Biochar Influences the Transformation and Translocation of Antimony in the Rhizosphere–Rice System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Materials
2.2. Soil Sampling, Biochar Preparation, and Subsequent Characterization
2.3. Pot Experiments
2.4. Sample Analysis
2.4.1. Environmental Factors of Rhizosphere Soil
2.4.2. Bioavailable Sb
2.4.3. The Sb Fractions
2.4.4. Sb Content in Plants
2.5. Statistical Analysis and Graphical Process
3. Results and Discussion
3.1. Properties of Soil and Biochar
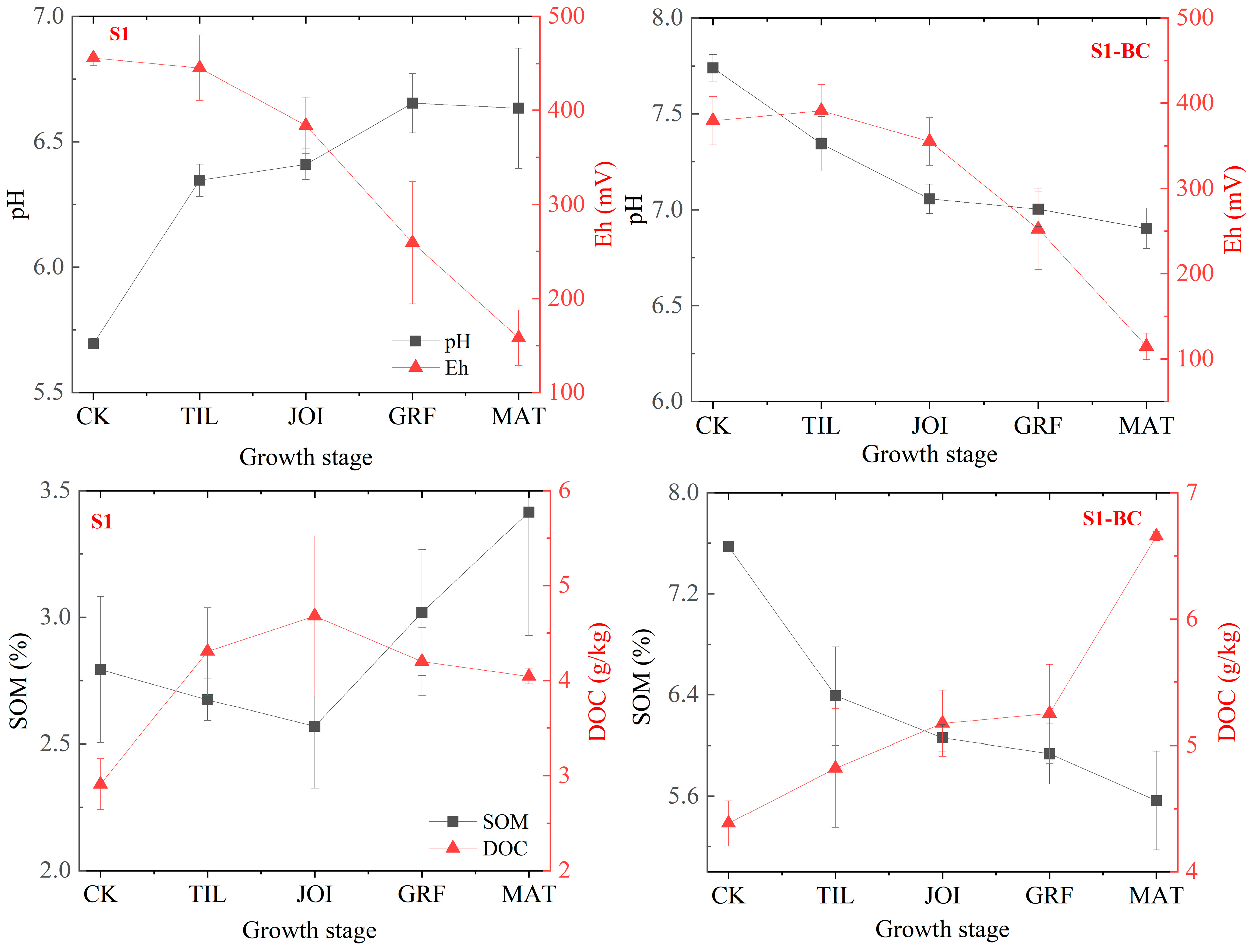
3.2. Variations in Environmental Factors in Rhizosphere
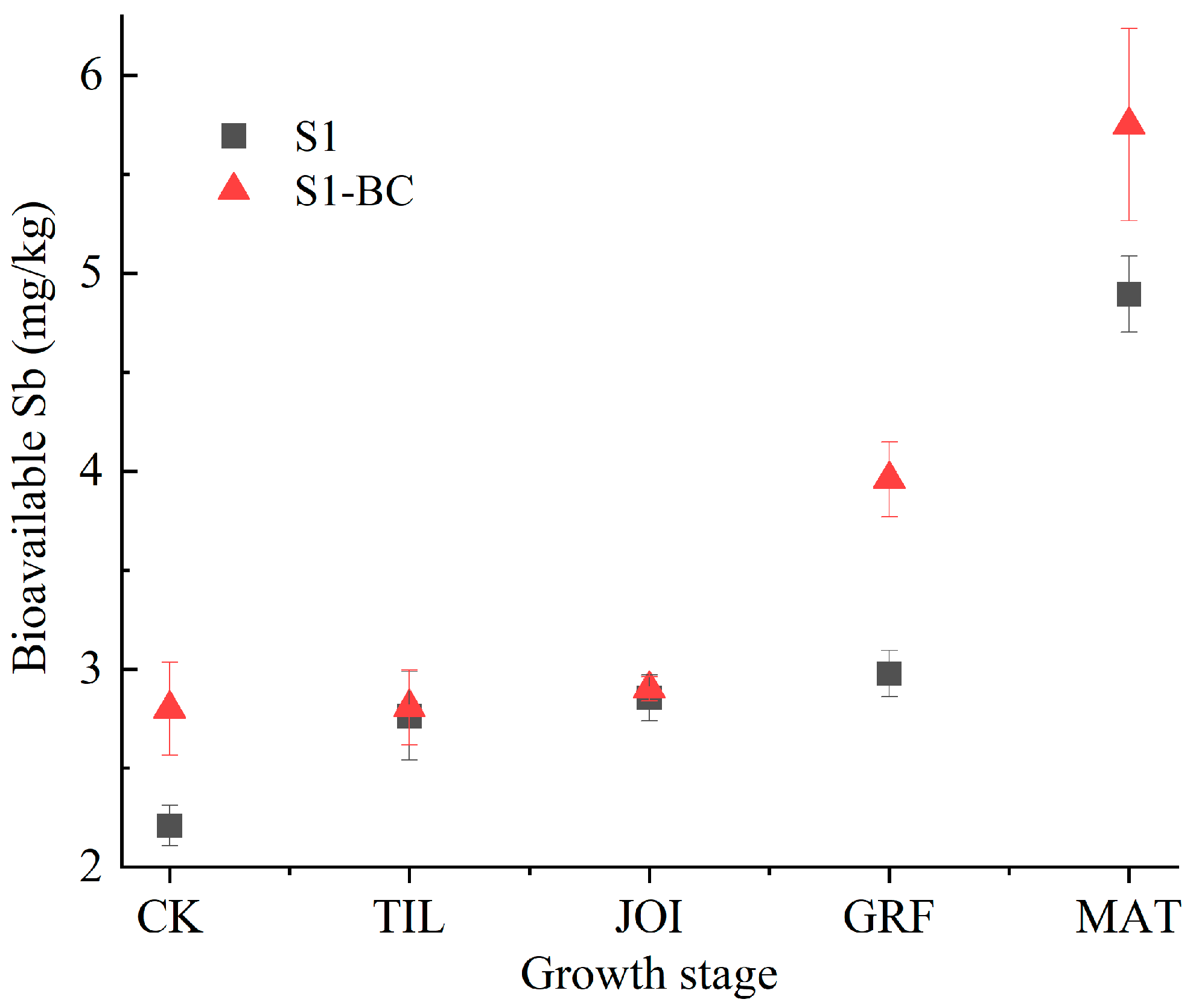
3.3. Bioavailable Sb in Rhizosphere
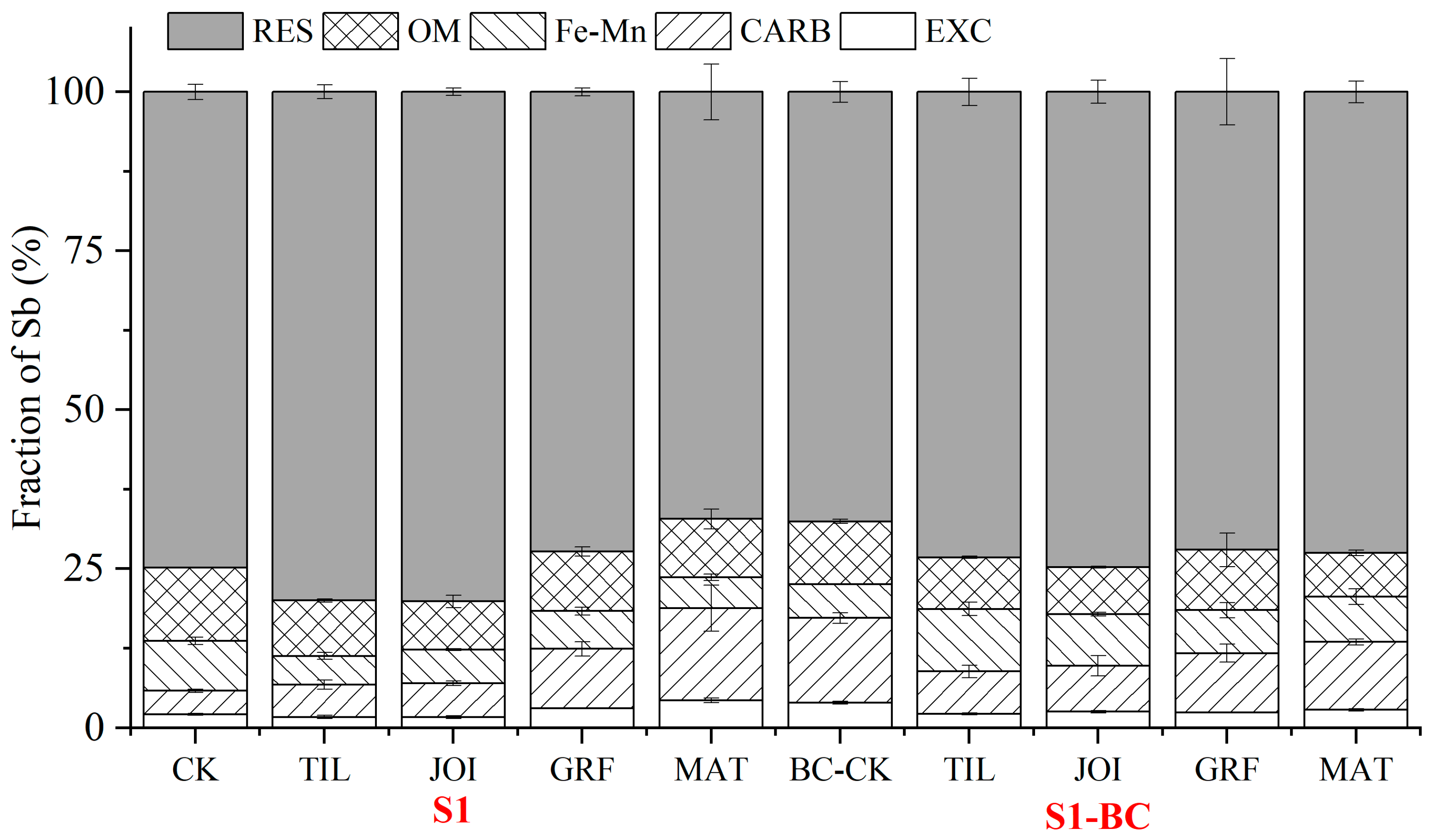
3.4. Redistribution of Sb Fractions in Rhizosphere
3.5. Sb Content in Rice Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, P.K.; Sonne, C.; Kim, K.H. Heavy metals and arsenic stress in food crops: Elucidating antioxidative defense mechanisms in hyperaccumulators for food security, agricultural sustainability, and human health. Sci. Total Environ. 2023, 874, 162327. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kumar, M.; Singh, E.; Kumar, A.; Singh, L.; Kumar, S.; Keerthanan, S.; Hoang, S.A.; El-Naggar, A.; Vithanage, M.; et al. Antimony contamination and its risk management in complex environmental settings: A review. Environ. Int. 2022, 158, 106908. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.G.; Hu, Q.; Zhao, F.J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Gautier di Confiengo, G.; Faga, M.G. Ecological transition in the field of brake pad manufacturing: An overview of the potential green constituents. Sustainability 2022, 14, 2508. [Google Scholar] [CrossRef]
