Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3′-Dichlorobiphenyl (PCB 11)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Dendritic Analyses
2.4. Axonal Outgrowth
2.5. Statistical Analyses
3. Results
3.1. PCB 11 Promotes Dendritic Arborization in a Sex, Concentration and Cell Specific Manner in Mouse Neurons
3.2. The Dendrite-Promoting Activity of PCB 11 Is Not Sex-Specific in Rat Neurons
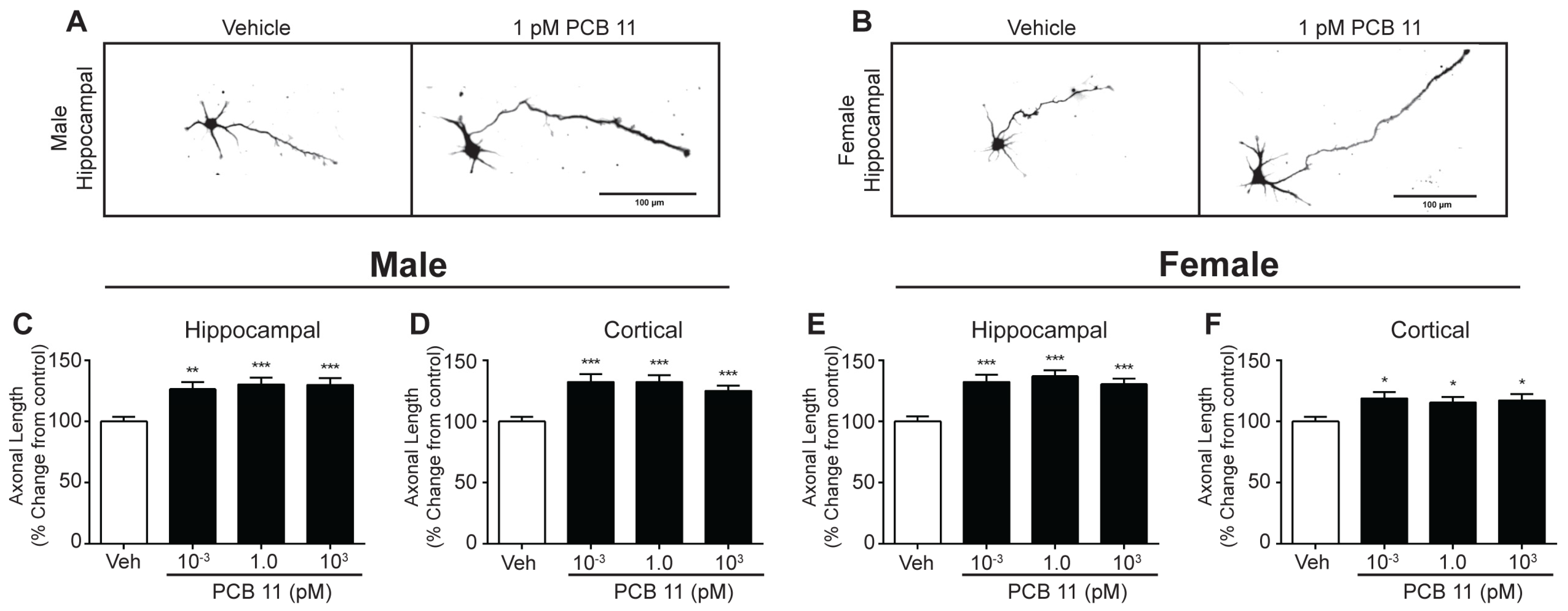
3.3. PCB 11 Promotes Axonal Growth Independent of Sex, Cell-Type, and Species
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsai, M.S.; Chen, M.H.; Lin, C.C.; Ng, S.; Hsieh, C.J.; Liu, C.Y.; Hsieh, W.S.; Chen, P.C. Children’s environmental health based on birth cohort studies of Asia. Sci. Total Environ. 2017, 609, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Bertrand, K.A.; Choi, A.L.; Hu, F.B.; Laden, F.; Grandjean, P.; Sun, Q. Persistent organic pollutants and type 2 diabetes: A prospective analysis in the nurses’ health study and meta-analysis. Environ. Health Perspect. 2013, 121, 153–161. [Google Scholar] [PubMed]
- Hertz-Picciotto, I.; Park, H.Y.; Dostal, M.; Kocan, A.; Trnovec, T.; Sram, R. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin. Pharmacol. Toxicol. 2008, 102, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.; Toninelli, G.; Filisetti, B.; Donato, F. Polychlorinated biphenyls and cancer: An epidemiological assessment. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2013, 31, 99–144. [Google Scholar] [CrossRef] [PubMed]
- Hopf, N.B.; Ruder, A.M.; Succop, P. Background levels of polychlorinated biphenyls in the U.S. population. Sci. Total Environ. 2009, 407, 6109–6119. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.Z.; Chen, H.Y.; Wang, S.J.; Wai, C.M.; Liao, W.; Chiu, K. Reductive dechlorination for remediation of polychlorinated biphenyls. Chemosphere 2012, 88, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, R.; Martinez-Carballo, E.; Gonzalez-Barreiro, C.; Rial-Otero, R.; Simal-Gandara, J. Distribution of polychlorinated biphenyls in both products and by-products of a mussel shell incinerator facility. Environ. Sci. Pollut. Res. Int. 2011, 18, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Greichus, Y.A.; Dohman, B.A. Polychlorinated biphenyl contamination of areas surrounding two transformer salvage companies, Colman, South Dakota-September 1977. Pestic. Monit. J. 1980, 14, 26–30. [Google Scholar] [PubMed]
