Research Progress on the Preparation of Iron-Manganese Modified Biochar and Its Application in Environmental Remediation
Abstract
1. Introduction
2. Preparation Method of Iron-Manganese-Modified Biochar
2.1. Impregnation Pyrolysis Method
2.2. Hydrothermal Synthesis
2.3. Co-Precipitation
2.4. Sol-Gel Method
2.5. Mechanical Ball Milling
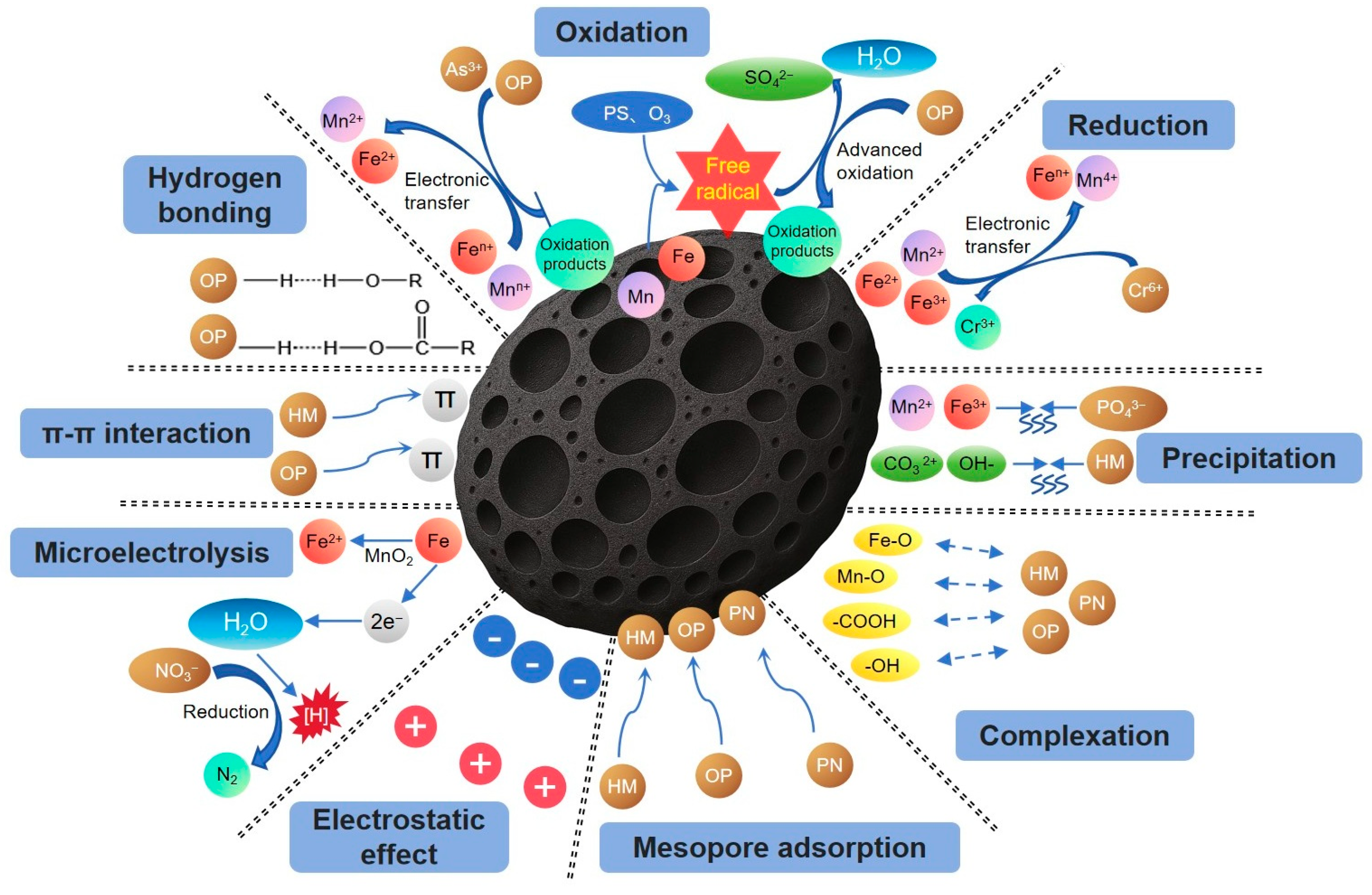
3. Removal of Heavy Metal Pollutants by Fe-Mn-Modified Biochar
3.1. Mesopore Adsorption
3.2. Reduction
3.3. Oxidation
3.4. Complexation
3.5. Electrostatic Effect
3.6. Precipitation
3.7. Cation-π Action
4. Removal of Organic Pollutants by Fe-Mn-Modified Biochar
4.1. Mesoporous Adsorption
4.2. Oxidation
4.3. Complexation
4.4. Hydrogen Bonding
4.5. Electrostatic Effect
4.6. π-π EDA Interaction
5. Removal of Inorganic Non-Metallic Salt Pollutants by Fe-Mn-Modified Biochar
5.1. Mesoporous Adsorption
5.2. Electrostatic Effect
5.3. Precipitation
5.4. Complexation
5.5. Microelectrolysis
6. Other Effects of Fe-Mn-Modified Biochar on the Environment
7. Prospects for Recycling of Iron-Manganese-Modified Biochar
7.1. Ultrapure Water Purification
7.2. Acid and Alkali Treatment
7.3. Magnetic Separation
7.4. Comparative Analysis of Regeneration Methods
8. Conclusions and Outlook
- Microstructural optimization and adsorption enhancement: To deepen understanding of the structure-activity relationship in FM-BC, advanced characterization techniques such as high-resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), and synchrotron radiation should be utilized. These tools can elucidate the links between crystal structure, pore architecture, elemental valence distribution, and adsorption behavior. By systematically regulating key preparation parameters—such as pyrolysis temperature, Fe/Mn molar ratio, and the type of activating agent—researchers can fine-tune the pore structure and surface functional properties of FM-BC. Such targeted modifications can lead to enhanced adsorption capacities and more stable pollutant immobilization, thereby minimizing the risk of secondary pollution due to leaching.
- Expansion of feedstocks and preparation methods: The feedstock base for FM-BC should be broadened beyond conventional agricultural and forestry residues. Unconventional biomass sources, such as algae, sewage sludge, and industrial organic waste, offer promising alternatives with unique physicochemical properties. Concurrently, the development of low-cost and energy-efficient synthesis methods, including co-pyrolysis with waste-derived additives, can reduce production costs and promote circular resource utilization. Capitalizing on the inherent functional groups and mineral compositions of these novel feedstocks may enable the fabrication of FM-BC with tailored adsorption functionalities, making it better suited for the removal of specific or complex pollutant mixtures.
- Engineering applications and field validation: To bridge the gap between laboratory findings and real-world deployment, greater emphasis must be placed on pilot-scale studies and field trials in contaminated sites such as mining regions, agricultural lands, and industrial zones. These studies should assess FM-BC’s long-term stability, pollutant retention performance, and ecological safety under variable environmental conditions. A particularly promising direction is the integration of FM-BC into permeable reactive barrier (PRB) systems. Given its porous structure and high pollutant affinity, FM-BC can serve as an effective filler material for continuous in situ groundwater remediation across large areas. Previous studies have demonstrated the feasibility of using metal-modified biochar in PRB systems for sustained contaminant removal, underscoring the engineering potential of FM-BC for site-specific applications.
