Evaluation of Second-Hand Exposure to Electronic Cigarette Vaping under a Real Scenario: Measurements of Ultrafine Particle Number Concentration and Size Distribution and Comparison with Traditional Tobacco Smoke
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design for Aerosol Characterization
2.3. Ethical Statement
3. Results and Discussion
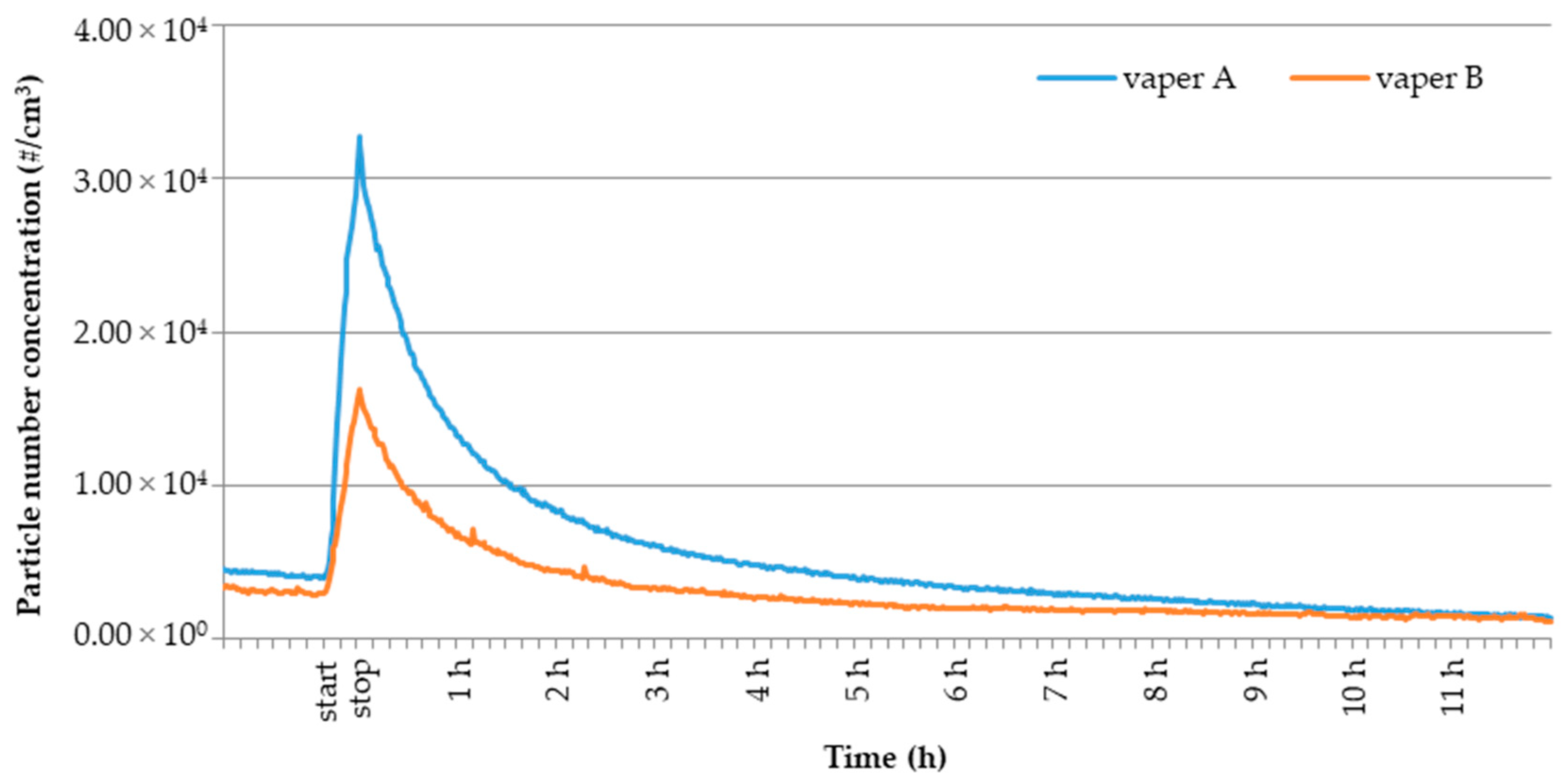
3.1. E-Cig Second-Hand Aerosol: Ultrafine Particle Number Concentration, Intra- and Inter-Vaper Variability
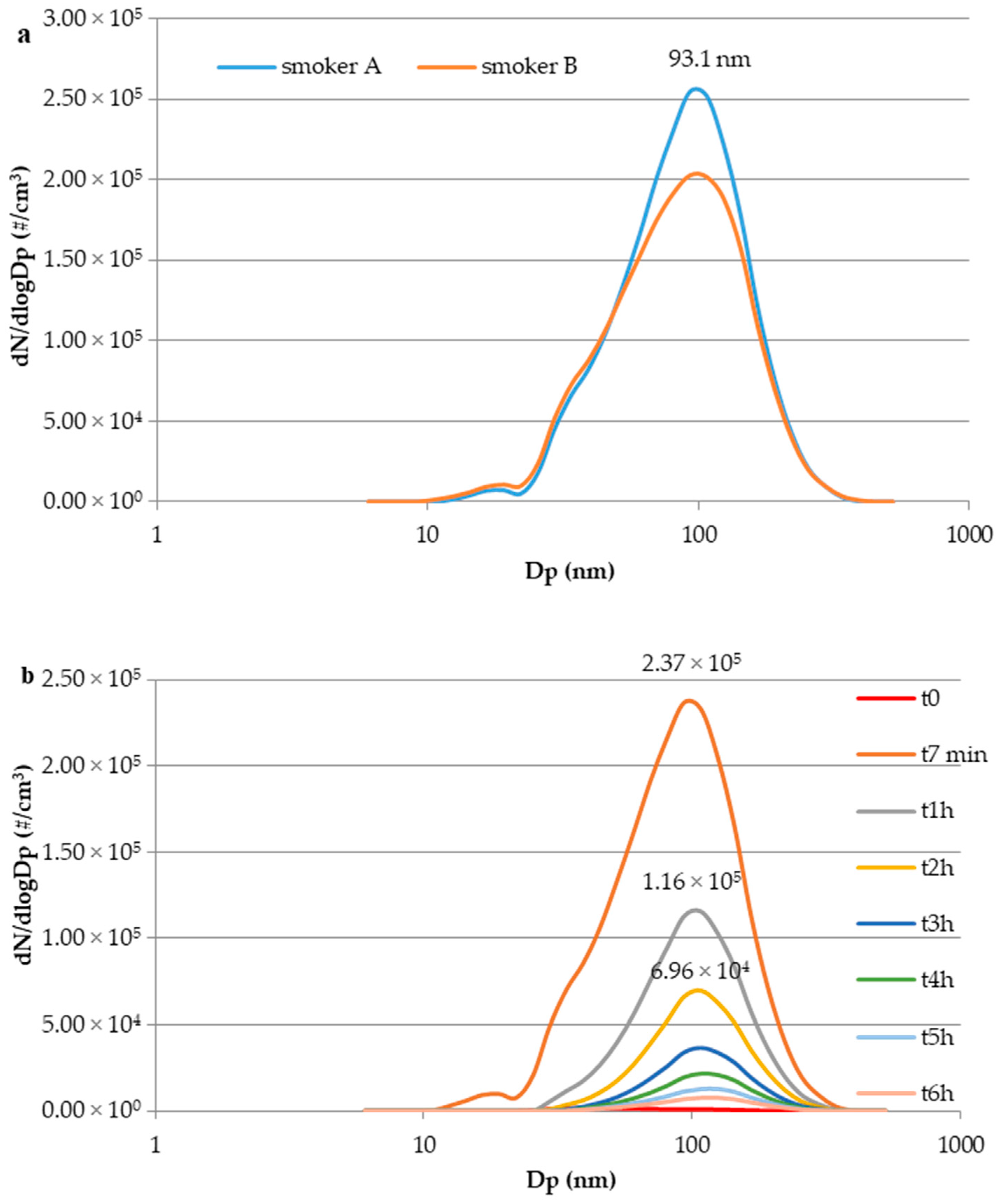
3.2. E-cig Second-Hand Aerosol: Temporal Profiles of UFPs Number Concentration
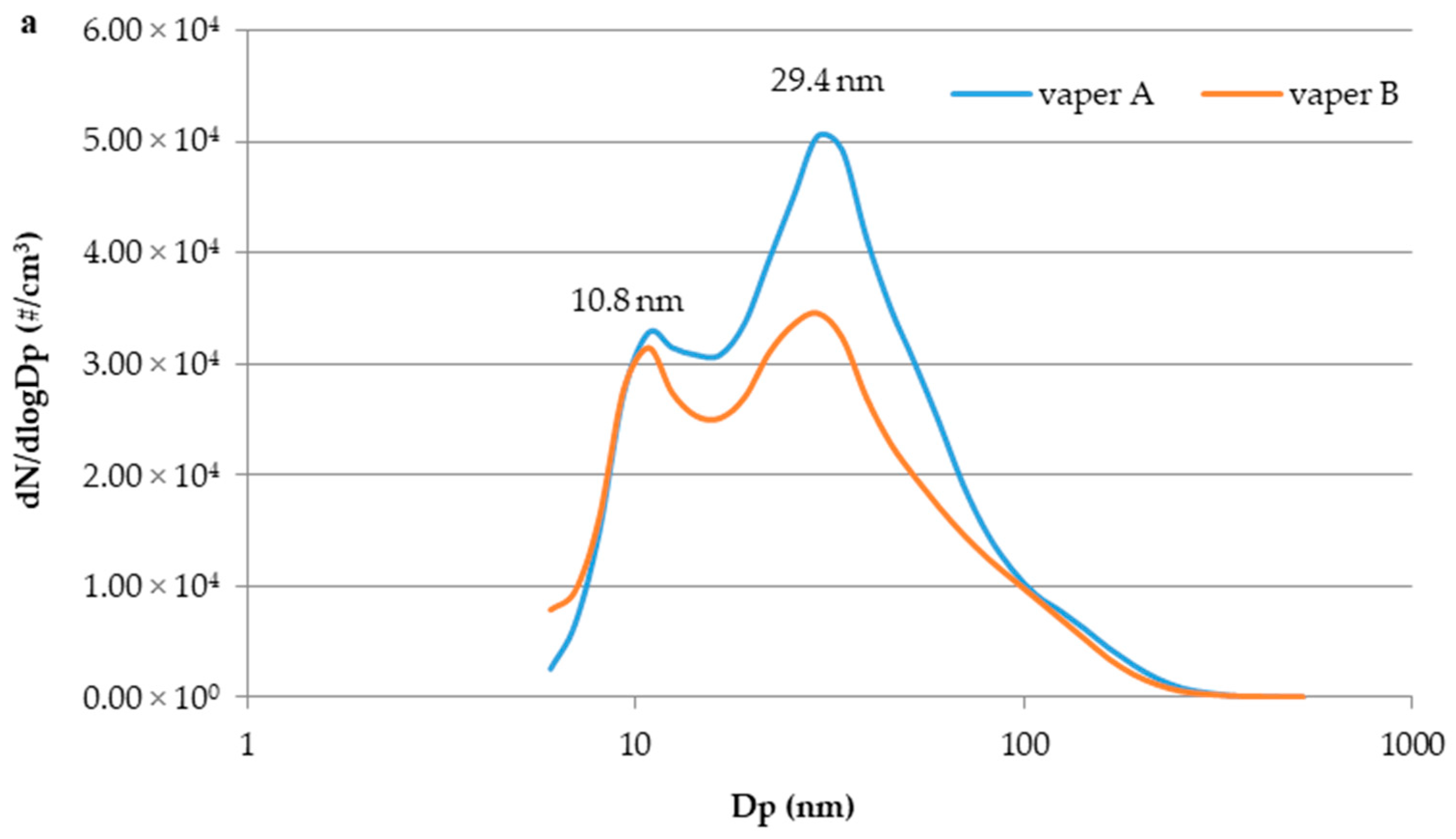
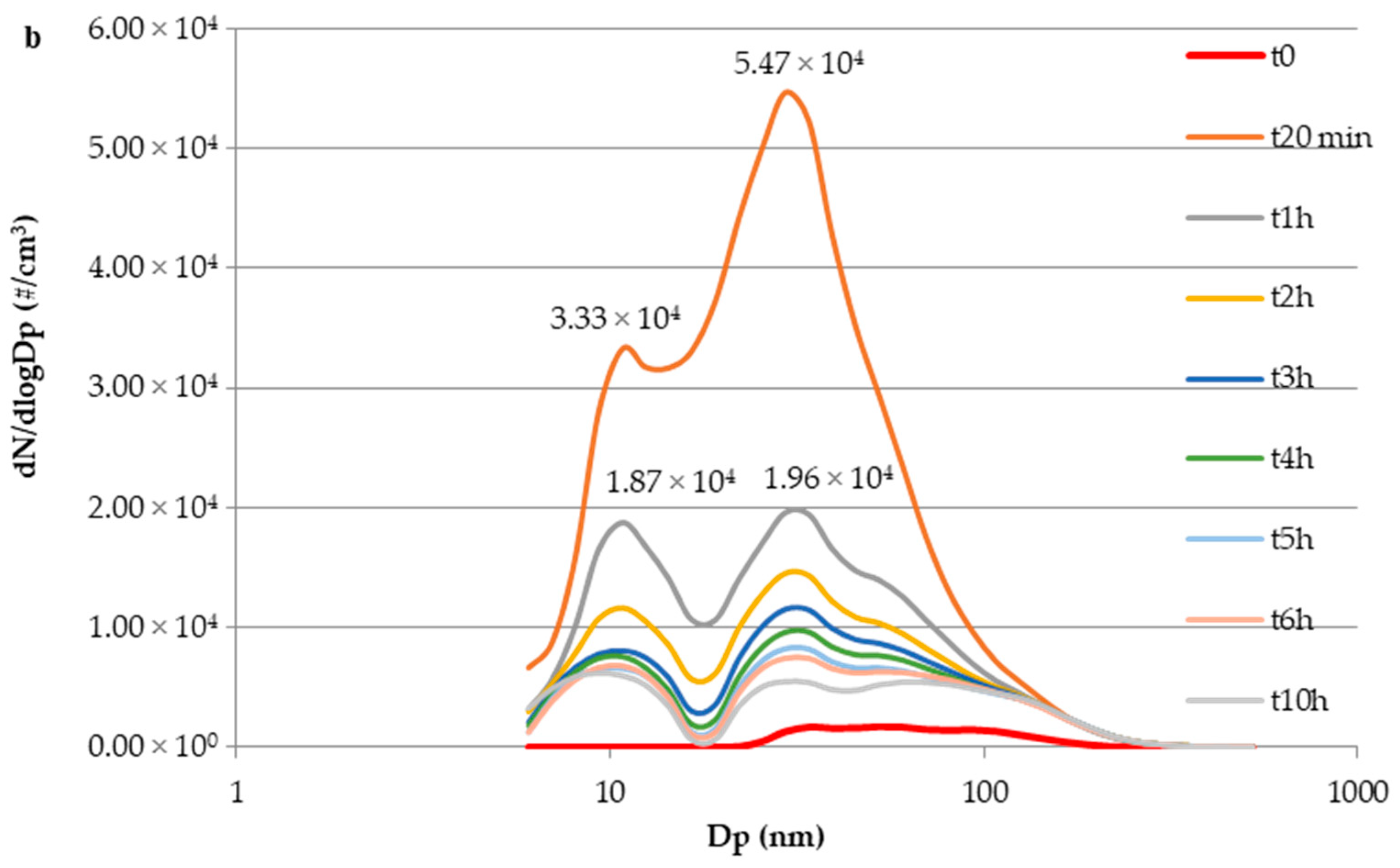
3.3. E-Cig Second-Hand Aerosol: Particle Size Distribution and Temporal Evolution
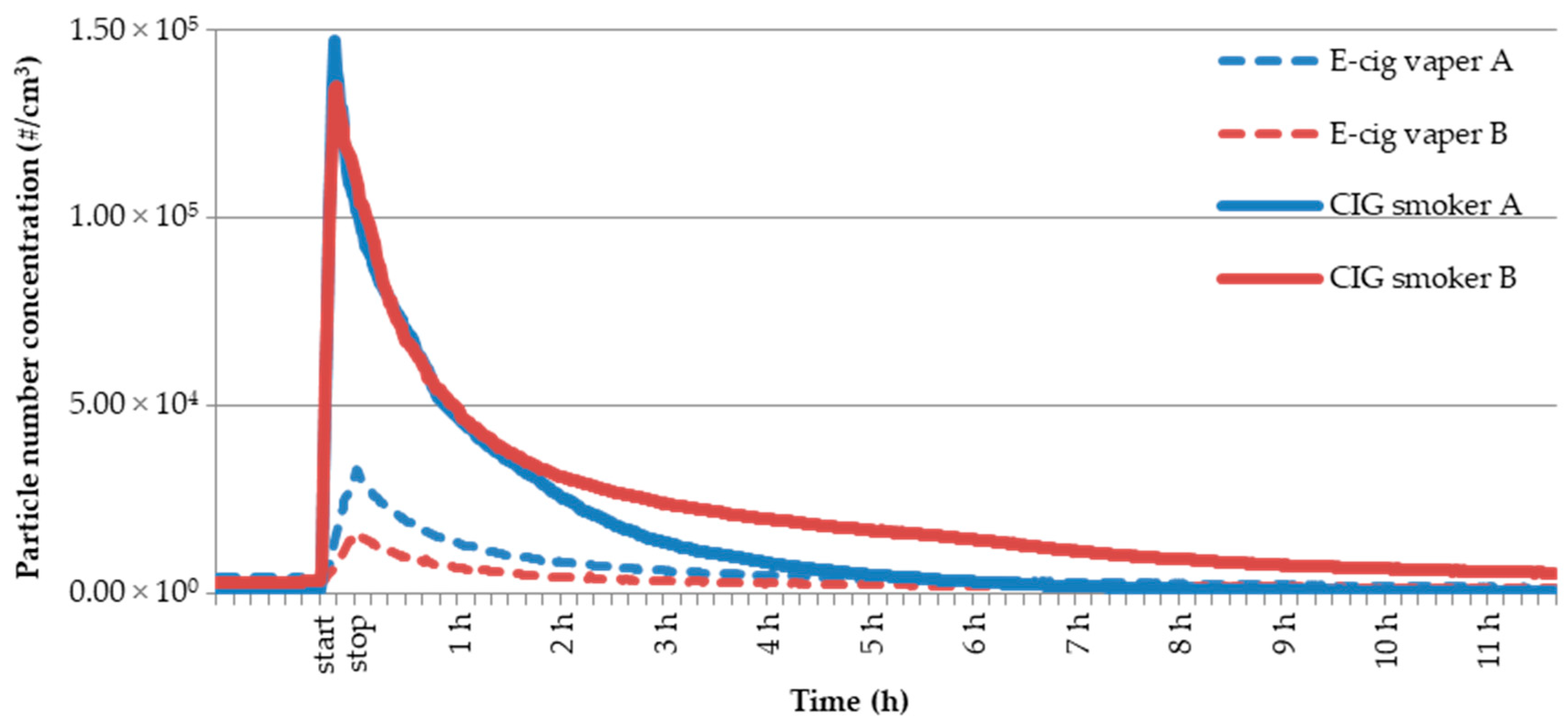
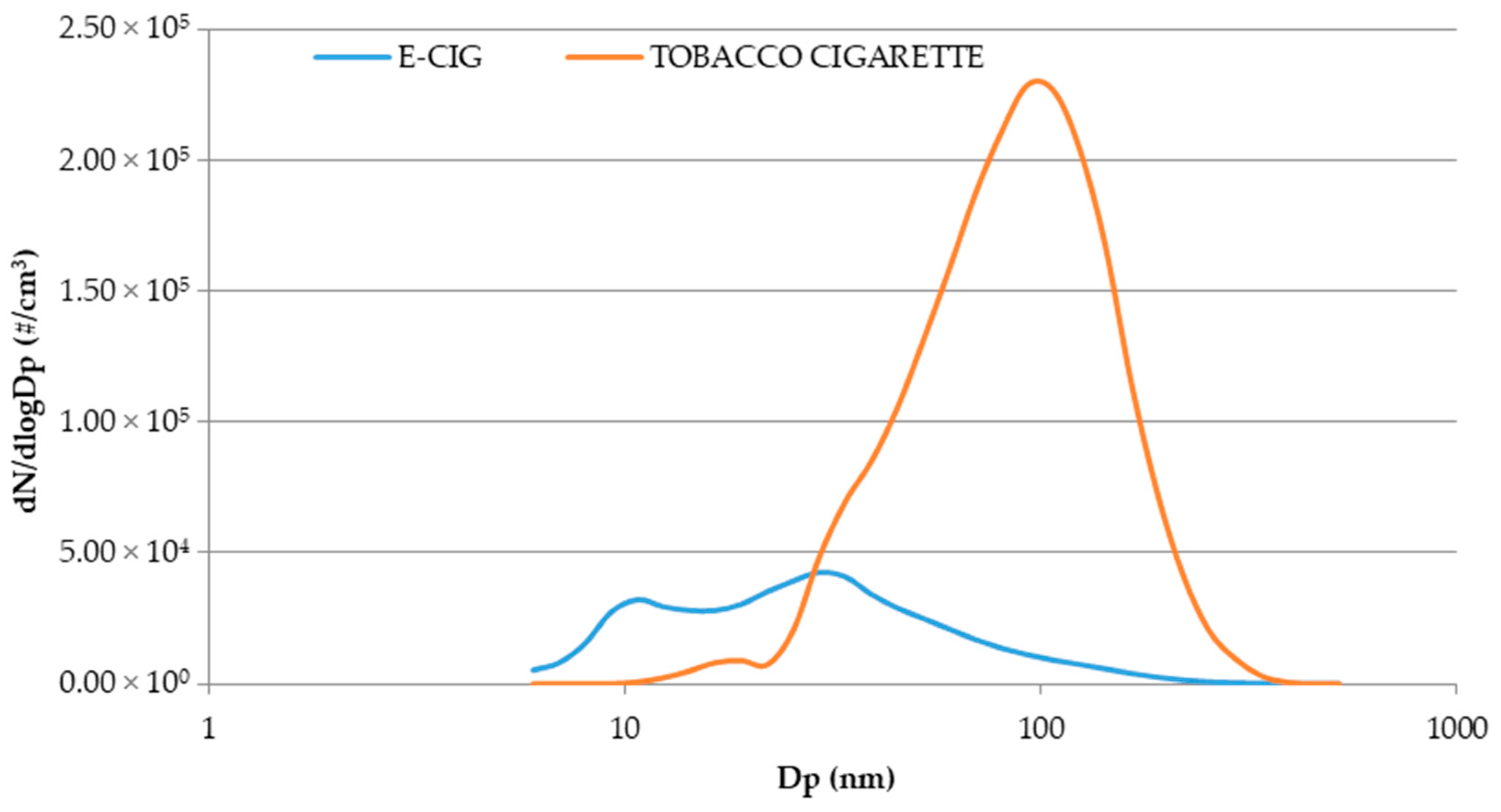
3.4. Comparison of E-Cig Second-Hand Aerosol with Environmental Tobacco Smoke
3.5. Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dockrell, M.; Morrison, R.; Bauld, L.; McNeill, A. E-cigarettes: Prevalence and attitudes in Great Britain. Nicot. Tob. Res. 2013, 15, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Romagna, G.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Characteristics, perceived side effects and benefits of electronic cigarette use: A worldwide survey of more than 19,000 consumers. Int. J. Environ. Res. Public Health 2014, 11, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directorate-General for Health and Food Safety and Coordinated by the Directorate-General for Communication. Special Eurobarometer 429. Attitudes of Europeans Towards Tobacco and Electronic Cigarettes. May 2005. Available online: https://ec.europa.eu/commfrontoffice/publicopinion/index.cfm/Survey/getSurveyDetail/search/429/surveyKy/2033 (accessed on 15 May 2005).
- Kitzen, J.M.; McConaha, J.L.; Bookser, M.L.; Pergolizzi, J.V.; Taylor, R.; Raffa, R.B. e-cigarettes for smoking cessation: Do they deliver? J. Clin. Pharm. Ther. 2019, 44, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, L.; Flacco, M.E.; Ferrante, M.; La Vecchia, C.; Siliquini, R.; Ricciardi, W.; Marzuillo, C.; Villari, P.; Fiore, M. Cohort study of electronic cigarette use: Effectiveness and safety at 24 months. Tob. Control 2017, 26, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Leventhal, A.M. Association of electronic cigarette vaping and subsequent smoking relapse among former smokers. Drug Alcohol Depend. 2019, 199, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Grana, R.A.; Glantz, S.A. E-cig use among Korean adolescents: A cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J. Adolesc. Health 2013, 54, 684–690. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Re-evaluation of propane-1,2-diol (E1520) as a food additive. EFSA J. 2018, 16, e05235. [Google Scholar] [CrossRef]