- Herrick, R.F.; Lefkowitz, D.J.; Weymouth, G.A. Soil contamination from PCB-containing buildings. Environ. Health Perspect. 2007, 115, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Herrick, R.F.; McClean, M.D.; Meeker, J.D.; Baxter, L.K.; Weymouth, G.A. An unrecognized source of PCB contamination in schools and other buildings. Environ. Health Perspect. 2004, 112, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Klosterhaus, S.; McKee, L.J.; Yee, D.; Kass, J.M.; Wong, A. Polychlorinated biphenyls in the exterior caulk of San Francisco Bay Area buildings, California, USA. Environ. Int. 2014, 66, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Melymuk, L.; Csiszar, S.A.; Giang, A.; Diamond, M.L.; Helm, P.A. Continuing sources of PCBs: The significance of building sealants. Environ. Int. 2010, 36, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Hornbuckle, K.C. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010, 44, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Ampleman, M.D.; Martinez, A.; DeWall, J.; Rawn, D.F.; Hornbuckle, K.C.; Thorne, P.S. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol. 2015, 49, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Chan-Hon-Tong, A.; Charles, M.A.; Forhan, A.; Heude, B.; Sirot, V. Exposure to food contaminants during pregnancy. Sci. Total Environ. 2013, 458–460, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Crispo Smith, S.; Park, J.S.; Petreas, M.X.; Rappaport, S.M.; Metayer, C. Concentrations of persistent organic pollutants in California women’s serum and residential dust. Environ. Res. 2015, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, E.; Mulvad, G.; Pedersen, H.S.; Ayotte, P.; Demers, A.; Weber, J.P.; Hansen, J.C. Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ. Health Perspect. 1999, 107, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Soechitram, S.D.; Athanasiadou, M.; Hovander, L.; Bergman, A.; Sauer, P.J. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ. Health Perspect. 