- Comprehensive policy and economic considerations: Economic feasibility is pivotal for the large-scale adoption of FM-BC. Cost reductions can be achieved by coupling low-cost raw materials with optimized pyrolysis and modification processes. In addition, FM-BC’s extended operational lifespan and reusability in field conditions may translate into lower life-cycle costs compared with conventional sorbents. Strategic alignment with supportive environmental policies, such as subsidies for green materials and clear regulatory frameworks, can facilitate market adoption. Standardized technical guidelines and regulatory clarity will streamline approval processes and encourage broader implementation. By uniting technological scalability (e.g., PRB integration), cost-effectiveness, and policy support, the industrialization of FM-BC can be accelerated—contributing to the development of robust, replicable models for environmental remediation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, F.; Wang, X.; Xu, C. Research Progress on Structural Characteristics, Structure-Application Relationships, and Environmental Application of Biochar-Supported Zero Valent Iron (ZVI-BC). Curr. Pollut. Rep. 2023, 9, 292–311. [Google Scholar] [CrossRef]
- Puglla, E.P.; Guaya, D.; Tituana, C.; Osorio, F.; García-Ruiz, M.J. Biochar from Agricultural By-Products for the Removal of Lead and Cadmium from Drinking Water. Water 2020, 12, 2933. [Google Scholar] [CrossRef]
- Gotore, O.; Itayama, T.; Dang, B.-T.; Nguyen, T.-D.; Ramaraj, R.; Osamu, N.; Shuji, T.; Maseda, H. Adsorption Analysis of Ciprofloxacin and Delafloxacin onto the Corn Cob Derived-Biochar under Different Pyrolysis Conditions. Biomass Conv. Bioref. 2024, 14, 10373–10388. [Google Scholar] [CrossRef]
- Dróżdż, D.; Malińska, K.; Wystalska, K.; Meers, E.; Robles-Aguilar, A. The Influence of Poultry Manure-Derived Biochar and Compost on Soil Properties and Plant Biomass Growth. Materials 2023, 16, 6314. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, T.; Tang, Y.; Chen, G. A “Seawater-in-Sludge” Approach for Capacitive Biochar Production via the Alkaline and Alkaline Earth Metals Activation. Front. Environ. Sci. Eng. 2021, 15, 3. [Google Scholar] [CrossRef]
- Muema, F.M.; Richardson, Y.; Keita, A.; Sawadogo, M. An Interdisciplinary Overview on Biochar Production Engineering and Its Agronomic Applications. Biomass Bioenergy 2024, 190, 107416. [Google Scholar] [CrossRef]
- Simić, M.; Petrović, J.; Koprivica, M.; Ercegović, M.; Dimitrijević, J.; Vuković, N.S.; Fiol, N. Efficient Adsorption of Lead on Hydro-Pyrochar Synthesized by Two-Step Conversion of Corn Cob in Magnesium Chloride Medium. Toxics 2025, 13, 459. [Google Scholar] [CrossRef]
- Awasthi, M.K. Engineered Biochar: A Multifunctional Material for Energy and Environment. Environ. Pollut. 2022, 298, 118831. [Google Scholar] [CrossRef]
- Fazeli Sangani, M.; Abrishamkesh, S.; Owens, G. Physicochemical Characteristics of Biochars Can Be Beneficially Manipulated Using Post-Pyrolyzed Particle Size Modification. Bioresour. Technol. 2020, 306, 123157. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the Preparation and Application of Modified Biochar for Improved Contaminant Removal from Water and Wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wang, L.; Liang, L.; Li, N.; Chen, G.; Guo, H.; Hou, L. A Mini-Review of Sludge-Derived Biochar (SDB) for Wastewater Treatment: Recent Advances in 2020–2025. Appl. Sci. 2025, 15, 6173. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Zhu, Z.; Wan, L.; Wang, X.; Hou, J.; Liu, S.; Fan, X. Preparation of Chitosan-Iron Oxide Modified Sludge-Based Biochar for Effective Removal of Tetracycline from Water: Performance and Mechanism. Environ. Sci. Pollut. Res. 2023, 31, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Mosa, A.; Natasha; Abdelrahman, H.; Niazi, N.K.; Antoniadis, V.; Shahid, M.; Song, H.; Kwon, E.E.; Rinklebe, J. Removal of Toxic Elements from Aqueous Environments Using Nano Zero-Valent Iron- and Iron Oxide-Modified Biochar: A Review. Biochar 2022, 4, 24. [Google Scholar] [CrossRef]
- Zhong, X.; Lai, Y.; Wang, X.; Wang, M.; Han, W.; Zhang, M.; Ji, H. Synthesis and Environmental Applications of Biochar-Supported Nano-Zero-Valent Iron Composites: A Review. Environ. Chem. Lett. 2024, 22, 1345–1363. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Y.; Li, X. Adsorption Performance and Mechanism of Iron-Loaded Biochar to Methyl Orange in the Presence of Cr6+ from Dye Wastewater. J. Hazard. Mater. 2021, 415, 125749. [Google Scholar] [CrossRef]
- Kang, J.-K.; Seo, E.-J.; Lee, C.-G.; Park, S.-J. Fe-Loaded Biochar Obtained from Food Waste for Enhanced Phosphate Adsorption and Its Adsorption Mechanism Study via Spectroscopic and Experimental Approach. J. Environ. Chem. Eng. 2021, 9, 105751. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Zhang, P.; Shen, J.; Wang, S.; Li, Y. Development of Iron-Based Biochar for Enhancing Nitrate Adsorption: Effects of Specific Surface Area, Electrostatic Force, and Functional Groups. Sci. Total Environ. 2023, 856, 159037. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, L.; Zhang, Q.; Cao, Y.; Zhang, Y.; Kang, X. Enhanced Cr(VI) Removal by Biochar-Loaded Zero-Valent Iron Coupled with Weak Magnetic Field. J. Water Process Eng. 2022, 47, 102732. [Google Scholar] [CrossRef]
- Liu, H.; Yao, R.; Yu, M.; Ye, Z.; Lu, Y.; Yu, X.; Tang, J.; Sun, J. Converting Waste into Treasure: Efficient Adsorption of Cr(VI) Using Iron-Modified Rice Straw Biochar. Toxics 2025, 13, 458. [Google Scholar] [CrossRef]
- Jiang, B.-N.; Zhang, Y.-Y.; Wang, Y.; Liu, H.; Zhang, Z.-Y.; Yang, Y.-J.; Song, H.-L. Microbial Biomass Stoichiometry and Proportion of Fe Organic Complexes Separately Shape the Heterogeneity of Mixotrophic Denitrification and Net N2O Sinks in Iron-Carbon Amended Ecological Ditch. Water Res. 2025, 272, 122945. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Yu, Q.; Chen, J.; Yu, J.; Fang, Z.; Qiu, X. Double-Edged Effect of Frequent Freeze-Thaw on the Stability of Zero-Valent Iron after Heavy Metal Remediation. J. Hazard. Mater. 2024, 465, 132977. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Li, L.; Shi, Y.; Zhang, Y.; Wei, L.; Dong, C.-L.; Lin, Z.; Su, J. Concurrently Boosting Activity and Stability of Oxygen Reduction Reaction Catalysts via Judiciously Crafting Fe-Mn Dual Atoms for Fuel Cells. Nano Micro Lett. 2025, 17, 88. [Google Scholar] [CrossRef]
