Benzo[a]pyrene-Induced Developmental Toxicity in Caenorhabditis elegans: Potential Involvement of Insulin/IGF Signaling and Collagen Gene Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Strains
2.2. Preparation and Exposure of B[a]P Solution
2.3. Body Length
2.4. Brood Size
2.5. Locomotor Behavioral Parameters
2.6. Lifespan Assay
2.7. ROS Detection
2.8. RNA Sequencing and Data Peocessing
2.9. RT-qPCR
2.10. Data Analysis
3. Results
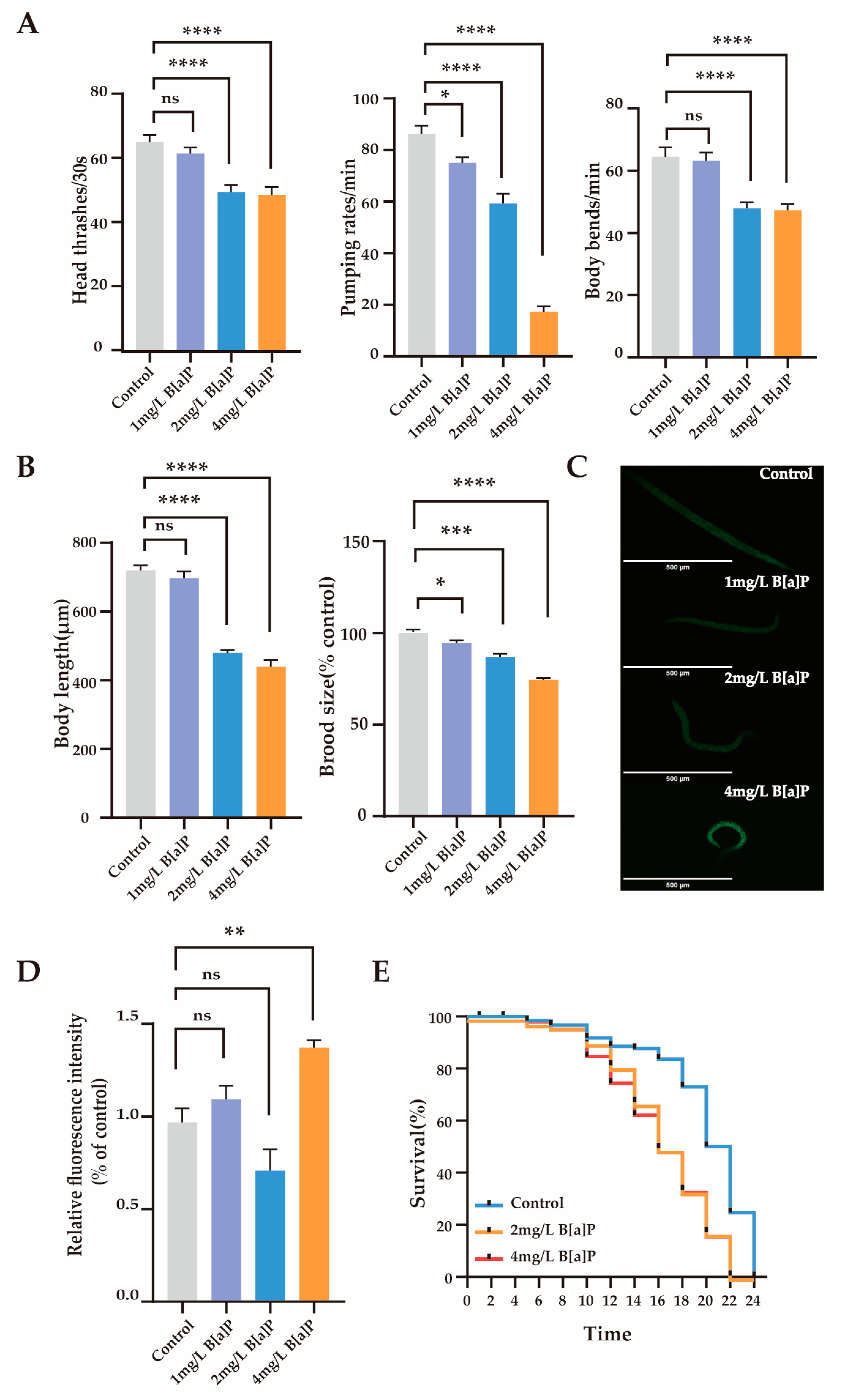
3.1. Effects of B[a]P on Physiological Parameters and Oxidative Stress in C. elegans
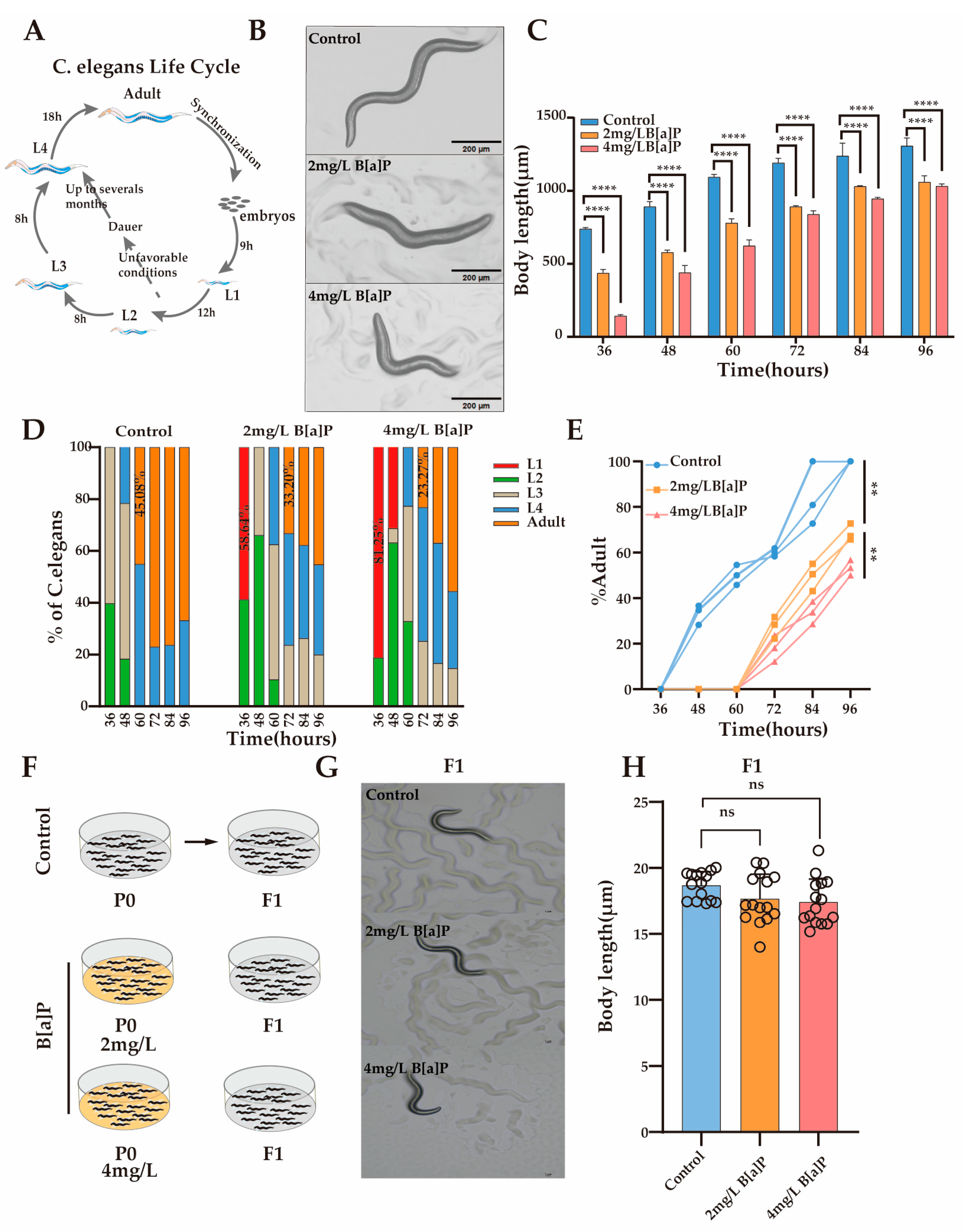
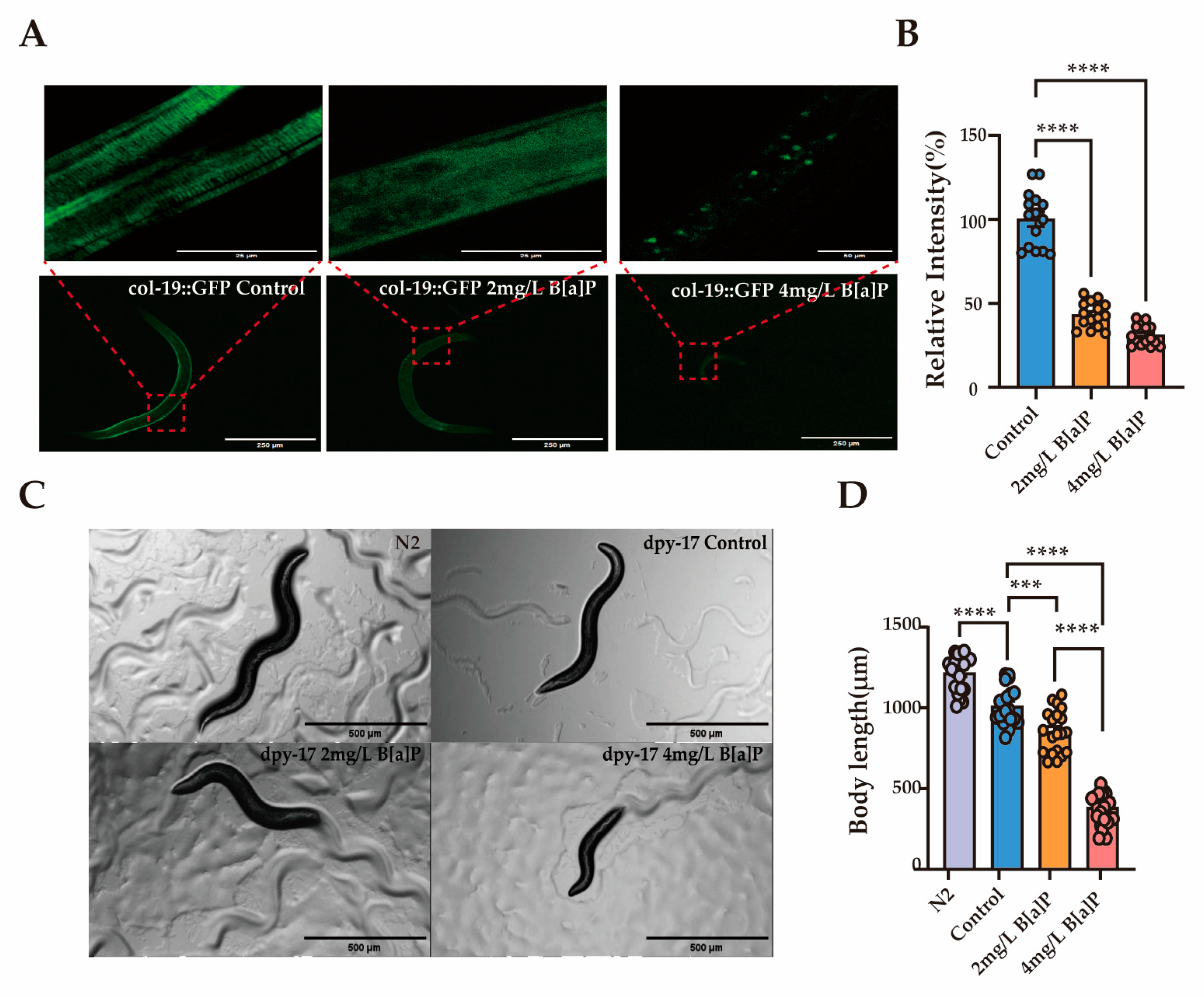
3.2. Effect of B[a]P on Development of C. elegans
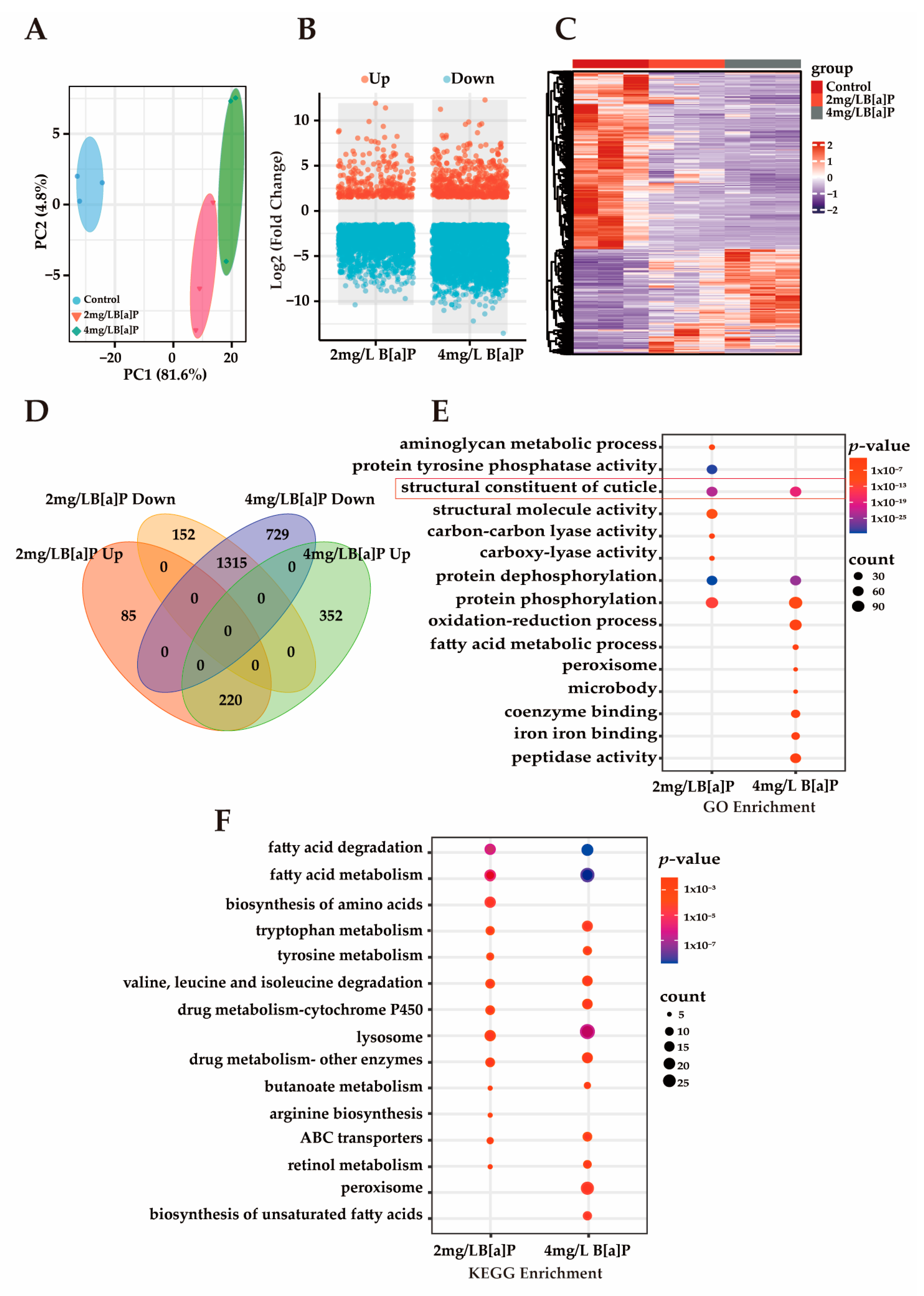
3.3. Effects of B[a]P Exposure on Gene Expression in C. eleganss
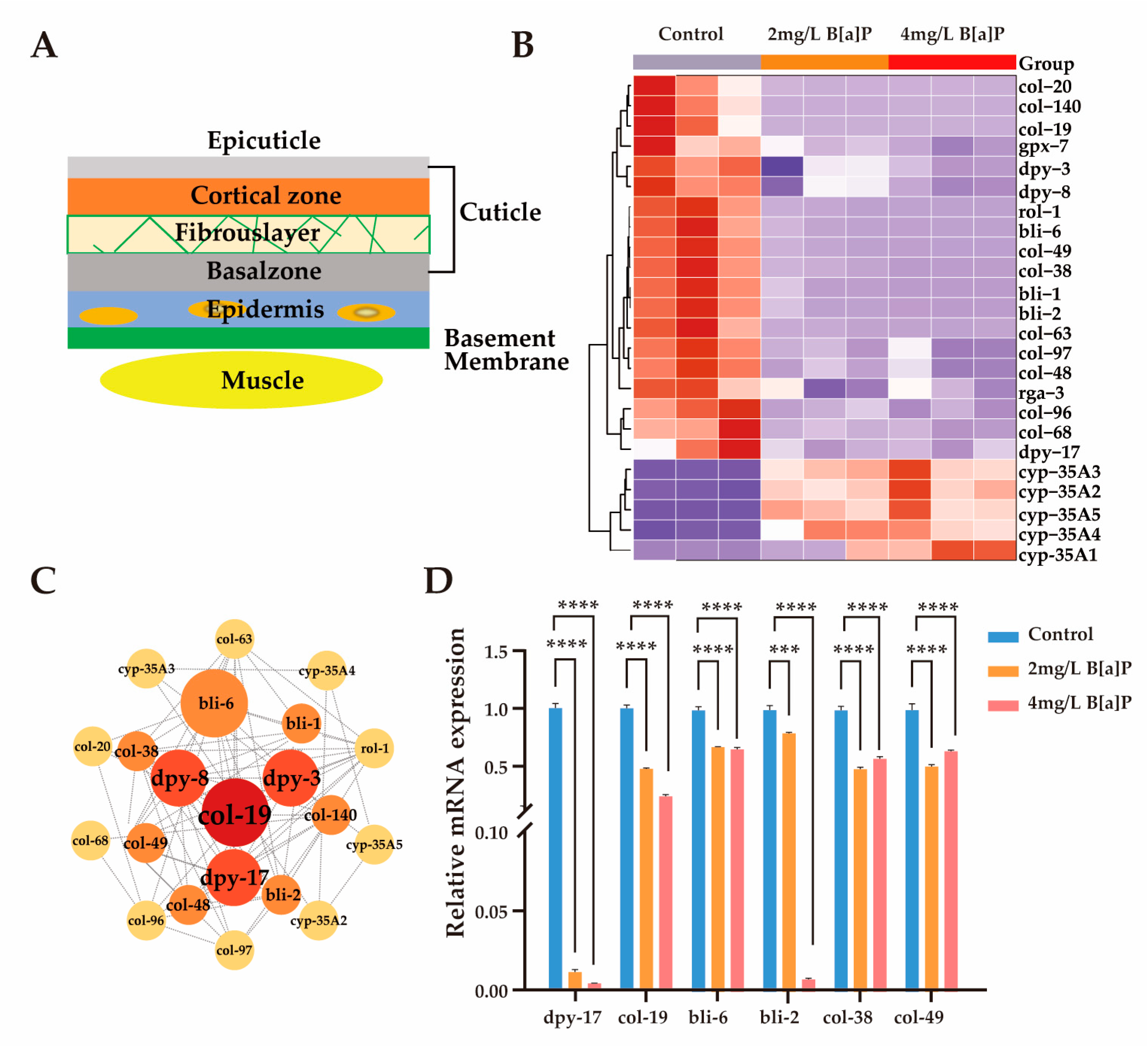
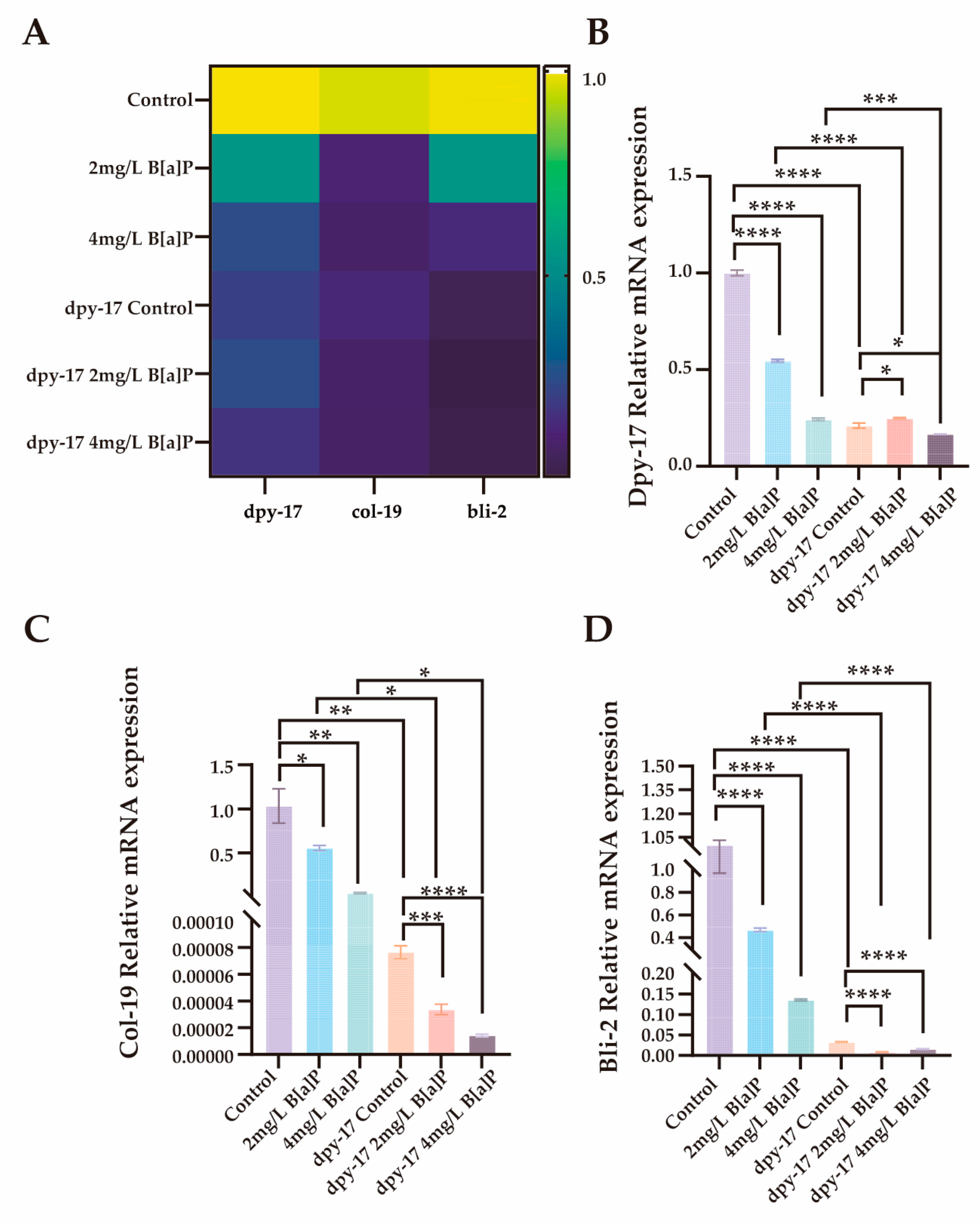
3.4. Effects of B[a]P on Cuticle Collagen Gene Expression in C. elegans
3.5. Molecular Mechanisms Underlying Developmental Toxicity of B[a]P
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Gao, P.; Da Silva, E.; Hou, L.; Denslow, N.D.; Xiang, P.; Ma, L.Q. Human exposure to polycyclic aromatic hydrocarbons: Metabolomics perspective. Environ. Int. 2018, 119, 466–477. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.; Lu, Y.; Yu, X.; Wang, J.; Sun, S.; Liu, M.; Li, D.; Li, Y.F.; Zhang, D. Uptake and translocation of polycyclic aromatic hydrocarbons (PAHs) and heavy metals by maize from soil irrigated with wastewater. Sci. Rep. 2017, 7, 12165. [Google Scholar] [CrossRef]
- Sánchez-Arévalo, C.M.; Olmo-García, L.; Fernández-Sánchez, J.F.; Carrasco-Pancorbo, A. Polycyclic aromatic hydrocarbons in edible oils: An overview on sample preparation, determination strategies, and relative abundance of prevalent compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3528–3573. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Sicińska, P. Influence of Benzo(a)pyrene on Different Epigenetic Processes. Int. J. Mol. Sci. 2021, 22, 13453. [Google Scholar] [CrossRef] [PubMed]
