Persistent Threats by Persistent Pollutants: Chemical Nature, Concerns and Future Policy Regarding PCBs—What Are We Heading For?
Abstract
:1. Introduction
2. Materials and Methods: Literature Review
3. History and Accidents throughout the 20th Century
4. Relationship between Exposure and Human Health: The Importance of Longitudinal Studies in Combination with In vitro/In vivo Research
5. Methods of PCB Destruction—Limiting Exposure
6. Regulations and Recommendations towards PCB Policy: Personal Perspectives
- After PCB use and manufacture were banned in 1979 (US), 1981 (UK), and 1986 (EU), PCB levels in biota started to decline (Jepson and Law, 2016), which shows the effectiveness of a ban policy.
- However, a complex and global problem of chemical pollution not only needs national policy actions, but also a worldwide response to internationally coordinated control measures. In February 1973, the Organization for Economic Co-operation and Development (OECD, 1973), was the first international organization advocating international action on polychlorinated biphenyls. Supported by the conclusions of the North Sea Ministerial Conferences, the OECD initiative cumulated in 2004, when the Stockholm Convention committed more than 90 signatory countries to phasing out or eliminating large stocks or other sources of the most hazardous persistent organic products (POPs), including PCBs.
- Since the 1970s, also, the knowledge base and the understanding of the mechanisms underlying the environmental health effects have deepened significantly. This particularly applies to the science supporting PCB policy design and decision making. The evolving list of health effects discussed in this paper provides an example. The first studies pointing to PCBs causing developmental effects, sexual maturation, and endocrine disruption date only from the 1980s. The elucidation of mechanisms that shed new light on the toxicology of the PCB congeners (e.g., PCB molecules with fewer chlorines are more susceptible to biotransformation) is even more recent.
- Define indicators for both control and response variables that will contribute qualifying the exposure and monitor progress towards reducing the pollution. The WHO/United Nation Environment Program (UNEP) experience with monitoring PCBs in maternal milk is encouraging, but in view of the progressing knowledge on action mechanisms and health effects, likely incomplete, and too superficial and too simple to address this complex problem.
- Develop new technologies and social instruments that mitigate PCB pollution, emphasizing a preventive approach.
- Coordinate pollution control and sustainability efforts. Future policy should not only make use of the most recent significant scientific progress (which necessitates more flexibility than in the past) but should also opt for equilibrated long-term solutions.
- Facilitate multiple control efforts involving scientists, civil society, government, and international organizations.
7. Conclusions
- By the 1930s, convincing occupational health evidence existed that PCBs could damage human health. Nevertheless, it took until the 1970s–1980s before a ban on these chemicals became gradually realized. With this almost half a century of latency, PCBs rank among the many chemicals for which early health warnings existed, while the policy was delayed. During this latency period, the (industrialized) world went through an impressive series of incidents, while an enormous environmental burden and delayed costs were built up. It is imperative for the future establishing mechanisms that this scientific evidence–policy latency period is shortened.
- As scientific data evolve and societal preparedness accepting policies increases, the PCB case shows that at least since the 1970s, a high level of proof existed pointing to the combined environmental and human health problems PCBs were responsible for. This environment–health nexus exists for many pollutants, and should be dealt with more intensively than before.
- PCBs illustrate the differentiated sources of pollution which necessitate a differentiated policy approach. While in OECD countries PCBs are closely associated with industrial pollution and a wide range of consumer applications, many developing countries face pollution from obsolete products and (illegal imported) waste. This necessitates not only international coordination, but also differentiation and flexibility of policies.
- The analysis of the health hazards and risks also entails elements of environmental health justice. Within one generation, the involuntary exposure of embryos and fetuses has different ethical consequences than the exposure of healthy industrial workers. The increasing insight into the genetic basis of PCB effects raises questions about how fair the next generations were treated during the past century.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hens, B.; Dyke, P.; Hens, L. What can we learn from ‘dioxin incidents’? IJEP 2016, 60, 34–62. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Hens, L.; Howard, V. (Eds.) Endocrine Disrupters: Environmental Health and Policies; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 2001; ISBN 978-0-7923-7056-7. [Google Scholar]
- Van Larebeke, N.; Hens, L.; Schepens, P.; Covaci, A.; Baeyens, J.; Everaert, K.; Bernheim, J.L.; Vlietinck, R.; De Poorter, G. The Belgian PCB and dioxin incident of January-June 1999: Exposure data and potential impact on health. Environ. Health Perspect. 2001, 109, 265–273. [Google Scholar] [CrossRef]
- Weber, R.; Gaus, C.; Tysklind, M.; Johnston, P.; Forter, M.; Hollert, H.; Heinisch, E.; Holoubek, I.; Lloyd-Smith, M.; Masunaga, S.; et al. Dioxin- and POP-contaminated sites—Contemporary and future relevance and challenges: Overview on background, aims and scope of the series. Environ. Sci. Pollut. Res. Int. 2008, 15, 363–393. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Buekens, A.; Liu, J.; Chen, T.; Lu, S.; Li, X.; Cen, K. Some technical issues in managing PCBs. Environ. Sci. Pollut. Res. Int. 2014, 21, 6448–6462. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters—What we have learned from Yusho disease. Environ. Res. 2001, 86, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W. The human Ah receptor: Hints from dioxin toxicities to deregulated target genes and physiological functions. Biol. Chem. 2013, 394, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.C.; He, G.; Tsutsumi, T.; Zhao, J.; Wirth, E.; Fulton, M.H.; Denison, M.S. Development of Species-Specific Ah Receptor-Responsive Third Generation CALUX Cell Lines with Enhanced Responsiveness and Improved Detection Limits. Environ. Sci. Technol. 2015, 49, 11903–11912. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ruiz, P. Polychlorinated biphenyls: New evidence from the last decade. Toxicol. Ind. Health 2015. [Google Scholar] [CrossRef] [PubMed]
- Ritter, R.; Scheringer, M.; MacLeod, M.; Moeckel, C.; Jones, K.C.; Hungerbühler, K. Intrinsic Human Elimination Half-Lives of Polychlorinated Biphenyls Derived from the Temporal Evolution of Cross-Sectional Biomonitoring Data from the United Kingdom. Environ. Health Perspect. 2011, 119, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.L.; Hicks, H.E.; De Rosa, C.T. Key Environmental Human Health Issues in the Great Lakes and St. Lawrence River Basins. Environ. Res. 1999, 80, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.; Cui, F.; Yin, Z.; Yang, Y.; Qiu, J.; Wang, C. Chiral PCB 91 and 149 Toxicity Testing in Embryo and Larvae (Danio rerio): Application of Targeted Metabolomics via UPLC-MS/MS. Sci. Rep. 2016, 6, 33481. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, N.; Sivaselvakumar, M.; Ramalingam, S. Fast and Parallel determination of PCB 77 and PCB 180 in plasma using Ultra Performance Liquid Chromatography (UPLC) with diode array detection (DAD): A Pharmacokinetic study in Swiss albino mouse. Biomed. Chromatogr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Beckmen, K.B.; Keogh, M.J.; Burek-Huntington, K.A.; Ylitalo, G.M.; Fadely, B.S.; Pitcher, K.W. Organochlorine contaminant concentrations in multiple tissues of free-ranging Steller sea lions (Eumetopias jubatus) in Alaska. Sci. Total Environ. 2016, 542, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Ulutaş, O.K.; Çok, I.; Darendeliler, F.; Aydin, B.; Çoban, A.; Henkelmann, B.; Schramm, K.-W. Blood levels of polychlorinated biphenlys and organochlorinated pesticides in women from Istanbul, Turkey. Environ. Monit. Assess. 2015, 187, 132. [Google Scholar] [CrossRef] [PubMed]
- Wöhrnschimmel, H.; Scheringer, M.; Bogdal, C.; Hung, H.; Salamova, A.; Venier, M.; Katsoyiannis, A.; Hites, R.A.; Hungerbuhler, K.; Fiedler, H. Ten years after entry into force of the Stockholm Convention: What do air monitoring data tell about its effectiveness? Environ. Pollut. 2016, 217, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef] [PubMed]
- The History of PCBs | America Unites 2017. Available online: https://www.coursehero.com/file/26529980/The-History-of-PCBs-America-Unitespdf/ (accessed on 30 August 2017).
