 | 0.97 |
| Rest of Upolu | 0.04 | −0.08 to 0.17 | 4.08 | −7.69 to 18.53 | 0.50 |
| Daily BPA Index a | |||||
|---|---|---|---|---|---|
| 95% CI ( ) | Percent Change b | 95% CI (Percent Change) | p | ||
| Age (years) | −0.02 | −0.08 to 0.03 | −1.98 | −7.69 to 3.05 | 0.37 |
| Sex | |||||
| Female | Reference | ||||
| Male | 0.08 | −0.03 to 0.18 | 8.33 | −2.96 to 19.72 | 0.14 |
| Dietary pattern | |||||
| More modern | Reference | ||||
| Less modern | −0.17 | −0.28 to −0.06 | −15.63 | −24.42 to −5.82 | 0.002 |
| Maternal Education (years) | −0.02 | −0.05 to 0.01 | −1.98 | −4.88 to 1.01 | 0.14 |
| Physical Activity | |||||
| More than peers | Reference | ||||
| About equal to peers | 0.15 | −0.01 to 0.39 | 16.18 | −1.00 to 47.70 | 0.07 |
| Less than peers | 0.19 | 0.03 to 0.27 | 20.92 | 3.05 to 31.00 | 0.02 |
| Annual Household Income | |||||
| <5000 talā | Reference | ||||
| 5000 to 9999 talā | 0.06 | −0.08 to 0.19 | 6.18 | −7.69 to 20.92 | 0.40 |
| ≥10,000 talā | −0.29 | −0.45 to −0.14 | −25.17 | −36.24 to −13.06 | 0.0003 |
| Household Assets (total number) | −0.01 | −0.02 to 0.003 | −1.00 | −1.98 to 0.30 | 0.15 |
| Region | |||||
| Apia Urban Area | Reference | ||||
| Northwest Upolu | −0.07 | −0.20 to 0.06 | −6.76 | −18.13 to 6.18 | 0.27 |
| Rest of Upolu | −0.04 | −0.18 to 0.09 | −3.92 | −16.47 to 9.42 | 0.51 |
| BMI (kg/m2) | AC (cm) | |||||
|---|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | |||
| Daily BPA Index a | 0.003 | −0.12 to 0.12 | 0.96 | −0.041 | −0.31 to 0.23 | 0.76 |
| Age (years) | 0.07 | −0.01 to 0.16 | 0.08 | 0.245 | 0.07 to 0.42 | 0.01 |
| Physical Activity | ||||||
| >0 MVPA minutes | Reference | Reference | ||||
| 0 MVPA minutes | −0.80 | −2.47 to 0.88 | 0.35 | −0.488 | −4.09 to 3.11 | 0.79 |
| Annual Household Income | ||||||
| <5000 talā | Reference | Reference | ||||
| 5000 to 9999 talā | −0.27 | −1.99 to 1.45 | 0.76 | −1.369 | −5.06 to 2.32 | 0.47 |
| ≥10,000 talā | 3.27 | 1.22 to 5.31 | 0.002 | 5.756 | 1.37 to 10.15 | 0.01 |
| Household Assets | 0.12 | −0.06 to 0.31 | 0.20 | 0.194 | −0.21 to 0.60 | 0.35 |
| BMI (kg/m2) | AC (cm) | |||||
|---|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | |||
| Daily BPA Index a | 0.02 | −0.02 to 0.06 | 0.42 | 0.05 | −0.05 to 0.15 | 0.33 |
| Age (years) | 0.20 | −0.004 to 0.41 | 0.06 | 1.90 | 1.39 to 2.41 | 1.5 × 10−10 |
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 0.22 | −0.17 to 0.62 | 0.27 | 0.44 | −0.54 to 1.41 | 0.38 |
| Dietary Pattern | ||||||
| More modern | Reference | Reference | ||||
| Less modern | −0.06 | −0.47 to 0.34 | 0.77 | −0.61 | −1.62 to 0.40 | 0.23 |
| Physical Activity | ||||||
| More active than peers | Reference | Reference | ||||
| About the same as peers | −0.22 | −0.68 to 0.24 | 0.35 | −0.10 | −1.24 to 1.05 | 0.87 |
| Less active than peers | −0.61 | −1.39 to 0.17 | 0.12 | −1.99 | −3.92 to −0.06 | 0.04 |
| Annual Household Income | ||||||
| <5000 talā | Reference | Reference | ||||
| 5000 to 9999 talā | −0.48 | −0.99 to 0.03 | 0.07 | −0.63 | −1.91 to 0.65 | 0.33 |
| ≥10,000 talā | −0.20 | −0.80 to 0.41 | 0.52 | 0.02 | −1.49 to 1.52 | 0.98 |
| Household Assets | 0.11 | 0.06 to 0.16 | 0.00004 | 0.21 | 0.08 to 0.34 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinsberg, L.W.; Bui, C.N.N.; Hartle, J.C.; Sereika, S.M.; Choy, C.C.; Wang, D.; Soti-Ulberg, C.; Naseri, T.; Reupena, M.S.; Duckham, R.L.; et al. Estimated Dietary Bisphenol-A Exposure and Adiposity in Samoan Mothers and Children. Toxics 2020, 8, 67. https://doi.org/10.3390/toxics8030067
Heinsberg LW, Bui CNN, Hartle JC, Sereika SM, Choy CC, Wang D, Soti-Ulberg C, Naseri T, Reupena MS, Duckham RL, et al. Estimated Dietary Bisphenol-A Exposure and Adiposity in Samoan Mothers and Children. Toxics. 2020; 8(3):67. https://doi.org/10.3390/toxics8030067
Chicago/Turabian StyleHeinsberg, Lacey W., Christina N.N. Bui, Jennifer C. Hartle, Susan M. Sereika, Courtney C. Choy, Dongqing Wang, Christina Soti-Ulberg, Take Naseri, Muagututia Sefuiva Reupena, Rachel L. Duckham, and et al. 2020. "Estimated Dietary Bisphenol-A Exposure and Adiposity in Samoan Mothers and Children" Toxics 8, no. 3: 67. https://doi.org/10.3390/toxics8030067
APA StyleHeinsberg, L. W., Bui, C. N. N., Hartle, J. C., Sereika, S. M., Choy, C. C., Wang, D., Soti-Ulberg, C., Naseri, T., Reupena, M. S., Duckham, R. L., Park, J. J., Hawley, N. L., & Deziel, N. C. (2020). Estimated Dietary Bisphenol-A Exposure and Adiposity in Samoan Mothers and Children. Toxics, 8(3), 67. https://doi.org/10.3390/toxics8030067