- Knafla, A.; Phillipps, K.A.; Brecher, R.W.; Petrovic, S.; Richardson, M. Development of a dermal cancer slope factor for benzo[a]pyrene. Regul. Toxicol. Pharmacol. 2006, 45, 159–168. [Google Scholar] [CrossRef]
- Hadrup, N.; Mielżyńska-Švach, D.; Kozłowska, A.; Campisi, M.; Pavanello, S.; Vogel, U. Association between a urinary biomarker for exposure to PAH and blood level of the acute phase protein serum amyloid A in coke oven workers. Environ. Health 2019, 18, 81. [Google Scholar] [CrossRef]
- Oliveira, M.; Capelas, S.; Delerue-Matos, C.; Morais, S. Grill Workers Exposure to Polycyclic Aromatic Hydrocarbons: Levels and Excretion Profiles of the Urinary Biomarkers. Int. J. Environ. Res. Public Health 2020, 18, 230. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Zhao, B.; Zhang, Y.; Liu, Z.; Xu, J.; Chen, Y.; Yang, Z.; Wang, F.; Wang, H.; et al. Human metabolic responses to chronic environmental polycyclic aromatic hydrocarbon exposure by a metabolomic approach. J. Proteome Res. 2015, 14, 2583–2593. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Mcelrath, T.F.; Pace, G.G.; Weller, D.; Zeng, L.; Pennathur, S.; Cantonwine, D.E.; Meeker, J.D. Correction to “Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women”. Environ. Sci. Technol. 2019, 53, 2269. [Google Scholar] [CrossRef]
- Drwal, E.; Rak, A.; Gregoraszczuk, E.L. Review: Polycyclic aromatic hydrocarbons (PAHs)-Action on placental function and health risks in future life of newborns. Toxicology 2019, 411, 133–142. [Google Scholar] [CrossRef]
- Duarte-Salles, T.; Mendez, M.A.; Meltzer, H.M.; Alexander, J.; Haugen, M. Dietary benzo(a)pyrene intake during pregnancy and birth weight: Associations modified by vitamin C intakes in the Norwegian Mother and Child Cohort Study (MoBa). Environ. Int. 2013, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Moreira, S.; De Lima Inocêncio, L.C.; Jorge, B.C.; Reis, A.C.C.; Hisano, H.; Arena, A.C. Effects of benzo(a)pyrene at environmentally relevant doses on embryo-fetal development in rats. Environ. Toxicol. 2021, 36, 831–839. [Google Scholar] [CrossRef]
