The Uptake and Translocation of Lead, Chromium, Cadmium, and Zinc by Tomato Plants Grown in Nutrient and Contaminated Nutrient Solutions: Implications for Food Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Materials
2.3. Experimental Conditions
2.4. Experimental Design
2.5. Analytical Procedures for the Determination of Elements in Nutrient Solutions and Plant Samples
2.6. Sample Preparation and Analysis for LA-ICP-MS Elemental Mapping
2.7. Calculation of the Translocation Factors
2.8. Dietary Exposure and Health Risk Assessment
3. Results and Discussion
3.1. Accuracy Check, Limits of Detection, and Limits of Quantification
3.2. Concentrations of Pb, Cr, Cd, and Zn in the Nutrient and Contaminated Nutrient Solutions During the Experiment
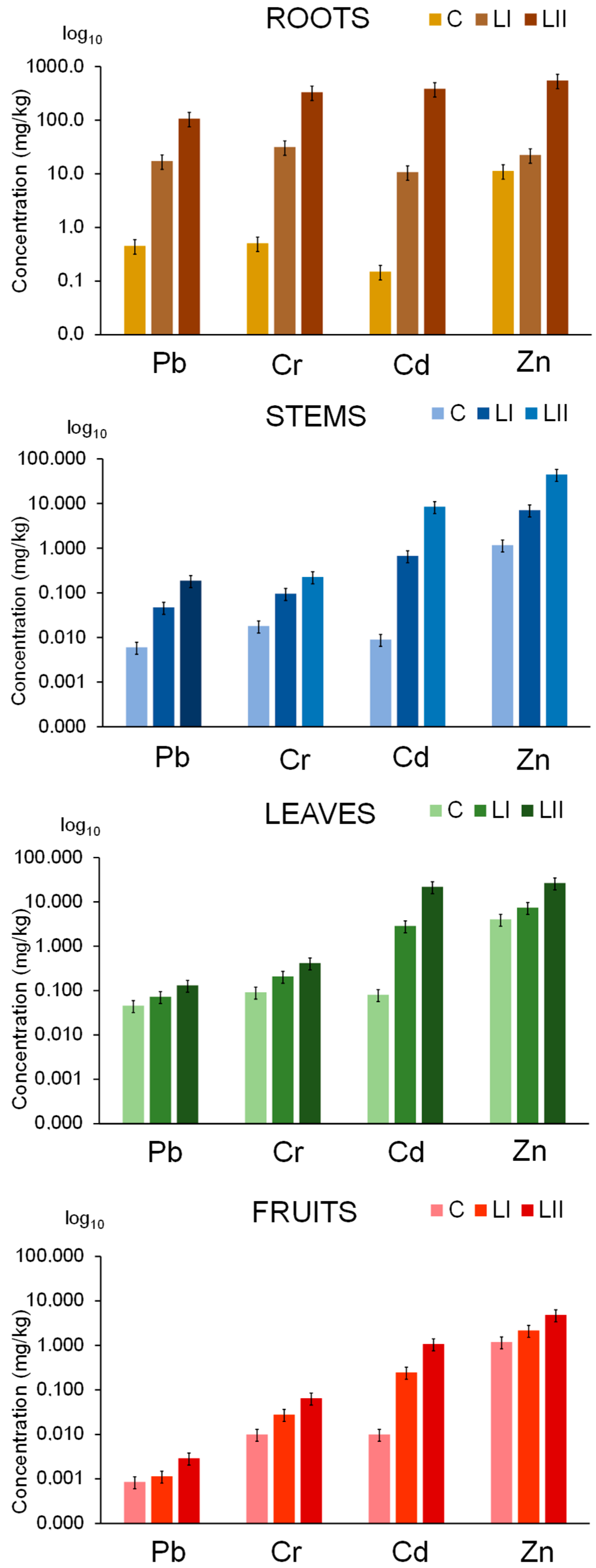
3.3. Uptake of Pb, Cr, Cd, and Zn in Tomato Plant from Nutrient and Contaminated Nutrient Solutions
3.4. Translocation of Pb, Cr, Cd, and Zn from the Roots to the Aerial Parts of the Tomato Plant
3.5. Health Risk Assessment
3.6. The Influence of Added Pb, Cr, Cd, and Zn Contaminants in the Nutrient Solution on the Uptake of Essential Elements in Tomato Plants
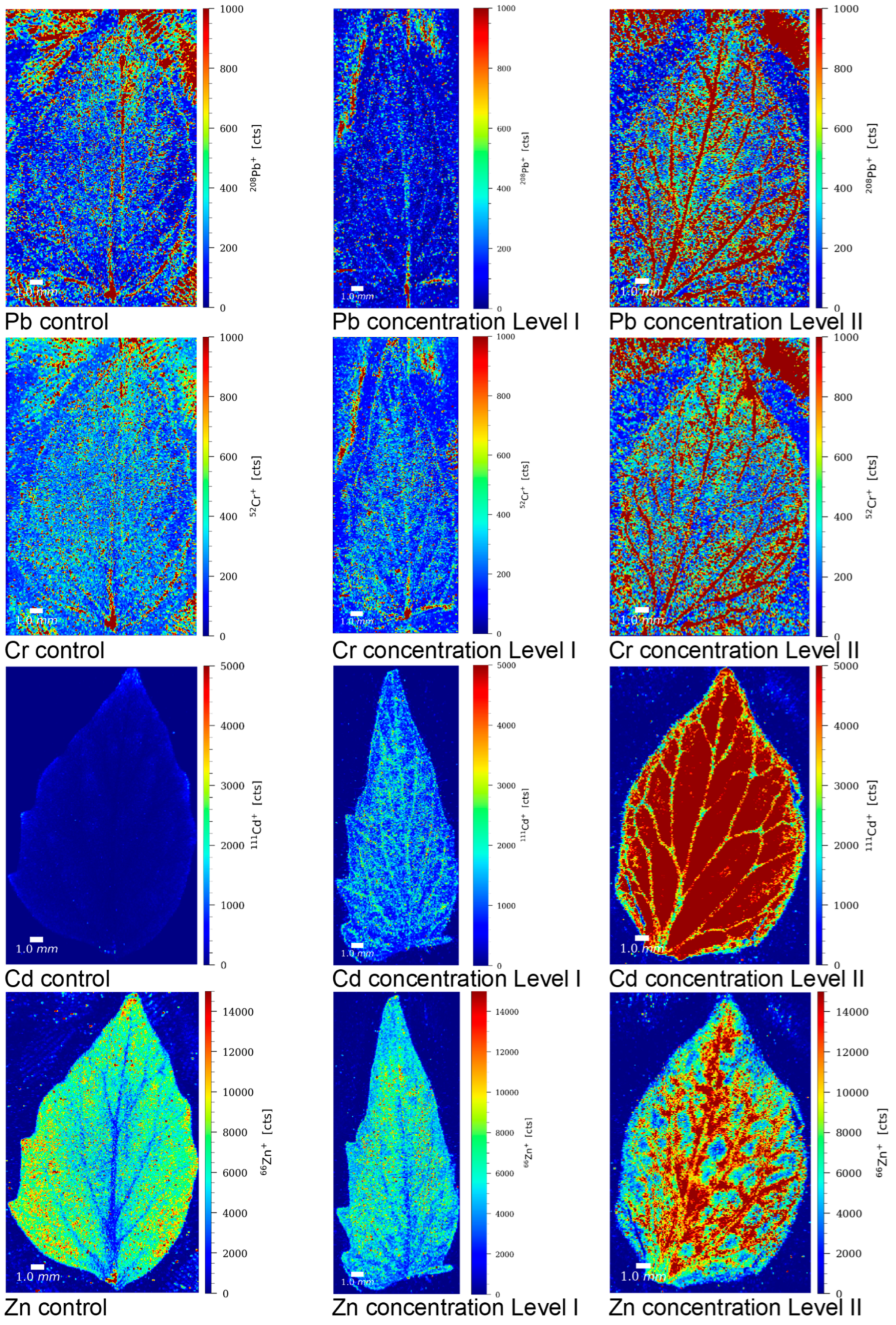
3.7. Imaging of Pb, Cr, Cd, and Zn in Tomato Leaves by LA-ICP-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarekegn, M.M.; Weldekidan, G.L. Concentration levels of heavy metals and selected ions in the irrigation water: The case of Little Akaki River, Addis Ababa, Ethiopia. In Environmental Impact and Remediation of Heavy Metals; Saleh, H.M., Hassan, A.I., Eds.; IntechOpen: London, UK, 2022; pp. 1–19. ISBN 978-1-80355-526-3. [Google Scholar] [CrossRef]
- Sharafi, K.; Mansouri, B.; Omer, A.K.; Bashardoust, P.; Ebrahimzadeh, G.; Sharifi, S.; Massahi, T.; Soleimani, H. Investigation of health risk assessment and the effect of various irrigation water on the accumulation of toxic metals in the most widely consumed vegetables in Iran. Sci. Rep. 2022, 12, 20806. [Google Scholar] [CrossRef]
- Abdulateef, A.A.; Naser, K.M. A study of irrigation water pollution by some heavy metals in Baghdad governorate. Conf. Ser. Earth Environ. Sci. 2021, 910, 012091. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Guo, S.; Zhao, M. Cadmium concentration and its typical input and output fluxes in agricultural soil downstream of a heavy metal sewage irrigation area. J. Hazard. Mater. 2021, 412, 25203. [Google Scholar] [CrossRef]
