Mercury Scenario in Fish from the Amazon Basin: Exploring the Interplay of Social Groups and Environmental Diversity
Highlights
- All risk models based on fish consumption studies exceeded the recommended MeHg intake limits.
- [Hg] in commercially important non-carnivorous fish remains understudied.
- Fish from lakes, reservoirs, and blackwater had higher Hg concentrations.
- Amazonian populations are at high risk of mercury exposure due to their high fish consumption rates, highlighting the need for targeted public health strategies.
- Monitoring non-carnivorous species may improve food safety strategies.
- Lakes, hydroelectric reservoirs, and blackwater are priority areas for Hg monitoring.
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review
2.2. Data Extraction
2.3. Risk Calculation
2.4. Meta-Analysis
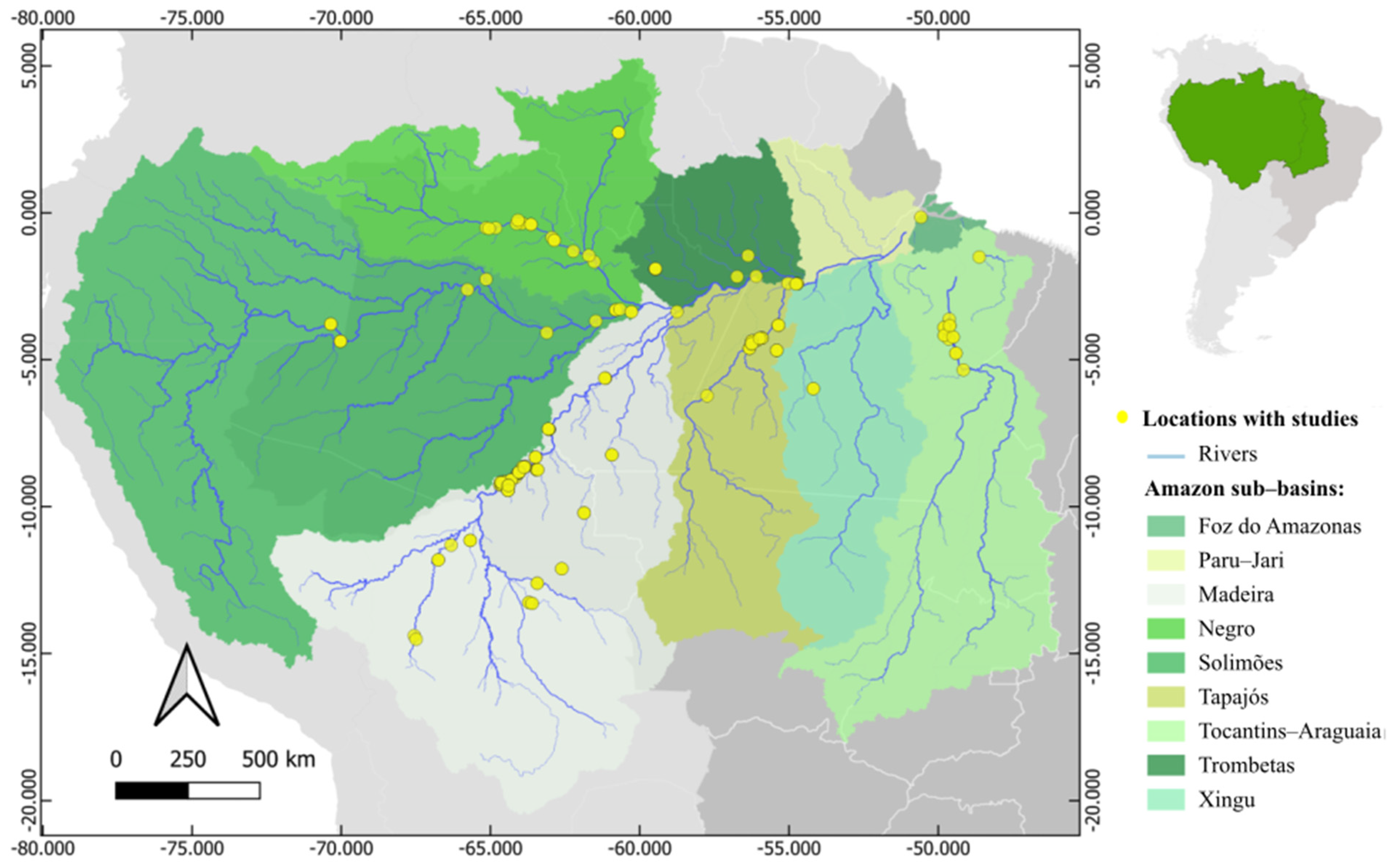
2.5. Maps
3. Results
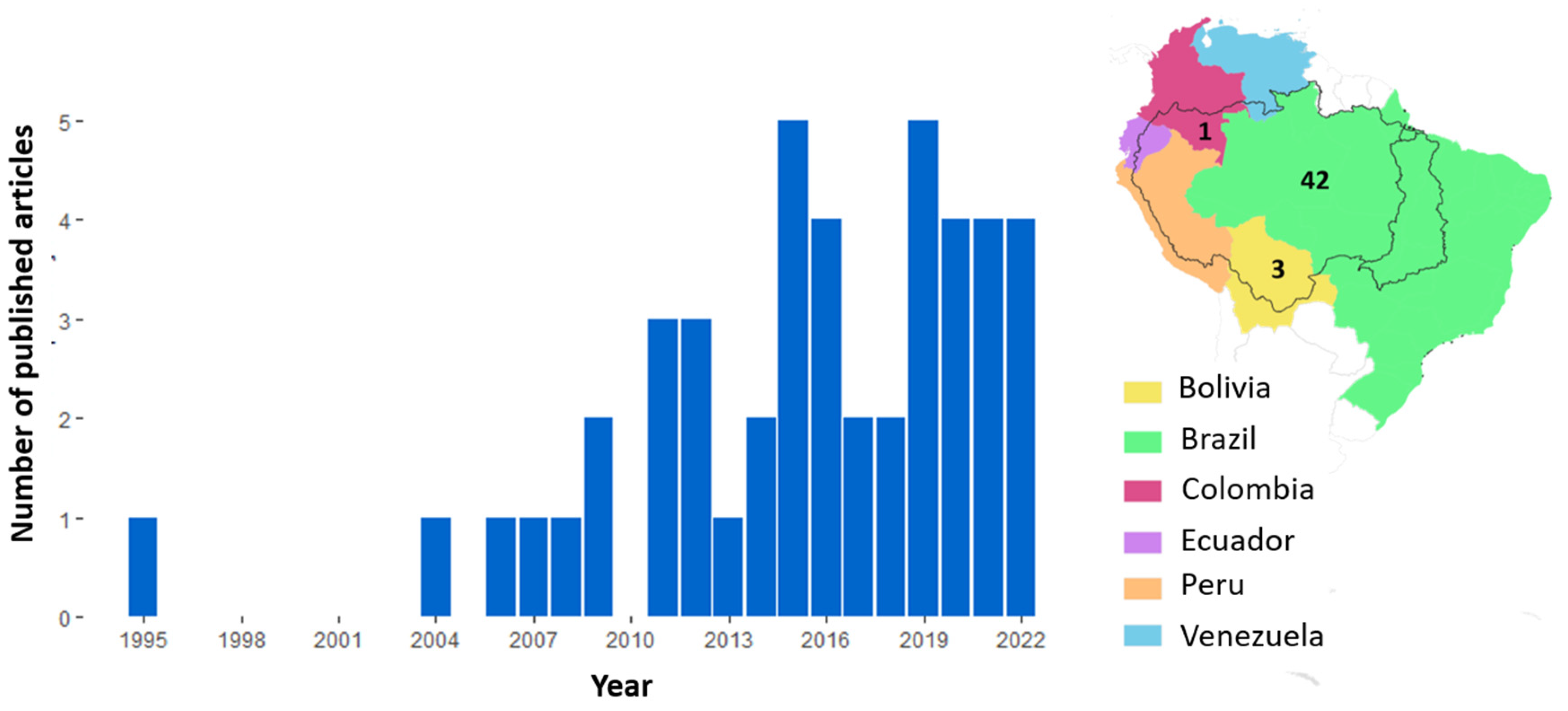
3.1. Systematic Review
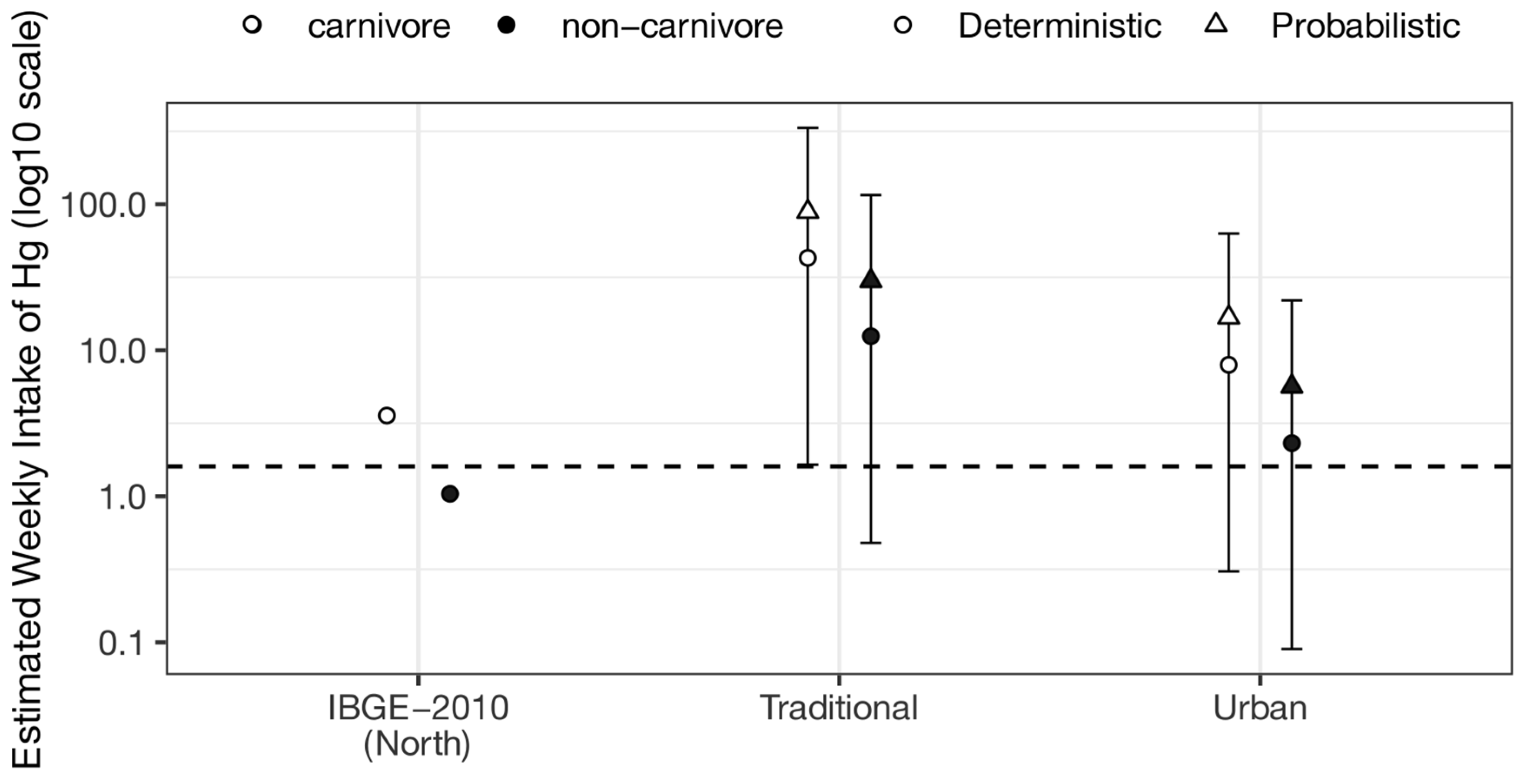
3.2. Risk Calculation
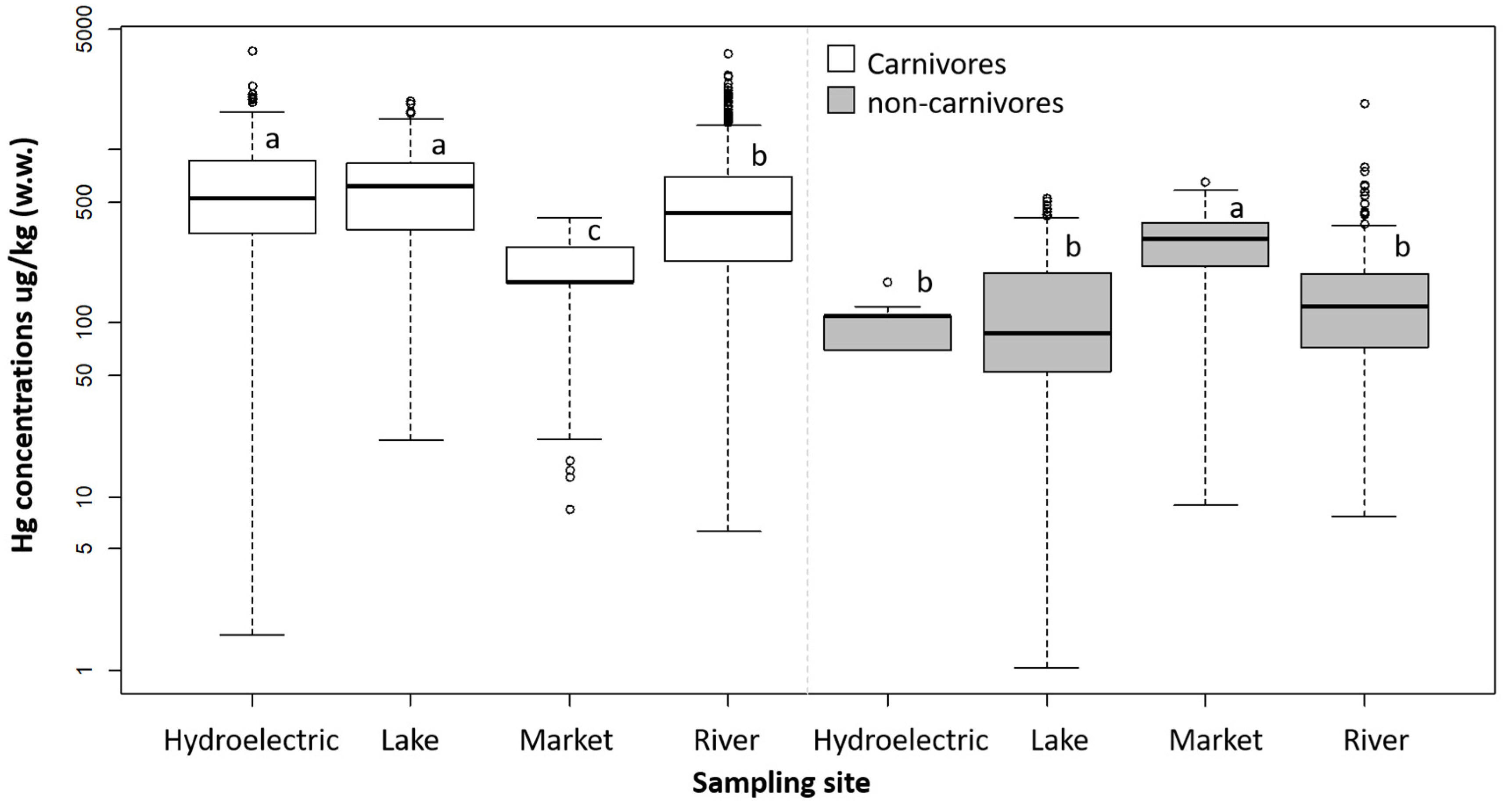
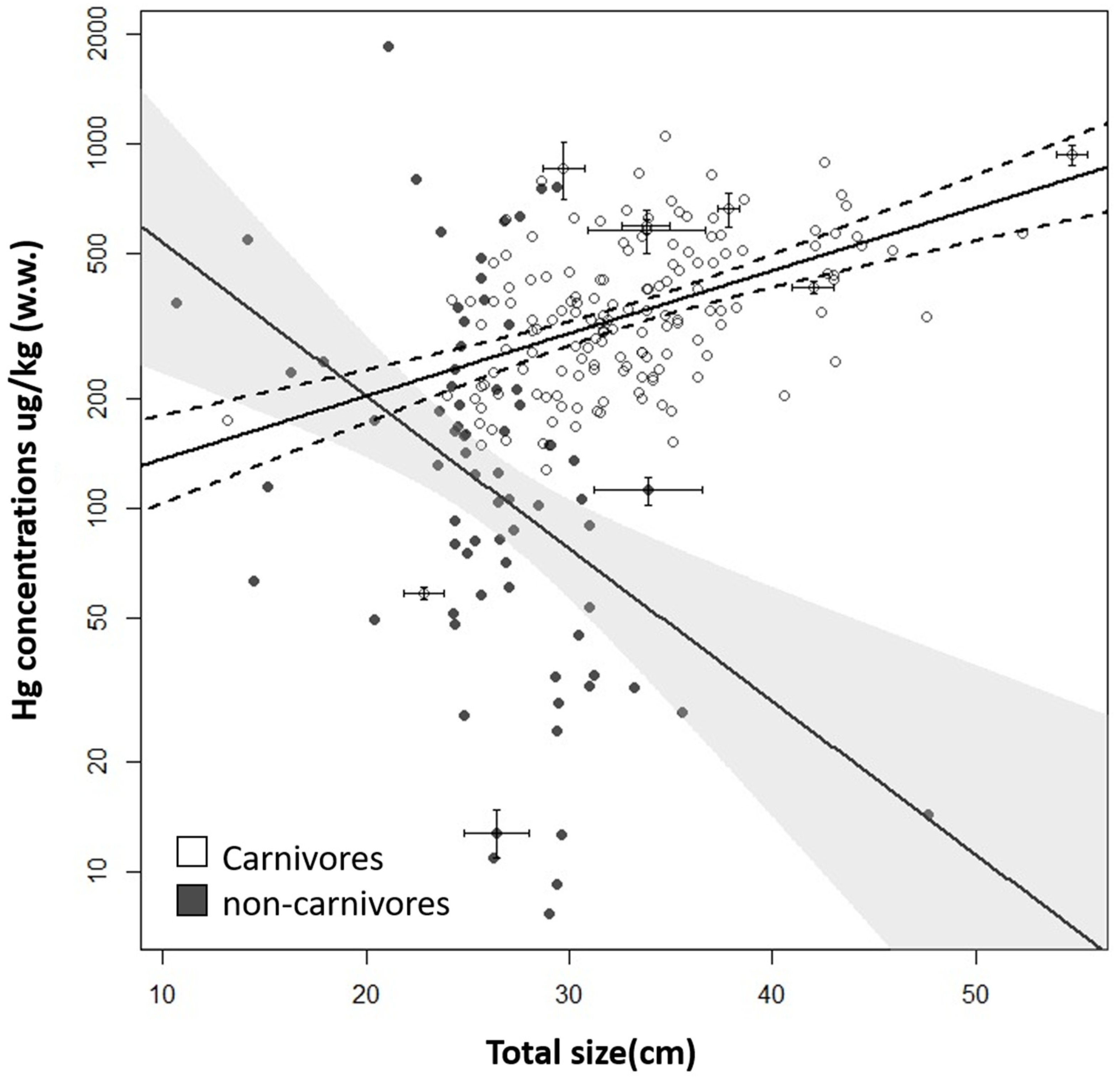
3.3. Influences on Hg Concentrations
4. Discussion
4.1. Systematic Review
4.2. Risk Calculation