- Harremoes, P. The Precautionary Principle in the 20th Century: Late Lessons from Early Warnings; Routledge: Abingdon-on-Thames, UK, 2013; ISBN 978-1-134-20778-7. [Google Scholar]
- Rypel, A.L.; Findlay, R.H.; Mitchell, J.B.; Bayne, D.R. Variations in PCB concentrations between genders of six warmwater fish species in Lake Logan Martin, Alabama, USA. Chemosphere 2007, 68, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Martinez, A.; Hornbuckle, K.C. Sedimentary Records of Non-Aroclor and Aroclor PCB mixtures in the Great Lakes. J. Great Lakes Res. 2011, 37, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.; Ceretti, E.; Covolo, L.; Donato, F. Do polychlorinated biphenyls cause cancer? A systematic review and meta-analysis of epidemiological studies on risk of cutaneous melanoma and non-Hodgkin lymphoma. Chemosphere 2017, 183, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Johnels, A.G.; Olsson, M.; Otterlind, G. DDT and PCB in Marine Animals from Swedish Waters. Nature 1969, 224, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Aminov, Z.; Haase, R.F.; Pavuk, M.; Carpenter, D.O.; Anniston Environmental Health Research Consortium. Analysis of the effects of exposure to polychlorinated biphenyls and chlorinated pesticides on serum lipid levels in residents of Anniston, Alabama. Environ. Health. 2013, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Persistent Organic Pollutants: A Global Issue, A Global Response. Available online: https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response (accessed on 29 May 2017).
- Panshin, S.Y.; Hites, R.A. Atmospheric concentrations of polychlorinated biphenyls in bloomington, indiana. Environ. Sci. Technol. 1994, 28, 2008–2013. [Google Scholar] [CrossRef] [PubMed]
- Custer, C.M.; Custer, T.W.; Hines, J.E. Adult tree swallow survival on the polychlorinated biphenyl-contaminated Hudson River, New York, USA, between 2006 and 2010. Environ. Toxicol. Chem. 2012, 31, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Nacci, D.E.; Hahn, M.E.; Karchner, S.I.; Jayaraman, S.; Mostello, C.; Miller, K.M.; Blackwell, C.G.; Nisbet, I.C.T. Integrating Monitoring and Genetic Methods to Infer Historical Risks of PCBs and DDE to Common and Roseate Terns Nesting Near the New Bedford Harbor Superfund Site (Massachusetts, USA). Environ. Sci. Technol. 2016, 50, 10226–10235. [Google Scholar] [CrossRef] [PubMed]
- Lee Pow, C.S.D.; Law, J.M.; Kwak, T.J.; Cope, W.G.; Rice, J.A.; Kullman, S.W.; Aday, D.D. Endocrine active contaminants in aquatic systems and intersex in common sport fishes. Environ. Toxicol. Chem. 2017, 36, 959–968. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Superfund Sites in Reuse in South Carolina. Available online: https://www.epa.gov/superfund-redevelopment-initiative/superfund-sites-reuse-south-carolina (accessed on 6 June 2017).
- Seattle Times Spokane Sues Monsanto over Spokane River Contamination. Available online: http://www.seattletimes.com/seattle-news/spokane-sues-monsanto-over-spokane-river-contamination/ (accessed on 6 June 2017).
- Gee, D. Late Lessons from Early Warnings: Towards realism and precaution with EMF? Pathophysiology 2009, 16, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, J.-F.; Robertson, L.W. 7th International PCB Workshop: Chemical mixtures in a complex world. Environ. Sci. Pollut. Res. Int. 2014, 21, 6269–6275. [Google Scholar] [CrossRef] [PubMed]
- Okoh, M.P. Exposure to Organo-Chlorinated Compound, PolyChlorinated Biphenyl (PCB), environmental and public health Implications: A Nigeria Case study. Int. J. Chem. Stud. 2015, 2, 14–21. [Google Scholar]
- Pedersen, E.B.; Ebbehøj, N.E.; Göen, T.; Meyer, H.W.; Jacobsen, P. Exposure to 27 polychlorinated biphenyls in the indoor environment of a workplace: A controlled bio-monitoring study. Int. Arch. Occup. Environ. Health 2016, 89, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Hens, L. Health Impact Assessment of Accidents with Environmental Carcinogens. In Cancer as an Environmental Disease; Springer: Dordrecht, The Netherlands, 2004; pp. 103–133. [Google Scholar]
- Hens, L.; Hens, B. Milieu-en gezondheidseffecten van benzeen en sommige verwanten: 150 jaar wijzigende inzichten. In Symposium 150 jaar Benzeen-Structuurformule: Ontstaan en Huidige Toepassingen ter Gelegenheid van 25 jaar KVCV—Sectie Historiek en 75 jaa; Koninklijke Vlaamse Chemie Vereniging vzw: Antwerp, Belgium, 2015; pp. 103–130. [Google Scholar]
- Morgan, M.; Deoraj, A.; Felty, Q.; Roy, D. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol. Cell. Endocrinol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Parada, H.; Wolff, M.S.; Engel, L.S.; Eng, S.M.; Khankari, N.K.; Neugut, A.I.; Teitelbaum, S.L.; Gammon, M.D. Polychlorinated biphenyls and their association with survival following breast cancer. Eur. J. Cancer 2016, 56, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Drinker, C.K.; Warren, M.F.; Bennett, G.A. The Problem of Possible Systemic Effects from Certain Chlorinated Hydrocarbons. J. Ind. Hyg. Toxicol. 1937, 19, 283–299. [Google Scholar]
- Fukushi, J.-I.; Tokunaga, S.; Nakashima, Y.; Motomura, G.; Mitoma, C.; Uchi, H.; Furue, M.; Iwamoto, Y. Effects of dioxin-related compounds on bone mineral density in patients affected by the Yusho incident. Chemosphere 2016, 145, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y. Approach to risk assessment of chlorinated dioxins from Yusho PCB poisoning. Chemosphere 1996, 32, 583–594. [Google Scholar] [CrossRef]
- Matsumoto, S.; Akahane, M.; Kanagawa, Y.; Kajiwara, J.; Mitoma, C.; Uchi, H.; Furue, M.; Imamura, T. Unexpectedly long half-lives of blood 2,3,4,7,8-pentachlorodibenzofuran (PeCDF) levels in Yusho patients. Environ. Health 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.L.; Hsin, J.W.; Hsu, C.C.; Chan, W.C.; Guo, Y.L. The immunologic evaluation of the Yucheng children. Chemosphere 1998, 37, 1855–1865. [Google Scholar] [CrossRef]