- Berihun, B.T.; Amare, D.E.; Raju, R.P.; Ayele, D.T.; Dagne, H. Determination of the level of metallic contamination in irrigation vegetables, the soil, and the water in Gondar city, Ethiopia. Nutr. Diet. Suppl. 2021, 13, 1–7. [Google Scholar] [CrossRef]
- Ahmed, M.; Matsumoto, M.; Ozaki, A.; Van Thinh, N.; Kurosawa, K. Heavy metal contamination of irrigation water, soil, and vegetables and the difference between dry and wet seasons near a multi-industry zone in Bangladesh. Water 2019, 11, 583. [Google Scholar] [CrossRef]
- Ferreira Camargo, M.A.; Aparecida Ribeiro Pereira, T. Evaluation of concentrations of heavy metals in water used in agricultural irrigation. Open Access Libr. 2018, 5, e4959. [Google Scholar] [CrossRef]
- Kovačič, A.; Andreasidou, E.; Brus, A.; Vehar, A.; Potočnik, D.; Jagodic Hudobivnik, M.; Heath, D.; Pintar, M.; Kacjan Maršič, N.; Ogrinc, N.; et al. Contaminant uptake in wastewater irrigated tomatoes. J. Hazard. Mater. 2023, 448, 130964. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Farhat, F.; Tariq, A.; Malik, Z.; Azis, R.B.; Kamran, M.; Elsharkawy, M.M.; Al-Hashimi, A.; Elshikh, M.S. Genetic behavior of tomato (Solanum lycopersicum L.) germplasm governing heavy metal tolerance and yield traits under wastewater irrigation. Plants 2022, 11, 2973. [Google Scholar] [CrossRef] [PubMed]
- Gashaye, D. Wastewater-irrigated urban vegetable farming in Ethiopia: A review on their potential contamination and health effects. Cogent Food Agric. 2020, 6, 1772629. [Google Scholar] [CrossRef]
- El-Azeem Ahmed, D.A.; Slima, D.F.; Al-Yasi, H.M.; Hassan, L.M.; Galal, T.M. Risk assessment of trace metals in Solanum lycopersicum L. (tomato) grown under wastewater irrigation conditions. Environ. Sci. Pollut. Res. 2023, 30, 42255–42266. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity, Rewiev. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Lauria, G.; Sinicropi, M.S.; Genchi, G. Lead toxicity, antioxidant defense and environment. Rev. Environ. Contam. Toxicol. 2015, 238, 45–67. [Google Scholar] [CrossRef]
- Prakash, S.; Kumar Verma, A. Arsenic: Its toxicity and impact on human health. Int. J. Biol. 2021, 3, 38–47. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Shreeya Bali, A.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Sahito, Z.A.; Zehra, A.; Yu, S.; Chen, S.; He, Z.; Yang, X. Chinese sapindaceous tree species (Sapindus mukorosii) exhibits lead tolerance and long-term phytoremediation potential for moderately contaminated soils. Chemosphere 2023, 338, 139376. [Google Scholar] [CrossRef]