- Amran, A.; Pigatto, L.; Pocock, R.; Gopal, S. Functions of the extracellular matrix in development: Lessons from Caenorhabditis elegans. Cell Signal. 2021, 84, 110006. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; Desimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Lamandé, S.R.; Bateman, J.F. Genetic Disorders of the Extracellular Matrix. Anat. Rec. 2020, 303, 1527–1542. [Google Scholar] [CrossRef]
- Su, H.; Karin, M. Collagen architecture and signaling orchestrate cancer development. Trends Cancer 2023, 9, 764–773. [Google Scholar] [CrossRef]
- Ahmed, S.; Nowlan, N.C. Initiation and emerging complexity of the collagen network during prenatal skeletal development. Eur. Cell Mater. 2020, 39, 136–155. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kao, T.W.; Wang, C.C.; Wu, C.J.; Zhou, Y.C.; Chen, W.L. Association between polycyclic aromatic hydrocarbons exposure and bone turnover in adults. Eur. J. Endocrinol. 2020, 182, 333–341. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Y.; Bian, S.; Zhao, C.; Jin, Y.; Yu, D.; Wu, X.; Zhang, D.; Cao, W.; Jing, F.; et al. Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005–2010. Environ. Pollut. 2018, 240, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Hua, X.; Wang, Z.; Li, L.; Li, Z.; Xiang, M.; Ding, P. Long-term toxicity of lindane through oxidative stress and cell apoptosis in Caenorhabditis elegans. Environ. Pollut. 2021, 272, 116036. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Pears, C.; Woollard, A. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 2017, 7, 9839. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J. Early life exposure of a biocide, CMIT/MIT causes metabolic toxicity via the O-GlcNAc transferase pathway in the nematode C. elegans. Toxicol. Appl. Pharmacol. 2019, 376, 1–8. [Google Scholar] [CrossRef]