- Crofton, K.M.; Kodavanti, P.R.; Derr-Yellin, E.C.; Casey, A.C.; Kehn, L.S. PCBs, thyroid hormones, and ototoxicity in rats: Cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol. Sci. 2000, 57, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, H.C.; Johnson, A.; McKnight, L.; Horinek, M.; Asbrock, C.; Burt, S.; Jolous-Jamshidi, B.; Meserve, L.A. Effects of polychlorinated biphenyls on maternal odor conditioning in rat pups. Physiol. Behav. 2007, 91, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Bandiera, S.; Sawyer, T.; Robertson, L.; Safe, L.; Parkinson, A.; Thomas, P.E.; Ryan, D.E.; Reik, L.M.; Levin, W.; et al. PCBs: Structure—Function relationships and mechanism of action. Environ. Health Perspect. 1985, 60, 47–56. [Google Scholar] [PubMed]
- World Health Organization. The International Programme on Chemical Safety (IPCS); World Health Organization: Geneva, The Netherlands, 2017. [Google Scholar]
- De Roos, A.J.; Hartge, P.; Lubin, J.H.; Colt, J.S.; Davis, S.; Cerhan, J.R.; Severson, R.K.; Cozen, W.; Patterson, D.G.; Needham, L.L.; et al. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin’s lymphoma. Cancer Res. 2005, 65, 11214–11226. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.M.; Vial, S.L.; Fuortes, L.J.; Guo, H.; Reedy, V.E.; Smith, E.M. Organochlorines and risk of prostate cancer. J. Occup. Environ. Med. 2003, 45, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Andersson, S.-O.; Carlberg, M.; Bohr, L.; van Bavel, B.; Lindström, G.; Björnfoth, H.; Ginman, C. Adipose tissue concentrations of persistent organic pollutants and the risk of prostate cancer. J. Occup. Environ. Med. 2006, 48, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.M.; Vial, S.L.; Fuortes, L.J.; Robertson, L.W.; Guo, H.; Reedy, V.E.; Smith, E.M. Comparison of proposed frameworks for grouping polychlorinated biphenyl congener data applied to a case-control pilot study of prostate cancer. Environ. Res. 2005, 98, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Charles, L.E.; Loomis, D.; Shy, C.M.; Newman, B.; Millikan, R.; Nylander-French, L.A.; Couper, D. Electromagnetic fields, polychlorinated biphenyls, and prostate cancer mortality in electric utility workers. Am. J. Epidemiol. 2003, 157, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; van Bavel, B.; Lindström, G.; Carlberg, M.; Dreifaldt, A.C.; Wijkström, H.; Starkhammar, H.; Eriksson, M.; Hallquist, A.; Kolmert, T. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ. Health Perspect. 2003, 111, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Van Bavel, B.; Lindström, G.; Carlberg, M.; Eriksson, M.; Dreifaldt, A.C.; Wijkström, H.; Starkhammar, H.; Hallquist, A.; Kolmert, T. Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int. J. Androl. 2004, 27, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Hoffman, H.J.; Klebanoff, M.A.; Brock, J.W.; Zhou, H.; Needham, L.; Adera, T.; Guo, X.; Gray, K.A. In utero exposure to polychlorinated biphenyls and sensorineural hearing loss in 8-year-old children. Neurotoxicol. Teratol. 2004, 26, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Alaluusua, S.; Kiviranta, H.; Leppäniemi, A.; Hölttä, P.; Lukinmaa, P.-L.; Lope, L.; Järvenpää, A.-L.; Renlund, M.; Toppari, J.; Virtanen, H.; et al. Natal and neonatal teeth in relation to environmental toxicants. Pediatr. Res. 2002, 52, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Roels, H.A.; Hoppenbrouwers, K.; Nawrot, T.; Thijs, L.; Vandermeulen, C.; Winneke, G.; Vanderschueren, D.; Staessen, J.A. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ. Health Perspect. 2002, 110, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Brucker-Davis, F.; Wagner-Mahler, K.; Delattre, I.; Ducot, B.; Ferrari, P.; Bongain, A.; Kurzenne, J.-Y.; Mas, J.-C.; Fénichel, P. Cryptorchidism Study Group from Nice Area Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum. Reprod. 2008, 23, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O.; Muttineni, J.; Karmaus, W. In utero exposure to organochlorines and age at menarche. Hum. Reprod. 2004, 19, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.; Sovcikova, E.; Kocan, A.; Wsolova, L.; Trnovec, T. Developmental dental defects in children exposed to PCBs in eastern Slovakia. Chemosphere 2007, 67, S350–S354. [Google Scholar] [CrossRef] [PubMed]
- Denham, M.; Schell, L.M.; Deane, G.; Gallo, M.V.; Ravenscroft, J.; DeCaprio, A.P. Akwesasne Task Force on the Environment Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among Akwesasne Mohawk girls. Pediatrics 2005, 115, e127–e134. [Google Scholar] [CrossRef] [PubMed]
- Riva, E.; Grandi, F.; Massetto, N.; Radaelli, G.; Giovannini, M.; Zetterström, R.; Agostoni, C. Polychlorinated biphenyls in colostral milk and visual function at 12 months of life. Acta Paediatr. 2004, 93, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.; Vrbic, V. Polychlorinated biphenyls cause developmental enamel defects in children. Caries Res. 2000, 34, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Mol, N.M.; Sørensen, N.; Weihe, P.; Andersson, A.-M.; Jørgensen, N.; Skakkebaek, N.E.; Keiding, N.; Grandjean, P. Spermaturia and serum hormone concentrations at the age of puberty in boys prenatally exposed to polychlorinated biphenyls. Eur. J. Endocrinol. 2002, 146, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Rignell-Hydbom, A.; Rylander, L.; Hagmar, L. Exposure to persistent organochlorine pollutants and type 2 diabetes mellitus. Hum. Exp. Toxicol. 2007, 26, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O.; Cameron, L.; Gardiner, J.; Deguire, P.; Karmaus, W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology 2006, 17, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Langer, P.; Tajtáková, M.; Guretzki, H.