- He, M.; Wang, X.; Wu, F.; Fu, Z. Antimony pollution in China. Sci. Total Environ. 2012, 421, 41–50. [Google Scholar] [CrossRef]
- Tang, H.; Hassan, M.U.; Nawaz, M.; Yang, W.; Liu, Y.; Yang, B. A review on sources of soil antimony pollution and recent progress on remediation of antimony polluted soils. Ecotoxicol. Environ. Saf. 2023, 266, 115583. [Google Scholar] [CrossRef]
- Diquattro, S.; Castaldi, P.; Ritch, S.; Juhasz, A.L.; Brunetti, G.; Scheckel, K.G.; Garau, G.; Lombi, E. Insights into the fate of antimony (Sb) in contaminated soils: Ageing influence on Sb mobility, bioavailability, bioaccessibility and speciation. Sci. Total Environ. 2021, 770, 145354. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Jaillard, B. Rhizosphere: A new frontier for soil biogeochemistry. J. Geochem. Explor. 2006, 88, 210–213. [Google Scholar] [CrossRef]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Rhizosphere: Its structure, bacterial diversity and significance. Rev. Environ. Sci. Bio-Technol. 2014, 13, 63–77. [Google Scholar] [CrossRef]
- Shen, J.; Bai, Y.; Wei, Z.; Chu, C.; Yuan, L.; Zhang, L.; Cui, Z.; Cong, W.; Zhang, F. Rhizobiont: An Interdisciplinary Innovation and Perspective for Harmonizing Resources, Environment, and Food Security. Acta Pedol. Sin. 2021, 58, 805–813, (In Chinese with English Abstract). [Google Scholar]