- Lukina, A.O.; Boutin, C.; Rowland, O.; Carpenter, D.J. Evaluating trivalent chromium toxicity on wild terrestrial and wetland plants. Chemosphere 2016, 162, 355–364. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants, Darwin review. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; McDonald, L.M.; Roy, M.; Lanzirotti, A.; Myneni, S.C.B. Uptake and speciation of zinc in edible plants grown in smelter contaminated soils. PLoS ONE 2020, 17, e0226180. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.E.A. New insights about cadmium impacts on tomato: Plant acclimation, nutritional changes, fruit quality and yield. Food Energy Secur. 2018, 7, e00131. [Google Scholar] [CrossRef]
- Akinci, I.E.; Akinci, S.; Kadir Yilmaz, K. Response of tomato (Solanum lycopersicum L.) to lead toxicity: Growth, element uptake, chlorophyll and water content. Afr. J. Agric. Res. 2010, 5, 416–423. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, H.; Jang, T. Irrigation Water Quality Standards for indirect wastewater reuse in agriculture: A contribution toward sustainable wastewater reuse in South Korea. Water 2016, 8, 169. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; incorporating the 1st addendum; IWA Publishing: London, UK, 2019; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 25 August 2025).
- WHO/SDE/WSH/03.04/17. Zinc in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2003. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/zinc.pdf?sfvrsn=9529d066_4 (accessed on 25 August 2025).
- Raza, B.; Hameed, A.; Saleem, M.Y. Fruit nutritional composition, antioxidant and biochemical profiling of diverse tomato (Solanum lycopersicum L.) genetic resource. Front. Plant Sci. 2022, 13, 1035163. [Google Scholar] [CrossRef]
- Bressy, F.C.; Brito, G.B.; Barbosa, I.S.; Teixeira, L.S.G.; Korn, M.G.A. Determination of trace element concentrations in tomato samples at different stages of maturation by ICP OES and ICP-MS following microwave-assisted digestion. Microchem. J. 2013, 109, 145–149. [Google Scholar] [CrossRef]
- Adesina, F.P.; Akinnifesi, O.J.; Akinyemi, M.I.; Adesina, T.F. Occurrence and uptake of heavy metals in soil and plant (tomato) associated with crude oil contamination. Trends Sci. 2022, 19, 5684. [Google Scholar] [CrossRef]
- Salem, N.M.; Albanna, L.S.; Awwad, A.M. Toxic heavy metals accumulation in tomato plant (Solanum lycopersicum). J. Agric. Biol. Sci. 2016, 11, 399–404. [Google Scholar]
- Trebolazabala, J.; Maguregui, M.; Morillas, H.; García-Fernandez, Z.; de Diego, A.; Madariaga, J.M. Uptake of metals by tomato plants (Solanum lycopersicum) and distribution inside the plant: Field experiments in Biscay (Basque Country). J. Food Compos. Anal. 2017, 59, 161–169. [Google Scholar] [CrossRef]