-J.; Kocan, A.; Petrík, J.; Chovancová, J.; Drobná, B.; Jursa, S.; Pavúk, M.; Trnovec, T.; et al. High prevalence of anti-glutamic acid decarboxylase (anti-GAD) antibodies in employees at a polychlorinated biphenyl production factory. Arch. Environ. Health 2002, 57, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Winneke, G.; Wilhelm, M.; Wittsiepe, J.; Lemm, F.; Fürst, P.; Ranft, U.; Imöhl, M.; Kraft, M.; Oesch-Bartlomowicz, B.; et al. Environmental exposure to dioxins and polychlorinated biphenyls reduce levels of gonadal hormones in newborns: Results from the Duisburg cohort study. Int. J. Hyg. Environ. Health 2008, 211, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Su, P.-H.; Jong, S.-B.; Guo, Y.L.; Chou, W.-L.; Päpke, O. In utero exposure to dioxins and polychlorinated biphenyls and its relations to thyroid function and growth hormone in newborns. Environ. Health Perspect. 2005, 113, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Rylander, L.; Rignell-Hydbom, A.; Hagmar, L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ. Health 2005, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Wang, S.-L.; Liao, P.-C.; Chen, H.Y.; Ko, Y.-C.; Lee, C.-C. Relationship between insulin sensitivity and exposure to dioxins and polychlorinated biphenyls in pregnant women. Environ. Res. 2008, 107, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Steffes, M.; Jacobs, D.R. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Environ. Health Perspect. 2007, 115, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-Y.; Hertz-Picciotto, I.; Petrik, J.; Palkovicova, L.; Kocan, A.; Trnovec, T. Prenatal PCB Exposure and Thymus Size at Birth in Neonates in Eastern Slovakia. Environ. Health Perspect. 2008, 116, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.F.; Hwang, S.-A.; Lambert, G.; Gomez, M.; Tarbell, A. PCB Exposure and in vivo CYP1A2 Activity among Native Americans. Environ. Health Perspect. 2005, 113, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Wallin, E.; Axmon, A.; Jönsson, B.A.G.; Akesson, H.; Janák, K.; Hagmar, L.; Bergman, A. Hydroxy-PCBs, PBDEs, and HBCDDs in serum from an elderly population of Swedish fishermen’s wives and associations with bone density. Environ. Sci. Technol. 2006, 40, 6282–6289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, E.; Rylander, L.; Jönssson, B.A.G.; Lundh, T.; Isaksson, A.; Hagmar, L. Exposure to CB-153 and p,p’-DDE and bone mineral density and bone metabolism markers in middle-aged and elderly men and women. Osteoporos. Int. 2005, 16, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.; Thomas, L.; Fattore, E.; Lind, P.M.; Alfven, T.; Hellström, L.; Håkansson, H.; Carubelli, G.; Fanelli, R.; Jarup, L. Bone mineral density changes in relation to environmental PCB exposure. Environ. Health Perspect. 2008, 116, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Glynn, A.W.; Michaëlsson, K.; Lind, P.M.; Wolk, A.; Aune, M.; Atuma, S.; Darnerud, P.O.; Mallmin, H. Organochlorines and bone mineral density in Swedish men from the general population. Osteoporos. Int. 2000, 11, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Hein, M.J.; Cassinelli, R.T.; Prince, M.M.; Nilsen, N.B.; Whelan, E.A.; Waters, M.A.; Ruder, A.M.; Schnorr, T.M. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology 2006, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H.M.; Marcus, M.; Tolbert, P.E.; Schuch, C.; Rubin, C.; Henderson, A.K.; Zhang, R.H.; Hertzberg, V.S. Time to menopause in relation to PBBs, PCBs, and smoking. Maturitas 2004, 49, 97–106. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dioxins and Their Effects on Human Health; World Health Organization: Geneva, The Netherlands, 2014. [Google Scholar]
- Steinberg, R.M.; Walker, D.M.; Juenger, T.E.; Woller, M.J.; Gore, A.C. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: Development, reproductive physiology, and second generational effects. Biol. Reprod. 2008, 78, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc. Soc. Exp. Biol. Med. 2000, 224, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, H.; Fastabend, A.; Hany, J.; Kaya, H.; Roth-Härer, A.; Dunemann, L.; Winneke, G. Reduced levels of 1,25-dihydroxyvitamin D(3) in rat dams and offspring after exposure to a reconstituted PCB mixture. Toxicol. Sci. 2000, 57, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Nyska, A.; Jokinen, M.P.; Brix, A.E.; Sells, D.M.; Wyde, M.E.; Orzech, D.; Haseman, J.K.; Flake, G.; Walker, N.J. Exocrine pancreatic pathology in female Harlan Sprague-Dawley rats after chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like compounds. Environ. Health Perspect. 2004, 112, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, D.L.; Meserve, L.A.; Bingman, V.P. Reduced growth of intra- and infra-pyramidal mossy fibers is produced by continuous exposure to polychlorinated biphenyl. Toxicology 1999, 138, 11–17. [Google Scholar] [CrossRef]