- Duplissis, A.; Medewar, A.; Hegarty, E.; Laing, A.; Shen, A.; Gomez, S.; Mondal, S.; Ben-Yakar, A. Machine learning-based analysis of microfluidic device immobilized C. elegans for automated developmental toxicity testing. Sci. Rep. 2025, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Luo, D.; Wang, H.; Liu, X.; Yang, M.; Tian, F.; Qin, S.; Liu, J.; Li, Y. Ameliorative effects of Bifidobacterium longum peptide-1 on benzo(α)pyrene induced oxidative damages via daf-16 in Caenorhabditis elegans. Cell Stress Chaperones 2023, 28, 909–920. [Google Scholar] [CrossRef]
- Chen, Y.; Abbass, M.; Brock, T.; Hobbs, G.; Ciufo, L.A.; Hopkins, C.; Arlt, V.M.; Stürzenbaum, S.R. Environmental carcinogen benzo[a]pyrene alters neutral lipid storage via a cyp-35A2 mediated pathway in Caenorhabditis elegans. Environ. Pollut. 2023, 339, 122731. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Rangsinth, P.; Warayanon, W.; Leung, G.P.; Chuchawankul, S.; Prasansuklab, A.; Tencomnao, T. Protective Effect of Aquilaria crassna Leaf Extract against Benzo[a]pyrene-Induced Toxicity in Neuronal Cells and Caenorhabditis elegans: Possible Active Constituent Includes Clionasterol. Nutrients 2023, 15, 3985. [Google Scholar] [CrossRef]
- Jeong, A.; Park, S.J.; Lee, E.J.; Kim, K.W. Nanoplastics exacerbate Parkinson’s disease symptoms in C. elegans and human cells. J. Hazard. Mater. 2024, 465, 133289. [Google Scholar] [CrossRef]
- White, J. Clues to basis of exploratory behaviour of the C. elegans snout from head somatotropy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Rothe, M.; Schunck, W.H.; Ruess, L.; Menzel, R. Serotonin-induced stereospecific formation and bioactivity of the eicosanoid 17,18-epoxyeicosatetraenoic acid in the regulation of pharyngeal pumping of C. elegans. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2023, 1868, 159304. [Google Scholar] [CrossRef]