4.3. Influences on Hg Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fearnside, P.M. Biodiversity as an environmental service in Brazil’s Amazonian forests: Risks, value and conservation. Environ. Conserv. 1999, 26, 305–321. [Google Scholar] [CrossRef]
- Fearnside, P.M. The intrinsic value of Amazon biodiversity. Biodivers. Conserv. 2021, 30, 1199–1202. [Google Scholar] [CrossRef]
- IBGE. 2022 Census: Population and Household Information by Census Tracts Support Public Management; Instituto Brasileiro de Geografia e Estatística. 2022. Available online: https://agenciadenoticias.ibge.gov.br/agencia-noticias/2012-agencia-de-noticias/noticias/39525-censo-2022-informacoes-de-populacao-e-domicilios-por-setores-censitarios-auxiliam-gestao-publica (accessed on 25 January 2025).
- Isaac, V.J.; Almeida, M.C.; Giarrizzo, T.; Deus, C.P.; Vale, R.; Klein, G.; Begossi, A. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. An. Acad. Bras. Ciênc. 2015, 87, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Pesquisa de Orçamentos Familiares 2008–2009: Análise do Consumo Alimentar Pessoal no Brasil; Instituto Brasileiro de Geografia e Estatística. 2010. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv50063.pdf (accessed on 3 July 2025).