- Brix, A.E.; Jokinen, M.P.; Walker, N.J.; Sells, D.M.; Nyska, A. Characterization of bronchiolar metaplasia of the alveolar epithelium in female Sprague-Dawley rats exposed to 3,3’,4,4’,5-pentachlorobiphenyl (PCB126). Toxicol. Pathol. 2004, 32, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Coburn, C.G.; Gillard, E.R.; Currás-Collazo, M.C. Dietary exposure to aroclor 1254 alters central and peripheral vasopressin release in response to dehydration in the rat. Toxicol. Sci. 2005, 84, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.N.; Chahoud, I. In utero exposure to low-dose 2,3’,4,4’,5-pentachlorobiphenyl (PCB 118) impairs male fertility and alters neurobehavior in rat offspring. Toxicology 2004, 202, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Orberg, J.; Edlund, U.-B.; Sjöblom, L.; Lind, L. The dioxin-like pollutant PCB 126 (3,3’,4,4’,5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol. Lett. 2004, 150, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Oskam, I.C.; Skaare, J.U.; Reksen, O.; Sweeney, T.; Dahl, E.; Farstad, W.; Ropstad, E. Effects of gestational and lactational exposure to low doses of PCBs 126 and 153 on anterior pituitary and gonadal hormones and on puberty in female goats. Reprod. Toxicol. 2004, 19, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.T.M.; Hoving, S.; van den Berg, J.H.J.; Weijers, B.M.; Swarts, H.J.; van der Beek, E.M.; Bergman, A.; Koeman, J.H.; Brouwer, A. Effects of in utero exposure to 4-hydroxy-2,3,3’,4’,5-pentachlorobiphenyl (4-OH-CB107) on developmental landmarks, steroid hormone levels, and female estrous cyclicity in rats. Toxicol. Sci. 2004, 82, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.L.; Cummings, J.A.; Clemens, L.G.; Nunez, A.A. Exposure to PCB 77 affects the maternal behavior of rats. Physiol. Behav. 2005, 84, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Walker, N.J.; Jokinen, M.P.; Brix, A.E.; Sells, D.M.; Marsh, T.; Wyde, M.E.; Orzech, D.; Haseman, J.K.; Nyska, A. Gingival carcinogenicity in female Harlan Sprague-Dawley rats following two-year oral treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like compounds. Toxicol. Sci. 2005, 83, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.A.; Nunez, A.A.; Clemens, L.G. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol. Behav. 2005, 85, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Pan, M.-H.; Li, L.-A.; Chen, C.-J.; Tsai, S.-S.; Guo, Y.L. Exposure in utero to 2,2’,3,3’,4,6’-hexachlorobiphenyl (PCB 132) impairs sperm function and alters testicular apoptosis-related gene expression in rat offspring. Toxicol. Appl. Pharmacol. 2007, 221, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kenet, T.; Froemke, R.C.; Schreiner, C.E.; Pessah, I.N.; Merzenich, M.M. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 7646–7651. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Lyche, J.L.; Ropstad, E.; Aleksandersen, M.; Rönn, M.; Skaare, J.U.; Larsson, S.; Orberg, J.; Lind, P.M. Perinatal exposure to PCB 153, but not PCB 126, alters bone tissue composition in female goat offspring. Toxicology 2006, 228, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Shirota, M.; Mukai, M.; Sakurada, Y.; Doyama, A.; Inoue, K.; Haishima, A.; Akahori, F.; Shirota, K. Effects of vertically transferred 3,3’,4,4’,5-pentachlorobiphenyl (PCB-126) on the reproductive development of female rats. J. Reprod. Dev. 2006, 52, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.M.; Juenger, T.E.; Gore, A.C. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm. Behav. 2007, 51, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Kopec, A.K.; Burgoon, L.D.; Ibrahim-Aibo, D.; Burg, A.R.; Lee, A.W.; Tashiro, C.; Potter, D.; Sharratt, B.; Harkema, J.R.; Rowlands, J.C.; et al. Automated dose-response analysis and comparative toxicogenomic evaluation of the hepatic effects elicited by TCDD, TCDF, and PCB126 in C57BL/6 mice. Toxicol. Sci. 2010, 118, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Orito, K.; Gotanda, N.; Murakami, M.; Ikeda, T.; Egashira, N.; Mishima, K.; Fujiwara, M. Prenatal exposure to 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) promotes anxiogenic behavior in rats. Tohoku J. Exp. Med. 2007, 212, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.A.; Clemens, L.G.; Nunez, A.A. Exposure to PCB 77 affects partner preference but not sexual behavior in the female rat. Physiol. Behav. 2008, 95, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Dziennis, S.; Yang, D.; Cheng, J.; Anderson, K.A.; Alkayed, N.J.; Hurn, P.D.; Lein, P.J. Developmental exposure to polychlorinated biphenyls influences stroke outcome in adult rats. Environ. Health Perspect. 2008, 116, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Sipka, S.; Eum, S.-Y.; Son, K.W.; Xu, S.; Gavalas, V.G.; Hennig, B.; Toborek, M. Oral Administration of Pcbs Induces Proinflammatory and Prometastatic Responses. Environ. Toxicol. Pharmacol. 2008, 25, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Rossberg, M.; Lendle, W.; Pfleiderer, G.; Tögel, A.; Dreher, E.-L.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack, H.; Cook, R.; et al. Chlorinated Hydrocarbons. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Wijnegem, Germany, 2000; ISBN 978-3-527-30673-2. [Google Scholar]
- Qi, Z.; Chen, T.; Bai, S.; Yan, M.; Lu, S.; Buekens, A.; Yan, J.; Bulmău, C.; Li, X. Effect of temperature and particle size on the thermal desorption of PCBs from contaminated soil. Environ. Sci. Pollut. Res. 2014, 21, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- Windt, W.D.; Aelterman, P.; Verstraete, W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ. Microbiol. 2005, 7, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Čvančarová, M.; Křesinová, Z.; Filipová, A.; Covino, S.; Cajthaml, T. Biodegradation of PCBs by ligninolytic fungi and characterization of the degradation products. Chemosphere 2012, 88, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.A.; Murray, J. Human biomonitoring of environmental chemicals—Early results of the 2007–2009 Canadian Health Measures Survey for males and females. Int. J. Hyg. Environ. Health 2012, 215, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Redding, L.E.; Sohn, M.D.; McKone, T.E.; Chen, J.-W.; Wang, S.-L.; Hsieh, D.P.H.; Yang, R.S.H. Population Physiologically Based Pharmacokinetic Modeling for the Human Lactational Transfer of PCB-153 with Consideration of Worldwide Human Biomonitoring Results. Environ. Health Perspect. 2008, 116, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
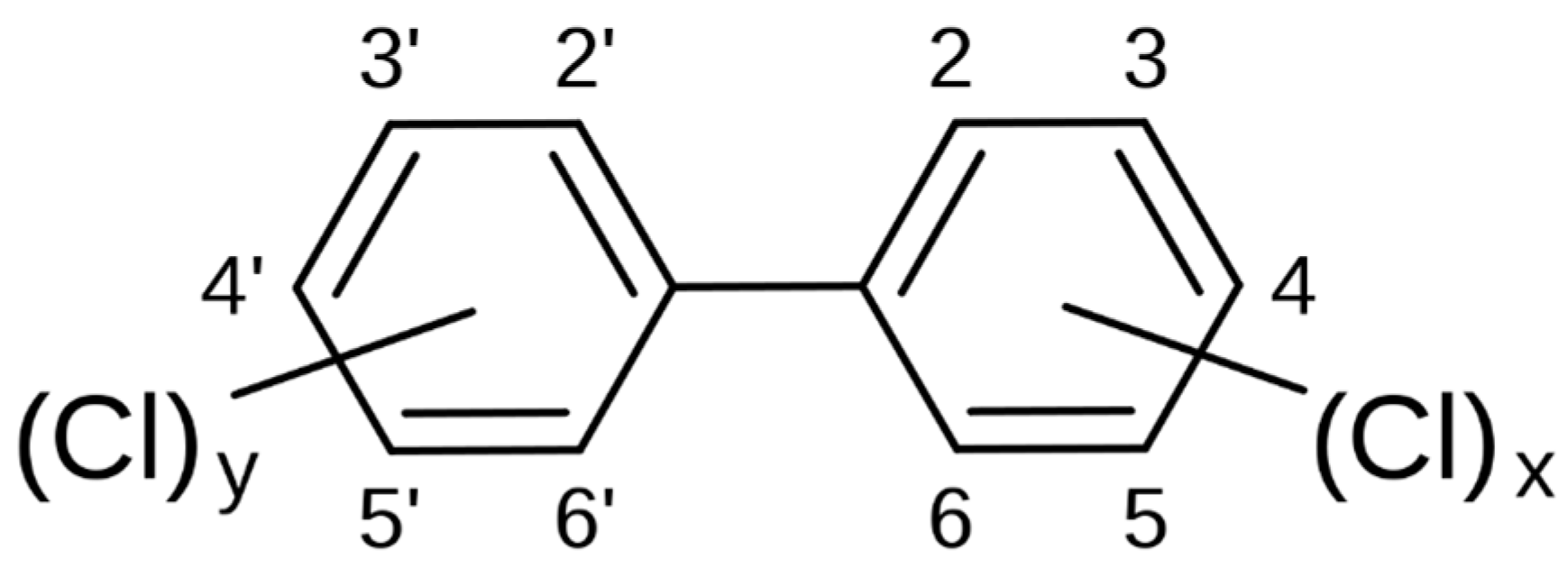