- Hassinan, C.W.; Sterrett, S.C.; Summy, B.; Khera, A.; Wang, A.; Bai, J. Dimensionality of locomotor behaviors in developing C. elegans. PLoS Comput. Biol. 2024, 20, e1011906. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Brooks, D.R.; Shepherd, S.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria extend lifespan in C. elegans. Aging 2023, 15, 11764–11781. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.R.; Balasubramanian, S.; Swerdlow, H.P.; Smith, G.P.; Milton, J.; Brown, C.G.; Hall, K.P.; Evers, D.J.; Barnes, C.L.; Bignell, H.R.; et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 2008, 456, 53–59. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Xu, J.; Du, P.; Liu, X.; Xu, X.; Ge, Y.; Zhang, C. Curcumin supplementation increases longevity and antioxidant capacity in Caenorhabditis elegans. Front. Pharmacol. 2023, 14, 1195490. [Google Scholar] [CrossRef]
- Qiu, Y.; Luo, L.; Yang, Y.; Kong, Y.; Li, Y.; Wang, D. Potential toxicity of nano polystyrene on lifespan and aging process of nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 705, 135918. [Google Scholar] [CrossRef]
- Stagaman, K.; Alexiev, A.; Sieler, M.J.; Hammer, A.; Kasschau, K.D.; Truong, L.; Tanguay, R.L.; Sharpton, T.J. The zebrafish gut microbiome influences benzo[a]pyrene developmental neurobehavioral toxicity. Sci. Rep. 2024, 14, 14618. [Google Scholar] [CrossRef]
- Zeb, R.; Yin, X.; Chen, F.; Wang, K.J. Chronic exposure to environmental concentrations of benzo[a]pyrene causes multifaceted toxic effects of developmental compromise, redox imbalance, and modulated transcriptional profiles in the early life stages of marine medaka (Oryzias melastigma). Aquat. Toxicol. 2024, 273, 107016. [Google Scholar] [CrossRef]
- Miao, R.; Li, M.; Zhang, Q.; Yang, C.; Wang, X. An ECM-to-Nucleus Signaling Pathway Activates Lysosomes for C. elegans Larval Development. Dev. Cell. 2020, 52, 21–37.e25. [Google Scholar] [CrossRef] [PubMed]
- Imanikia, S.; Taylor, R.C. Molty-Level Regulation: Lysosomes Participate in Developmental ECM Remodeling in C. elegans. Dev. Cell. 2020, 52, 1–2. [Google Scholar] [CrossRef]
- Uppaluri, S.; Brangwynne, C.P. A size threshold governs Caenorhabditis elegans developmental progression. Proc. Biol. Sci. 2015, 282, 20151283. [Google Scholar]