- Fatoba, P.O.; Adepoju, A.O.; Okewole, G.A. Heavy metal accumulation in the fruits of tomato and okra irrigated with industrial waste effluents. J. Ind. Pollut. Control 2012, 28, 103–107. [Google Scholar]
- Anagaw, M.; Amare Zereffa, A.; Firmechale, D.; Murthy, A. Determination of heavy metals in tomato and its support soil samples from horticulture and floriculture industrial area, Ziway, Ethiopia. Res. Dev. Material Sci. 2019, 10, RDMS.000729.2019. [Google Scholar] [CrossRef]
- Somda, M.K.; Kabore, D.; Mogmenga, I.; Ouattara, C.A.T.; Ouattara, A.; Dabire, Y.; Nikiema, M.; Mihin, H.B.; Akakpo, A.Y.; Ouedraogo, Q.; et al. Health risk assessment of heavy metals and microbial quality of local tomato (Solanum lycopersicum) of Ouagadougou, Burkina Faso. J. Environ. Prot. 2019, 10, 942–957. [Google Scholar] [CrossRef]
- Sulaiman, M.B.; Maigari, I.A.; Yahaya, Y. Health risk assessment of heavy metals accumulation in tomatoes irrigation farms at Kwadon, Gombe, Nigeria. ATBU J. Sci. 2019, 7, 271–280. [Google Scholar]
- Tabassam, Q.; Ahmad, M.S.A.; Alvi, A.K.; Awais, M.; Kaushik, P.; El-Sheikh, M.A. Accumulation of different metals in tomato (Lycopersicon esculentum L.) fruits irrigated with wastewater. Appl. Sci. 2023, 13, 9711. [Google Scholar] [CrossRef]
- Yaradua, A.I.; Alhassan, A.J.; Nasir, A.; Bala, M.; Usman, A.; Idi, A.; Muhammad, I.U.; Yaro, S.A.; Muhammad, I. Heavy metal burden and evaluation of human health risks in tomato fruits cultivated in Katsina State, North West Nigeria. Asian Food Sci. J. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2021/1323; Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Certain Foodstuffs. Official Journal of the European Union: Luxembourg, 2021; L 288/13–L 288/18.
- Commission Regulation (EU) 2021/1317; Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Official Journal of the European Union: Luxembourg, 2021; L 286/1–L 286/4.
- Nguyen, N.T.; McInturf, S.A.; Mendoza-Cózatl, D.G. Hydroponics: A versatile system to study nutrient allocation and plant responses to nutrient availability and exposure to toxic elements. J. Vis. Exp. 2016, 113, e54317. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rahman, A.; Azam, H.; Kim, H.S.; Kwon, M.J. Characterizing nutrient uptake kinetics for efficient crop production during Solanum lycopersicum var. cerasiforme Alef. growth in a closed indoor hydroponic system. PLoS ONE 2017, 12, e0177041. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villegas, N.; Del Carmen Rivera-Cruz, M.; Cadenas-Pliego, G.; Juárez-Maldonado, A.; Meza-Figueroa, D.; González-Moscoso, M. SiO2 nanoparticles improve nutrient uptake in tomato plants developed in the presence of arsenic. Rev. Bio Cienc. 2021, 8, e1084. [Google Scholar] [CrossRef]