- Lin, X.; Ge, Q.; Zhou, X.; Wang, Y.; Zhu, C.; Liu, K.; Wan, J. Enhancement of Electron Transfer Between Fe/Mn Promotes Efficient Activation of Peroxomonosulfate by FeMn-NBC. Water 2025, 17, 1700. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Shi, Y.; He, Y.-L.; Yang, S.-R.; Yu, S.-Y.; Xiong, Z.; Zhang, H.; Yao, G.; He, C.-S.; Lai, B. Synthesis of Fe-Mn-Based Materials and Their Applications in Advanced Oxidation Processes for Wastewater Decontamination: A Review. Ind. Eng. Chem. Res. 2023, 62, 10828–10848. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J.; Chen, S.; Wang, J.; Yang, Y. Enhanced Removal of Heavy Metal Ions from Aqueous Solution Using Manganese Dioxide-Loaded Biochar: Behavior and Mechanism. Sci. Rep. 2020, 10, 6067. [Google Scholar] [CrossRef] [PubMed]
- Guillemet-Fritsch, S.; Navrotsky, A.; Tailhades, P.; Coradin, H.; Wang, M. Thermochemistry of Iron Manganese Oxide Spinels. J. Solid. State Chem. 2005, 178, 106–113. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, G.; Liu, L.; He, F.; Arinzechi, C.; Liao, Q.; Yang, W.; Si, M. Simultaneous Immobilization of Lead, Cadmium and Arsenic in Soil by Iron-Manganese Modified Biochar. Front. Environ. Sci. 2023, 11, 1281341. [Google Scholar] [CrossRef]
- Fu, N.; Shen, Y.; Allen, A.R.; Song, L.; Ozaki, A.; Lin, S. Mn-Catalyzed Electrochemical Chloroalkylation of Alkenes. ACS Catal. 2019, 9, 746–754. [Google Scholar] [CrossRef]
- Liang, Y.; Tao, R.; Zhao, B.; Meng, Z.; Cheng, Y.; Yang, F.; Lei, H.; Kong, L. Roles of Iron and Manganese in Bimetallic Biochar Composites for Efficient Persulfate Activation and Atrazine Removal. Biochar 2024, 6, 41. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Ma, X.; Cao, T.; Xu, J.; Feng, H.; Diao, R.; Qi, F.; Huang, H.; Ma, P. Efficient Preparation of Biomass-Based Ultra-Thin 2D Porous Carbon Materials by In Situ Template-Activation And Its Application in Sodium Ion Capacitors. Adv. Funct. Mater. 2024, 34, 2310717. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, S.-F.; Huang, B.-C.; Shen, X.-C.; Chen, W.-J.; Zhou, T.-P.; Cheng, H.-Y.; Cheng, B.-H.; Wu, C.-Z.; Li, W.-W.; et al. Sustainable Production of Value-Added Carbon Nanomaterials from Biomass Pyrolysis. Nat. Sustain. 2020, 3, 753–760. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z.; Chau, H.W. Arsenic Removal in Aqueous Solution by a Novel Fe-Mn Modified Biochar Composite: Characterization and Mechanism. Ecotoxicol. Environ. Saf. 2017, 144, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from Aqueous Solutions by Ferromanganese Binary Oxide-Biochar Composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, B.; Yuan, C. Adsorption of Atrazine by Fe-Mn-Modified Biochar: The Dominant Mechanism of π-π Interaction and Pore Structure. Agronomy 2022, 12, 3097. [Google Scholar] [CrossRef]
- Hyväluoma, J.; Hannula, M.; Arstila, K.; Wang, H.; Kulju, S.; Rasa, K. Effects of Pyrolysis Temperature on the Hydrologically Relevant Porosity of Willow Biochar. J. Anal. Appl. Pyrolysis 2018, 134, 446–453. [Google Scholar] [CrossRef]
- Mabagala, F.S.; Wang, T.; Feng, Q.; Zeng, X.; He, C.; Wu, C.; Zhang, N.; Su, S. Application of Iron-Bimetal Biochar for As and Cd Reduction and Soil Organic Carbon Preservation Under Varying Moisture. Agriculture 2025, 15, 1114. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Lee, Y.J. Facile One-Pot Hydrothermal Synthesis of Cubic Spinel-Type Manganese Ferrite/Biochar Composites for Environmental Remediation of Heavy Metals from Aqueous Solutions. Bioresour. Technol. 2018, 261, 1–9. [Google Scholar] [CrossRef]
- Jang, E.-S.; Ryu, D.-Y.; Kim, D. Hydrothermal Carbonization Improves the Quality of Biochar Derived from Livestock Manure by Removing Inorganic Matter. Chemosphere 2022, 305, 135391. [Google Scholar] [CrossRef]
- Deng, P.; Yuan, W.; Wang, J.; Li, L.; Zhou, Y.; Beiyuan, J.; Xu, H.; Jiang, S.; Tan, Z.; Gao, Y.; et al. Enhanced Passivation of Thallium, Vanadium and Arsenic in Contaminated Soils: Critical Role of Fe-Mn-Biochar. Biochar 2024, 6, 61. [Google Scholar] [CrossRef]
- Lian, B.; Wu, J.; Zhao, K.; Ye, Z.; Yuan, F. Novel Insight into the Adsorption Mechanism of Fe-Mn Oxide-Microbe Combined Biochar for Cd(II) and As(III). Environ. Sci. 2022, 43, 1584–1595. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Y.; Niu, X.; Wu, B.; Liu, X.; Hao, F.; Ma, Z.; Cai, H.; Liu, Y. The Efficient Degradation of Oxytetracycline in Wastewater Using Fe/Mn-Modified Magnetic Oak Biochar: Pathways and Mechanistic Investigation. Magnetochemistry 2025, 11, 49. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Xu, B. Principle, Advance and Current Application Situation of Sol-Gel Method. Chem. Ind. Eng. 2009, 26, 273–277. [Google Scholar]
- Han, J.; Guo, J.; Zhang, L.; Wang, W.; Li, Y.; Sun, P.; Jiang, Q. Adsorption Test of Biochar-MnFe2O4 to Zn2+ and Cu2+. Water Resour. Prot. 2020, 36, 59–64. [Google Scholar]
- Che, N.; Qu, J.; Wang, J.; Liu, N.; Li, C.; Liu, Y. Adsorption of Phosphate onto Agricultural Waste Biochars with Ferrite/Manganese Modified-Ball-Milled Treatment and Its Reuse in Saline Soil. Sci. Total Environ. 2024, 915, 169841. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Chen, J.; Chen, H.; Gao, B. MgO Modified Biochar Produced through Ball Milling: A Dual-Functional Adsorbent for Removal of Different Contaminants. Chemosphere 2020, 243, 125344. [Google Scholar] [CrossRef]
- Zhao, F.; Shan, R.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y. Magnetically Recyclable Loofah Biochar by KMnO4 Modification for Adsorption of Cu(II) from Aqueous Solutions. ACS Omega 2022, 7, 8844–8853. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Li, C.; Chen, Y.; Zheng, L.; Ding, D.; Shan, S. Synergistic Mechanism of Iron Manganese Supported Biochar for Arsenic Remediation and Enzyme Activity in Contaminated Soil. J. Environ. Manag. 2023, 347, 119127. [Google Scholar] [CrossRef]