- Czogala, J.; Goniewicz, M.L.; Fidelus, B.; Zielinska-Danch, W.; Travers, M.J.; Sobczak, A. Secondhand exposure to vapors from electronic cigarettes. Nicot. Tob. Res. 2014, 16, 655–662. [Google Scholar] [CrossRef]
- Famele, M.; Palmisani, J.; Ferranti, C.; Abenavoli, C.; Palleschi, L.; Mancinelli, R.; Fidente, R.M.; de Gennaro, G.; Draisci, R. Liquid chromatography with tandem mass spectrometry method for the determination of nicotine and minor tobacco alkaloids in electronic cigarette refill liquids and second-hand generated aerosol. J. Sep. Sci. 2017, 40, 1049–1056. [Google Scholar] [CrossRef]
- Geiss, O.; Bianchi, I.; Barahona, F.; Barrero-Moreno, J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int. J. Hyg. Environ. Health 2015, 218, 169–180. [Google Scholar] [CrossRef]
- Lee, M.-S.; LeBouf, R.F.; Son, Y.-S.; Koutrakis, P.; Christiani, D.C. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ. Health 2017, 16, 1–10. [Google Scholar] [CrossRef]
- McAuley, T.R.; Hopke, P.K.; Zhao, J.; Babaian, S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal. Toxicol. 2012, 24, 850–857. [Google Scholar] [CrossRef]
- Qu, Y.; Szulejko, J.E.; Kim, K.-H.; Jo, S.-H. The effect of varying battery voltage output on the emission rate of carbonyls released from e-cigarette smoke. Microchem. J. 2019, 145, 47–54. [Google Scholar] [CrossRef]
- O’Connell, G.; Colard, S.; Cahours, X.; Pritchard, J.D. An assessment of indoor air quality before, during and after unrestricted use of e-cigarettes in a small room. Int. J. Environ. Res. Public Health 2015, 12, 4889–4907. [Google Scholar] [CrossRef] [PubMed]