- López-Millán, A.-F.; Sagardoy, R.; Solanas, M.; Abadía, A.; Abadía, J. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ. Exp. Bot. 2009, 65, 376–385. [Google Scholar] [CrossRef]
- Degryse, F.; Smolders, E. Cadmium and nickel uptake by tomato and spinach seedlings: Plant or transport control? Environ. Chem. 2012, 9, 48–54. [Google Scholar] [CrossRef]
- Kumar, P.; Edelstein, M.; Cardarelli, M.; Ferri, E.; Colla, G. Grafting affects growth, yield, nutrient uptake, and partitioning under cadmium stress in tomato. HortScience 2015, 50, 1654–1661. [Google Scholar] [CrossRef]
- Azariz, L.; Elblidi, S.; Fekhaoui, M.; Yahyaoui, A. Uptake and accumulation of lead in Lycopersicon esculentum and Phaseolus vulgaris L. planted on organic hydroponics. Int. J. Environ. Anal. Chem. 2021, 101, 2242–2254. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Liu, S.; Shi, J.; Wang, J.; Dai, Y.; Li, H.; Li, J.; Liu, X.; Chen, X.; Wang, Z.; Zhang, P. Interactions Between Microplastics and Heavy Metals in Aquatic Environments: A Review. Front. Microbiol. 2021, 12, 652520. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manag. 2022, 302, 113995. [Google Scholar] [CrossRef]
- Chen, P.; Hsiao, M.; Xiao, L.; Liu, Z. Adsorption behavior of heavy metals onto microplastics derived from conventional and biodegradable commercial plastic products. Sci. Total Environ. 2024, 951, 175537. [Google Scholar] [CrossRef]
- Marković, S.; Levstek, L.; Žigon, D.; Ščančar, J.; Milačič, R. Speciation and bio-imaging of chromium in taraxacum officinale using HPLC post-column ID-ICP-MS, high resolution MS and laser ablation ICP-MS techniques. Front. Chem. 2022, 10, 863387. [Google Scholar] [CrossRef] [PubMed]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, 8th ed.; CRC Press: Boca Raton, FL, USA, 2022; 642p. [Google Scholar]
- Kämtz, F.L. Uber die Ableitung mittlerer Resultate aus meteorologischen Beobachtungen. Repert. Für Meteorol. 1860, 1, 107–134. [Google Scholar]
- Dorais, M. The use of supplemental lighting for vegetable crop production: Light intensity, crop response, nutrition, crop management, cultural practices. In Proceedings of the Canadian Greenhouse Conference, Niagara Falls, ON, Canada, 9 October 2003; Volume 9, pp. 115–133. [Google Scholar]
