Coffee By-Products Studied by the Planar Ames Bioassay with pH Indicator Endpoint Using the 2LabsToGo-Eco
Highlights
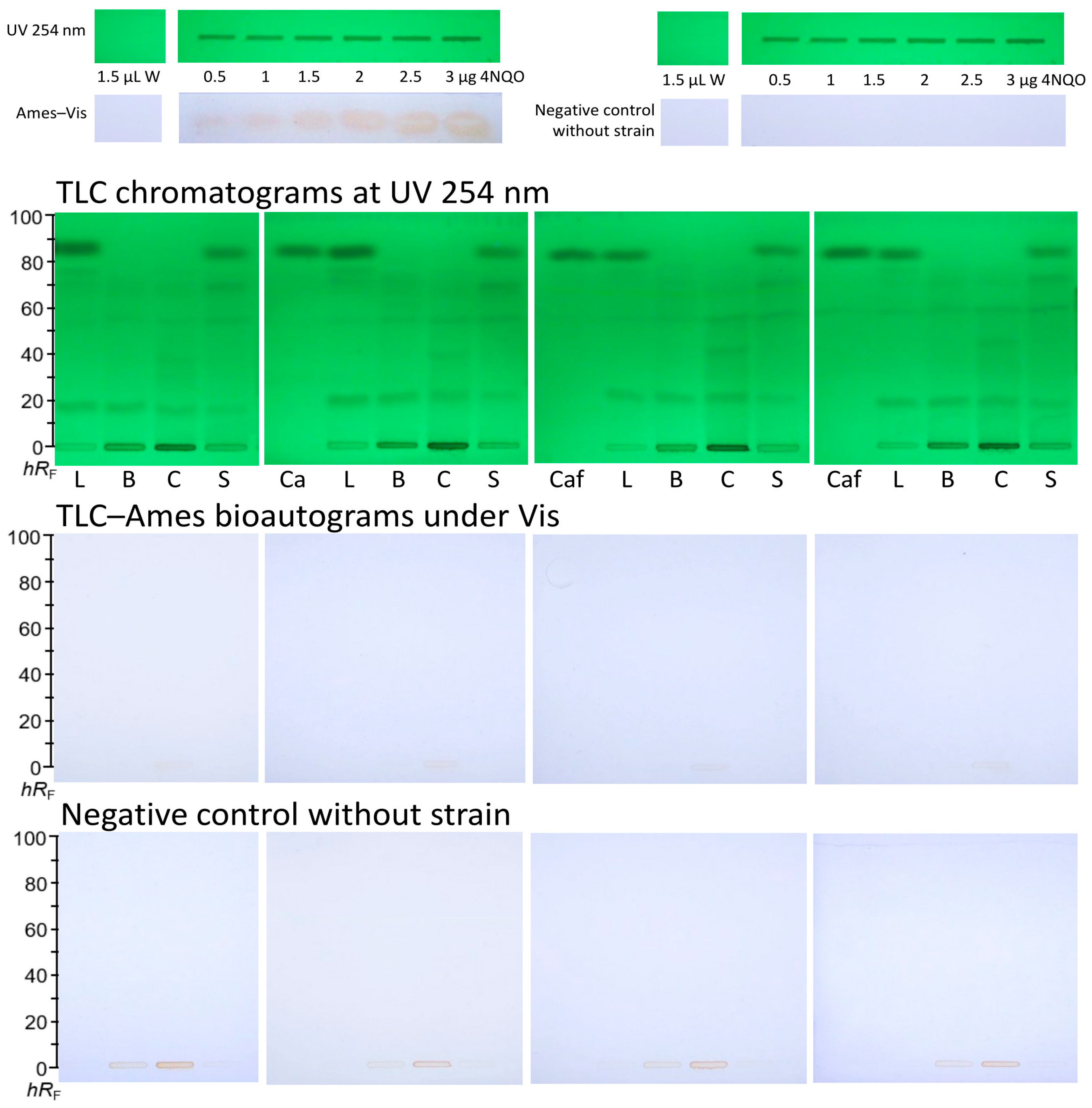
- Coffee by-products (leaves, blossoms, cherries, and silverskin) did not show mutagenic zones in the planar Ames bioassay with Salmonella Typhimurium TA98.