- Visser, W.F.; Klerx, W.N.; Cremers, H.W.J.M.; Ramlal, R.; Schwillens, P.L.; Talhout, R. The health risks of electronic cigarette use to bystanders. Int. J. Environ. Res. Public Health 2019, 16, 1525. [Google Scholar] [CrossRef]
- Schripp, T.; Markewitz, D.; Uhde, E.; Salthammer, T. Does e-cig consumption cause passive vaping? Indoor Air 2013, 23, 25–31. [Google Scholar] [CrossRef]
- Avino, P.; Scungio, M.; Stabile, L.; Cortellessa, G.; Buonanno, G.; Manigrasso, M. Second-hand aerosol from tobacco and electronic cigarettes: Evaluation of the smoker emission rates and doses and lung cancer risk of passive smokers and vapers. Sci. Total Environ. 2018, 642, 137–147. [Google Scholar] [CrossRef]
- Maloney, J.C.; Thompson, M.K.; Oldham, M.J.; Stiff, C.L.; Lilly, P.D.; Patskan, G.J.; Shafer, K.H.; Sarkar, M.A. Insights from two industrial hygiene pilot e-cigarette passive vaping studies. J. Occup. Environ. Hyg. 2016, 13, 275–283. [Google Scholar] [CrossRef]
- Nguyen, C.; Li, L.; Sen, C.A.; Ronquillo, E.; Zhu, Y. Fine and ultrafine particles concentrations in vape shops. Atmos. Environ. 2019, 211, 159–169. [Google Scholar] [CrossRef]
- Protano, C.; Manigrasso, M.; Avino, P.; Vitali, M. Second-hand smoke generated by combustion and electronic smoking devices used in real scenarios: Ultrafine particle pollution and age-related dose assessment. Environ. Int. 2017, 107, 190–195. [Google Scholar] [CrossRef]