- World Health Organization (WHO). Guidelines for the Safe Use of Wastewater, Excreta and Greywater: Volume II–Wastewater Use in Agriculture; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Council of the European Communities. Council Directive 91/271/EEC of 21 May 1991 concerning urban wastewater treatment. Off. J. Eur. Communities 1991, 135, 40–52. [Google Scholar]
- Carnesecchi, E.; Mostrag, A.; Ciacci, A.; Roncaglioni, A.; Tarkhov, A.; Gibin, D.; Sartori, L.; Benfenati, E.; Yang, C.; Dorne, J.L.C.M. OpenFoodTox: EFSA’s Chemical Hazards Database (Version 6); Zenodo: Meyrin, Switzerland, 2023. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. The release of root exudates as affected by the plant nutrient status. J. Plant Nutr. Soil Sci. 2007, 170, 299–311. [Google Scholar] [CrossRef]
- Nimal Perera, W.; Hefter, G.; Sipos, P.M. An Investigation of the lead(II)-hydroxide system. Inorg. Chem. 2001, 40, 3974–3978. [Google Scholar] [CrossRef]
- Metze, D.; Jakubowski, N.; Klockow, D. Handbook of Elemental Speciation II–Species in the Environment, Food, Medicine and Occupational Health. Speciation of Chromium in Environment and Food, ed.; Cornelis, R., Caruso, J., Crews, H., Heumann, K., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 120–135. [Google Scholar]
- Seregin, I.V.; Kozhevnikova, A.D. The Role of low-molecular-weight organic acids in metal homeostasis in plants. Int. J. Mol. Sci. 2024, 25, 9542. [Google Scholar] [CrossRef]
- Sinha, A.; Basak, A.; Mondol, S.A. Understanding heavy metal accumulation in crops: Sources, plant responses, tolerance mechanisms, and environmental effects. J. Environ. Sci. Health C Toxicol. Carcinog. 2025, 43, 269–294. [Google Scholar] [CrossRef]
- Gupta, M.; Dwivedi, V.; Kumar, S.; Patel, A.; Niazie, P.; Kumar Yadav, V. Lead toxicity in plants: Mechanistic insights into toxicity, physiological responses of plants and mitigation strategies, Review. Plant Signal Behav. 2024, 19, e2365576. [Google Scholar] [CrossRef] [PubMed]
- Melamed, R.; Cao, X.; Chen, M.; Ma, L.Q. Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci. Total Environ. 2003, 305, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for chromium. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- National Institutes of Health, Office of Dietary Supplements. Zinc–Fact Sheet for Consumers. 2023. Available online: https://ods.od.nih.gov/factsheets/Zinc-Consumer/ (accessed on 14 August 2025).