| Year | State/Country | Source |
|---|---|---|
| 1929–1971 | Alabama, USA | PCB-containing materials were leached into Snow Creek, spread out over the Choccolocco Creek and Logan Martin Lake. |
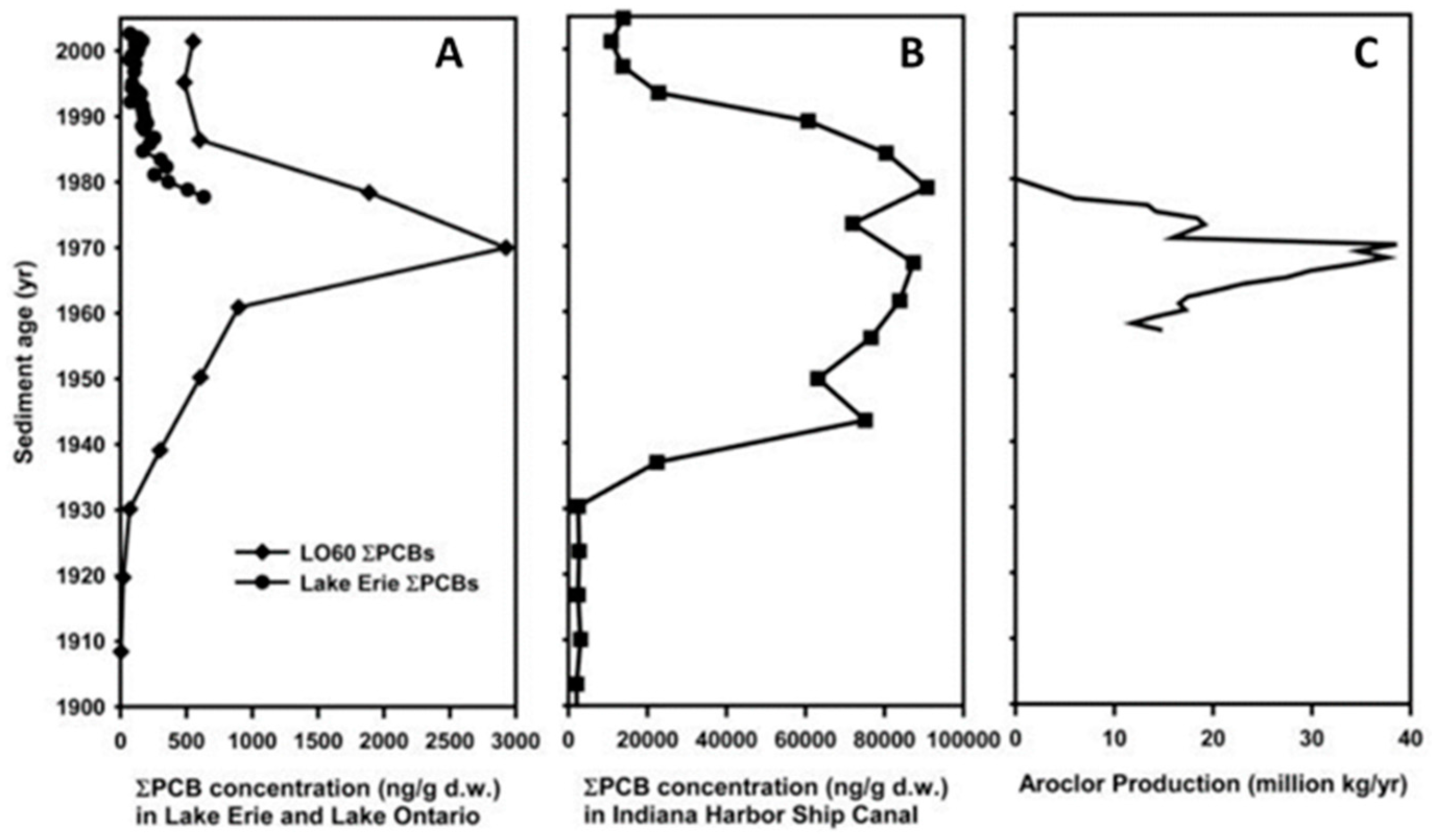
| 1929–1971 | The Midwest States and Canada | Peak concentrations of Aroclor® 1248 and 1254 in the seventies as observed in Lake Erie, Lake Ontario and in Indiana Harbor Ship Canal, similar to the production of Aroclor® during those days. |
| 1950–1977 | Bloomington, Indiana | Westinghouse Electric Company produced large amounts of PCBs, which were still detectable in environmental sources (air and soils) until 1993. |
| 1947–1977 | Pittsfield, Massachusetts; Lockport, New York | Dumping of PCBs by General Electric, both sites now listed as Superfund sites to be decontaminated. |
| 1978 | North Carolina | One of the largest PCB dumps (31,000 gallons of PCB-contaminated oil) along the roadsides. |
| 1970s | South Carolina | Sangama Weston Plant dumped PCB-contaminated waste into the Twelve Miles Creek. |
| 1970s | Washington (State) | PCB contamination in river soil from industrial discharges. |
| 1970s | The Netherlands | Contamination coming from industrial plants in Germany, France, and Switzerland into the Rhine River. |
| 1970s–1980s | Germany | Immensely high concentrations of PCBs in the milk of breastfeeding women. |
| 1974 | France | Contamination of PCBs in soft cheese. |
| 1999 | Belgium | Chicken feed was contaminated with PCBs from a decommissioned electricity transformer. |
| 1970s up until now | Nigeria | 3400 tons of PCB-contaminated waste present in Nigeria throughout the years, produced by industrial processes. |
| Health Effect/Outcome | Levels of Exposure | Outcome | Reference |
|---|---|---|---|
| Cancer/Non-Hodgkin’s lymphoma | PCBs and other organochlorines | PCBs 156,180, and 194 associated with increased risk of non-Hodgkin’s lymphoma | [49] |
| Cancer/Prostate cancer | 30 PCBs and 18 organochlorine pesticide | PCB 180 was associated with an increase in risk of prostate cancer | [50] |
| Cancer/PSA levels | PCBs and other POPs (Chlordane, DDE) | In cases with PCB 153 > than the median concentration among controls, the OR = 3.15 (95% CI, 1.04–9.54) | [51] |
| Cancer/Prostate | 30 PCB congeners in serum | Odds of high exposure group > twice that of lowest exposure group | [52] |
| Cancer/Prostate | Both high exposure to electromagnetic fields and PCBs | No association after adjusting for confounders | [53] |
| Cancer/Testicular/Seminoma | 38 PCB congeners, DDT, hexachlorobenzene, chlordane | PCBs yielded odds ratio 3.8, 95% CI, 1.4–10 among case mothers | [54] |
| Cancer/Testicular Cancer | 37 PCBs exposure | The concentrations of PCBs are higher in mothers to patients with testicular cancer | [55] |
| Developmental/Sensorineural hearing loss (SNHL) | 2.8 μg/L serum total PCBs; mothers in 3rd trimester | The mean of mother’s serum PCB concentrations not related to the adjusted odds of SNHL | [56] |
| Development of natal and neonatal teeth | TEQ 11.9 pg/g fat PCDD/F, TEQ 7.24 pg/g fat | No association | [57] |
| Developmental; sexual maturation | PCBs (138, 153, 180), Dioxin-like compounds | Doubling of serum PCB 153 and dioxin-like chemicals significantly affected sexual maturation clarify | [58] |
| Developmental; reproduction effects | French cohort | At birth, cryptorchidism associated with higher prenatal exposure to PCBs. | [59] |
| Developmental/Age at menarche in offspring | PCBs and DDTs. Retrospective cohort study for two generations | No association with maternal PCB exposure | [60] |
| Developmental/Gingival health by standard dental indices and enamel by FDI index | Children living near industrial area contaminated with PCBs | Enamel defects in deciduous teeth significantly high in higher exposed children (Chi-Sq = 8.35; p = 0.03). For permanent teeth with any enamel defects (Chi-Sq = 7.237; p = 0.027). The extent of enamel defects is significantly greater in high PCB exposure group (Chi-Sq = 10.714; p = 0.005) | [61] |
| Developmental/Onset of menses | 16 PCB congeners | PCBs levels are significant predictors of menarcheal status | [62] |
| Developmental/Visual function | Breastfed for 4 months and examined at 12 month of age | P100 with latency evoked potentials (VEPs) at 60 min. related to PCB 180 (r = −0.504) | [63] |
| Developmental/Dental enamel | Concentration of PBCs in diet | Enamel development defects were found in 71.3% exposed vs. 49.5% control | [64] |
| Developmental/Hormone levels and sexual differentiation | Prenatal exposure to PCBs. Umbilical cord specimens were collected. | 20 boys with cryptorchidism; other 58 with spermaturia. | [65] |
| Endocrine/Type 2 diabetes mellitus | POPs/PCB153 | Odds Ratio = 1.6; 95% CI, 1.0–2.7 associated with an increase of PCB-153 of 100 ng/g lipid | [66] |
| Endocrine/Type 2 diabetes mellitus | PCBs exposure | Positive linear association of PCB levels with diabetes at the time of enrollment in women | [67] |
| Endocrine/Thyroid | Retrospective study | Anti-GAD was 4 times higher than that of all controls | [68] |
| Endocrine/Testosterone and estradiol | PCBs concentrations 149 ng/g in blood and 177 ng/g in milk | Testosterone and estradiol levels were less in babies with high PCB concentrations | [69] |
| Endocrine/Thyroid and growth hormones | 118 pregnant women (ages 25–34 years); Placental and cord blood samples. 12 dioxin-like PCBs | Significant negative associations between FT4, TSH and the increase of non-ortho PCBs (r = −0.2; p < 0.05) | [70] |
| Endocrine/Diabetes mellitus | PCBs 153 | PCB 153 significantly associated with diabetes (an increase of 100 ng/g lipid corresponded to OR =1.16, 95% CI, 1.03–1.32, p = 0.03) | [71] |