- Ruprecht, A.A.; De Marco, C.; Pozzi, P.; Munarini, E.; Mazza, R.; Angellotti, G.; Turla, F.; Boffi, R. Comparison between particulate matter and ultrafine particle emission by electronic and normal cigarettes in real-life conditions. Tumori 2014, 100, e24–e27. [Google Scholar] [CrossRef] [PubMed]
- Schober, W.; Szendrei, K.; Matzen, W.; Osiander-Fuchs, H.; Heitmann, D.; Schettgen, T.; Jörres, R.A.; Fromme, H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 2014, 217, 628–637. [Google Scholar] [CrossRef]
- Scungio, M.; Stabile, L.; Buonanno, G. Measurements of electronic cigarette-generated particles for the evaluation of lung cancer risk of active and passive users. J. Aerosol Sci. 2018, 115, 1–11. [Google Scholar] [CrossRef]
- Volesky, K.D.; Maki, A.; Scherf, C.; Watson, L.; Van Ryswyk, K.; Fraser, B.; Weichenthal, S.A.; Cassol, E.; Villeneuve, P.J. The influence of three e-cigarette models on indoor fine and ultrafine particulate matter concentrations under real-world conditions. Environ. Pollut. 2018, 243, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Nguyen, C.; Lin, C.-H.; Middlekauff, H.R.; Peters, K.; Moheimani, R.; Guo, Q.; Zhu, Y. Characteristics of secondhand electronic cigarette aerosols from active human use. Aerosol Sci. Technol. 2017, 51, u1368–u1376. [Google Scholar] [CrossRef]