- Yin, G.; Song, X.; Tao, L.; Sarkar, B.; Sarmah, A.K.; Zhang, W.; Lin, Q.; Xiao, R.; Liu, Q.; Wang, H. Novel Fe-Mn Binary Oxide-Biochar as an Adsorbent for Removing Cd(II) from Aqueous Solutions. Chem. Eng. J. 2020, 389, 124465. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, Q.; Li, B.; Zhou, T.; Cen, Q.; Liu, Z.; Zhou, Y. Insights into Remediation of Cadmium and Lead Contaminated-Soil by Fe-Mn Modified Biochar. J. Environ. Chem. Eng. 2024, 12, 112771. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G.; Xing, B. Facile Synthesis of Multifunctional Bone Biochar Composites Decorated with Fe/Mn Oxide Micro-Nanoparticles: Physicochemical Properties, Heavy Metals Sorption Behavior and Mechanism. J. Hazard. Mater. 2020, 399, 123067. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luo, Y.; Yi, Y.; Su, S.; Qin, W. Peroxymonosulfate Activation by Fe-Mn Co-Doped Biochar for Carbamazepine Degradation. RSC Adv. 2024, 14, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, J.; Xiang, Y.; Jia, M.; Xiong, W.; Yang, Z.; Peng, H.; Ye, Y. Fe/Mn Modified Biochar as Electrode Particles in Electrochemical System for Efficient Anaerobic Sludge Digestion. Chem. Eng. J. 2023, 472, 144754. [Google Scholar] [CrossRef]
- Liu, J.; Ren, S.; Cao, J.; Tsang, D.C.W.; Beiyuan, J.; Peng, Y.; Fang, F.; She, J.; Yin, M.; Shen, N.; et al. Highly Efficient Removal of Thallium in Wastewater by MnFe2O4-Biochar Composite. J. Hazard. Mater. 2021, 401, 123311. [Google Scholar] [CrossRef]
- Yu, C.; Yang, J. Removal of Cr(VI) in Wastewater by Fe-Mn Oxide Loaded Sludge Biochar. RSC Adv. 2024, 14, 11746–11757. [Google Scholar] [CrossRef]
- Wang, H.; Xia, H.; Chen, Q.; Liu, R.; Zhang, Y. Enhanced Removal Capacities and Mechanisms of Mn/Fe-Loaded Biochar Composites Functionalized with Chitosan Schiff-Base or Hydroxyl toward Pb(II) and Cd(II) from Aqueous Solutions. J. Environ. Chem. Eng. 2023, 11, 111132. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Wan, Y.; Creamer, A.E. Sorption of Arsenate onto Magnetic Iron-Manganese (Fe-Mn) Biochar Composites. RSC Adv. 2015, 5, 67971–67978. [Google Scholar] [CrossRef]
- Qu, J.; Che, N.; Niu, G.; Liu, L.; Li, C.; Liu, Y. Iron/Manganese Binary Metal Oxide-Biochar Nano-Composites with High Adsorption Capacities of Cd2+: Preparation and Adsorption Mechanisms. J. Water Process. Eng. 2023, 51, 103332. [Google Scholar] [CrossRef]
- Lin, L.; Huang, Q.; Lian, F.; Liu, Z.; Song, Z. Effect of humic acid and pH on the adsorption of arsenic(III) on biochar-ferro manganese oxide composite material. J. Agro Environ. Sci. 2017, 36, 387–393. [Google Scholar]
- Verma, L.; Singh, J. Arsenic Adsorption from Aqueous Solution and Groundwater Using Monometallic (Fe) and Bimetallic (Fe/Mn) Tectona Biochar Synthesized from Plant Refuse: Mechanism, Isotherm, and Kinetic Study. Environ. Eng. Res. 2022, 28, 220110. [Google Scholar] [CrossRef]
- Tan, W.-T.; Zhou, H.; Tang, S.-F.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Enhancing Cd(II) Adsorption on Rice Straw Biochar by Modification of Iron and Manganese Oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, T.; Dong, J.; Chen, G.; Li, Z.; Zhou, J.; Chen, Z. Enhanced Cr (VI) Reduction and Removal by Fe/Mn Oxide Biochar Composites under Acidic Simulated Wastewater. Environ. Sci. Pollut. Res. 2022, 30, 31489–31500. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, W.; Gao, L.; Liang, X.; Yang, Q. Removal of Hexavalent Chromium in Water by Chitosan-Modified Enteromorpha Prolifera Biochar Loaded with Iron-Manganese Oxides: Application Performances and Reaction Mechanisms. Mater. Chem. Phys. 2024, 317, 129189. [Google Scholar] [CrossRef]
- Zhu, Y.; Dai, W.; Deng, K.; Pan, T.; Guan, Z. Efficient Removal of Cr(VI) from Aqueous Solution by Fe-Mn Oxide-Modified Biochar. Water Air Soil Pollut. 2020, 231, 61. [Google Scholar] [CrossRef]
- Sun, T.; Sun, Y.; Xu, Y.; Wang, L.; Liang, X. Effective Removal of Hg2+ and Cd2+ in Aqueous Systems by Fe-Mn Oxide Modified Biochar: A Combined Experimental and DFT Calculation. Desalination 2023, 549, 116306. [Google Scholar] [CrossRef]
- Tang, S.-F.; Zhou, H.; Tan, W.-T.; Huang, J.-G.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Adsorption Characteristics and Mechanisms of Fe-Mn Oxide Modified Biochar for Pb(II) in Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 8420. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Liu, R.; Zhang, Y.; Zhang, Y. Synthesis and Application of Starch-Stablized Fe-Mn/Biochar Composites for the Removal of Lead from Water and Soil. Chemosphere 2022, 305, 135494. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, M.; Xu, X.; Cao, X.; Ippolito, J.A.; Mohanty, S.K.; Ni, B.-J.; Xu, S.; Tsang, D.C.W. Electron Donation of Fe-Mn Biochar for Chromium(VI) Immobilization: Key Roles of Embedded Zero-Valent Iron Clusters within Iron-Manganese Oxide. J. Hazard. Mater. 2023, 456, 131632. [Google Scholar] [CrossRef]
- Xie, X.; Cao, M.; Tu, S.; Xiong, S.; Zheng, M. Adsorption Performance of Cd(II) and As(III) in Aqueous Solution by Iron-Manganese Modified Biochar Synthesized via Microwave-Assisted Low-Temperature Oxidation. J. Environ. Chem. Eng. 2025, 13, 118073. [Google Scholar] [CrossRef]
- Han, J.; Zhao, C.; Yang, M.; Ye, M.; Li, Y.; Zhou, K.; Zhang, J.; Song, P. Comparative Remediation of Arsenic and Antimony Co-Contaminated Soil by Iron- and Manganese-Modified Activated Carbon and Biochar. Toxics 2024, 12, 740. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Wang, L. Enhanced Hexavalent Chromium (Cr(VI)) Removal from Aqueous Solution by Fe-Mn Oxide-Modified Cattail Biochar: Adsorption Characteristics and Mechanism. Chem. Ecol. 2020, 36, 138–154. [Google Scholar] [CrossRef]
- Chu, J.; Xin, S.; He, Y.; Gao, X.; Zhou, X.; Zhang, Y.; Liu, X.; Zhang, X.; Wang, S. Enhanced Adsorption of Hexavalent Chromium from Aqueous Solutions by Iron- Manganese Modified Cedarwood Biochar: Synthesis, Performance, Mechanism, and Variables. Bull. Environ. Contam. Toxicol. 2023, 111, 43. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L. Removal Mechanisms of Cd from Water and Soil Using Fe-Mn Oxides Modified Biochar. Environ. Res. 2022, 212, 113406. [Google Scholar] [CrossRef]
- Liang, M.; Xu, S.; Zhu, Y.; Chen, X.; Deng, Z.; Yan, L.; He, H. Preparation and Characterization of Fe-Mn Binary Oxide/Mulberry Stem Biochar Composite Adsorbent and Adsorption of Cr(VI) from Aqueous Solution. Int. J. Environ. Res. Public Health 2020, 17, 676. [Google Scholar] [CrossRef]