| Endocrine/Diabetes mellitus/Insulin sensitivity | 12 PCB congeners exposure | PCBs (123,126 and 169) were significant associated with insulin activity (r = −0.34, p < 0.05) | [72] |
| Endocrine/Type 2 diabetes | Persistent organic pollutants (POPs); 19 POPs in 5 subclasses | Association observed between HOMA-IR and two non-dioxin-like PCBs | [73] |
| Immunological/Antibodies to tetanus and diphtheria toxoids | Two cohorts from the Faroe Islands, mother serum (during pregnancy) and milk PCB levels were analyzed. Antibodies for tetanus and diphtheria were measured. | For each doubling of PCBs serum concentration, antibodies for diphtheria toxoid decreased by 24.4% at age 18 months (95% CI, 1.63–41.9; p = 0.04). Ab for tetanus toxoid decreased by 16.5% at age 7 y (95% CI, 1.51–29.3; p = 0.03) | [74] |
| Immunological/Rheumatoid arthritis | Cross-sectional study, 1721; 20 years or more of age; dioxin and non- dioxin-like (DL) PCBs | Odds ratios 1.0, 2.1, 3.5, and 2.9 across quartiles of DL PCBs. ODs for non-dioxin-like PCBs quartiles are 1.0, 1.6, 2.6, and 2.5. p for trends = 0.02. Men: no clear association | [73] |
| Immunological/Thymus atrophy | 15 PCB congeners in neonates | Smaller thymus | [75] |
| Metabolism/Enzyme biomarker/ | PCBs exposures via food (serum PCB concentrations) | Positive association with the serum levels of 9 PCB congeners | [76] |
| Musculoskeletal | This is part of the study of Swedish fisherman’s wives | No association found between PCB-153 and OH-PCBs and bone mineral density or biochemical markers of bone metabolism | [77] |
| Musculoskeletal/Bone mineral density (BMD) | Swedish fishermen and their wives | After adjustment for age and body mass index, the significant negative relationship between PCB-153 and BMD was not valid anymore | [78] |
| Musculoskeletal/Bone mineral density (BMD) | 5 dioxin-like PCBs and 3 non-dioxin- like PCBs blood levels. | Male odds ratio negatively associated with BMD 1.6 (95% CI, 1.01–1.2) per 10 pg/mL CB-118 | [79] |
| Musculoskeletal/Bone mineral density | Persistent organochlorines (PCBs, DDT) | PCBs were not associated with significant effects on bone density | [80] |
| Neurological/Neurodegenerative diseases. | PCB levels of workers were about 10 times higher than the PCB levels in community | Overall no significant effects (SMR = 1.40, 1.11, and 1.26, respectively. Women’s amyotrophic lateral (SMR = 2.26; 95% CI, 1.08–4.15) | [81] |
| Reproductive/Time to menopause | Halogenated biphenyl (PCBs, PBB) blood samples | No association with either PCBs or PBB | [82] |
| Species | Study Designs | Health Effects (Findings) | Reference |
|---|---|---|---|
| Rats (Sprague-Dawley) | Aroclor 1221 (0, 0.1, 1, or 10 mg/kg). In utero exposed female offspring (F1) and (F2); Gd 16 and 18 | In both generations, litter sex ratio was skewed toward females | [84] |
| Mice (CD-1) | Aroclor 1016, fed 50 µg/kg/d; Gd 16–18; offspring examined at D3, D21, and D60 | Increase prostate size, anogenital distance, decrease epididymal weight | [85] |
| Rats | Diets containing 0, 5, 20, or 40 mg PCBs/kg diet; exposure started 50 days before mating and terminated at birth | Reduced 1,25-dihydroxycholecalciferol during pregnancy | [86] |
| Rats (Sprague-Dawley) | 2 years, Gavage, PCB 126. Control corn oil-acetone vehicle | Cytoplasmic vacuolation, chronic active inflammation, atrophy in exocrine pancreas | [87] |
| Rats (Sprague-Dawley) | 125 ppm Aroclor 1254 in diet, pregnant rats | Reduce growth of hippocampal intra-and infrapyramidal (II-P) mossy fiber | [88] |
| Rats (22–24/dose) clarify | 0 or 6 mg/kg A1254 (p.o. in corn oil) GD6-PND 21. Cross-fostered the offspring resulted in 4 groups (ctrl/ctrl; A1254/A/1254 perinatal exposure; A1254/ctrl, prenatal exposure only; ctrl/A1254, postnatal exposure only | Permanent hearing deficits in A1254/A1254 and ctrl/A1254 groups | [45] |
| Rats (Sprague-Dawley) | Females, gavage exposure to PCB 126 | Bronchiolar metaplasia | [89] |
| Adult male rats | A1254 (diet) (30 mg/kg/day for 15 days) | Dehydrated PCB-fed rats had 863% increase in plasma vasopressin (VP); for the dehydrated control, a 241% increase in VP | [90] |
| Rats (Sprague-Dawley) | Single dose Gavage of 375 ug PCB 118/kg on GD 6 | Hyperactivity and smaller testes, epididymides, seminal vesicles, decrease in sperm and spermatid numbers in offspring on PND 170. | [91] |
| Rats (females) | 40 rats exposed to PCB 126 alone, vehicle, ovariectomy, or sham operation (2 × 2 factorial design) for 12 weeks | PCB 126 increases heart weight and serum cholesterol in both groups. PCB 126 increases blood pressure in sham-operated rats only. | [92] |
| Goat (kids) | Goat kids exposed to PCB 153 and PCB 126 during gestation and lactation. The average PCB concentrations in goat kids’ fat at age of 9 months were 5800 ng/g and 0.49 ng/g fat weight for PCB 153 and PCB 126 respectively. | At puberty, low LH, delayed puberty, higher progesterone level in the group exposed to PCB 153. PCB 126 has no effect at these levels | [93] |
| Pregnant Rats | 0, 0.5, and 5.0 mg/kg bt of 4-OH-CB107 or Aroclor 1254 (25 mg/kg bt) during GD 10-GD16 | At 0.5 and 5.0 mg of 4-OH-PCB 107 a significant prolongation of the estrous. A 50% increase in plasma estradiol levels in female offspring in animals treated with 5 mg 4-OH-CB107/kg body weight. Aroclor 1254 treatment had no significant effects on estradiol levels. | [94] |
| Female Rats (Sprague-Dawley) | Binary mixture of 1000 ng/kg PCB 126 + 1000 ng/kg PCB 153; PCB 126 = PCB 118 (216 and 360 ng TCDD equivalent/kg) | Hyperplasia of respiratory epithelium and metaplasia of olfactory epithelium, acute inflammatory exudates observed within the lumen of nasal cavity on the affected area | [87] |
| Long-Evans 5 day Pregnant Rats | 2 or 4 mg/kg/subcutaneous injection of PCB 77 on GD 6–18 | Nursing time was reduced in both treatments. At 4 mg/kg body weight, the amount of licking time and pup mortality were increased | [95] |