2004, 112, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, S.A.; Bos, A.F.; Sauer, P.J.; Roze, E. Developmental neurotoxicity of persistent organic pollutants: An update on childhood outcome. Arch. Toxicol. 2015, 89, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, S.A.; Soechitram, S.D.; Sauer, P.J.; Bos, A.F. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with neurological functioning in 3-month-old infants. Toxicol. Sci. 2014, 142, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Altshul, L.M.; Korrick, S.A. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ. Health Perspect. 2012, 120, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.A.; Sjodin, A.; Yoshida, C.K.; Zerbo, O.; Kharrazi, M.; Windham, G.C. Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ. Health Perspect. 2016, 125, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Eubig, P.A.; Aguiar, A.; Schantz, S.L. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 2010, 118, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.; Wittsiepe, J.; Kasper-Sonnenberg, M.; Schoneck, N.; Scholmerich, A.; Wilhelm, M. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: Results from the Duisburg Birth Cohort Study. Int. J. Hyg. Environ. Health 2015, 218, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nowack, N.; Wittsiepe, J.; Kasper-Sonnenberg, M.; Wilhelm, M.; Scholmerich, A. Influence of Low-Level Prenatal Exposure to PCDD/Fs and PCBs on Empathizing, Systemizing and Autistic Traits: Results from the Duisburg Birth Cohort Study. PLoS ONE 2015, 10, e0129906. [Google Scholar] [CrossRef] [PubMed]
- Sealey, L.A.; Hughes, B.W.; Sriskanda, A.N.; Guest, J.R.; Gibson, A.D.; Johnson-Williams, L.; Pace, D.G.; Bagasra, O. Environmental factors in the development of autism spectrum disorders. Environ. Int. 2016, 88, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Frame, G.M.; Cochran, J.W.; Bowadt, S.S. Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J. Sep. Sci. 1996, 19, 657–668. [Google Scholar] [CrossRef]