- Xu, L.; He, Z.; Wei, X.; Shang, Y.; Shi, J.; Jin, X.; Bai, X.; Shi, X.; Jin, P. Facile-Prepared Fe/Mn Co-Doped Biochar Is an Efficient Catalyst for Mediating the Degradation of Aqueous Ibuprofen via Catalytic Ozonation. Chem. Eng. J. 2023, 461, 142028. [Google Scholar] [CrossRef]
- Tao, H.; Ge, H.; Shi, J.; Liu, X.; Guo, W.; Zhang, M.; Meng, Y.; Li, X. The Characteristics of Oestrone Mobility in Water and Soil by the Addition of Ca-Biochar and Fe-Mn-Biochar Derived from Litchi Chinensis Sonn. Environ. Geochem. Health 2020, 42, 1601–1615. [Google Scholar] [CrossRef]
- Liu, J.; Luo, K.; Xiao, Q.; Zhang, Z.; Wu, C.; Wang, X. The Removal of Ciprofloxacin by Fe-Mn Oxides-Loaded Biochar Composite. Environ. Sci. Technol. 2019, 42, 61–67. [Google Scholar] [CrossRef]
- Chu, D.; Dong, H.; Li, Y.; Jin, Z.; Xiao, J.; Xiang, S.; Dong, Q.; Hou, X. Enhanced Activation of Sulfite by a Mixture of Zero-Valent Fe-Mn Bimetallic Nanoparticles and Biochar for Degradation of Sulfamethazine in Water. Sep. Purif. Technol. 2022, 285, 120315. [Google Scholar] [CrossRef]
- Hou, D.; Wang, P.; Zhang, P.; Fan, C.; Cao, K.; Zou, J. Enhanced Peroxymonosulfate Activation by Biogenic Iron-Manganese Oxide on Biochar: Singlet Oxygen Generation and Synergistic Mechanism. Chem. Eng. J. 2024, 497, 154460. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Wan, Y.; Bai, X.; Chen, H.; Zheng, Y.; Zhang, Y.; Yang, Y.; Fan, Y.; Gao, B. Removal of Sulfamethoxazole Using Fe-Mn Biochar Filtration Columns: Influence of Co-Existing Polystyrene Microplastics. J. Clean. Prod. 2024, 477, 143877. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Tang, J.; Deng, Z.; Zeng, C.; Zhang, Z. An In-Situ Electrogenerated Persulfate and Its Activation by Functionalized Sludge Biochar for Efficient Degradation of Sulfamethoxazole. J. Clean. Prod. 2023, 423, 138768. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, H.; Du, Y.; Cheng, Q.; Liu, Y.; Ma, J.; Yang, S.; Lin, H. Unraveling How Fe-Mn Modified Biochar Mitigates Sulfamonomethoxine in Soil Water: The Activated Biodegradation and Hydroxyl Radicals Formation. J. Hazard. Mater. 2024, 465, 133490. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, Q.; Wang, Z.; Gao, B.; Fan, Z.; Li, M.; Hao, H.; Wei, X.; Zhong, M. Degradation of Anthraquinone Dye Reactive Blue 19 Using Persulfate Activated with Fe/Mn Modified Biochar: Radical/Non-Radical Mechanisms and Fixed-Bed Reactor Study. Sci. Total Environ. 2021, 758, 143584. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Song, Z.; Xu, Y.; Gao, M. Response of Soil Characteristics to Biochar and Fe-Mn Oxide-Modified Biochar Application in Phthalate-Contaminated Fluvo-Aquic Soils. Ecotoxicol. Environ. Saf. 2021, 225, 112755. [Google Scholar] [CrossRef]
- Xu, Y.; Song, Z.; Chang, X.; Guo, Z.; Gao, M. Effects of Fe-Mn Oxide-Modified Biochar Composite Applications on Phthalate Esters (PAEs) Accumulation in Wheat Grains and Grain Quality under PAEs-Polluted Brown Soil. Ecotoxicol. Environ. Saf. 2021, 208, 111624. [Google Scholar] [CrossRef]
- Gan, Y.; Cao, Q. Degradation of Rhodamine B by Activating Potassium Persulfate with Biochar-Loaded Fe-Mn Oxides. J. Southwest Univ. (Nat. Sci. Ed.) 2024, 46, 127–136. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Chen, Y.; Shen, S.; Xue, J.; Ma, Y.; Zheng, L.; Wu, L.; Zhang, Z.; Yang, L. Iron-Manganese Oxide Loaded Sludge Biochar as a Novel Periodate Activator for Thiacloprid Efficient Degradation over a Wide pH Range. Sep. Purif. Technol. 2022, 288, 120703. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Z.; Chen, X.; Xi, S.; Cui, K.; Li, J.; Dong, D.; Wu, F.; Wu, Z. Efficient Degradation of Thiamethoxam Pesticide in Water by Iron and Manganese Oxide Composite Biochar Activated Persulfate. Chem. Eng. J. 2023, 473, 145051. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Y.; Lv, K.; Zhu, C.; Guan, X.; Xie, B.; Zou, X.; Luo, X.; Zhou, Y. N-Doped Biochar-Fe/Mn as a Superior Peroxymonosulfate Activator for Enhanced Bisphenol a Degradation. Water Res. 2025, 278, 123399. [Google Scholar] [CrossRef]
- Li, Y.; Lin, D.; Li, Y.; Jiang, P.; Fang, X.; Yu, B. Nonradical-Dominated Peroxymonosulfate Activation through Bimetallic Fe/Mn-Loaded Hydroxyl-Rich Biochar for Efficient Degradation of Tetracycline. Nano Res. 2023, 16, 155–165. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Z.; Jiang, X.; Li, J.; Xiao, K.; Xu, W.; Chen, X.; Liang, J.; Lin, Z.; Li, M.; et al. NaOH-Modified Biochar Supported Fe/Mn Bimetallic Composites as Efficient Peroxymonosulfate Activator for Enhance Tetracycline Removal. Chem. Eng. J. 2023, 454, 139949. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, X.; Xie, R.; Zhang, Y.; Jin, Y.; Jiang, W. A Novel Porous Biochar-Supported Fe-Mn Composite as a Persulfate Activator for the Removal of Acid Red 88. Sep. Purif. Technol. 2020, 250, 117232. [Google Scholar] [CrossRef]
- Alazba, A.A.; Shafiq, M.; Amin, M.T. Transforming Conocarpus Hedge Wasteinto a Highly Effective Iron/ManganeseNanocomposite Biochar for Efficient MethyleneBlue Dye Removal from Aqueous Solution. Pol. J. Environ. Stud. 2025, 34, 3033–3045. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, Z.; Zhou, Y.; Wei, Y.; Long, X.; He, Y.; Zhi, D.; Yang, J.; Luo, L. A Sustainable Ferromanganese Biochar Adsorbent for Effective Levofloxacin Removal from Aqueous Medium. Chemosphere 2019, 237, 124464. [Google Scholar] [CrossRef]
- Jiao, G.; Zhou, H.; Li, X.; Liu, J.; She, D. Degradation of Oxytetracycline by Iron-Manganese Modified Industrial Lignin-Based Biochar Activated Peroxy-Disulfate: Pathway and Mechanistic Analysis. Bioresour. Technol. 2023, 384, 129357. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Song, C.; Liu, Z. A Method for Repairing Soil Contaminated with Chlorinated Organic Compounds by Low-Temperature Plasma Coupled with Iron-Manganese Oxides-Biochar. China:CN 118988960 A, 22 November 2024. [Google Scholar]
- Fu, C.; Zhou, M.; Song, W.; Yang, G.; Feng, P.; Chulalaksananukul, W.; Zhu, S.; Huang, K.; Wang, Z. Innovative Iron-manganese Modified Microalgae Biochar for Efficient Phosphate Iron Removal from Water: Preparation and Adsorption Mechanisms. J. Water Process. Eng. 2024, 66, 106051. [Google Scholar] [CrossRef]