- Hot water extraction most effectively simulated human consumption conditions and yielded the highest number of detectable compounds.
- The 2LabsToGo-Eco system successfully performed TLC-coupled bioassays with good reproducibility and verified positive control responses.
- The findings provide additional safety data for Coffea leaves and blossoms, supporting their potential use as novel food ingredients.
- The portable 2LabsToGo-Eco technology offers a powerful, sustainable screening option for toxicological assessment of coffee by-products amid increasing valorization interest.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Extraction of Coffee By-Product Plant Materials
2.3. Preparation of the Solutions and of the Salmonella Suspension
2.4. 2LabsToGo-Eco Analysis
2.5. Planar Ames–Vis Bioassay
3. Results
3.1. Optimization of Extraction and Mobile Phase for TLC Analysis of Coffee By-Products
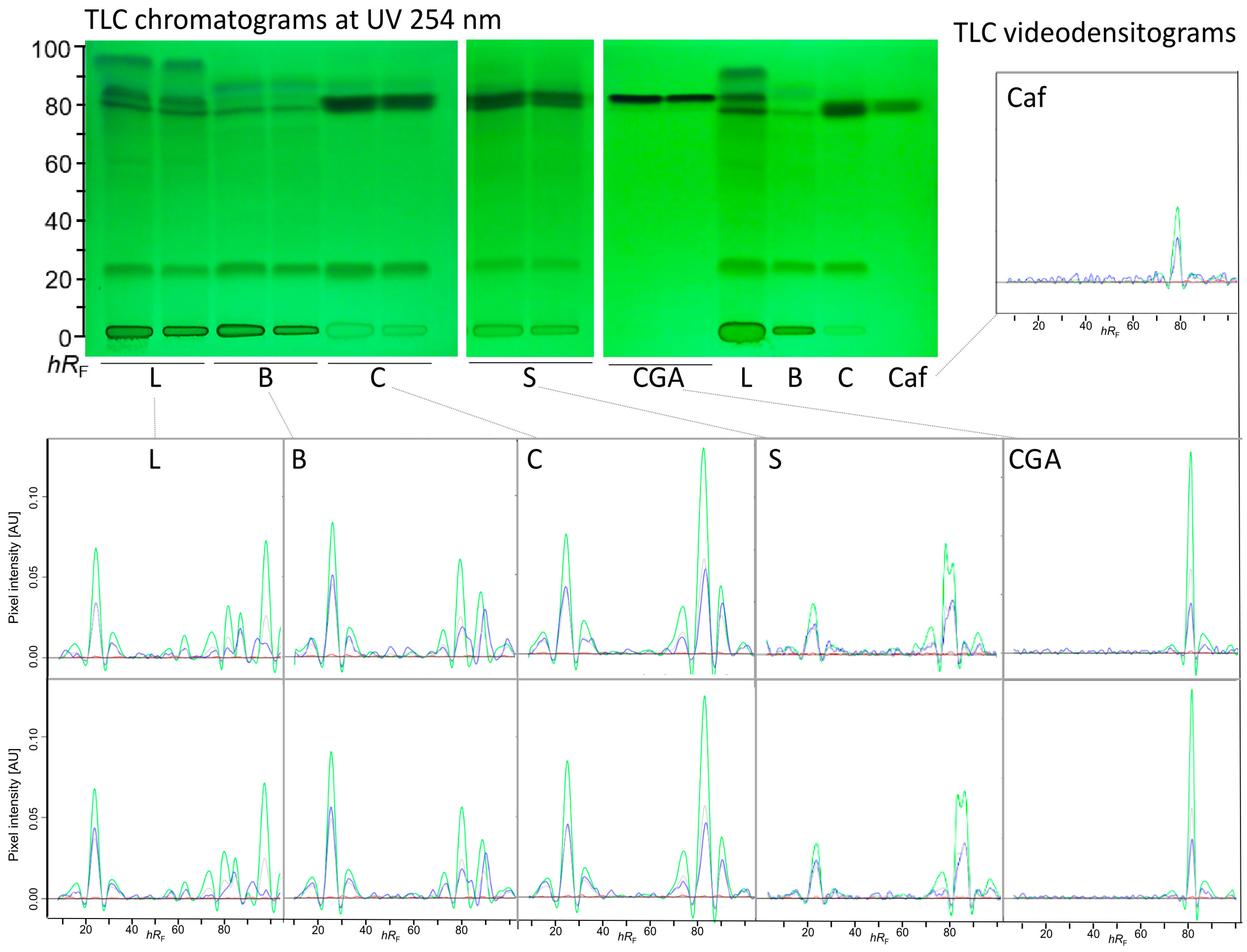
3.2. Repeatability of the 2LabsToGo-Eco Analysis
3.3. Reproducibility of the TLC–Ames–Vis Bioautograms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blinová, L.; Sirotiak, M.; Bartošová, A.; Soldán, M. Utilization of waste from coffee production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P. State of the Art in Coffee Processing By-Products. In Handbook of Coffee Processing by-Products; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 1–26. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting cork by-products to ecofriendly cork bioactive ingredients: Novel pharmaceutical and cosmetics applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Kull, A.-K.; Lachenmeier, D.W. Comprehensive Update on European Union Labeling Standards for Coffee and Its By-Products. Proceedings 2024, 109, 19. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies; Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Test No. 487: In Vitro Mammalian Cell Micronucleus Test; OECD Publishing: Paris, France, 2023; pp. 1–29. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Test No. 471: Bacterial Reverse Mutation Test; OECD Publishing: Paris, France, 2020; pp. 1–11. [Google Scholar] [CrossRef]
- Wexler, P.; Anderson, B.D. Encyclopedia of Toxicology; Academic Press: San Diego, CA, USA, 2005; pp. 46–48. [Google Scholar] [CrossRef]
- Kier, L.D. Use of the Ames test in toxicology. Regul. Toxicol. Pharmacol. 1985, 5, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Bjørseth, A.; Eidså, G.; Gether, J.; Landmark, L.; Møller, M. Detection of mutagens in complex samples by the Salmonella assay applied directly on thin-layer chromatography plates. Science 1982, 215, 87–89. [Google Scholar] [CrossRef]
- Schilter, B.; Burnett, K.; Eskes, C.; Geurts, L.; Jacquet, M.; Kirchnawy, C.; Oldring, P.; Pieper, G.; Pinter, E.; Tacker, M. Value and limitation of in vitro bioassays to support the application of the threshold of toxicological concern to prioritise unidentified chemicals in food contact materials. Food Addit. Contam. Part A 2019, 36, 1903–1936. [Google Scholar] [CrossRef]