- Choi, S.D.; Baek, S.Y.; Chang, Y.S.; Wania, F.; Ikonomou, M.G.; Yoon, Y.J.; Park, B.K.; Hong, S. Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean Arctic and Antarctic research stations: Implications for long-range transport and local pollution. Environ. Sci. Technol. 2008, 42, 7125–7131. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Belton, T.J.; Rodenburg, L.A. Source apportionment of polychlorinated biphenyls in the tidal Delaware River. Environ. Sci. Technol. 2008, 42, 4044–4051. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Martinez, A.; Hornbuckle, K.C. Discovery of non-aroclor PCB (3,3’-dichlorobiphenyl) in Chicago air. Environ. Sci. Technol. 2008, 42, 7873–7877. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Li, Y.; Wang, T.; Wang, P.; Zhang, H.; Zhang, Q.; Jiang, G. The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3’-dichlorobiphenyl (PCB 11). Chemosphere 2014, 98, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Chen, X.; Kass, P.H.; Puschner, B. Polychlorinated biphenyl and polybrominated diphenyl ether profiles in serum from cattle, sheep, and goats across California. Chemosphere 2017, 181, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Y.; Dang, K.; Puschner, B. Quantification of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Commercial Cows’ Milk from California by Gas Chromatography-Triple Quadruple Mass Spectrometry. PLoS ONE 2017, 12, e0170129. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.X.; Hornbuckle, K.C.; Thorne, P.S. Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ. Sci. Technol. 2015, 49, 8105–8112. [Google Scholar] [CrossRef] [PubMed]
- Marek, R.F.; Thorne, P.S.; Wang, K.; Dewall, J.; Hornbuckle, K.C. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol. 2013, 47, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Keil, K.P.; Chen, H.; Hayakawa, K.; Li, X.; Lin, Y.; Lehmler, H.J.; Puschner, B.; Lein, P.J. Detection of 3,3’-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons. Toxicol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Libersat, F.; Duch, C. Mechanisms of dendritic maturation. Mol. Neurobiol. 2004, 29, 303–320. [Google Scholar] [CrossRef]
- Menon, S.; Gupton, S.L. Building Blocks of Functioning Brain: Cytoskeletal Dynamics in Neuronal Development. Int. Rev. Cell Mol. Biol. 2016, 322, 183–245. [Google Scholar] [PubMed]
- Scott, E.K.; Luo, L. How do dendrites take their shape? Nat. Neurosci. 2001, 4, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Copf, T. Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments. Neurosci. Biobehav. Rev. 2016, 68, 946–978. [Google Scholar] [CrossRef] [PubMed]
