Composting as a Sustainable Approach for Managing Mercury-Contaminated Aquatic Biomass
Abstract
1. Introduction
2. Materials and Methods
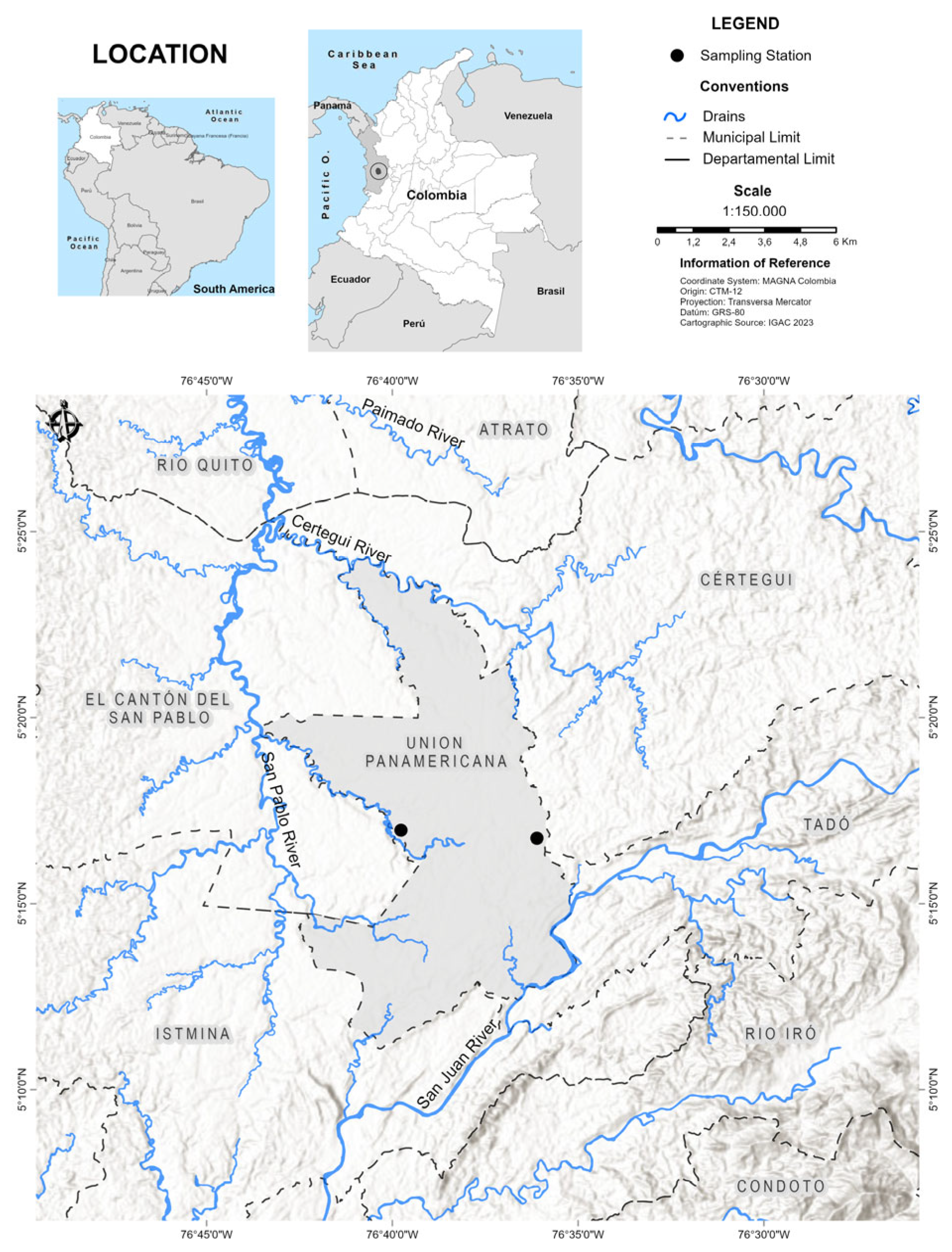
2.1. Contaminated Biomass Samples
2.2. Composting Process
2.3. Monitoring of Physicochemical Parameters During Composting Process
Biomass Loss During Composting
2.4. Analysis of Total Mercury Concentrations
2.4.1. Mass Balance for Total Mercury
2.4.2. Sequential Extraction of Mercury in the Compost
2.5. Analysis of Compost Quality
2.6. Statistical Analysis
3. Results and Discussion
3.1. Behavior of the Physicochemical Parameters During Composting
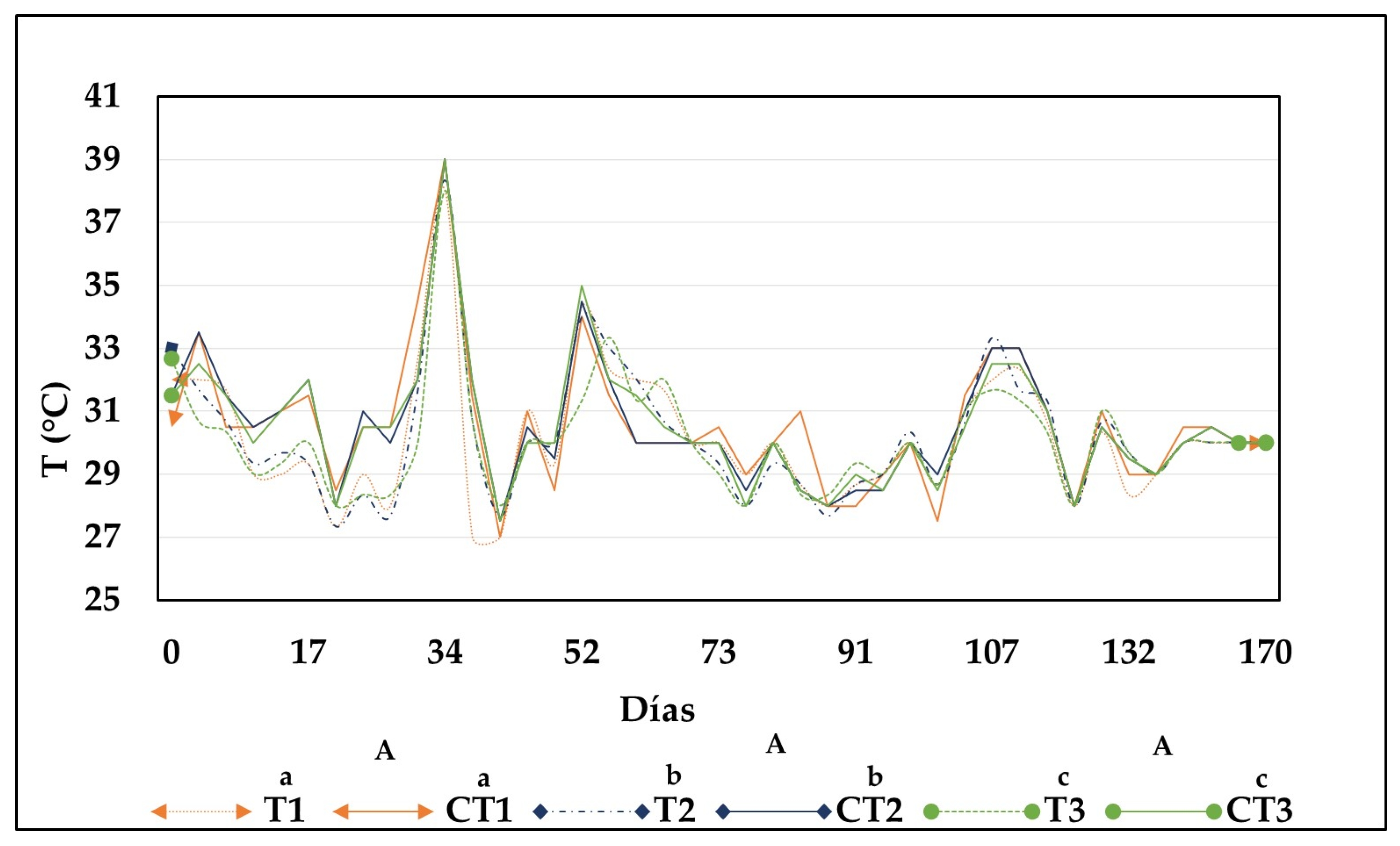
3.1.1. Temperature
3.1.2. pH
3.1.3. Electrical Conductivity
3.1.4. Biomass Losses During the Composting Process
3.2. Analysis of Hg Concentrations During Composting
Mass Balance of Total Mercury
3.3. Characterization and Composition of Compost
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, J.; Díez, S.; Morales-Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M.P. Mercury distribution in different environmental matrices in aquatic systems of abandoned gold mines, Western Colombia: Focus on human health. J. Hazard. Mater. 