- Zhao, X.; Tian, P.; Sun, Z.; Liu, S.; Wang, Q.; Zeng, Z. Rhizosphere effects on soil organic carbon processes in terrestrial ecosystems: A meta-analysis. Geoderma 2022, 412, 115739. [Google Scholar] [CrossRef]
- Meier, I.C.; Pritchard, S.G.; Brzostek, E.R.; McCormack, M.L.; Phillips, R.P. The rhizosphere and hyphosphere differ in their impacts on carbon and nitrogen cycling in forests exposed to elevated CO2. New Phytol. 2015, 205, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Piao, S.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, M.K.; Zhuang, S.Y.; Chiang, P.N. Chemical and physical properties of rhizosphere and bulk soils of three tea plants cultivated in Ultisols. Geoderma 2006, 136, 378–387. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, M.; Cao, Y.; Wang, J.; Tang, M.; Ban, Y. Comparisons of soil properties, enzyme activities and microbial communities in heavy metal contaminated bulk and rhizosphere soils of Robinia pseudoacacia L. in the Northern foot of Qinling Mountain. Forests 2017, 8, 430. [Google Scholar] [CrossRef]
- Cheng, H.; Kong, T.; Lv, G.; Wang, D.; Zhang, L. Ecological stoichiometric characteristics of rhizosphere and non-rhizosphere soil of Pinus sylvestris var. mongolica plantations at different ages. J. Zhejiang A&F Univ. 2021, 38, 1058–1065, (In Chinese with English Abstract). [Google Scholar]
- Fang, W.; Yang, Y.; Wang, H.; Yang, D.; Luo, J.; Williams, P. Rice rhizospheric effects on the bioavailability of toxic trace elements during land application of biochar. Environ. Sci. Technol. 2021, 55, 7344–7354. [Google Scholar] [CrossRef]