- National Institute of Health. Demand for Scientific Update Regarding the Risk Associated to E-cigs Containing Nicotine, Rome, Italy. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1882_allegato.pdf (accessed on 20 December 2012).
- European Parliament. Directive 2014/40/EU of the European Parliament and of the Council of 3 April 2014 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Concerning the Manufacture, Presentation and Sale of Tobacco and Related Products and Repealing Directive 2001/37/EC. Off. J. Eur. Union 2014, 127, 1–38. [Google Scholar]
- d’Ambrosio Alfano, F.R.; Dell’Isola, M.; Ficco, G.; Tassini, F. Experimental analysis of air tightness in Mediterranean buildings using the fan pressurization method. Build. Environ. 2012, 53, 16–25. [Google Scholar] [CrossRef]
- He, C.; Morawska, L.; Hitchins, J.; Gilbert, D. Contribution from indoor sources to particle number and mass concentrations in residential homes. Atmos. Environ. 2004, 38, 3405–3415. [Google Scholar] [CrossRef]
- Aszyk, J.; Woźniak, M.K.; Kubica, P.; Kot-Wasik, A.; Wasik, A. Concentration levels of selected analytes in the gas phase of an e-cigarette aerosol. Microchem. J. 2019, 148, 17–724. [Google Scholar] [CrossRef]
- CORESTA. CORESTA Recommended Method No 81. Routine Analytical Machine for E Cigarette Aerosol Generation and Collection—Definitions and Standard Conditions. Available online: https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf (accessed on 15 June 2015).