- Rainer, B.; Mayrhofer, E.; Redl, M.; Dolak, I.; Mislivececk, D.; Czerny, T.; Kirchnawy, C.; Marin-Kuan, M.; Schilter, B.; Tacker, M. Mutagenicity assessment of food contact material migrates with the Ames MPF assay. Food Addit. Contam. Part A 2019, 36, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, K.; Lemme, J.; Morlock, G.E. Ames Assay Transferred from the Microtiter Plate to the Planar Assay Format. J. Xenobiotics 2025, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Monazzah, M.; Lachenmeier, D.W. Genotoxicity of Coffee, Coffee By-Products, and Coffee Bioactive Compounds: Contradictory Evidence from In Vitro Studies. Toxics 2025, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Albertini, S.; Friederich, U.; Schlatter, C.; Würgler, F.E. The influence of roasting procedure on the formation of mutagenic compounds in coffee. Food Chem. Toxicol. 1985, 23, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Shane, B.S.; Troxclair, A.M.; McMillin, D.J.; Henry, C.B. Comparative mutagenicity of nine brands of coffee to Salmonella Typhimurium TA100, TA102, and TA104. Environ. Mol. Mutagen. 1988, 11, 195–206. [Google Scholar] [CrossRef]
- Fujita, Y.; Wakabayashi, K.; Nagao, M.; Sugimura, T. Characteristics of major mutagenicity of instant coffee. Mutat. Res. Lett. 1985, 142, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-S.; Chen, P.-W.; Wang, J.-Y.; Kuo, T.-C. Assessment of Cellular Mutagenicity of Americano Coffees from Popular Coffee Chains. J. Food Prot. 2017, 80, 1489–1495. [Google Scholar] [CrossRef]
- Duarte, M.P.; Laires, A.; Gaspar, J.; Oliveira, J.S.; Rueff, J. Genotoxicity of instant coffee and of some phenolic compounds present in coffee upon nitrosation. Teratog. Carcinog. Mutagen. 2000, 20, 241–249. [Google Scholar] [CrossRef]
- Kato, T.; Hiramoto, K.; Kikugawa, K. Possible occurrence of new mutagens with the DNA breaking activity in coffee. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1994, 306, 9–17. [Google Scholar] [CrossRef]
- Bravo, J.; Arbillaga, L.; de Peña, M.P.; Cid, C. Antioxidant and genoprotective effects of spent coffee extracts in human cells. Food Chem. Toxicol. 2013, 60, 397–403. [Google Scholar] [CrossRef]
- Monente, C.; Bravo, J.; Vitas, A.I.; Arbillaga, L.; de Peña, M.P.; Cid, C. Coffee and spent coffee extracts protect against cell mutagens and inhibit growth of food-borne pathogen microorganisms. J. Funct. Foods 2015, 12, 365–374. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honório, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef]
- Heimbach, J.T.; Marone, P.A.; Hunter, J.M.; Nemzer, B.V.; Stanley, S.M.; Kennepohl, E. Safety studies on products from whole coffee fruit. Food Chem. Toxicol. 2010, 48, 2517–2525. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; Del Castillo, M.D.; Morales, P. Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef]