- Beiyuan, J.; Wu, X.; Ruan, B.; Chen, Z.; Liu, J.; Wang, J.; Li, J.; Xu, W.; Yuan, W.; Wang, H. Highly Efficient Removal of Aqueous Phosphate via Iron-Manganese Fabricated Biochar: Performance and Mechanism. Chemosphere 2024, 364, 143207. [Google Scholar] [CrossRef]
- Sun, T.; Gao, F.; Lin, L.; Li, R.; Dong, L. Adsorption of Low-Concentration Phosphorus from Water by Composite Metal Modified Biochar. Environ. Sci. 2020, 41, 784–791. [Google Scholar] [CrossRef]
- Sun, Y.; Ju, K.; Cao, Y.; Zhang, X.; Yang, G.; Li, X.; Wan, Q. Enhanced Nitrate Removal and Nitrogen-Selective Conversion Mechanism of a Combined Sponge Iron/Biochar/Manganese Sand System. Chem. Eng. Res. Des. 2022, 181, 343–353. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, A.; Zhang, Y.; Shi, L.; Zhang, X. Characteristic of Nitrate Adsorption in Aqueous Solution by Iron and Manganese Oxide/Biochar Composite. Environ. Sci. 2018, 39, 1220–1232. [Google Scholar] [CrossRef]
- Li, X.; Bin, B.; Gong, Y.; Wu, Z.; Zeng, X. Construction of iron-manganese modified mulberry branch biochar and the adsorption of aqueous phosphorus. Ind. Water Treat. 2025, 45, 175–184. [Google Scholar] [CrossRef]
- Jia, L.; Liu, H.; Kong, Q.; Li, M.; Wu, S.; Wu, H. Interactions of High-Rate Nitrate Reduction and Heavy Metal Mitigation in Iron-Carbon-Based Constructed Wetlands for Purifying Contaminated Groundwater. Water Res. 2020, 169, 115285. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Liu, J.; Xue, J. A Review of Research Advances in the Effects of Biochar on Soil Nitrogen Cycling and Its Functional Microorganisms. J. Ecol. Rural. Environ. 2022, 38, 689–701. [Google Scholar] [CrossRef]
- Ma, S.; Ma, S.; Yin, W.; Wang, S.; Sheng, H.; Wang, X. Effects of Biochar on the Availability of Trace Elements in Different Types of Soil. Toxics 2025, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chang, X.; Xu, Y.; Guo, Z.; Song, Z. Effects of Fe-Mn Impregnated Biochar on Enzymatic Activity and Bacterial Community in Phthalate-Polluted Brown Soil Planted with Wheat. Environ. Pollut. 2021, 284, 117179. [Google Scholar] [CrossRef]
- Sun, T.; Gao, G.; Yang, W.; Sun, Y.; Huang, Q.; Wang, L.; Liang, X. High-Efficiency Remediation of Hg and Cd Co-Contaminated Paddy Soils by Fe-Mn Oxide Modified Biochar and Its Microbial Community Responses. Biochar 2024, 6, 57. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Liu, X.; Qiu, W.; Song, Z. Effects of Fe-Mn Modified Biochar Composite Treatment on the Properties of As-Polluted Paddy Soil. Environ. Pollut. 2019, 244, 600–607. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.; Zhang, R.; Sheng, G. Research Progress of Manganese-Loaded Biochar Preparation and Its Application in Environmental Remediation. Chem. Ind. Eng. Prog. 2023, 42, 4385–4397. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Li, Y.; Chen, X. Solidification/stabilization of As in soil using biochar loaded with ferric manganese binary oxides(fmbo). Environ. Eng. 2022, 40, 118–125. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, D.; Liang, M.; Tang, S.; Li, H.; Zhang, T. Preparation of mulberry Stem Activated Carbon/Fe-Mn Oxide Composite Sorbent and Its Effects on the Adsorption of Cr(VI). Environ. Chem. 2016, 35, 783–792. [Google Scholar] [CrossRef]
| Biomass | Preparation Method | Iron Source | Manganese Source | Specific Surface Area/m2·g−1 | Average Pore Size/nm | Iron Mass Fraction/% | Manganese Mass Fraction/% | Oxygen Mass Fraction/% | References |
|---|---|---|---|---|---|---|---|---|---|
| Corn stover | Impregnation pyrolysis | Fe(NO3)3 | KMnO4 | 208.6 | 2.76 | 1.11 | 7.43 | 6.9 | [33] |
| Loofah | Impregnation pyrolysis | Fe(NO3)3·9H2O | KMnO4 | 187.11 | 2.91 | 35.79 | 22.38 | 12.29 | [47] |
| Bamboo | Impregnation pyrolysis | FeCl3 | KMnO4 | 200.88 | / | / | / | / | [48] |
| Wolfsbane straw | Impregnation pyrolysis | Fe(NO3)3 | KMnO4 | 8.80 | 9.67 | / | / | / | [49] |
| Fava bean straw | Impregnation pyrolysis | FeCl3·6H2O | KMnO4 | 24.29 | 14.02 | 16.0 | 30.3 | 51.46 | [50] |
| Waste bone meal | Impregnation pyrolysis | Fe(NO3)3 | KMnO4 | 287.58 | 6.53 | 5.42 | 10.1 | 39.2 | [51] |
| Algae | Hydrothermal synthesis | FeCl3·6H2O | MnCl2-6H2O | 180.2 | 6.11 | 52.5 | 14.1 | / | [38] |
| Soybean powder | Hydrothermal synthesis | Iron powder | KMnO4 | / | / | / | / | / | [52] |
| Peanut blight | Hydrothermal synthesis | FeCl3·6H2O | MnCl2·4H2O | 99.05 | / | 12.96 | 20.42 | / | [53] |
| Banana leaf | Co-precipitation method | FeSO4·7H2O | KMnO4 | 187.03 | 9.18 | 37.62 | 12.34 | 19.12 | [54] |
| Sludge | Co-precipitation | FeCl3·6H2O | MnCl2·5H2O | 67.34 | 15.60 | / | / | / | [55] |
| Corn kernel | Co-precipitation method | FeCl3·6H2O | MnSO4·H2O | 192.41 | / | / | / | / | [56] |
| Pine | Co-precipitation method | FeCl3·6H2O | MnCl2·4H2O | 280 | 0.175 | 9.13 | 4.85 | 48.84 | [57] |
| Hickory bushes | Sol-gel method | FeSO4·7H2O | MnSO4, KMnO4 | / | / | / | / | / | [41] |
| Corn stover | Sol-gel method | Fe(NO3)3 | Mn(NO3)2 | / | / | / | / | / | [44] |
| Cotton straw, corn stover, and rice husk | Mechanical ball milling and co-precipitation | FeSO4 | KMnO4 | 264.48 | 4.37 | / | / | / | [58] |
| Cotton straw, corn stover, and rice husk | Mechanical ball milling method and co-precipitation method | FeSO4 | KMnO4 | 226.5–331.5 | / | / | / | / | [45] |
| Heavy Metals | Biomass Raw Material | Preparation Method | Modification Conditions | Reaction Conditions | Adsorption Amount (mg/g) | Adsorption Rate | Removal Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| As(Ⅲ) | Corn stover | Impregnation pyrolysis | Fe/Mn mass ratio of 1:4, pyrolysis temperature of 600 °C, and N2 atmosphere | 25 °C, pH = 3 | 8.39 | / | Mesoporous adsorption, oxidation, complexation, and electrostatic interaction | [59] |
| Corn stems | Impregnation pyrolysis | Fe/Mn mass ratio of 1:4, pyrolysis temperature of 620 °C, and N2 atmosphere | pH = 7 | 8.25 | / | Mesoporous adsorption, oxidation, complexation, and electrostatic interaction | [33] | |
| Ironwood | Impregnation pyrolysis | Fe/Mn mass ratio of 1:1 and pyrolysis temperature of 800 °C | 25 °C, pH = 9 | 1.89 | 90.35% of the total amount of the product | Mesoporous adsorption, oxidation, complexation, and electrostatic forces | [60] | |