- Palmisani, J.; N&rgaard, A.W.; Kofoed-S&rensen, V.; Clausen, P.A.; de Gennaro, G.; Wolkoff, P. Evaluation of ozone-initiated VOC and particle emissions from a carpet deodorizer. In Proceedings of the Healthy Buildings Europe 201, HB 2017, International Society of Indoor Air Quality and Climate, Lublin, Poland, 2–5 July 2017; ISBN 978-837947260-4. [Google Scholar]
- de Gennaro, G.; Dambruoso, P.R.; Di Gilio, A.; Di Palma, V.; Marzocca, A.; Tutino, M. Discontinuous and continuous indoor air quality monitoring in homes with fireplaces or wood stoves as heating system. Int. J. Environ. Res. Public Health 2016, 13, 78. [Google Scholar] [CrossRef]
- Fuoco, F.C.; Buonanno, G.; Stabile, L.; Vigo, P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut. 2014, 18, 523–529. [Google Scholar] [CrossRef]
- Mikheev, V.B.; Ivanov, A.; Lucas, E.A.; South, P.L.; Colijn, H.O.; Clark, P.I. Aerosol size distribution measurement of electronic cigarette emissions using combined differential mobility and inertial impaction methods: Smoking machine and puff topofìgraphy influence. Aerosol Sci. Technol. 2018, 52, 1233–1248. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A.; Dechsakulthon, F. Nanoparticles: A review of particle toxicology following inhalation exposure. Inhal. Toxicol. Int. Forum Respir. Res. 2012, 24, 125–135. [Google Scholar] [CrossRef]
- Frederix, E.M.A.; Kuczaj, A.K.; Nordlund, M.; Bělka, M.; Lizal, F.; Jedelsky, J.; Elcner, J.; Jicha, M.; Geurts, B.J. Simulation of size-dependent aerosol deposition in a realistic model of the upper human airways. J. Aerosol Sci. 2018, 115, 29–45. [Google Scholar] [CrossRef]
- Blank, F.; Gehr, P.; Rutishauser, R.R. In vitro human lung cell culture models to study the toxic potential of nanoparticles. In Nanotoxicity: From In Vivo and In Vitro Models to Health Risks; Sahu, S., Casciano, D., Eds.; Wiley: Chichester, UK, 2009; pp. 379–395. [Google Scholar]
- Bekö, G.; Morrison, G.; Weschler, C.J.; Koch, H.M.; Pälmke, C.; Salthammer, T.; Schripp, T.; Eftekhari, A.; Toftum, J.; Clausen, G. Dermal uptake of nicotine air and clothing: Experimental verification. Indoor Air 2018, 28, 247–257. [Google Scholar] [CrossRef]
| E-Liquid ID | Propylen Glycol (%) | Glycerol (%) | Water (%) | Flavour | Nicotine (mg/mL, * mg/g) | Manufacture (Country) |
|---|---|---|---|---|---|---|
| 01 | not declared | not declared | not declared | Mint | 0 | Italy |
| 02 | not declared | not declared | not declared | Cuban cigar | 0 | Italy |
| 03 | not declared | not declared | not declared | Rhum | 0 | Italy |
| 04 | 50 | 40 | 5–10 | Biscuit | 0 | Italy |
| 05 | 50 | 40 | 5–10 | Tobacco USA | 0 | Italy |
| 06 | 50 | 40 | 5–10 | Tobacco USA | 18 | Italy |
| 07 | 50 | 40 | 5–10 | Anise | 0 | Italy |
| 08 | not declared | not declared | not declared | Mint | 18 | Italy |
| 09 | 75 | 25 | / | Almond | 11 * | China |
| 10 | not declared | not declared | not declared | Liquirice | 18 | Italy |
| 11 | 50 | 40 | 5–10 | Anise | 18 | Italy |
| 12 | 75 | 25 | / | Virginia tobacco | 18 * | China |
| 13 | 75 | 25 | / | Cigar | 18 * | China |
| 14 | >80 | <20 | not declared | Habanos Cigar | 0 | France |
| 15 | >80 | <20 | not declared | Cuban cigar | 0 | UK |
| E-Liquid ID | Vaper A | Vaper B | ||||
|---|---|---|---|---|---|---|
| UFPs Concentration Vaping Start (#/cm3) | UFPs Concentration Vaping Stop (#/cm3) | Δ (#/cm3) | UFPs Concentration Vaping Start (#/cm3) | UFPs Concentration Vaping Stop (#/cm3) | Δ (#/cm3) | |
| 01 | 7.24 × 103 | 2.15 × 104 | 1.43 × 104 | 1.21 × 104 | 3.71 × 104 | 2.50 × 104 |
| 02 | 8.99 × 103 | 2.27 × 104 | 1.37 × 104 | 9.04 × 103 | 1.56 × 104 | 6.56 × 103 |