- Cabezudo, I.; Salazar, M.O.; Ramallo, I.A.; Le Furlan, R. Effect-directed analysis in food by thin-layer chromatography assays. Food Chem. 2022, 390, 132937. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. Chemical safety screening of products–better proactive. J. Chromatogr A 2025, 1752, 465946. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. Planar chromatographic super-hyphenations for rapid dereplication. Phytochem. Rev. 2025, 24, 1–12. [Google Scholar] [CrossRef]
- Morlock, G.E.; Heil, J.; Inarejos-Garcia, A.M.; Maeder, J. Effect-directed profiling of powdered tea extracts for catechins, theaflavins, flavonols and caffeine. Antioxidants 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-Garcia, A.M.; Heil, J.; Martorell, P.; Álvarez, B.; Llopis, S.; Helbig, I.; Liu, J.; Quebbeman, B.; Nemeth, T.; Holmgren, D. Effect-directed, chemical and taxonomic profiling of peppermint proprietary varieties and corresponding leaf extracts. Antioxidants 2023, 12, 476. [Google Scholar] [CrossRef]
- Morlock, G.E.; Heil, J. Fast unmasking hazards of safe perfumes. J. Chromatogr. A 2025, 1754, 465959. [Google Scholar] [CrossRef]
- Windisch, M.; Kittinger, C.; Heil, J.; Morlock, G.E. Simple performance of the planar SOS-Umu-C–FLD genotoxicity bioassay shown for perfume and packaging material analysis. JPC J. Planar Chromatogr. Mod. TLC 2023, 36, 513–520. [Google Scholar] [CrossRef]
- Oresanya, I.O.; Orhan, I.E.; Heil, J.; Morlock, G.E. African Under-Utilized Medicinal Leafy Vegetables Studied by Microtiter Plate Assays and High-Performance Thin-Layer Chromatography–Planar Assays. Molecules 2024, 29, 733. [Google Scholar] [CrossRef] [PubMed]
- Spiliotopoulos, D.; Koelbert, C. Assessment of the miniaturized liquid Ames microplate format (MPF™) for a selection of the test items from the recommended list of genotoxic and non-genotoxic chemicals. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 856–857, 503218. [Google Scholar] [CrossRef]
- Meyer, D.; Marin-Kuan, M.; Debon, E.; Serrant, P.; Cottet-Fontannaz, C.; Schilter, B.; Morlock, G.E. Detection of low levels of genotoxic compounds in food contact materials using an alternative HPTLC-SOS-Umu-C assay. ALTEX 2021, 38, 387–397. [Google Scholar] [CrossRef]
- Azadniya, E.; Mollergues, J.; Stroheker, T.; Billerbeck, K.; Morlock, G.E. New incorporation of the S9 metabolizing system into methods for detecting acetylcholinesterase inhibition. Anal. Chim. Acta 2020, 1129, 76–84. [Google Scholar] [CrossRef]
- Fichou, D.; Morlock, G.E. Powerful artificial neural network for planar chromatographic image evaluation, shown for denoising and feature extraction. Anal. Chem. 2018, 90, 6984–6991. [Google Scholar] [CrossRef]
- Fichou, D.; Ristivojevic, P.; Morlock, G.E. Proof-of-principle of rTLC, an open-source software developed for image evaluation and multivariate analysis of planar chromatograms. Anal. Chem. 2016, 88, 12494–12501. [Google Scholar] [CrossRef]