2021, 404, 124080. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Gorito, A.M.; Ribeiro, A.R.; Almeida, C.M.R.; Silva, A.M.T. A review on the application of constructed wetlands for the removal of priority substances and contaminants of emerging concern listed in recently launched EU legislation. Environ. Pollut. 2017, 227, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Guittonny-Philippe, A.; Petit, M.-E.; Masotti, V.; Monnier, Y.; Malleret, L.; Coulomb, B.; Combroux, I.; Baumberger, T.; Viglione, J.; Laffont-Schwob, I. Selection of wild macrophytes for use in constructed wetlands for phytoremediation of contaminant mixtures. J. Environ. Manag. 2015, 147, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Pratas, J.; Paulo, C.; Favas, P.J.C.; Venkatachalam, P. Potential of aquatic plants for phytofiltration of uranium-contaminated waters in laboratory conditions. Ecol. Eng. 2014, 69, 170–176. [Google Scholar] [CrossRef]
- Gunathilakae, N.; Yapa, N.; Hettiarachchi, R. Effect of arbuscular mycorrhizal fungi on the cadmium phytoremediation potential of Eichhornia crassipes (Mart.) Solms. Groundw. Sustain. Dev. 2018, 7, 477–482. [Google Scholar] [CrossRef]
- Iha, D.S.; Bianchini, I. Phytoremediation of Cd, Ni, Pb and Zn by Salvinia minima. Int. J. Phytoremediat. 2015, 17, 929–935. [Google Scholar] [CrossRef]
- Daud, M.; Ali, S.; Abbas, Z.; Zaheer, I.; Riaz, M.; Malik, A.; Zhu, S. Potential of Duckweed (Lemna minor) for the Phytoremediation of Landfill Leachate. J. Chem. 2018, 2018, 3951540. [Google Scholar] [CrossRef]
- Abbas, Z.; Arooj, F.; Ali, S.; Zaheer, I.E.; Rizwan, M.; Riaz, M.A. Phytoremediation of landfill leachate waste contaminants through floating bed technique using water hyacinth and water lettuce. Int. J. Phytoremediat. 2019, 21, 1356–1367. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Rodríguez, J.S.L.; Furtado, A.T.; Martin, A.A. Minería del platino y el oro en Chocó: Pobreza, riqueza natural e informalidad. Rev. Econ. Inst. 2019, 22, 241–268. [Google Scholar] [CrossRef]
- Balcázar Suárez, A.M. Bioacumulación de Mercurio en Anuros de Desarrollo Directo e Indirecto—Los Efectos de la Minería en la Región Megadiversa del Chocó, Colombia. 2024. Available online: https://hdl.handle.net/1992/75006 (accessed on 3 June 2025).