- Engle, E.C. Human genetic disorders of axon guidance. Cold Spring Harb. Perspect. Biol. 2010, 2, a001784. [Google Scholar] [CrossRef] [PubMed]
- Keown, C.L.; Shih, P.; Nair, A.; Peterson, N.; Mulvey, M.E.; Muller, R.A. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep. 2013, 5, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. Multifaceted origins of sex differences in the brain. Philos. Trans. R. Soc. Lond B Biol. Sci. 2016, 371, 20150106. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Takanashi, H.; Nagasawa, M.; Kikusui, T. Sex differences in spatiotemporal expression of AR, ERalpha, and ERbeta mRNA in the perinatal mouse brain. Neurosci. Lett. 2015, 584, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Lein, P.J. Overview of the role of environmental factors in neurodevelopmental disorders. In Environmental Factors in Neurodevelopmental and Neurodegenerative Disorders; Elsevier: Oxford, UK, 2015; pp. 3–20. [Google Scholar]
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu. Rev. Public Health 2017, 38, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Wayman, G.A.; Bose, D.D.; Yang, D.; Lesiak, A.; Bruun, D.; Impey, S.; Ledoux, V.; Pessah, I.N.; Lein, P.J. PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ. Health Perspect. 2012, 120, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Keil, K.P.; Mehta, V.; Abler, L.L.; Joshi, P.S.; Schmitz, C.T.; Vezina, C.M. Visualization and quantification of mouse prostate development by in situ hybridization. Differentiation 2012, 84, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Wayman, G.A.; Impey, S.; Marks, D.; Saneyoshi, T.; Grant, W.F.; Derkach, V.; Soderling, T.R. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 2006, 50, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Keil, K.P.; Sethi, S.; Wilson, M.D.; Chen, H.; Lein, P.J. In vivo and in vitro sex differences in the dendritic morphology of developing murine hippocampal and cortical neurons. Sci. Rep. 2017, 7, 8486. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar] [PubMed]