- Oliveira, R.C.; Dórea, J.G.; Bernardi, J.V.; Bastos, W.R.; Almeida, R.; Manzatto, Â.G. Fish consumption by traditional subsistence villagers of the Rio Madeira (Amazon): Impact on hair mercury. Ann. Hum. Biol. 2010, 37, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.N.L. Do Rio Para o Prato: O Que Influencia o Consumo de Peixe na Amazônia? Master’s Dissertation, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2024. Available online: https://repositorio.ufmg.br/handle/1843/80091 (accessed on 3 July 2025).
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Frazzoli, C.; Mantovani, A. Toxicants exposures as novel zoonoses: Reflections on sustainable development, food safety and veterinary public health. Zoonoses Public Health 2010, 57, e136–e142. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mercury and Health; WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 7 October 2024).
- Guimarães, J.R. Mercury in the Amazon: Problem or opportunity? A commentary on 30 years of research on the subject. Elem. Sci. Anth. 2020, 8, 032. [Google Scholar] [CrossRef]
- Kinghorn, A.; Solomon, P.; Chan, H.M. Temporal and spatial trends of mercury in fish collected in the English–Wabigoon river system in Ontario, Canada. Sci. Total Environ. 2007, 372, 615–623. [Google Scholar] [CrossRef]
- Rodriguez-Levy, I.E.; Van Damme, P.A.; Carvajal-Vallejos, F.M.; Bervoets, L. Trace element accumulation in different edible fish species from the Bolivian Amazon and the risk for human consumption. Heliyon 2022, 8, e11531. [Google Scholar] [CrossRef]
- Begossi, A.; Salivonchyk, S.V.; Hallwass, G.; Hanazaki, N.; Lopes, P.F.M.; Silvano, R.A.M.; Dumaresq, D.; Pittock, J. Fish consumption on the Amazon: A review of biodiversity, hydropower and food security issues. Braz. J. Biol. 2018, 79, 345–357. [Google Scholar] [CrossRef]
- Lechler, P.J.; Miller, J.R.; Lacerda, L.D.D.; Vinson, D.; Bonzongo, J.C.; Lyons, W.B.; Warwick, J.J. Elevated mercury concentrations in soils, sediments, water, and fish of the Madeira River basin, Brazilian Amazon: A function of natural enrichments? Sci. Total Environ. 2000, 260, 87–96. [Google Scholar] [CrossRef]
- Fadini, P.S.; Jardim, W.F. Is the Negro River Basin (Amazon) impacted by naturally occurring mercury? Sci. Total Environ. 2001, 275, 71–82. [Google Scholar] [CrossRef]
- Marshall, B.G.; Forsberg, B.R.; Thomé-Souza, M.; Peleja, R.; Moreira, M.Z.; Freitas, C.E.C.D. Evidence of mercury biomagnification in the food chain of the cardinal tetra Paracheirodon axelrodi (Osteichthyes: Characidae) in the Rio Negro, central Amazon, Brazil. J. Fish Biol. 2016, 89, 220–240. [Google Scholar] [CrossRef]
- Siqueira, G.W.; Aprile, F.; Irion, G.; Braga, E.S. Mercury in the Amazon basin: Human influence or natural geological pattern? J. S. Am. Earth Sci. 2018, 86, 193–199. [Google Scholar] [CrossRef]
- Pestana, I.A.; Rezende, C.E.; Almeida, R.; Lacerda, L.D.; Bastos, W.R. Let’s talk about mercury contamination in the Amazon (again): The case of the floating gold miners’ village on the Madeira River. Extract. Ind. Soc. 2022, 11, 101122. [Google Scholar] [CrossRef]
- Forsberg, B.R.; Melack, J.M.; Dunne, T.; Barthem, R.B.; Goulding, M.; Paiva, R.C.D.; Sorribas, M.V.; Silva, U.L., Jr.; Weisser, S. The potential impact of new Andean dams on Amazon fluvial ecosystems. PLoS ONE 2017, 12, e0182254. [Google Scholar] [CrossRef] [PubMed]
- Crespo-López, M.E.; Arrifano, G.P.; Augusto-Oliveira, M.; Macchi, B.M.; Lima, R.R.; do Nascimento, J.L.M.; Souza, C.B. Mercury in the Amazon: The danger of a single story. Ecotoxicol. Environ. Saf. 2023, 256, 114895. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Wang, W.X. Why mercury concentration increases with fish size? Biokinetic explanation. Environ. Pollut. 2012, 163, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sioli, H. Tropical rivers as expressions of their terrestrial environments. In Tropical Ecological Systems: Trends in Terrestrial and Aquatic Research; Springer: Berlin/Heidelberg, Germany, 1975; pp. 275–288. [Google Scholar]
- Silva-Forsberg, M.C.; Forsberg, B.R.; Zeidemann, V.K. Mercury contamination in humans linked to river chemistry in the Amazon Basin. Ambio 1999, 28, 519–521. [Google Scholar]
- Belger, L.; Forsberg, B.R. Factors controlling Hg levels in two predatory fish species in the Negro river basin, Brazilian Amazon. Sci. Total Environ. 2006, 367, 451–459. [Google Scholar] [CrossRef]