| As(V) | Pine | Impregnation pyrolysis | Fe/Mn molar ratio of 1:2, pyrolysis temperature of 600 °C, co-precipitation temperature of 80 °C, and N2 atmosphere | 25 °C, pH = 7.5 | 3.44 | / | Oxidation, complexation, and electrostatic interaction | [57] |
| Cd(II) | Cotton straw, corn stover, and rice husk | Mechanical ball milling and co-precipitation | Fe/Mn mass ratio of 0.5:3, pyrolysis temperature of 500 °C, and N2 atmosphere | 25 °C, pH = 5 | 131.03 | 96.85% | Complexation, electrostatic interaction, precipitation, and cation-π interaction | [58] |
| Rice straw | Impregnation pyrolysis | Fe/Mn molar ratio of 3:5 and pyrolysis temperature of 300 °C | 25 °C, pH = 5 | 120.77 | 95.20%, pH = 5 120.77 | Complexation, electrostatic interaction, precipitation, and cation-π interaction | [61] | |
| Wolfsbane straw | Impregnation pyrolysis | Fe/Mn mass ratio of 1:4, pyrolysis temperature of 600 °C, and N2 atmosphere | 25 °C, pH = 5 | 95.23 | / | Complexation, electrostatic interaction, precipitation, and cation-π interaction | [49] | |
| Cr(VI) | Lotus seed | Impregnation pyrolysis | Pyrolysis temperature of 600 °C and N2 atmosphere | 25 °C, pH = 1.5 | 21.25 | 99% of the total amount of the product | Mesoporous adsorption, reduction, complexation, electrostatic interaction, and precipitation | [62] |
| Seaweed | Impregnation pyrolysis | Fe/Mn molar ratio of 1:3, pyrolysis temperature of 500 °C, and N2 atmosphere | 30 °C, pH = 3 | 104.5 | 98.90%. | Mesoporous adsorption, reduction, complexation, electrostatic interaction, and precipitation | [63] | |
| Corn stover | Impregnation pyrolysis | Fe/Mn molar ratio of 1:3, pyrolysis temperature of 400 °C, and N2 atmosphere | 25 °C, pH = 2 | 118.03 | 91.79%. | Mesoporous adsorption, reduction, complexation, and electrostatic interaction | [64] | |
| Cu(II) | Loofah | Impregnation pyrolysis | Using Fe (NO3)3·9H2O and KMnO4 impregnation, pyrolysis temperature of 600 °C, and N2 atmosphere | 25 °C, pH = 5.5 | 47.64 | 92.50% | Mesoporous adsorption, complexation, and electrostatic interaction | [47] |
| Corn stover | Impregnation pyrolysis | Fe/Mn mass ratio of 1:3, pyrolysis temperature of 600 °C, and N2 atmosphere | 25 °C, pH = 2.0 | 64.9 | 91.79%. | Mesoporous adsorption, complexation, and electrostatic interaction | [34] | |
| Undaria pinnatifida root | Hydrothermal synthesis | Fe/Mn molar ratio of 2:1 and 453 K (180 °C) hydrothermal for 10 h | 25 °C, pH = 5 | 295.2 | / | Mesoporous adsorption and electrostatic interaction | [38] | |
| Hg(II) | Corn stover | Impregnation pyrolysis | Fe/Mn mass ratio of 0.5:3, pyrolysis temperature of 600 °C, and N2 atmosphere | 25 °C, pH = 7 | 86.82 | 72.34%. | Mesoporous adsorption, complexation, electrostatic interaction, and precipitation | [65] |
| Pb(II) | Rice straw | Impregnation pyrolysis | Fe/Mn molar ratio of 2:5 and pyrolysis temperature of 300 °C | 25 °C, pH = 7 | 165.88 | 90.42% of the total amount | Mesopore adsorption, complexation, electrostatic interaction, precipitation, and cation-π interaction | [66] |
| Corn stover | Co-precipitation method | Pyrolysis temperature of 350 °C and N2 atmosphere | 25 °C, pH = 5 | 190.17 | / | Mesoporous adsorption, complexation, and electrostatic interaction | [67] | |
| Corn kernel | Co-precipitation | Pyrolysis temperature of 850 °C and N2 atmosphere | 25 °C, pH = 5 | 196.69 | / | Mesoporous adsorption, complexation, electrostatic interaction, and precipitation | [56] | |
| Tl(I) | Banana leaf | Co-precipitation | Fe/Mn molar ratio of 2:1 (MnFe2O4), pyrolysis temperature of 500 °C, and N2 atmosphere | 25 °C, pH = 6 | 170.55 | 99%. | Mesoporous adsorption, oxidation, and complexation | [54] |
| Zn(II) | Corn stover | Sol-gel method | Pyrolysis temperature of 300 °C | 25 °C, pH = 5 | / | / | Mesoporous adsorption and complexation | [44] |
| Organic Pollutants | Biomass Raw Material | Preparation Method | Modification Conditions | Reaction Conditions | Adsorption Amount (mg/g) | Adsorption Rate (%) | Oxidizing Agent | Removal Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Atrazine | Rice straw | Impregnation pyrolysis | Fe/Mn molar ratio of 3:1, pyrolysis temperature of 500 °C, and N2 atmosphere | 25 °C and pH = 7 | / | 96.70% | Persulfate | Mesoporous adsorption, oxidation (·OH, SO4−·, and 1O2), and complexation | [30] |
| Ibuprofen | Sawdust | Impregnation pyrolysis | Pyrolysis temperature of 800 °C and N2 atmosphere | 24 °C and pH = 7 | / | 95% | Ozone | Mesoporous adsorption, oxidation (·OH and SO4−·), and hydrogen bonding | [75] |
| Estrone | Litchi wood | Impregnation pyrolysis | Pyrolysis temperature of 650 °C and N2 atmosphere | 25 °C and pH = 3 | 4.18 | 91.50% | / | Mesoporous adsorption, hydrogen bonding, π-π EDA interaction, and complexation | [76] |
| Ciprofloxacin | Sludge | Impregnation pyrolysis | Fe/Mn molar ratio of 0.5:1, pyrolysis temperature of 500 °C, and N2 atmosphere | 25 °C and pH = 5 | / | 80.85% | / | Mesoporous adsorption, oxidation (·OH and 1O2), and electrostatic interaction | [77] |
| Sulfamethoxazole | Corn stover | Co-precipitation | Fe/Mn molar ratio of 2:1, BC pyrolysis temperature of 800 °C, and N2 atmosphere | 25 °C and 3 ≤ pH ≤ 9 | / | 92% | Sulfites | Mesoporous adsorption and oxidation (·OH and SO4−·) | [78] |
| Peanut shells | Co-precipitation method | Pyrolysis temperature of 500 °C, and N2 atmosphere | 25 °C and 3 ≤ pH ≤ 11 | / | 100% | Peroxymonosulfate | Mesoporous adsorption and oxidation (·OH and 1O2) | [79] | |
| Bamboo waste | Impregnation pyrolysis | Fe/Mn molar ratio of 3:2, pyrolysis temperature of 800 °C, and N2 atmosphere | 25 °C and pH ≈ 5.6 | / | 97.90% | Peroxymonosulfate | Oxidation (·OH and 1O2), electrostatic interaction, hydrogen bonding, and π-π EDA effect | [80] | |
| Sludge | Co-precipitation | Pyrolysis temperature of 600 °C and N2 atmosphere | 25 °C and 3 ≤ pH ≤ 11 | / | 98.80% | Persulfate | Mesoporous adsorption, oxidation (·OH and 1O2), and hydrogen bonding | [81] | |
| Sulfamethoxazole | Rice straw | Hydrothermal synthesis | Pyrolysis temperature of 500 °C | 25 °C and natural pH | / | 83.80% | / | Mesoporous adsorption and oxidation (·OH) | [82] |