- Ma, L.; Liu, Y.; Sahito, Z.A.; Liu, C.; Li, Z.; Yu, C.; Feng, Y.; Guo, W. Intraspecific variation in tomato: Impact on production quality and cadmium phytoremediation efficiency in intercropping systems with hyperaccumulating plant. Ecotoxicol. Environ. Saf. 2024, 282, 116715. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Singh, N.K.; Patel, M. Tiwari, M.R.; Rai, U.N. Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol. Environ. Saf. 2011, 74, 1670–1677. [Google Scholar] [CrossRef] [PubMed]


| 1st sampling at the start of the experiment | ||||||
| Samples | Pb (ng/mL) | Cr (ng/mL) | Cd (ng/mL) | Zn (ng/mL) | pH | The volume (L) of nutrient solution or contaminated nutrient solutions added at the start of the experiment |
| C-1a | 0.622 | 0.519 | 0.283 | 20.7 | 7.1 | 40 |
| LI-1a | 90.7 | 107 | 54.0 | 125 | 6.7 | 40 |
| LII-1a | 541 | 1166 | 591 | 1144 | 6.7 | 40 |
| 2nd sampling after 7 days | ||||||
| Samples | Pb (ng/mL) | Cr (ng/mL) | Cd (ng/mL) | Zn (ng/mL) | pH | The volume (L) of nutrient solution or contaminated nutrient solutions replenished to 40 L |
| C-2a | / | / | / | / | 8.0 | 2.0 |
| C-2b | / | / | / | / | 7.0 | 2.0 |
| LI- 2a | 1.25 | 0.594 | 32.7 | 50.4 | 7.5 | 2.0 |
| LI-2b | 6.2 | 6.11 | 34.6 | 56.2 | 7.3 | 2.0 |
| LII- 2a | 0.71 | 0.72 | 74.9 | 71.3 | 7.5 | 2.0 |
| LII-2b | 21.7 | 53.3 | 104 | 130 | 7.3 | 2.0 |
| 3rd sampling after 14 days | ||||||
| Samples | Pb (ng/mL) | Cr (ng/mL) | Cd (ng/mL) | Zn (ng/mL) | pH | The volume (L) of nutrient solution or contaminated nutrient solutions replenished to 40 L |
| C-3a | / | / | / | / | 7.7 | 10 |
| C-3b | / | / | / | / | 7.0 | 10 |
| LI-3a | 2.14 | 0.705 | 35.7 | 59.7 | 7.3 | 10 |
| LI-3b | 27.4 | 32.7 | 40.7 | 72.4 | 6.8 | 10 |
| LII-3a | 2.10 | 1.19 | 82.4 | 105 | 7.2 | 10 |
| LII-3b | 101 | 301 | 192 | 298 | 6.7 | 10 |
| 4th sampling after 21 days | ||||||
| Samples | Pb (ng/mL) | Cr (ng/mL) | Cd (ng/mL) | Zn (ng/mL) | pH | The volume (L) of nutrient solution or contaminated nutrient solutions replenished to 40 L |
| C-4a | / | / | / | / | 7.4 | 15 |
| C-4b | / | / | / | / | 7.1 | 15 |
| LI-4a | 12.4 | 0.896 | 41.6 | 75.0 | 7.3 | 15 |
| LI-4b | 36.9 | 47.1 | 43.0 | 83.7 | 6.6 | 15 |
| LII-4a | 17.6 | 12.3 | 119 | 151 | 7.1 | 15 |
| LII-4b | 88.7 | 320 | 233 | 315 | 6.8 | 15 |
| 5th sampling after 28 days | ||||||
| Samples | Pb (ng/mL) | Cr (ng/mL) | Cd (ng/mL) | Zn (ng/mL) | pH | The volume (L) of nutrient solution or contaminated nutrient solutions at the end of the experiment |
| C-5a | / | / | / | / | 7.5 | 25 |
| C-5b | / | / | / | / | 6.7 | 25 |
| LI- 5a | 47.3 | 6.5 | 119 | 339 | 7.9 | 25 |