- Fan, C.; Cui, Y.; Zhang, Q.; Yin, N.; Cai, X.; Yuan, X.; Senadheera, S.; Cho, Y.; Ok, Y.S. A critical review of the interactions between rhizosphere and biochar during the remediation of metal (loid) contaminated soils. Biochar 2023, 5, 87. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, M.; He, L.; Zhang, Z.; Gustave, W.; Vithanage, M.; Niazi, N.K.; Chen, B.; Zhang, X.; Wang, H.; et al. Challenges in safe environmental applications of biochar: Identifying risks and unintended consequence. Biochar 2025, 7, 12. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; El-Sherbini, M.A.; Selim, E.M. Effects of biochar on soil properties, heavy metal availability and uptake, and growth of summer squash grown in metal-contaminated soil. Sci. Hortic. 2022, 301, 111097. [Google Scholar] [CrossRef]
- Hua, L.; Wu, C.; Zhang, H.; Cao, L.; Wei, T.; Guo, J. Biochar-induced changes in soil microbial affect species of antimony in contaminated soils. Chemosphere 2021, 263, 127795. [Google Scholar] [CrossRef] [PubMed]
- Itam, D.H.; Horsfall, I.T.; Ekiyor, T.H. Application of Biochar in Soil Remediation: A Decade of Scientometrics and Systematic Review from 2014–2024. Results Eng. 2024, 23, 102757. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd Allah, E.F.; et al. Biochar production and characteristics, its impacts on soil health, crop production, and yield enhancement: A review. Plants 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, J.; Wang, L.; Lin, Z.; Dai, J.; Wang, R.; Yu, Y.; Liu, H.; Rensing, C.; Feng, R. Factors influencing the uptake and speciation transformation of antimony in the soil-plant system, and the redistribution and toxicity of antimony in plants. Sci. Total Environ. 2020, 738, 140232. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; El-Naggar, A.; Wang, H.; Du Laing, G.; Alessi, D.S.; Ok, Y.S. Redox-induced mobilization of Ag, Sb, Sn, and Tl in the dissolved, colloidal and solid phase of a biochar-treated and un-treated mining soil. Environ. Int. 2020, 140, 05754. [Google Scholar] [CrossRef]
- Meng, F.; Huang, Q.; Cai, Y.; Xiao, L.; Wang, T.; Li, X.; Wu, W.; Yuan, G. A comparative assessment of humic acid and biochar altering cadmium and arsenic fractions in a paddy soil. J. Soils. Sediments. 2023, 23, 845–855. [Google Scholar] [CrossRef]
- Qian, B.; Liu, L.; Xiao, X. Comparative tests on different methods for content of soil organic matter. J. Hohai Univ. Nat. Sci. 2011, 39, 34–38, (In Chinese with English Abstract). [Google Scholar]
- Xiao, L.; Yuan, G.; Bi, D.; Wei, J.; Shen, G. Equipment and technology of field preparation of biochars from agricultural and forest residues under aerobic conditions with water-fire coupled method. Trans. Chin. Soc. Agric. Eng. 2019, 35, 239–244, (In Chinese with English Abstract). [Google Scholar]
- IHSS. Available online: https://humic-substances.org/acidic-functional-groups-of-ihss-samples/#products (accessed on 2 February 2024).