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, J.; Díez, S.; Morales-Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M.P. Distribution of chemical forms of mercury in sediments from abandoned ponds created during former gold mining operations in Colombia. Chemosphere 2020, 258, 127319. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Zhao, J.; Xia, D.; Pan, F.; Liu, C.; Kyzas, G.Z.; Fu, J. Palygorskite changes heavy metal bioavailability and microbial functional diversity in sewage sludge composting. Environ. Technol. 2015, 36, 2855–2862. [Google Scholar] [CrossRef]
- Attiogbe, F.K.; Ayim, N.Y.K.; Martey, J. Effectiveness of black soldier fly larvae in composting mercury contaminated organic waste. Sci. Afr. 2019, 6, e00205. [Google Scholar] [CrossRef]
- Wu, S.; He, H.; Inthapanya, X.; Yang, C.; Lu, L.; Zeng, G.; Han, Z. Role of biochar on composting of organic wastes and remediation of contaminated soils-a review. Environ. Sci. Pollut. Res. Int. 2017, 24, 16560–16577. [Google Scholar] [CrossRef]
- Vargas-Pineda, O.I.; Trujillo-González, J.M.; Torres-Mora, M.A.; Vargas-Pineda, O.I.; Trujillo-González, J.M.; Torres-Mora, M.A. El compostaje, una alternativa para el aprovechamiento de residuos orgánicos en las centrales de abastecimiento. Orinoquia 2019, 23, 123–129. [Google Scholar] [CrossRef]
- Akber, S.; Joardar, J. Co-Composting of Water Hyacinth with Cattle Manure and Its Effects on Growth and Yield of Okra | Semantic Scholar [Internet]. 2020. Available online: https://www.semanticscholar.org/paper/Co-Composting-of-Water-Hyacinth-with-Cattle-Manure-Akber-Joardar/7eafce511dacf821ee41ee9df1bde945d3c06017 (accessed on 12 April 2025).
- Rastogi, M.; Nandal, M.; Khosla, B. Microbes as vital additives for solid waste composting. Heliyon 2020, 6, e03343. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, O.; Moraes, L.; de Carvalho, G.; Marques, E.C.; Lorena, R.B. Biomass reduction of organic materials in a domestic composting system. BIOFIX Sci. J. 2021, 6, 98–102. [Google Scholar]
- USEPA. Method 3015A for Use of Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils [Internet]. 2007. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3051a.pdf (accessed on 26 June 2025).
- Bloom, N.S.; Preus, E.; Katon, J.; Hiltner, M. Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediments and soils. Anal. Chim. Acta 2003, 479, 233–248. [Google Scholar] [CrossRef]
- Caraballo-Laza, M.J.; Laza-Durante, M.; Durante-Yanez, E.; Ossa-Henao, D. Valorización de biomasa acuática contaminada con mercurio proveniente de cuerpos de agua de la mojana, en el Departamento de Sucre, mediante la técnica de compostaje. In Alternativas Basadas en la Naturaleza para la Recuperación de Ecosistemas Degradados por Minería Aurífera bajo un Enfoque de Economía Circular; Zenodo: Geneva, Switzerland, 2025; pp. 125–164. [Google Scholar] [CrossRef]
- NTC-5167; Agricultural Industry Products. Organic Products Used as Fertilizers and Soil Amendments. ICONTEC: Bogota, Colombia, 2022. Available online: https://tienda.icontec.org/gp-ntc-productos-para-la-industria-agricola-productos-organicos-usados-como-abonos-o-fertilizantes-y-enmiendas-o-acondicionadores-de-suelo-ntc5167-2022.html (accessed on 12 April 2025).
- Gusain, R.; Pandey, B.; Suthar, S. Composting as a sustainable option for managing biomass of aquatic weed Pistia: A biological hazard to aquatic system. J. Clean. Prod. 2018, 177, 803–812. [Google Scholar] [CrossRef]
- Makan, A.; Fadili, A.; Oubenali, M. Interaction of physicochemical parameters during pressurized in-vessel composting of food waste. Bioresour. Technol. Rep. 2020, 10, 100350. [Google Scholar] [CrossRef]
- Janowska, B.; Szymański, K.; Sidełko, R.; Siebielska, I.; Walendzik, B. Assessment of mobility and bioavailability of mercury compounds in sewage sludge and composts. Environ. Res. 2017, 156, 394–403. [Google Scholar] [CrossRef]
- Alkarimiah, R.; Suja, F. Effects of Technical Factors Towards Achieving the Thermophilic Temperature Stage in Composting Process and the Benefits of Closed Rector System Compared to Conventional Method—A Mini Review. Appl. Ecol. Environ. Res. 2019, 17, 9979–9996. [Google Scholar] [CrossRef]
- Michel, F.; O’Neill, T.; Rynk, R.; Gilbert, J.; Wisbaum, S.; Halbach, T. Chapter 5—Passively aerated composting methods, including turned windrows. In The Composting Handbook; Rynk, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 159–196. ISBN 978-0-323-85602-7. Available online: https://www.sciencedirect.com/science/article/pii/B9780323856027000029 (accessed on 12 April 2025).