- Stojanovski, K.; Großhans, H.; Towbin, B.D. Coupling of growth rate and developmental tempo reduces body size heterogeneity in C. elegans. Nat. Commun. 2022, 13, 3132. [Google Scholar] [CrossRef]
- Page, A.P.; Johnstone, I.L. The cuticle. WormBook 2007, 19, 1–15. [Google Scholar] [CrossRef]
- Cho, J.Y.; Choi, T.W.; Kim, S.H.; Ahnn, J.; Lee, S.K. Morphological Characterization of small, dumpy, and long Phenotypes in Caenorhabditis elegans. Mol. Cells. 2021, 44, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.D.; Xu, S. The Caenorhabditis elegans epidermis as a model skin. II: Differentiation and physiological roles. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 879–902. [Google Scholar] [CrossRef]
- Abbass, M.; Chen, Y.; Arlt, V.M.; Stürzenbaum, S.R. Benzo[a]pyrene and Caenorhabditis elegans: Defining the genotoxic potential in an organism lacking the classical CYP1A1 pathway. Arch. Toxicol. 2021, 95, 1055–1069. [Google Scholar] [CrossRef]
- Hada, K.; Asahina, M.; Hasegawa, H.; Kanaho, Y.; Slack, F.J.; Niwa, R. The nuclear receptor gene nhr-25 plays multiple roles in the Caenorhabditis elegans heterochronic gene network to control the larva-to-adult transition. Dev. Biol. 2010, 344, 1100–1109. [Google Scholar] [CrossRef]
- Birnbaum, S.K.; Cohen, J.D.; Belfi, A.; Murray, J.I.; Adams, J.R.G.; Chisholm, A.D.; Sundaram, M.V. The proprotein convertase BLI-4 promotes collagen secretion prior to assembly of the Caenorhabditis elegans cuticle. PLoS Genet. 2023, 19, e1010944. [Google Scholar] [CrossRef]
- Lakdawala, M.F.; Madhu, B.; Faure, L.; Vora, M.; Padgett, R.W.; Gumienny, T.L. Genetic interactions between the DBL-1/BMP-like pathway and dpy body size-associated genes in Caenorhabditis elegans. Mol. Biol. Cell. 2019, 30, 3151–3160. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.R.G.; Pooranachithra, M.; Jyo, E.M.; Zheng, S.L.; Goncharov, A.; Crew, J.R.; Kramer, J.M.; Jin, Y.; Ernst, A.M.; Chisholm, A.D. Nanoscale patterning of collagens in C. elegans apical extracellular matrix. Nat. Commun. 2023, 14, 7506. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, K.S.; Sun, X.; Williams, P.L.; Wang, J.S.; Tang, L. Toxicogenomic responses to zearalenone in Caenorhabditis elegans reveal possible molecular mechanisms of reproductive toxicity. Food Chem. Toxicol. 2018, 122, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Colman, M.J.; Dansen, T.B.; Burgering, B.M.T. FOXO transcription factors as mediators of stress adaptation. Nat. Rev. Mol. Cell Biol. 2024, 25, 46–64. [Google Scholar] [CrossRef]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry extract promoted longevity and stress tolerance via the insulin/IGF signaling pathway and DAF-16 in Caenorhabditis elegans. Food Funct. 2020, 11, 3598–3609. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Meng, H.; Jiang, M.; Zhu, Z.; Guan, X.; Bai, Y.; Wang, C.; Zhou, Y.; Hong, S.; Xiao, Y.; et al. The interaction effects of zinc and polygenic risk score with benzo[a]pyrene exposure on lung cancer risk: A prospective case-cohort study among Chinese populations. Environ. Res. 2024, 250, 118539. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, R.; Yu, J.; Xing, C.; Zhuang, C.; Qu, Z. Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis. Nutrients 2023, 15, 491. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Q.; Zhang, Y.; Huang, Y.; Liu, Q.; Chen, M.; Wang, C. Benzo(a)pyrene exposure impacts cerebrovascular development in zebrafish embryos and the antagonistic effect of berberine. Sci. Total Environ. 2024, 949, 174980. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Du, L.; Zhao, J.; Ci, X.; Yin, J.; Niu, Q.; Mo, Y.; Zhang, Q.; Nie, J. Maternal Exposure of SD Rats to Benzo[a]Pyrene Impairs Neurobehavior and Hippocampal Synaptic Ultrastructure in Offspring via Downregulating Synaptic-Associated Factors. Environ Toxicol. 2025. [Google Scholar] [CrossRef]
- Luo, S.; Li, J.; Zhou, Y.; Zhai, Z.; Li, Q.; Huang, Z.; He, W.; Zhong, K.; Kong, B.; Xia, Z.; et al. Diisooctyl phthalate (DIOP) exposure leads to cell apoptosis to HUVEC cells and toxicity to Caenorhabditis elegans through increasing the oxidative stress. Ecotoxicol. Environ. Saf. 2025, 290, 117594. [Google Scholar] [CrossRef]