- Silva, S.F.; Oliveira, D.C.; Pereira, J.P.G.; Castro, S.P.; Costa, B.N.S.; Lima, M.O. Seasonal variation of mercury in commercial fishes of the Amazon Triple Frontier, Western Amazon Basin. Ecol. Indic. 2019, 106, 105549. [Google Scholar] [CrossRef]
- Paiva, T.C.; Dary, E.P.; Pestana, I.A.; Amadio, S.A.; Malm, O.; Kasper, D. Flood-pulse and trophic position modulate mercury concentrations in fishes from an Amazon floodplain lake. Environ. Res. 2022, 215, 114307. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.; Forsberg, B.R.; do Amaral Kehrig, H.; Amaral, J.H.F.; Bastos, W.R.; Malm, O. Mercury in black-waters of the Amazon. In Igapó (Black-Water Flooded Forests) of the Amazon Basin; Springer International Publishing: Cham, Switzerland, 2018; pp. 39–56. [Google Scholar]
- Arrifano, G.P.; Martín-Doimeadios, R.C.R.; Jiménez-Moreno, M.; Ramírez-Mateos, V.; da Silva, N.F.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; do Nascimento, J.L.M.; et al. Large-scale projects in the Amazon and human exposure to mercury: The case-study of the Tucuruí Dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Biomonitoring of mercury, cadmium and selenium in fish and the population of Puerto Nariño, at the southern corner of the Colombian Amazon. Arch. Environ. Contam. Toxicol. 2020, 79, 354–370. [Google Scholar] [CrossRef]
- Fiocruz. Daily Mercury Intake Exceeds Safe Limits in Six Amazon States. 2023. Available online: https://portal.fiocruz.br/noticia/ingestao-diaria-de-mercurio-excede-os-limites-seguros-em-seis-estados-na-amazonia (accessed on 7 October 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Pierezan, M.D.; Hoff, R.B.; Marsico, E.T.; Verruck, S. Total mercury and methylmercury levels in Brazilian Amazon fish: A scope review with meta-analysis and local population health risk assessment. J. Trace Elem. Miner. 2024, 10, 100196. [Google Scholar] [CrossRef]
- Portier, K.; Tolson, J.K.; Roberts, S.M. Body weight distributions for risk assessment. Risk Anal. 2007, 27, 11–26. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Sixty-Seventh Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA): Methyl Mercury; IPCS—International Programme on Chemical Safety: Geneva, Switzerland, 2007; pp. 269–315. [Google Scholar]
- Pouillot, R.; Delignette-Muller, M.L. Evaluating variability and uncertainty separately in microbial quantitative risk assessment using two R packages. Int. J. Food Microbiol. 2010, 142, 330–340. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.r-project.org/ (accessed on 3 July 2025).
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2025; Available online: http://www.posit.co/ (accessed on 3 July 2025).
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-7. 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 3 July 2025).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Altman, N.; Krzywinski, M. Regression diagnostics. Nat. Methods 2016, 13, 385–386. [Google Scholar] [CrossRef]
- Porvari, P. Mercury levels of fish in Tucuruí hydroelectric reservoir and in River Moju in Amazonia, in the state of Pará, Brazil. Sci. Total Environ. 1995, 175, 109–117. [Google Scholar] [CrossRef]
- Castilhos, Z.C.; Almonsy, N.; Souto, P.S.; Pereira da Silva, L.C.C.; Linde, A.R.; Bidone, E.D. Bioassessment of ecological risk of Amazonian ichthyofauna to mercury. Bull. Environ. Contam. Toxicol. 2004, 72, 671. [Google Scholar] [CrossRef]
- World Health Organization. IPCC Environmental Health Criteria 101: Methylmercury; International Programme of Chemical Safety; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- ANVISA. Resolução RDC N° 42, de 29 de Agosto de 2013—Dispõe Sobre o Regulamento Técnico MERCOSUL Sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos; Agência Nacional de Vigilância Sanitária, Ministério da Saúde: Brasília, Brazil, 2013; p. 17.
- U.S. Environmental Protection Agency (USEPA). National Recommended Water Quality Criteria: Human Health Criteria Table. 2010. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-human-health-criteria-table (accessed on 3 July 2025).
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: An evaluation of human exposure. J. Trace Elem. Med. Biol. 2018, 48, 196–201. [Google Scholar] [CrossRef]
- ÁGUASAMAZÔNICAS. Basin. Available online: https://pt.aguasamazonicas.org/bacia (accessed on 26 January 2025).