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.; Casman, E.; Lowry, G. CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef]
- Zheng, M.; Feng, L.; He, J.; Chen, M.; Zhang, J.; Zhang, M.; Wang, J. Delayed geochemical hazard: A tool for risk assessment of heavy metal polluted sites and case study. J. Hazard. Mater. 2015, 287, 197–206. [Google Scholar] [CrossRef]
- Rezapour, S.; Atashpaz, B.; Moghaddam, S.; Kalavrouziotis, I.; Damalas, C. Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere 2019, 231, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Besold, J.; Eberle, A.; Noël, V.; Kujala, K.; Kumar, N.; Scheinost, A.C.; Pacheco, J.L.; Fendorf, S.; Planer-Friedrich, B. Antimonite binding to natural organic matter: Spectroscopic evidence from a mine water impacted peatland. Environ. Sci. Technol. 2019, 53, 10792–10802. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal (loid) s in soil: A review. Environ. Sci. Process. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, J.; Xie, X.; Zhang, Y.; Jing, C. Acidity-dependent mobilization of antimony and arsenic in sediments near a mining area. J. Hazard. Mater. 2022, 426, 127790. [Google Scholar] [CrossRef]
- Meng, F.; Huang, Q.; Cai, Y.; Li, F.; Yuan, G. Effects of biowaste-derived biochar on the dynamic behavior of cadmium fractions in soils. Environ. Sci. Pollut. Res. 2022, 29, 59043–59051. [Google Scholar] [CrossRef]
- Costa, L.; Zopfi, J.; Alewell, C.; Lehmann, M.F.; Lenz, M. Antimony Mobility in Soils: Current Understanding and Future Research Directions. Environ. Sci. Process. Impacts 2025, 27, 833–848. [Google Scholar] [CrossRef]
- Jayalath, N.; Mosley, L.; Fitzpatrick, R.; Marschner, P. Addition of organic matter influences pH changes in reduced and oxidised acid sulfate soils. Geoderma 2016, 262, 125–132. [Google Scholar] [CrossRef]
- Vives-Peris, V.; De Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R. Metal availability in contaminated soils: I. Effects of floodingand organic matter on changes in Eh, pH and solubility of Cd, Ni and Zn. Nutr. Cycl. Agroecosys. 2001, 61, 247–255. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and soil organic matter decomposition rates–synthesis of current knowledge and a way forward. Glob. Change Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Gmach, M.; Cherubin, M.; Kaiser, K.; Cerri, C. Processes that influence dissolved organic matter in the soil: A review. Sci. Agr. 2019, 77, e20180164. [Google Scholar] [CrossRef]
- Tsolis, V.; Barouchas, P. Biochar as soil amendment: The effect of biochar on soil properties using VIS-NIR diffuse reflectance spectroscopy, biochar aging and soil microbiology—A review. Land 2023, 12, 1580. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.S.; Hur, J. Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: A review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef]
- Pan, Y.; Koopmans, G.; Bonten, L.; Song, J.; Luo, Y.; Temminghoff, E.; Comans, R. Influence of pH on the redox chemistry of metal (hydr) oxides and organic matter in paddy soils. J. Soils. Sediments. 2014, 14, 1713–1726. [Google Scholar] [CrossRef]
- Pierart, A.; Shahid, M.; Séjalon-Delmas, N.; Dumat, C. Antimony bioavailability: Knowledge and research perspectives for sustainable agricultures. J. Hazard. Mater. 2015, 289, 219–234. [Google Scholar] [CrossRef]
- Hockmann, K.; Lenz, M.; Tandy, S.; Nachtegaal, M.; Janousch, M.; Schulin, R. Release of antimony from contaminated soil induced by redox changes. J. Hazard. Mater. 2014, 275, 215–221. [Google Scholar] [CrossRef]