| Activated Blue 19 | Sludge | Impregnation pyrolysis | Fe/Mn molar ratio 1:1 and pyrolysis temperature of 600 °C | 25 °C and 3 ≤ pH ≤ 9 | / | 98.33% | Persulfate | Mesoporous adsorption, oxidation (·OH), and complexation | [83] |
| Carbamazepine | Soybean powder | Hydrothermal synthesis | Fe powder 0.17 mol/L + KMnO4, pyrolysis temperature of 600 °C, and N2 atmosphere | 25 °C and pH ≈ 7 | / | 99% | Peroxymonosulfate | Oxidation (·OH and 1O2), π-π EDA effect, and hydrogen bonding effect | [52] |
| Dibutyl phthalate, Bis(2-ethylhexyl) phthalate | Corn stover | Impregnation pyrolysis | Fe/Mn mass ratio of 1:6, pyrolysis temperature of 600 °C, and N2 atmosphere | Room temperature | / | / | / | Mesoporous adsorption and electrostatic interaction | [84] |
| Corn stover | Impregnation pyrolysis | Pyrolysis temperature of 600 °C | Natural temperature and natural pH | / | / | / | Mesoporous adsorption, electrostatic interaction, and complexation | [85] | |
| Rhodamine B | Straw | Sol-gel method | Fe/Mn molar ratio of 2:1 and pyrolysis temperature of 300 °C | Room temperature and pH = 7 | / | 100% | Potassium persulfate | Oxidation (·OH and SO4−·), π-π EDA action, and complexation | [86] |
| Thiacloprid | Sludge | Co-precipitation method | Pyrolysis temperature of 600 °C and N2 atmosphere | 25 °C and 3 ≤ pH ≤ 11 | / | 94.10% | Periodate | Mesoporous adsorption and oxidation (·OH and IO3·) | [87] |
| Thiamethoxam | Straw | Sol-gel method | Fe/Mn molar ratio of 2:1 and pyrolysis temperature of 600 °C | Room temperature and natural pH | / | 99% | Potassium persulfate | Mesoporous adsorption, oxidation (·OH and SO4−·), and surface complexation | [88] |
| Bisphenol A | Straw | Impregnation pyrolysis | Pyrolysis temperature of 800 °C and N2 atmosphere | 20 °C and 3 ≤ pH ≤ 10 | / | 100% | Peroxymonosulfate | Mesoporous adsorption and oxidation (·OH, SO4−·, and 1O2) | [89] |
| Tetracycline | Rice straw | Hydrothermal synthesis | Pyrolysis temperature 600 °C and N2 atmosphere | 25 °C and 5 ≤ pH ≤ 9 | / | 85% | Peroxymonosulfate | Mesoporous adsorption and oxidation (·OH and 1O2) | [90] |
| Platycodon grandiflorum twigs | Impregnation pyrolysis | Pyrolysis temperature of 800 °C and N2 atmosphere | 25 °C and 2.29 ≤ pH ≤ 11.43 | / | 97.90% | Peroxymonosulfate | Oxidation (·OH and 1O2), electrostatic interaction, hydrogen bonding, and π-π EDA interaction | [91] | |
| Acid red 88 | Cedar sawdust | Impregnation pyrolysis | Fe/Mn mass ratio of 1:1, microwave radiation power of 200 W, and N2 atmosphere | 25 °C | / | 98.84% | Persulfate | Mesoporous adsorption and oxidation (·OH and SO4−·) | [92] |
| Methylene blue | Alder | Impregnation pyrolysis | Fe/Mn molar ratio of 2:1, pyrolysis temperature of 800 °C, and N2 atmosphere | 25 °C and 3 ≤ pH ≤ 10 | 97.41 | 97.41 | / | Mesoporous adsorption, complexation, hydrogen bonding, and π-π EDA effect | [93] |
| Anaerobic sludge | Peanut shells | Hydrothermal synthesis | Pyrolysis temperature 800 °C and N2 atmosphere | 37 °C and natural pH | / | / | // | Mesoporous adsorption and oxidation | [53] |
| Levofloxacin | Wine lees waste | Impregnation pyrolysis method and co-precipitation method | Pyrolysis temperature of 800 °C and N2 atmosphere | 25 °C and pH = 5 | 181 | 91.50% | / | Mesoporous adsorption, hydrogen bonding, π-π EDA effect, and salinization effect | [94] |
| Phosphate/Nitrate | Biomass Raw Material | Preparation Method | Modification Conditions | Optimal Reaction Conditions | Maximum Adsorption Amount (mg/g) | Maximum Adsorption Rate | Removal Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Phosphate | Cotton straw, corn stover, and rice husk | Impregnation pyrolysis and mechanical ball milling | Fe/Mn coating (ball milling for 6 h) and N2 atmosphere | 24.85 °C and pH = 3 | 53.3 | 94.72%. | Mesoporous adsorption, complexation, electrostatic interaction, and precipitation | [45] |
| Microalgae | Impregnation pyrolysis | Fe/biomass = 1.25 (w/w), Mn/biomass = 1.10 (w/w), and pyrolysis temperature of 650 °C, N2 atmosphere, and EDTA chelation | 25 °C and pH = 7 | 23.23 | 91.60% | Mesoporous adsorption, complexation, electrostatic interaction, and precipitation | [97] | |
| Rice straw | Impregnation method | Fe/Mn molar ratio of 3:1 | Room Temperature and pH = 6 | 135.88 | - | Complexation, electrostatic interaction, and precipitation | [98] | |
| Fruit shell (apricot shell) | Impregnation method | Fe/Mn molar ratio of 1:1 and drying temperature of 378 K | 25 °C and 4 ≤ pH ≤ 10 | 4.69 (under 10 mg/L phosphorus concentration) | 93.24% | Complexation and electrostatic interaction | [99] | |
| Nitrate | Coconut shell | Microelectrolysis | Shell iron/biochar/manganese sand = 6:2:1 (mass ratio) to construct a microelectrolysis system | 27 °C and pH = 7 | / | 80.30% | Microelectrolysis and complexation | [100] |
| Wheat straw | Impregnation pyrolysis | Fe/Mn molar ratio of 1:1, pyrolysis temperature of 400 °C, and N2 atmosphere | 25 °C and 1 ≤ pH ≤ 9 | 37.36 | 78.70% | Mesoporous adsorption, complexation, and electrostatic interaction | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Xu, X.; He, A.; Zhang, Y.; Che, R.; Yang, L.; Wei, J.; Wang, F.; Hua, J.; Shi, J. Research Progress on the Preparation of Iron-Manganese Modified Biochar and Its Application in Environmental Remediation. Toxics 2025, 13, 618. https://doi.org/10.3390/toxics13080618
Liu C, Xu X, He A, Zhang Y, Che R, Yang L, Wei J, Wang F, Hua J, Shi J. Research Progress on the Preparation of Iron-Manganese Modified Biochar and Its Application in Environmental Remediation. Toxics. 2025; 13(8):618. https://doi.org/10.3390/toxics13080618
Chicago/Turabian StyleLiu, Chang, Xiaowei Xu, Anfei He, Yuanzheng Zhang, Ruijie Che, Lu Yang, Jing Wei, Fenghe Wang, Jing Hua, and Jiaqi Shi. 2025. "Research Progress on the Preparation of Iron-Manganese Modified Biochar and Its Application in Environmental Remediation" Toxics 13, no. 8: 618. https://doi.org/10.3390/toxics13080618
APA StyleLiu, C., Xu, X., He, A., Zhang, Y., Che, R., Yang, L., Wei, J., Wang, F., Hua, J., & Shi, J. (2025). Research Progress on the Preparation of Iron-Manganese Modified Biochar and Its Application in Environmental Remediation. Toxics, 13(8), 618. https://doi.org/10.3390/toxics13080618