- Cella-Ribeiro, A.; Torrente-Vilara, G.; Lima-Filho, J.A.; Doria, C.R.C. Ecologia e Biologia de Peixes do Rio Madeira; EDUFRO: Porto Velho, Brazil, 2016. [Google Scholar]
- Beltran-Pedreros, S.; Zuanon, J.; Leite, R.G.; Peleja, J.R.P.; Mendonça, A.B.; Forsberg, B.R. Mercury bioaccumulation in fish of commercial importance from different trophic categories in an Amazon floodplain lake. Neotrop. Ichthyol. 2011, 9, 901–908. [Google Scholar] [CrossRef]
- de Vasconcellos, A.C.S.; Ferreira, S.R.B.; de Sousa, C.C.; de Oliveira, M.W.; de Oliveira Lima, M.; Basta, P.C. Health risk assessment attributed to consumption of fish contaminated with mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics 2022, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.C.S.; Braga, C.P.; de Oliveira, G.; Padilha, C.D.C.F.; de Moraes, P.M.; Zara, L.F.; Padilha, P.D.M. Mercury exposure: Protein biomarkers of mercury exposure in Jaraqui fish from the Amazon region. Biol. Trace Elem. Res. 2018, 183, 164–171. [Google Scholar] [CrossRef]
- Vasconcellos, A.C.S.D.; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N.D.M.; Lima, M.D.O.; Basta, P.C. Health risk assessment of mercury exposure from fish consumption in Munduruku indigenous communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 7940. [Google Scholar] [CrossRef]
- Gomes, D.F.; Moreira, R.A.; Sanches, N.A.O.; Do Vale, C.A.; Daam, M.A.; Gorni, G.R.; Bastos, W.R. Dynamics of (total and methyl) mercury in sediment, fish, and crocodiles in an Amazonian Lake and risk assessment of fish consumption to the local population. Environ. Monit. Assess. 2020, 192, 101. [Google Scholar] [CrossRef] [PubMed]
- Basta, P.C.; de Vasconcellos, A.C.S.; Hallwass, G.; Yokota, D.; Pinto, D.d.O.d.R.; de Aguiar, D.S.; de Souza, C.C.; Oliveira-da-Costa, M. Risk assessment of mercury-contaminated fish consumption in the Brazilian Amazon: An ecological study. Toxics 2023, 11, 800. [Google Scholar] [CrossRef]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Júnior, R.A.B.; Oliveira, F.L.C.; Sucupira, M.C.A.; Ortolani, E.L.; López-Alonso, M. Toxic and essential trace element concentrations in fish species in the Lower Amazon, Brazil. Sci. Total Environ. 2020, 732, 138983. [Google Scholar] [CrossRef]
- Mendes, R.A.; Lima, M.O.; Deus, R.J.; Medeiros, A.C.; Faial, K.C.; Jesus, I.M.; Santos, L.S. Assessment of DDT and mercury levels in fish and sediments in the Iriri River, Brazil: Distribution and ecological risk. J. Environ. Sci. Health B 2019, 54, 915–924. [Google Scholar]
- Fearnside, P.M. Brazil’s Samuel Dam: Lessons for hydroelectric development policy and the environment in Amazonia. Environ. Manag. 2005, 35, 1–19. [Google Scholar] [CrossRef]
- Bilodeau, F.; Therrien, J.; Schetagne, R. Intensity and duration of effects of impoundment on mercury levels in fishes of hydroelectric reservoirs in northern Québec (Canada). Inland Waters 2017, 7, 493–503. [Google Scholar] [CrossRef]
- Paiva, T.C.; Pestana, I.A.; Oliveira, B.C.V.; Malm, O.; Rezende, C.E.; Kasper, D. Temporal variation of mercury levels in fish, soil, and sediments in an Amazon reservoir: Insights from 35 years of river impoundment in Pará State, Brazil. Environ. Monit. Assess. 2024, 196, 1089. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Kasper, D.; da Silva, S.A.A.; Lázaro, W.L.; Muniz, C.C.; Carvalho, G.S.; Ignácio, Á.R.A. Cascade reservoirs affect mercury concentrations in fish from Teles Pires river, Brazilian Amazon. Ecotoxicology 2025, 34, 444–455. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; de Matos Macchi, B.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.S.; Lucotte, M.; Paquet, S.; Davidson, R. Influence of ecological factors and of land use on mercury levels in fish in the Tapajós River basin, Amazon. Environ. Res. 2009, 109, 432–446. [Google Scholar] [CrossRef]
- Pope, Q.; Rand, M.D. Variation in methylmercury metabolism and elimination in humans: Physiological pharmacokinetic modeling highlights the role of gut biotransformation, skeletal muscle, and hair. Toxicol. Sci. 2021, 180, 26–37. [Google Scholar] [CrossRef]
- Faial, K.; Deus, R.; Deus, S.; Neves, R.; Jesus, I.; Santos, E.; Brasil, D. Mercury levels assessment in hair of riverside inhabitants of the Tapajós River, Pará State, Amazon, Brazil: Fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 2015, 30, 66–76. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Feng, X. Mercury and selenium interactions in human blood in the Wanshan mercury mining area, China. Sci. Total Environ. 2016, 573, 376–381. [Google Scholar] [CrossRef]
- Mendes, V.A.; Carvalho, D.P.; Almeida, R.; Recktenvald, M.C.N.D.N.; Pedrosa, O.P.; Sousa-Filho, I.F.; Bastos, W.R. Mercury in blood, hair, and feces from subsistence fish-eating riverines of the Madeira River Basin (Western Amazon). J. Trace Elem. Med. Biol. 2021, 67, 126773. [Google Scholar] [CrossRef]
- Passos, C.J.S.; Silva, D.S.; Lemire, M.; Fillion, M.; Guimaraes, J.R.D.; Lucotte, M.; Mergler, D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 76–87. [Google Scholar] [CrossRef]
- Mendes, V.A. Crescimento Linear e Estado Nutricional de Crianças e Adolescentes Ribeirinhos de Comunidades no Baixo Rio Madeira, Expostos ao Mercúrio. Master’s Dissertation, Universidade Federal de Rondônia, Porto Velho, RO, Brazil, 2018. [Google Scholar]
- Pedrosa, O.P. Estudo Prospectivo do Estado de Saúde de Uma População Ribeirinha da Amazônia Brasileira. Doctoral Thesis, Universidade Federal de Rondônia, Porto Velho, Brazil, 2018; p. 137. [Google Scholar]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Total and methyl mercury distribution in water, sediment, plankton and fish along the Tapajós River basin in the Brazilian Amazon. Chemosphere 2019, 235, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.C.; Forsberg, B.R.; Kasper, D.; Amaral, J.H.; de Vasconcelos, M.R.; de Sousa, O.P.; Bastos, W.R. The influence of inundation and lake morphometry on the dynamics of mercury in the water and plankton in an Amazon floodplain lake. Hydrobiologia 2017, 790, 35–48. [Google Scholar] [CrossRef]
- Willacker, J.J.; Eagles-Smith, C.A.; Lutz, M.A.; Tate, M.T.; Lepak, J.M.; Ackerman, J.T. Reservoirs and water management influence fish mercury concentrations in the western United States and Canada. Sci. Total Environ. 2016, 568, 739–748. [Google Scholar] [CrossRef]
- Santos, R.R.V. Aquaculture in the Amazonian Context: Evolution and Productive Specialization in the State of Pará. Master’s Thesis, Universidade Federal Rural da Amazônia, Belém, Brazil, 2021. [Google Scholar]
- IDAM. In 15 Years, Aquaculture Grows in Amazonas. Available online: https://www.idam.am.gov.br/em-15-anos-piscicultura-cresce-no-amazonas/ (accessed on 20 December 2024).