| 03 | 8.28 × 103 | 3.18 × 104 | 2.35 × 104 | 8.33 × 103 | 1.56 × 104 | 7.27 × 103 |
| 04 | 1.04 × 104 | 4.65 × 104 | 3.61 × 104 | 8.77 × 103 | 4.16 × 104 | 3.28 × 104 |
| 05 | 1.49 × 104 | 5.50 × 104 | 4.01 × 104 | 7.97 × 103 | 2.41 × 104 | 1.61 × 104 |
| 06 | 1.22 × 104 | 4.94 × 104 | 3.72 × 104 | 9.55 × 103 | 2.69 × 104 | 1.74 × 104 |
| 07 | 1.14 × 104 | 3.91 × 104 | 2.77 × 104 | 1.13 × 104 | 2.70 × 104 | 1.57 × 104 |
| 08 | 9.24 × 103 | 4.26 × 104 | 3.34 × 104 | 1.15 × 104 | 2.43 × 104 | 1.28 × 104 |
| 09 | 7.41 × 103 | 3.82 × 104 | 3.08 × 104 | 9.07 × 103 | 2.72 × 104 | 1.81 × 104 |
| 10 | 1.13 × 104 | 4.13 × 104 | 3.00 × 104 | 1.01 × 104 | 3.75 × 104 | 2.74 × 104 |
| 11 | 8.53 × 103 | 3.50 × 104 | 2.65 × 104 | 1.08 × 104 | 2.54 × 104 | 1.46 × 104 |
| 12 | 1.09 × 104 | 3.58 × 104 | 2.49 × 104 | 1.28 × 104 | 2.98 × 104 | 1.70 × 104 |
| 13 | 7.11 × 103 | 3.16 × 104 | 2.45 × 104 | 1.02 × 104 | 2.68 × 104 | 1.66 × 104 |
| 13 (2) * | 8.35 × 103 | 2.88 × 104 | 2.05 × 104 | 1.15 × 104 | 3.05 × 104 | 1.90 × 104 |
| 13 (3) * | 8.03 × 103 | 2.74 × 104 | 1.94 × 104 | 1.60 × 104 | 2.94 × 104 | 1.34 × 104 |
| 14 | 8.87 × 103 | 3.72 × 104 | 2.83 × 104 | 1.38 × 104 | 3.85 × 104 | 2.47 × 104 |
| 14 (2) * | 1.12 × 104 | 4.37 × 104 | 3.25 × 104 | 1.42 × 104 | 3.69 × 104 | 2.27 × 104 |
| 14 (3) * | 1.17 × 104 | 4.75 × 104 | 3.58 × 104 | 1.41 × 104 | 3.76 × 104 | 2.35 × 104 |
| 15 | 1.25 × 104 | 5.11 × 104 | 3.86 × 104 | 1.14 × 104 | 3.49 × 104 | 2.35 × 104 |
| Tobacco Exp. | Smoker A | Smoker B | ||||
|---|---|---|---|---|---|---|
| UFPs Concentration Smoking Start (#/cm3) | UFPs Concentration Smoking Stop (#/cm3) | Δ (#/cm3) | UFPs Concentration Smoking Start (#/cm3) | UFPs Concentration Smoking Stop (#/cm3) | Δ (#/cm3) | |
| exp. 1 | 9.43 × 102 | 1.47 × 105 | 1.46 × 105 | 3.81 × 103 | 1.16 × 105 | 1.12 × 105 |
| exp. 2 | 2.10 × 103 | 1.33 × 105 | 1.31 × 105 | 5.74 × 103 | 1.31 × 105 | 1.25 × 105 |
| exp. 3 | 2.70 × 103 | 1.41 × 105 | 1.38 × 105 | 3.31 × 103 | 1.35 × 105 | 1.32 × 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmisani, J.; Di Gilio, A.; Palmieri, L.; Abenavoli, C.; Famele, M.; Draisci, R.; de Gennaro, G. Evaluation of Second-Hand Exposure to Electronic Cigarette Vaping under a Real Scenario: Measurements of Ultrafine Particle Number Concentration and Size Distribution and Comparison with Traditional Tobacco Smoke. Toxics 2019, 7, 59. https://doi.org/10.3390/toxics7040059
Palmisani J, Di Gilio A, Palmieri L, Abenavoli C, Famele M, Draisci R, de Gennaro G. Evaluation of Second-Hand Exposure to Electronic Cigarette Vaping under a Real Scenario: Measurements of Ultrafine Particle Number Concentration and Size Distribution and Comparison with Traditional Tobacco Smoke. Toxics. 2019; 7(4):59. https://doi.org/10.3390/toxics7040059
Chicago/Turabian StylePalmisani, Jolanda, Alessia Di Gilio, Laura Palmieri, Carmelo Abenavoli, Marco Famele, Rosa Draisci, and Gianluigi de Gennaro. 2019. "Evaluation of Second-Hand Exposure to Electronic Cigarette Vaping under a Real Scenario: Measurements of Ultrafine Particle Number Concentration and Size Distribution and Comparison with Traditional Tobacco Smoke" Toxics 7, no. 4: 59. https://doi.org/10.3390/toxics7040059
APA StylePalmisani, J., Di Gilio, A., Palmieri, L., Abenavoli, C., Famele, M., Draisci, R., & de Gennaro, G. (2019). Evaluation of Second-Hand Exposure to Electronic Cigarette Vaping under a Real Scenario: Measurements of Ultrafine Particle Number Concentration and Size Distribution and Comparison with Traditional Tobacco Smoke. Toxics, 7(4), 59. https://doi.org/10.3390/toxics7040059