- Yang, D.; Kania-Korwel, I.; Ghogha, A.; Chen, H.; Stamou, M.; Bose, D.D.; Pessah, I.N.; Lehmler, H.J.; Lein, P.J. PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol. Sci. 2014, 138, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Meijering, E.; Jacob, M.; Sarria, J.C.; Steiner, P.; Hirling, H.; Unser, M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytom. A 2004, 58, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [PubMed]
- Lein, P.J.; Banker, G.A.; Higgins, D. Laminin selectively enhances axonal growth and accelerates the development of polarity by hippocampal neurons in culture. Brain Res. Dev. Brain Res. 1992, 69, 191–197. [Google Scholar] [CrossRef]
- Francis, C.; Natarajan, S.; Lee, M.T.; Khaladkar, M.; Buckley, P.T.; Sul, J.Y.; Eberwine, J.; Kim, J. Divergence of RNA localization between rat and mouse neurons reveals the potential for rapid brain evolution. BMC Genom. 2014, 15, 883. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Kawano, J.; Yanai, A.; Fujinaga, R.; Tanaka, M.; Watanabe, Y.; Shinoda, K. Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci. Res. 2004, 49, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.R.; Kokubu, K.; Islam, M.N.; Matsuo, C.; Yanai, A.; Wroblewski, G.; Fujinaga, R.; Shinoda, K. Species differences in androgen receptor expression in the medial preoptic and anterior hypothalamic areas of adult male and female rodents. Neuroscience 2015, 284, 943–961. [Google Scholar] [CrossRef] [PubMed]
- Nugent, B.M.; Schwarz, J.M.; McCarthy, M.M. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: Implications for sexual differentiation. Horm. Behav. 2011, 59, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Konkle, A.T.; Zup, S.L.; McCarthy, M.M. Impact of sex and hormones on new cells in the developing rat hippocampus: A novel source of sex dimorphism? Eur. J. Neurosci. 2008, 27, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Sex hormone modulation of neural development in vitro. Horm. Behav. 1994, 28, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Flor, S.; He, X.; Lehmler, H.J.; Ludewig, G. Estrogenicity and androgenicity screening of PCB sulfate monoesters in human breast cancer MCF-7 cells. Environ. Sci. Pollut. Res. Int. 2016, 23, 2186–2200. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Anezaki, K.; Kojima, H. Effects of unintentional PCBs in pigments and chemical products on transcriptional activity via aryl hydrocarbon and nuclear hormone receptors. Environ. Pollut. 2017, 227, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Baj, G.; Patrizio, A.; Montalbano, A.; Sciancalepore, M.; Tongiorgi, E. Developmental and maintenance defects in Rett syndrome neurons identified by a new mouse staging system in vitro. Front. Cell. Neurosci. 2014, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.M.; Waddell, J.; McCarthy, M.M. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol. Sex Differ. 2010, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Mullins, S.E.; Koenig, J.I. Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. J. Comp. Neurol. 2013, 521, 1828–1843. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Sethi, S.; Lein, P.J.; Keil, K.P. Valid statistical approaches for analyzing sholl data: Mixed effects versus simple linear models. J. Neurosci. Methods 2017, 279, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Koss, W.A.; Frick, K.M. Sex differences in hippocampal function. J. Neurosci. Res. 2017, 95, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Juraska, J.M. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol. Aging 2002, 23, 579–588. [Google Scholar] [CrossRef]
- Maier, D.L.; Mani, S.; Donovan, S.L.; Soppet, D.; Tessarollo, L.; McCasland, J.S.; Meiri, K.F. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 9397–9402. [Google Scholar] [CrossRef] [PubMed]
- Berger-Sweeney, J.; Hohmann, C.F. Behavioral consequences of abnormal cortical development: Insights into developmental disabilities. Behav. Brain Res. 1997, 86, 121–142. [Google Scholar] [CrossRef]
- Cremer, H.; Chazal, G.; Goridis, C.; Represa, A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol. Cell. Neurosci. 1997, 8, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.H.; Hwan Kim, S.; Lee, S.Y.; Jang, C.G. Lactational and postnatal exposure to polychlorinated biphenyls induces sex-specific anxiolytic behavior and cognitive deficit in mice offspring. Synapse 2011, 65, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, K.; Swinnen, S.; Wenderoth, N. Sex differences in Autism: A resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci. 2016, 11, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Bourgeron, T. A synaptic trek to autism. Curr. Opin. Neurobiol. 