- Agência Pará. Aquaculture Growth in Pará Surpasses the National Average. 2024. Available online: https://www.agenciapara.com.br/noticia/51723/crescimento-da-aquicultura-no-para-supera-a-media-nacional (accessed on 20 December 2024).
- FAPERJ. O Tucunaré é Originalmente Uma Espécie Amazônica, 2024. Available online: https://siteantigo.faperj.br/?id=4212.2.4#:~:text=O%20tucunar%C3%A9%20%C3%A9%20originalmente%20uma,1990%2C%20em%20v%C3%A1rias%20regi%C3%B5es%20brasileiras (accessed on 3 July 2025).
- Lacerda, L.D.D.; Costa, B.G.B.C.; Lopes, D.N.; Oliveira, K.; Bezerra, M.F.; Bastos, W.R. Mercury in indigenous, introduced and farmed fish from the semiarid region of the Jaguaribe River basin, NE Brazil. Bull. Environ. Contam. Toxicol. 2014, 93, 31–35. [Google Scholar] [CrossRef]
- Custódio, F.B.; Andrade, A.M.G.; Guidi, L.R.; Leal, C.A.; Gloria, M.B.A. Total mercury in commercial fishes and estimation of Brazilian dietary exposure to methylmercury. J. Trace Elem. Med. Biol. 2020, 62, 126641. [Google Scholar] [CrossRef]
- Kasper, D.; Botaro, D.; Palermo, E.F.A.; Malm, O. Mercúrio em peixes-fontes e contaminação. Oecol. Bras. 2007, 11, 228–239. [Google Scholar] [CrossRef]
- Vasconcelos, M.R.R.; Brito, B.C.; Forsberg, B.R.; Freitas Goch, Y.G.; Malm, O.; Melo, S.; Kasper, D. Export and bioaccumulation of methylmercury in streams draining distinct soils in the Central Brazilian Amazon, 2012–2013. J. Trace Elem. Miner. 2022, 2, 100014. [Google Scholar] [CrossRef]
- Roulet, M.; Maury-Brachet, R. Le mercure dans les organismes aquatiques amazoniens. In Le Mercure en Amazonie; Carmouze, J.P., Lucotte, M., Boudou, A., Eds.; IRD Éditions: Paris, France, 2001; pp. 203–271. [Google Scholar]
- Pouilly, M.; Pérez, T.; Rejas, D.; Guzman, F.; Crespo, G.; Duprey, J.L.; Guimarães, J.R.D. Mercury bioaccumulation patterns in fish from the Iténez River basin, Bolivian Amazon. Ecotoxicol. Environ. Saf. 2012, 83, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Rebelo, M.D.F.; Fonseca, M.D.F.; Almeida, R.D.; Malm, O. A description of mercury in fishes from the Madeira River Basin, Amazon, Brazil. Acta Amaz. 2008, 38, 431–438. [Google Scholar] [CrossRef]
- Rodríguez Martín-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Arrifano, G.P.F.; Herculano, A.M.; Crespo-López, M.E. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ. Sci. Pollut. Res. 2014, 21, 7466–7479. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, T.d.C.; Pestana, I.A.; Miranda, L.N.L.; de Carvalho, G.O.; Bastos, W.R.; Kasper, D. Mercury Scenario in Fish from the Amazon Basin: Exploring the Interplay of Social Groups and Environmental Diversity. Toxics 2025, 13, 580. https://doi.org/10.3390/toxics13070580
Paiva TdC, Pestana IA, Miranda LNL, de Carvalho GO, Bastos WR, Kasper D. Mercury Scenario in Fish from the Amazon Basin: Exploring the Interplay of Social Groups and Environmental Diversity. Toxics. 2025; 13(7):580. https://doi.org/10.3390/toxics13070580
Chicago/Turabian StylePaiva, Thaís de Castro, Inácio Abreu Pestana, Lorena Nascimento Leite Miranda, Gabriel Oliveira de Carvalho, Wanderley Rodrigues Bastos, and Daniele Kasper. 2025. "Mercury Scenario in Fish from the Amazon Basin: Exploring the Interplay of Social Groups and Environmental Diversity" Toxics 13, no. 7: 580. https://doi.org/10.3390/toxics13070580
APA StylePaiva, T. d. C., Pestana, I. A., Miranda, L. N. L., de Carvalho, G. O., Bastos, W. R., & Kasper, D. (2025). Mercury Scenario in Fish from the Amazon Basin: Exploring the Interplay of Social Groups and Environmental Diversity. Toxics, 13(7), 580. https://doi.org/10.3390/toxics13070580