| LI- 5b | 59.5 | 45.1 | 61.0 | 161 | 6.9 | 25 |
| LII-5a | 64.3 | 34.3 | 262 | 547 | 8.1 | 25 |
| LII-5b | 207 | 324 | 351 | 839 | 6.9 | 25 |
| 6th sampling after 35 days at the end of the experiment | ||||||
| Samples | Pb (ng/mL) | Cr (ng/mL) | Cd (ng/mL) | Zn (ng/mL) | pH | The volume (L) of nutrient solution or contaminated nutrient solutions at the end of the experiment |
| C-6a | 0.413 | 0.191 | 1.23 | 48.7 | 7.4 | 17 |
| LI- 6a | 35.4 | 4.60 | 78.2 | 159 | 7.7 | 17 |
| LII-6a | 42.6 | 32.4 | 178 | 541 | 7.8 | 17 |
| Concentration Level | Pb | ||
|---|---|---|---|
| TFroot → stem | TFroot → leaf | TFroot → fruit | |
| C | 0.083 | 0.326 | 0.014 |
| LI | 0.010 | 0.010 | 0.0004 |
| LII | 0.0042 | 0.0023 | 0.0002 |
| Cr | |||
| C | 0.141 | 0.398 | 0.145 |
| LI | 0.0081 | 0.016 | 0.0086 |
| LII | 0.0017 | 0.0023 | 0.0014 |
| Cd | |||
| C | 0.355 | 1.81 | 0.738 |
| LI | 0.257 | 0.691 | 0.217 |
| LII | 0.054 | 0.109 | 0.020 |
| Zn | |||
| C | 0.450 | 0.932 | 1.04 |
| LI | 1.34 | 0.850 | 0.938 |
| LII | 0.198 | 0.092 | 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milačič Ščančar, R.; Kozlica, K.; Marković, S.; Leban, P.; Vidmar, J.; Heath, E.; Kacjan Maršić, N.; Železnikar, Š.; Ščančar, J. The Uptake and Translocation of Lead, Chromium, Cadmium, and Zinc by Tomato Plants Grown in Nutrient and Contaminated Nutrient Solutions: Implications for Food Safety. Toxics 2025, 13, 738. https://doi.org/10.3390/toxics13090738
Milačič Ščančar R, Kozlica K, Marković S, Leban P, Vidmar J, Heath E, Kacjan Maršić N, Železnikar Š, Ščančar J. The Uptake and Translocation of Lead, Chromium, Cadmium, and Zinc by Tomato Plants Grown in Nutrient and Contaminated Nutrient Solutions: Implications for Food Safety. Toxics. 2025; 13(9):738. https://doi.org/10.3390/toxics13090738
Chicago/Turabian StyleMilačič Ščančar, Radmila, Katarina Kozlica, Stefan Marković, Pia Leban, Janja Vidmar, Ester Heath, Nina Kacjan Maršić, Špela Železnikar, and Janez Ščančar. 2025. "The Uptake and Translocation of Lead, Chromium, Cadmium, and Zinc by Tomato Plants Grown in Nutrient and Contaminated Nutrient Solutions: Implications for Food Safety" Toxics 13, no. 9: 738. https://doi.org/10.3390/toxics13090738
APA StyleMilačič Ščančar, R., Kozlica, K., Marković, S., Leban, P., Vidmar, J., Heath, E., Kacjan Maršić, N., Železnikar, Š., & Ščančar, J. (2025). The Uptake and Translocation of Lead, Chromium, Cadmium, and Zinc by Tomato Plants Grown in Nutrient and Contaminated Nutrient Solutions: Implications for Food Safety. Toxics, 13(9), 738. https://doi.org/10.3390/toxics13090738