- Nakamaru, Y.M.; Peinado, F.J. Effect of soil organic matter on antimony bioavailability after the remediation process. Environ. Pollut. 2017, 228, 425–432. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, J.G.; Kim, T.; Alessi, D.S.; Baek, K. Mobility of arsenic in soil amended with biochar derived from biomass with different lignin contents: Relationships between lignin content and dissolved organic matter leaching. Chem. Eng. J. 2020, 393, 124687. [Google Scholar] [CrossRef]
- Zhang, X.; Su, C.; Liu, X.; Liu, Z.; Gu, P.; Deng, M.; Liu, Q. Periodical changes of dissolved organic matter (DOM) properties induced by biochar application and its impact on downward migration of heavy metals under flood conditions. J. Clean. Prod. 2020, 275, 123787. [Google Scholar] [CrossRef]
- Hasan, M.; Kausar, D.; Akhter, G.; Shah, M. Evaluation of the mobility and pollution index of selected essential/toxic metals in paddy soil by sequential extraction method. Ecotoxicol. Environ. Saf. 2018, 147, 283–291. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Y.; Zhong, Y.; Guan, Y.; Chen, Q.; Du, W.; Liu, G. Biological calcium carbonate enhanced the ability of biochar to passivate antimony and lead in soil. Environ. Sci. Process. Impacts 2023, 25, 1365–1373. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, P.; Ye, Z.; Wen, B.; Beckie, R.D.; Zhou, A.; Zhou, Z.; Zhou, J. Antimony mobility in soil near historical waste rock at the world’s largest Sb mine, Central China. Sci. Total Environ. 2024, 921, 171194. [Google Scholar] [CrossRef]
- Ren, J.H.; Ma, L.Q.; Sun, H.J.; Cai, F.; Luo, J. Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci. Total Environ. 2014, 475, 83–89. [Google Scholar] [CrossRef]
- Meng, F.; Huang, Q.; Chen, W.; Cai, Y.; Yuan, G. Biochar affects the transformation and migration behavior of As and Cd in the rhizosphere-rice system at various growth stages. J. Soils. Sediments 2025, 25, 662–674. [Google Scholar] [CrossRef]
- Quan, G.; Fan, Q.; Sun, J.; Cui, L.; Wang, H.; Gao, B.; Yan, J. Characteristics of organo-mineral complexes in contaminated soils with long-term biochar application. J. Hazard. Mater. 2020, 384, 121265. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, C.; Gan, Y.; Wang, S. Impact of biochars on the iron plaque formation and the antimony accumulation in rice seedings. Bull. Environ. Contam. Toxicol. 2022, 109, 1088–1094. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Rahim, H.; Akbar, W.; Alatalo, J. A comprehensive literature review on cadmium (Cd) status in the soil environment and its immobilization by biochar-based materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Xu, M.; Gao, P.; Wu, J.; Ma, J.; Zhang, X.; Yang, G.; Long, L.; Chen, C.; Song, C.; Xiao, Y. Biochar promotes arsenic sequestration on iron plaques and cell walls in rice roots. Chemosphere 2022, 288, 132422. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Meng, F.; Chen, W.; Cai, Y.; Xiao, E. Biochar Influences the Transformation and Translocation of Antimony in the Rhizosphere–Rice System. Toxics 2025, 13, 389. https://doi.org/10.3390/toxics13050389
Huang Q, Meng F, Chen W, Cai Y, Xiao E. Biochar Influences the Transformation and Translocation of Antimony in the Rhizosphere–Rice System. Toxics. 2025; 13(5):389. https://doi.org/10.3390/toxics13050389
Chicago/Turabian StyleHuang, Qiuxiang, Fande Meng, Wenzhe Chen, Yongbing Cai, and Enzong Xiao. 2025. "Biochar Influences the Transformation and Translocation of Antimony in the Rhizosphere–Rice System" Toxics 13, no. 5: 389. https://doi.org/10.3390/toxics13050389
APA StyleHuang, Q., Meng, F., Chen, W., Cai, Y., & Xiao, E. (2025). Biochar Influences the Transformation and Translocation of Antimony in the Rhizosphere–Rice System. Toxics, 13(5), 389. https://doi.org/10.3390/toxics13050389