2009, 19, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Uddin, L.Q.; Khouzam, A.; Phillips, J.; Gaillard, W.D.; Kenworthy, L.E.; Yerys, B.E.; Vaidya, C.J.; Menon, V. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013, 5, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Martien, K.M.; Gagnidze, K.; Pfaff, D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr. Rev. 2014, 35, 961–991. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B. The intersection of neurotoxicology and endocrine disruption. Neurotoxicology 2012, 33, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Comfort, N.; Re, D.B. Sex-Specific Neurotoxic Effects of Organophosphate Pesticides Across the Life Course. Curr. Environ. Health Rep. 2017, 4, 392–404. [Google Scholar] [CrossRef] [PubMed]
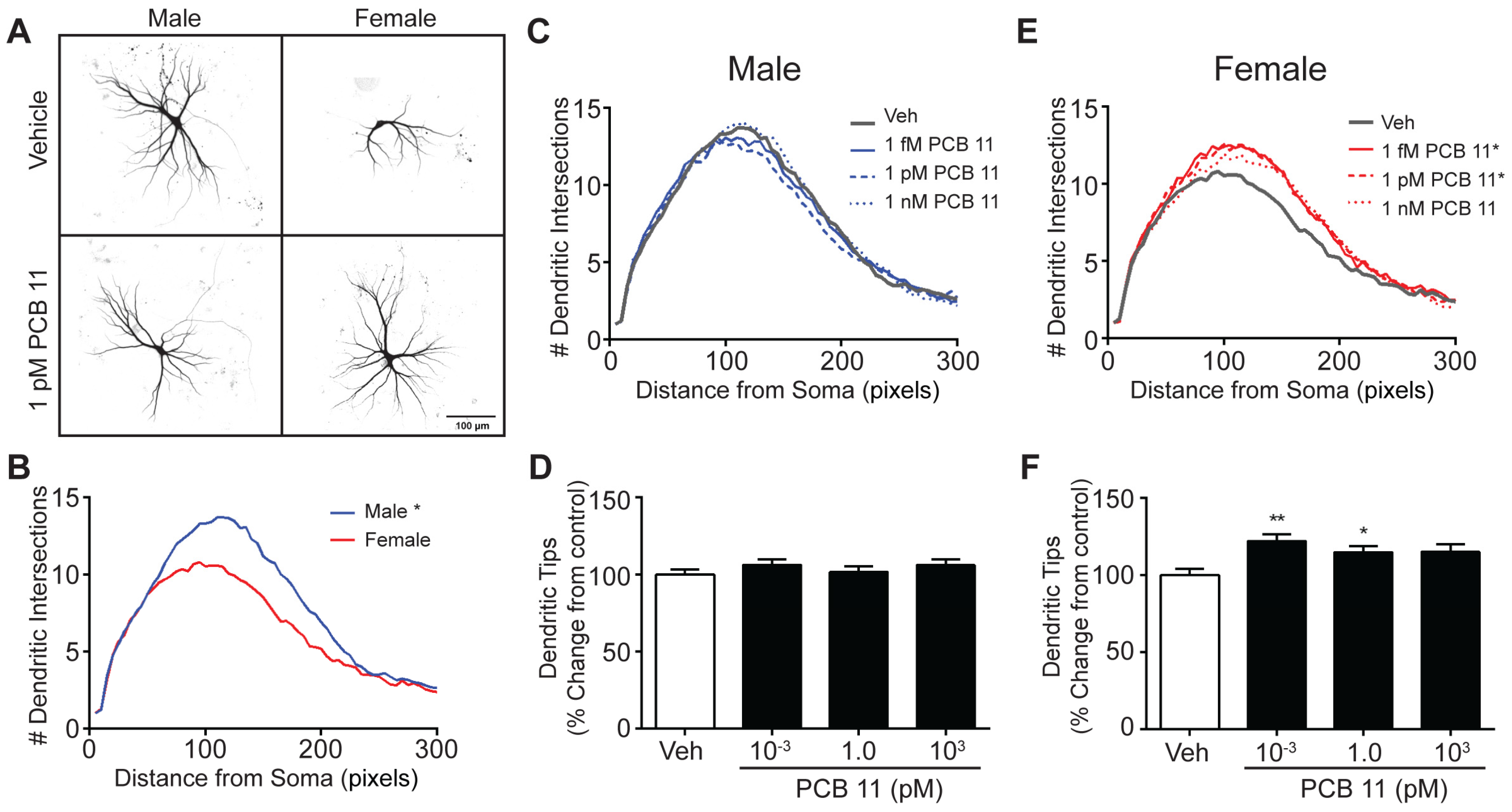
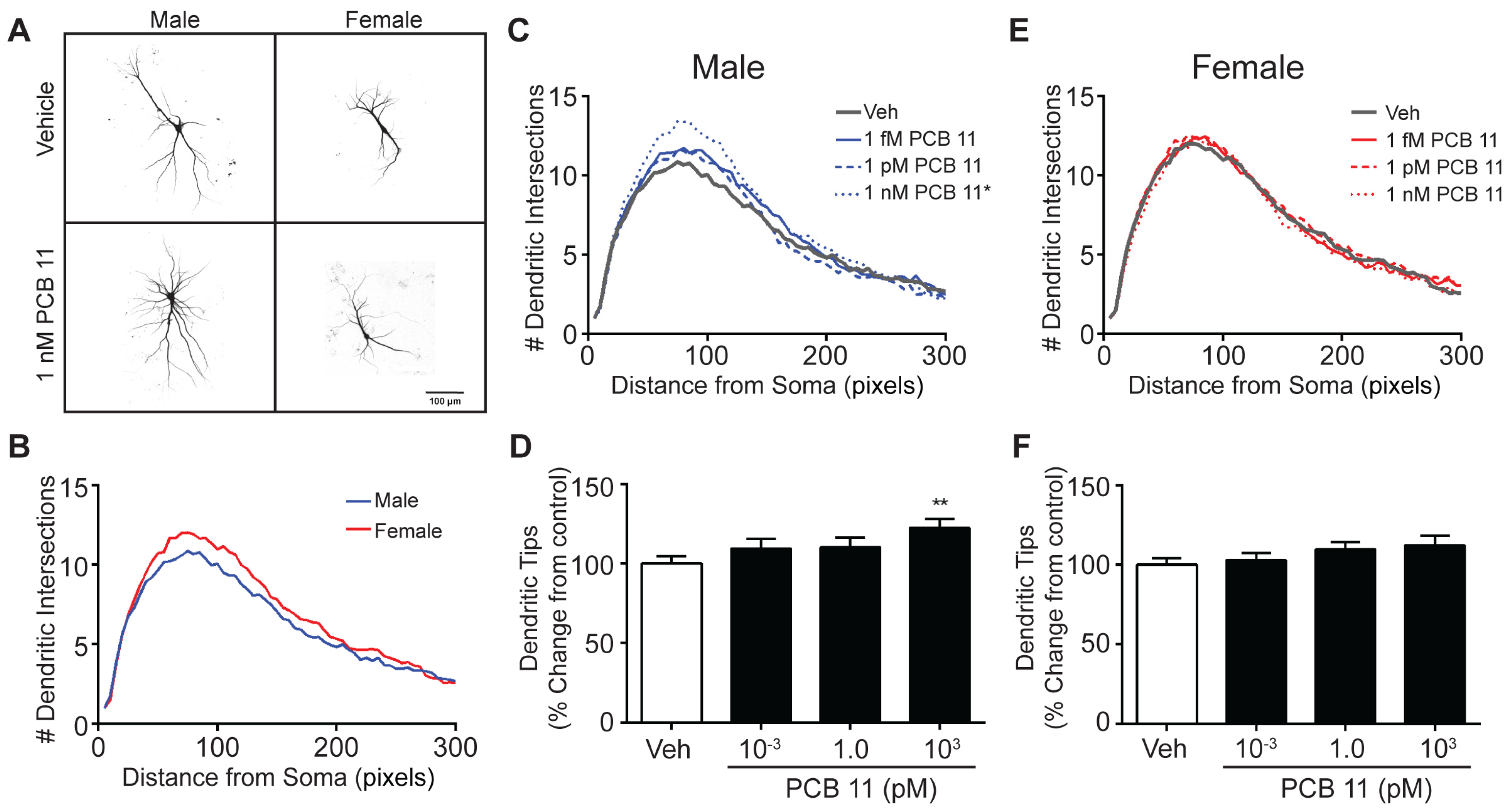
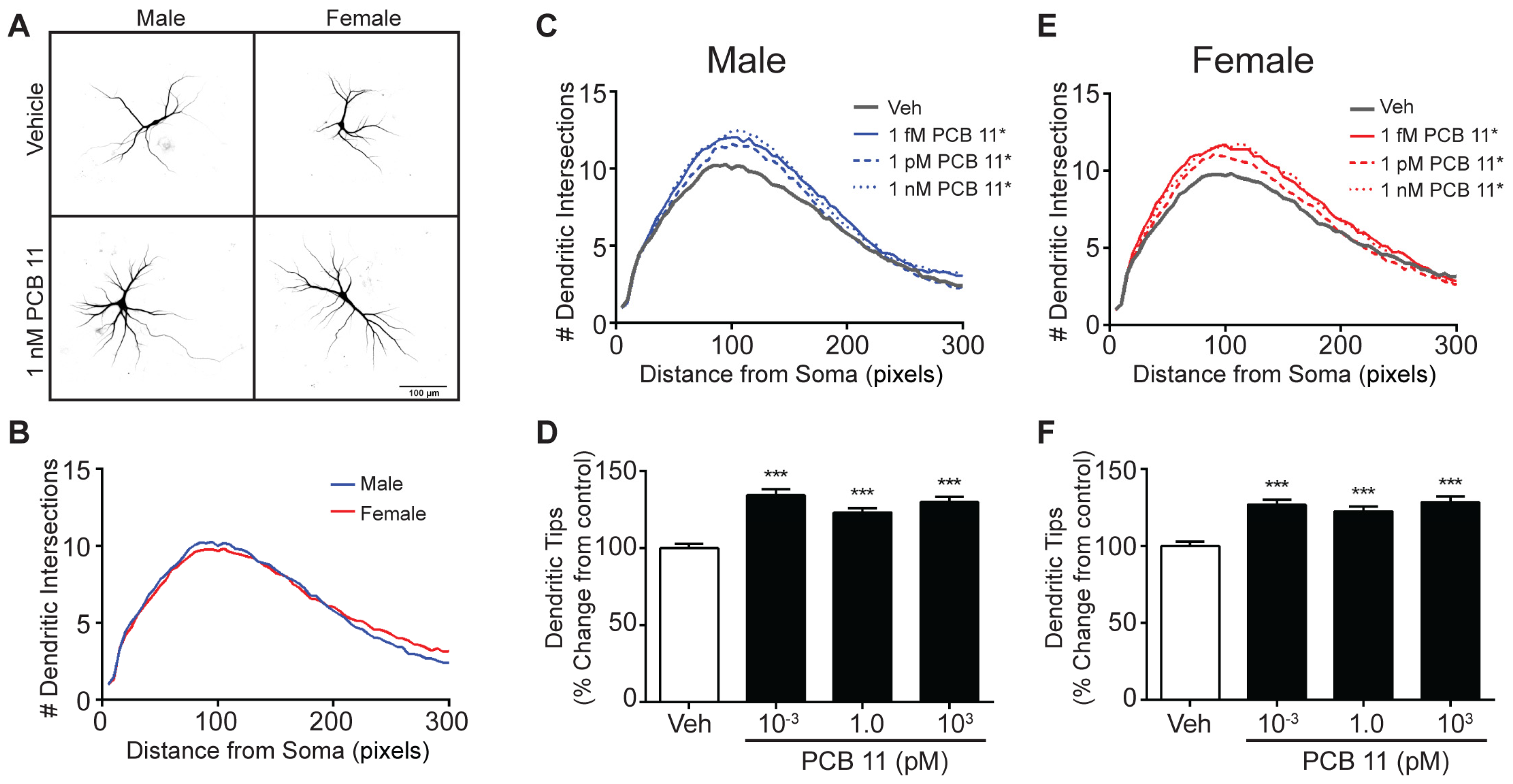
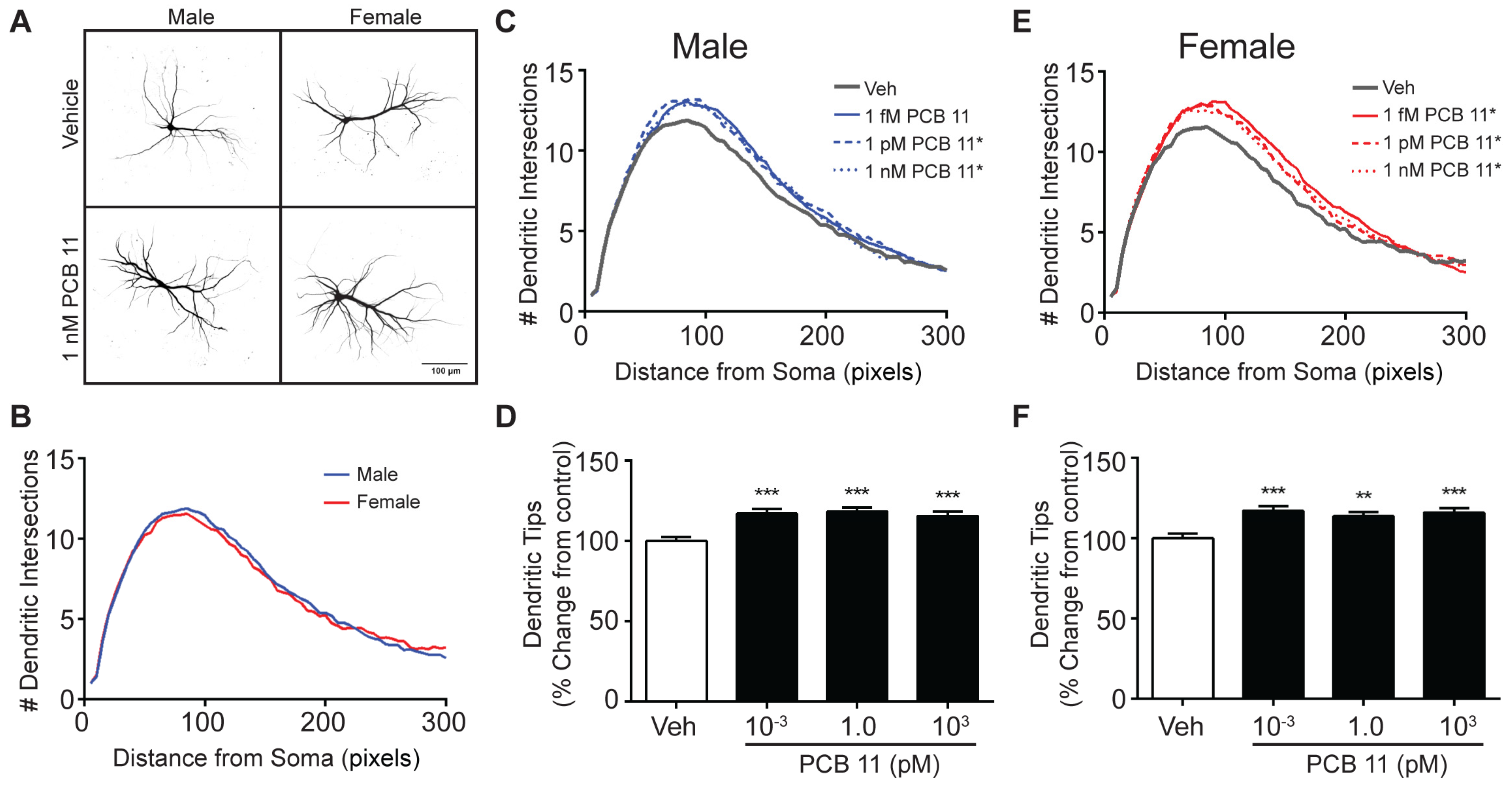

| Male Mouse | Female Mouse | Male Rat | Female Rat | |||||
|---|---|---|---|---|---|---|---|---|
| Hippocampal | Cortical | Hippocampal | Cortical | Hippocampal | Cortical | Hippocampal | Cortical | |
| Axon | ↑ All Concentrations Tested (1 fM–1 nM) | |||||||
| Dendrite | - | ↑ 1 nM | ↑ 1 fM, 1 pM | - | ↑ All Concentrations Tested | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethi, S.; Keil, K.P.; Lein, P.J. Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3′-Dichlorobiphenyl (PCB 11). Toxics 2018, 6, 4. https://doi.org/10.3390/toxics6010004
Sethi S, Keil KP, Lein PJ. Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3′-Dichlorobiphenyl (PCB 11). Toxics. 2018; 6(1):4. https://doi.org/10.3390/toxics6010004
Chicago/Turabian StyleSethi, Sunjay, Kimberly P. Keil, and Pamela J. Lein. 2018. "Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3′-Dichlorobiphenyl (PCB 11)" Toxics 6, no. 1: 4. https://doi.org/10.3390/toxics6010004
APA StyleSethi, S., Keil, K. P., & Lein, P. J. (2018). Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3′-Dichlorobiphenyl (PCB 11). Toxics, 6(1), 4. https://doi.org/10.3390/toxics6010004