- Afonso, S.; Arrobas, M.; Pereira, E.L.; Rodrigues, M.Â. Recycling nutrient-rich hop leaves by composting with wheat straw and farmyard manure in suitable mixtures. J. Environ. Manag. 2021, 284, 112105. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Wang, X.; Selvam, A. 4—Improving Compost Quality by Controlling Nitrogen Loss During Composting. In Current Developments in Biotechnology and Bioengineering; Wong, J.W.-C., Tyagi, R.D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–82. ISBN 978-0-444-63664-5. Available online: https://www.sciencedirect.com/science/article/pii/B9780444636645000046 (accessed on 12 April 2025).
- Zhang, L.; Sun, X. Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manag. 2016, 48, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Debernardi-Vazquez, T.d.J.; Aguilar-Rivera, N.; Nuñez-Pastrana, R. Composting of Byproducts from the Orange (Citrus sinensis (L.) Osbeck) and Sugarcane (Saccharum spp. hybrids) Agroindustries. Ing. E Investig. 2020, 40, 81–88. [Google Scholar] [CrossRef]
- Alavi, N.; Daneshpajou, M.; Shirmardi, M.; Goudarzi, G.; Neisi, A.; Babaei, A.A. Investigating the efficiency of co-composting and vermicomposting of vinasse with the mixture of cow manure wastes, bagasse, and natural zeolite. Waste Manag. 2017, 69, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compost Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Vergara, S.E.; Silver, W.L. Greenhouse gas emissions from windrow composting of organic wastes: Patterns and emissions factors. Environ. Res. Lett. 2019, 14, 124027. [Google Scholar] [CrossRef]
- Yeo, D.; Dongo, K.; Mertenat, A.; Lüssenhop, P.; Körner, I.; Zurbrügg, C. Material Flows and Greenhouse Gas Emissions Reduction Potential of Decentralized Composting in Sub-Saharan Africa: A Case Study in Tiassalé, Côte d’Ivoire. Int. J. Environ. Res. Public Health 2020, 17, 7229. [Google Scholar] [CrossRef]
- Rivas-Nichorzon, M.; Silva-Acuña, R. Calidad Física y Química de tres Compost, Elaborados con Residuos de Jardinería, Pergamino de Café y Bora (Eichhornia Crassipes). 2020. Available online: https://www.redalyc.org/articulo.oa?id=582661898009 (accessed on 12 April 2025).
- Lin, C.; Cheruiyot, N.K.; Bui, X.-T.; Ngo, H.H. Composting and its application in bioremediation of organic contaminants. Bioengineered 2022, 13, 1073–1089. [Google Scholar] [CrossRef]
- Manea, E.E.; Bumbac, C.; Dinu, L.R.; Bumbac, M.; Nicolescu, C.M. Composting as a Sustainable Solution for Organic Solid Waste Management: Current Practices and Potential Improvements. Sustainability 2024, 16, 6329. [Google Scholar] [CrossRef]
- Marrugo-Madrid, S.; Salas-Moreno, M.; Gutiérrez-Mosquera, H.; Salazar-Camacho, C.; Marrugo-Negrete, J.; Díez, S. Assessment of dissolved mercury by diffusive gradients in thin films devices in abandoned ponds impacted by small scale gold mining. Environ. Res. 2022, 208, 112633. [Google Scholar] [CrossRef]
- McCartney, D.; Zhang, Y.; Grant, C. Characterization of Compost Produced at a Golf Course: Impact of Historic Mercury Accumulations in Putting Green Soil. Compost Sci. Util. 2001, 9, 73–91. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Wang, C.; Wei, J.; Wei, Y.; Chen, M. Understanding of mercury and methylmercury transformation in sludge composting by metagenomic analysis. Water Res. 2022, 226, 119204. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, L.; Geng, Y.; Wang, N.; Mao, Y.; Cai, Y. Occurrence, speciation and fate of mercury in the sewage sludge of China. Ecotoxicol. Environ. Saf. 2019, 186, 109787. [Google Scholar] [CrossRef]