- Fichou, D.; Yüce, I.; Morlock, G.E. eicCluster software, an open-source in silico tool, and on-surface syntheses, an in situ concept, both exploited for signal highlighting in high-resolution mass spectrometry to ease structure elucidation in planar chromatography. J. Chromatogr. A 2018, 1577, 101–108. [Google Scholar] [CrossRef]
- Sereshti, H.; Poursorkh, Z.; Aliakbarzadeh, G.; Zarre, S. Quality control of saffron and evaluation of potential adulteration by means of thin layer chromatography-image analysis and chemometrics methods. Food Control 2018, 90, 48–57. [Google Scholar] [CrossRef]
- Fichou, D.; Morlock, G.E. quanTLC, an online open-source solution for videodensitometric quantification. J. Chromatogr. A 2018, 1560, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Klingelhöfer, I.; Morlock, G.E. Sharp-bounded zones link to the effect in planar chromatography-bioassay-mass spectrometry. J. Chromatogr. A 2014, 1360, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Azadniya, E.; Goldoni, L.; Bandiera, T.; Morlock, G.E. Same analytical method for both (bio) assay and zone isolation to identify/quantify bioactive compounds by quantitative nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2020, 1628, 461434. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Lu, C.; Zhou, S.; Tian, G.; He, L.; Bao, Y.; Fauconnier, M.-L.; Xiao, H.; Zheng, J. Simultaneous determination of 14 bioactive citrus flavonoids using thin-layer chromatography combined with surface enhanced Raman spectroscopy. Food Chem. 2021, 338, 128115. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. High-performance thin-layer chromatography combined with effect-directed assays and high-resolution mass spectrometry as an emerging hyphenated technology: A tutorial review. Anal. Chim. Acta 2021, 1180, 338644. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.; Ronzheimer, A.; Friz, M.; Morlock, G.E. Multiplex planar bioassay with reduced diffusion on normal phase, identifying androgens, verified antiandrogens and synergists in botanicals via 12D hyphenation. Food Chem. 2022, 395, 133610. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. Miniaturized planar chromatography using office peripherals—Office chromatography. J. Chromatogr. A 2015, 1382, 87–96. [Google Scholar] [CrossRef]
- Romero, M.C.O.; Jakob, K.; Schmidt, J.; Nimmerfroh, T.; Schwack, W.; Morlock, G.E. Consolidating two laboratories into the most sustainable lab of the future: 2LabsToGo-Eco. Anal. Chim. Acta 2025, 1367, 344103. [Google Scholar] [CrossRef]
- Morlock, G.E.; Koch, J.; Schwack, W. Miniaturized open-source 2LabsToGo screening of lactose-free dairy products and saccharide-containing foods. J. Chromatogr. A 2023, 1688, 463720. [Google Scholar] [CrossRef]