| Rats (Sprague-Dawley) | PCB 126 + PCB 153; PCB 126 + PCB 118; PCB 126 alone; PCB 153 alone; TCDD + PCB 126 + PeCDF; By Gavage/2 years | In all mixtures, the incidences of gingival squamous cell hyperplasia were increased significantly. In TCCD, PCB 126, and PCB 126 + PCB 153 treated groups, squamous cell carcinoma increased significantly. | [96] |
| Pregnant Rat | 2 mg/kg PCB 77 Gd 6–18 and gestation | Increase frequency of nursing bouts and amount of maternal auto-grooming | [97] |
| Rats | PCBs on maternal odor conditioning in rat pups 12–14 days of age | Significantly depressed the preference for the maternal-associated cue, but did not impair discrimination for a novel odor | [46] |
| Rats | Pregnant rats administered single doses of PCB 132 at 1 or 10 mg/kg on a gestational day 15. Male offspring were assessed on postnatal day 84 | Decreased cauda epididymal weight, epididymal sperm count, and motile epididymal sperm count in adult offspring. The spermatozoa of PCB 132-exposed offspring produced significantly higher levels of ROS than the controls. Low-dose PCB 132 group, p53 was significantly induced and caspase-3 was inhibited. High-dose group, activation of caspase-3 and -9 was significantly increased, while the expressions of Fas, Bax, bcl-2, and p53 genes were significantly decreased. | [98] |
| Rats | Noncoplanar PCBs were fed to rat dams during gestation and throughout three subsequent nursing weeks | Abnormal development of the primary auditory cortex (A1) | [99] |
| Female Goat | Goat dams were orally dosed with PCB 153 in corn oil (98 µg/kg body wt/day) or PCB 126 (49 ng/kg body wt/day) from day 60 of gestation until delivery. The offspring were exposed to PCB in utero and through maternal milk. The suckling period lasted for 6 weeks. | Perinatal exposure to PCB 153, but not PCB 126, resulted in altered bone composition in female goat offspring | [100] |
| Female Rats (Sprague-Dawley) | Daily oral administration of vehicle (corn oil) or 1 or 3 μg/kg of PCB-126 from 2 weeks prior to mating with intact males until 20 days after delivery, examined from birth until puberty | Direct effect on the ovary and adverse effects female puberty by altering the morphological and functional development of the female reproductive system | [101] |
| Adult Female Rats (Sprague-Dawley) | Prenatal exposure to the PCB mixture Aroclor 1221 on adult female | Mating trial pacing, vocalizations, ambulation, and the female’s likelihood to mate. Were these impaired? | [102] |
| Female Mice (C57BL/6) | Temporal analysis, mice were orally gavaged with PCB126 or sesame oil as the vehicle and sacrificed after 2, 4, 8, 12, 18, 24, 72, 120, or 168 h. In the dose-response study, mice were gavaged with 0.3, 1, 3, 10, 30, 100, 300, 1000 μg/kg PCB126, 30 or 100 μg/kg TCDD and sacrificed after 72 h | 251 and 367 genes were differentially expressed by PCB 126 at one or more time points or doses, respectively, significantly less than elicited by TCDD. At 300 μg/kg PCB 126 elicited a subset of weaker effects compared with 30 μg/kg TCDD in immature, ovariectomized C57BL/6 mice. | [103] |
| Rats | Pregnant rats were treated orally with PCB 126 at a dose of 30 µg/kg or corn oil, its vehicle, on gestational day 15, and their male offspring were subjected to locomotor activity and anxiety-related test, social interaction, and rotating test at 4–5 weeks old | % time spent in the center, social interaction time, and the number of rearing were significantly reduced in PCB treated group | [104] |
| Female Rats | Pre- and/or postnatal exposure to PCB 77. Pregnant rats were treated with oil or PCB dissolved in oil (2 mg/kg b.w.) on gestation days 6–18 and then given pups that had been exposed to either the oil vehicle or PCB during gestation. Female offspring were monitored until adulthood. | None of the treatments (preferred to exposed) affected female sexual behavior | [105] |
| Adult Rats | PCB mixture Aroclor 1254 (A1254) at 0.1 or 1 mg/kg/day in the maternal diet throughout gestation and lactation. Focal cerebral ischemia was induced at 6–8 weeks of age via middle cerebral artery occlusion, and infarct size was measured in the cerebral cortex and striatum at 22 hr of reperfusion. | Significantly decreased striatal infarct in females and males at 0.1 and 1 mg/kg/day, respectively. Effects of developmental A1254 exposure on Bcl2 and Cyp2C11 expression did not correlate with effects on infarct volume. | [106] |
| Mice | Single gavage dose (150 µmol/kg body weight) of PCB 77, PCB 104, PCB 153 (as a mixture) | Induction of pro-inflammatory mediators in livers, lungs, and brains. The strongest expression of pro-inflammatory proteins occurred 24 h following the PCB administration independent of the class of PCB. | [107] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hens, B.; Hens, L. Persistent Threats by Persistent Pollutants: Chemical Nature, Concerns and Future Policy Regarding PCBs—What Are We Heading For? Toxics 2018, 6, 1. https://doi.org/10.3390/toxics6010001
Hens B, Hens L. Persistent Threats by Persistent Pollutants: Chemical Nature, Concerns and Future Policy Regarding PCBs—What Are We Heading For? Toxics. 2018; 6(1):1. https://doi.org/10.3390/toxics6010001
Chicago/Turabian StyleHens, Bart, and Luc Hens. 2018. "Persistent Threats by Persistent Pollutants: Chemical Nature, Concerns and Future Policy Regarding PCBs—What Are We Heading For?" Toxics 6, no. 1: 1. https://doi.org/10.3390/toxics6010001
APA StyleHens, B., & Hens, L. (2018). Persistent Threats by Persistent Pollutants: Chemical Nature, Concerns and Future Policy Regarding PCBs—What Are We Heading For? Toxics, 6(1), 1. https://doi.org/10.3390/toxics6010001