- Kanzler, C.R.; Lian, P.; Trainer, E.L.; Yang, X.; Govind, N.; Parks, J.M.; Graham, A.M. Emerging investigator series: Methylmercury speciation and dimethylmercury production in sulfidic solutions. Environ. Sci. Process. Impacts 2018, 20, 584–594. [Google Scholar] [CrossRef]
- Jiang, T.; Bravo, A.; Skyllberg, U.; Björn, E.; Wang, D.; Yan, H. Influence of dissolved organic matter (DOM) characteristics on dissolved mercury (Hg) species composition in sediment porewater of lakes from southwest China. Water Res. 2018, 146, 146–158. [Google Scholar] [CrossRef]
- Xu, S.; Li, L.; Zhan, J.; Guo, X. Variation and factors on heavy metal speciation during co-composting of rural sewage sludge and typical rural organic solid waste. J. Environ. Manag. 2022, 306, 114418. [Google Scholar] [CrossRef]
- Sardroudi, N.; Sorolla, S.; Casas, C.; Bacardit, A. A Study of the Composting Capacity of Different Kinds of Leathers, Leatherette and Alternative Materials. Sustainability 2024, 16, 2324. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Zhang, C.; Zhang, Y.; Huang, D.; Yan, S.; Sun, T.; Mao, Y.; Cai, Y. Influence of dissolved organic matter on methylmercury transformation during aerobic composting of municipal sewage sludge under different C/N ratios. J. Environ. Sci. 2022, 119, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Cheng, L.; Ma, B.; Cai, Y. The fate of mercury in municipal wastewater treatment plants in China: Significance and implications for environmental cycling. J. Hazard. Mater. 2016, 306, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, T.; Driscoll, C.T.; Zhang, H.; Zhang, X. Dimethylmercury in Floodwaters of Mercury Contaminated Rice Paddies. Environ. Sci. Technol. 2019, 53, 9453–9461. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Mou, Z.; Cooper, C.J.; Johnston, R.C.; Brooks, S.C.; Gu, B.; Parks, J. M Mechanistic Investigation of Dimethylmercury Formation Mediated by a Sulfide Mineral Surface. J. Phys. Chem. A 2021, 125, 5397–5405. [Google Scholar] [CrossRef] [PubMed]
- Barkay, T.; Gu, B. Demethylation-The Other Side of the Mercury Methylation Coin: A Critical Review. ACS Environ. Au 2022, 2, 77–97. [Google Scholar] [CrossRef]
- Oliveira, J.; Vasques, I.; Lima, F.R.D.; Job, M.T.P.; Carvalho, G.S.; Oliveira, T.S.; Marques, J.J. Synthesis and characterization of adsorbent materials for the retention of elemental mercury from contaminated tropical soils. Int. J. Environ. Sci. Technol. 2022, 20, 6221–6232. [Google Scholar] [CrossRef]
- Monroy, A.; Prada, L. Optimización del Proceso de Compostaje para el Aprovechamiento de los Residuos Orgánicos Generados en el Parque Jaime Duque Ubicado en el Municipio de Tocancipá, Cundinamarca [Internet]. 2019. Available online: https://ciencia.lasalle.edu.co/items/15129ecc-c594-4dae-9348-a3cb31646d4b (accessed on 12 April 2025).
- Guo, W.; Zhou, Y.; Zhu, N.; Hu, H.; Shen, W.; Huang, X.; Zhang, T.; Wu, P.; Li, Z. On site composting of food waste: A pilot scale case study in China. Resour. Conserv. Recycl. 2018, 132, 130–138. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Alawlaqi, B.; Alhajeri, S. Characterization of physicochemical parameters and bacterial diversity of composted organic food wastes in Dubai. Heliyon 2023, 9, e16426. [Google Scholar] [CrossRef]
- García, O.; Veiga, M.M.; Cordy, P.; Suescún, O.E.; Molina, J.M.; Roeser, M. Artisanal gold mining in Antioquia, Colombia: A successful case of mercury reduction. J. Clean. Prod. 2015, 90, 244–252. [Google Scholar] [CrossRef]
- Navia, C. Evaluation of Different Formulations from Waste Composting Crop Tomato (Solanum lycopersicum) [Internet]. 2013. Available online: http://www.scielo.org.co/scielo.php?script=sci_abstract&pid=S1692-35612013000300019 (accessed on 12 April 2025).