- Sing, L.; Schwack, W.; Göttsche, R.; Morlock, G.E. 2LabsToGo─ Recipe for Building Your Own Chromatography Equipment Including Biological Assay and Effect Detection. Anal. Chem. 2022, 94, 14554–14564. [Google Scholar] [CrossRef]
- Jakob, K.; Schwack, W.; Morlock, G.E. All-in-one 2LabsToGo system for analysis of ergot alkaloids in whole rye. Food Chem. 2024, 453, 139593. [Google Scholar] [CrossRef]
- Schade, F.; Schwack, W.; Demirbas, Y.; Morlock, G.E. Open-source all-in-one LabToGo Office Chromatography. Anal. Chim. Acta 2021, 1174, 338702. [Google Scholar] [CrossRef]
- Fichou, D.; Morlock, G.E. Office chromatography: Miniaturized all-in-one open-source system for planar chromatography. Anal. Chem. 2018, 90, 12647–12654. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Genet. Toxicol. Environ. Mutagen 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Chamyuang, S.; Owatworakit, A.; Intatha, U.; Duangphet, S. Coffee pectin production: An alternative way for agricultural waste management in coffee farms. Sci. Asia 2021, 47S, 90–95. [Google Scholar] [CrossRef]
- Blumenthal, P.; Steger, M.C.; Einfalt, D.; Rieke-Zapp, J.; Quintanilla Bellucci, A.; Sommerfeld, K.; Schwarz, S.; Lachenmeier, D.W. Methanol mitigation during manufacturing of fruit spirits with special consideration of novel coffee cherry spirits. Molecules 2021, 26, 2585. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J. 2011, 9, 2379. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens; Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A. Guidance on the scientific requirements for an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8961. [Google Scholar] [CrossRef] [PubMed]
- Hoelzl, C.; Knasmüller, S.; Wagner, K.-H.; Elbling, L.; Huber, W.; Kager, N.; Ferk, F.; Ehrlich, V.; Nersesyan, A.; Neubauer, O.; et al. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol. Nutr. Food Res. 2010, 54, 1722–1733. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monazzah, M.; Herrmann, C.; Morlock, G.E.; Fuchs, J.; Lachenmeier, D.W. Coffee By-Products Studied by the Planar Ames Bioassay with pH Indicator Endpoint Using the 2LabsToGo-Eco. Toxics 2025, 13, 739. https://doi.org/10.3390/toxics13090739
Monazzah M, Herrmann C, Morlock GE, Fuchs J, Lachenmeier DW. Coffee By-Products Studied by the Planar Ames Bioassay with pH Indicator Endpoint Using the 2LabsToGo-Eco. Toxics. 2025; 13(9):739. https://doi.org/10.3390/toxics13090739
Chicago/Turabian StyleMonazzah, Maryam, Cedric Herrmann, Gertrud E. Morlock, Jannika Fuchs, and Dirk W. Lachenmeier. 2025. "Coffee By-Products Studied by the Planar Ames Bioassay with pH Indicator Endpoint Using the 2LabsToGo-Eco" Toxics 13, no. 9: 739. https://doi.org/10.3390/toxics13090739
APA StyleMonazzah, M., Herrmann, C., Morlock, G. E., Fuchs, J., & Lachenmeier, D. W. (2025). Coffee By-Products Studied by the Planar Ames Bioassay with pH Indicator Endpoint Using the 2LabsToGo-Eco. Toxics, 13(9), 739. https://doi.org/10.3390/toxics13090739