- Castro, G.; Marth, C. Evaluación de Enmiendas en el Proceso de Compostaje de Residuos de Curtiembres [Internet]. 2016. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1909-04552016000100006 (accessed on 12 April 2025).



| Treatments | * CB (kg) | * NCB (kg) | * OR (kg) | * M (kg) | * RH (kg) | Final Weight (kg) |
|---|---|---|---|---|---|---|
| T1 | 2.5 | 0 | 6 | 1.5 | 0.5 | 10.5 |
| CT1 | 0 | 2.5 | 6 | 1.5 | 0.5 | 10.5 |
| T2 | 5 | 0 | 3.5 | 1.5 | 0.5 | 10.5 |
| CT2 | 0 | 5 | 3.5 | 1.5 | 0.5 | 10.5 |
| T3 | 7.5 | 0 | 1 | 1.5 | 0.5 | 10.5 |
| CT3 | 0 | 7.5 | 1 | 1.5 | 0.5 | 10.5 |
| Treatments | mi * (kg) | mf * (kg) | VL * (L) | Biomass Losses (kg) | Biomass Losses (%) |
|---|---|---|---|---|---|
| T1 | 10.5 ± 0.1 | 2.84 ± 0.05 | 2.46 ± 0.02 | 5.21 ± 0.05 | 72.99 ± 1.50 a |
| CT1 | 10.5 ± 0.1 | 2.88 ± 0.01 | 2.42 ± 0.03 | 5.20 ± 0.04 | 72.58 ± 1.19 a |
| T2 | 10.5 ± 0.1 | 3.40 ± 0.06 | 1.20 ± 0.10 | 5.90 ± 0.16 | 67.66 ± 2.04 a |
| CT2 | 10.5 ± 0.1 | 3.50 ± 0.07 | 1.16 ± 0.01 | 5.84 ± 0.08 | 66.67 ± 1.69 a |
| T3 | 10.5 ± 0.1 | 4.78 ± 0.12 | 0.10 ± 0.01 | 5.62 ± 0.12 | 54.47 ± 1.11 b |
| CT3 | 10.5 ± 0.1 | 4.79 ± 0.06 | 0.15 ± 0.06 | 5.54 ± 0.01 | 54.40 ± 1.59 b |
| CB Treatments | Initial Concentration (µg·kg−1) | Final Concentration (µg·kg−1) |
|---|---|---|
| T1 | 97.96 ± 1.81 a | 112.87 ± 1.92 b |
| T2 | 176.44 ± 1.90 c | 216.65 ± 1.16 d |
| T3 | 247.55 ± 1.69 e | 314.15 ± 1.54 f |
| Treatments | [Hgi] × mi µgHg | [Hgf] × mf µgHg | [HgL] × VL µgHg | mHg lost mass (%) |
|---|---|---|---|---|
| T1 | 1028.6 ± 1.06 | 320.1 ± 1.33 | 9.51 ± 0.11 | 68.8 ± 1.68 a |
| T2 | 1852.6 ± 0.20 | 735.6 ± 1.10 | 5.56 ± 0.41 | 60.2 ± 0.80 a |
| T3 | 2599.5 ± 1.75 | 1501.9 ± 1.11 | 0.68 ± 0.02 | 42.2 ± 1.11 b |
| Parameters | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| T1 | Control T1 | T2 | Control T2 | T3 | Control T3 | NTC-5167 | |
| Moisture (%) | 33.7 ± 2.29 a | 30.3 ± 1.18 a | 39.4 ± 1.49 b | 41.3 ± 1.34 b | 47.2 ± 0.98 c | 44.6 ± 1.11 d | Max. 25 |
| Moisture retention (%) | 132 ± 1.27 a | 137 ± 1.19 b | 119.5 ± 2.45 c | 124 ± 1.8 c | 103.8 ± 1.32 d | 110.7 ± 1.44 e | Min. 100 |
| pH | 7.09 ± 1.19 a | 7.70 ± 1.10 bc | 7.56 ± 1.41 b | 7.87 ± 1.08 c | 7.1 ± 1.03 a | 7.65 ± 2.33 b | Between 4 and 9 |
| Ash (%) | 14.05 ± 1.67 a | 17.7 ± 1.19 b | 13.55 ± 0.08 a | 11.8 ± 0.76 c | 12.19 ± 1 c | 10.7 ± 0.60 c | Max. 60 |
| CIC (meq·100 g−1) | 42.45 ± 0.67 a | 47.1 ± 0.67 b | 38.25 ± 0.38 c | 34.8 ± 0.22 d | 35.5 ± 0.43 e | 33.7 ± 0.12 e | Min. 30 |
| COOT (%) | 14.95 ± 1.38 a | 13.9 ± 1.7 a | 11 ± 2.03 ab | 9.91 ± 0.56 b | 10.81 ± 1 b | 11.9 ± 0.88 ab | Min. 15 |
| Relación C/N | 15 ± 1.76 a | 13 ± 0.97 a | 17 ± 0.36 b | 19 ± 0.73 bc | 18 ± 0.75 b | 21 ± 0.87 c | Max. 25 |
| TN (%) | 0.623 ± 0.8 ab | 0.770 ± 1.66 ab | 0.557 ± 0.01 a | 0.428 ± 0.01 b | 0.580 ± 0.83 ab | 0.700 ± 0.50 ab | Min. 1 |
| TP (%) | 0.282 ± 0.7 abc | 0.384 ± 0.3 abc | 0.346 ± 0.1 a | 0.209± 0.13 abc | 0.159 ± 0.02 b | 0.181± 0.05 c | Min. 1 |
| TK (%) | 1.074 ± 0.69 a | 1.580 ± 0.3 a | 1.145 ± 0.06 b | 0.854 ± 0.25 b | 0.582 ± 0.48 b | 0.807 ± 0.73 ab | Min. 1 |
| Total silicon (%) | 10.1 ± 0.69 a | 18.6 ± 0.9 b | 8.2 ± 0.13 cd | 7.9 ± 0.21 c | 8.5 ± 0.28 d | 7.8 ± 0.31 c | Max. 50 |
| THg (µg·kg−1) | 112.87 ± 1.92 a | - | 216.65 ± 1.16 b | - | 314.15 ± 1.54 c | - | Max. 17.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caraballo-Laza, M.J.; Ossa-Henao, D.M.; Urango-Cardenas, I.; Rosso-Pinto, M.; Guimarães, J.R.D.; Paternina-Uribe, R.; Palacios-Torres, Y.; Marrugo-Negrete, J. Composting as a Sustainable Approach for Managing Mercury-Contaminated Aquatic Biomass. Toxics 2025, 13, 553. https://doi.org/10.3390/toxics13070553
Caraballo-Laza MJ, Ossa-Henao DM, Urango-Cardenas I, Rosso-Pinto M, Guimarães JRD, Paternina-Uribe R, Palacios-Torres Y, Marrugo-Negrete J. Composting as a Sustainable Approach for Managing Mercury-Contaminated Aquatic Biomass. Toxics. 2025; 13(7):553. https://doi.org/10.3390/toxics13070553
Chicago/Turabian StyleCaraballo-Laza, María José, Diana Marcela Ossa-Henao, Iván Urango-Cardenas, Mauricio Rosso-Pinto, Jean Remy Davée Guimarães, Roberth Paternina-Uribe, Yuber Palacios-Torres, and José Marrugo-Negrete. 2025. "Composting as a Sustainable Approach for Managing Mercury-Contaminated Aquatic Biomass" Toxics 13, no. 7: 553. https://doi.org/10.3390/toxics13070553
APA StyleCaraballo-Laza, M. J., Ossa-Henao, D. M., Urango-Cardenas, I., Rosso-Pinto, M., Guimarães, J. R. D., Paternina-Uribe, R., Palacios-Torres, Y., & Marrugo-Negrete, J. (2025). Composting as a Sustainable Approach for Managing Mercury-Contaminated Aquatic Biomass. Toxics, 13(7), 553. https://doi.org/10.3390/toxics13070553