- Wu, T.; Xu, H.; Liang, X.; Tang, M. Caenorhabditis elegans as a complete model organism for biosafety assessments of nanoparticles. Chemosphere 2019, 221, 708–726. [Google Scholar] [CrossRef] [PubMed]
- Jorge, B.C.; Reis, A.C.C.; Stein, J.; Balin, P.D.S.; Sterde, É.T.; Barbosa, M.G.; De Aquino, A.M.; Kassuya, C.A.L.; Arena, A.C. Parental exposure to benzo(a)pyrene in the peripubertal period impacts reproductive aspects of the F1 generation in rats. Reprod. Toxicol. 2021, 100, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Jorge, B.C.; Stein, J.; Reis, A.C.C.; Bueno, J.N.; Paschoalini, B.R.; Moreira, S.D.S.; Manoel, B.M.; Arena, A.C. Paternal low-dose benzo(a)pyrene exposure in rats impairs sexual development and fertility of the paternal lineage in F2 generation: A transgenerational study. Toxicology 2023, 494, 153585. [Google Scholar] [CrossRef] [PubMed]
- Van Gilst, M.R.; Hadjivassiliou, H.; Yamamoto, K.R. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc. Natl. Acad. Sci. USA 2005, 102, 13496–13501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Shi, Y.; Zhou, Y.; Ge, Y. Benzo[a]pyrene-Induced Developmental Toxicity in Caenorhabditis elegans: Potential Involvement of Insulin/IGF Signaling and Collagen Gene Dysregulation. Toxics 2025, 13, 384. https://doi.org/10.3390/toxics13050384
Zhou J, Shi Y, Zhou Y, Ge Y. Benzo[a]pyrene-Induced Developmental Toxicity in Caenorhabditis elegans: Potential Involvement of Insulin/IGF Signaling and Collagen Gene Dysregulation. Toxics. 2025; 13(5):384. https://doi.org/10.3390/toxics13050384
Chicago/Turabian StyleZhou, Jinjin, Yage Shi, Yanfeng Zhou, and Yang Ge. 2025. "Benzo[a]pyrene-Induced Developmental Toxicity in Caenorhabditis elegans: Potential Involvement of Insulin/IGF Signaling and Collagen Gene Dysregulation" Toxics 13, no. 5: 384. https://doi.org/10.3390/toxics13050384
APA StyleZhou, J., Shi, Y., Zhou, Y., & Ge, Y. (2025). Benzo[a]pyrene-Induced Developmental Toxicity in Caenorhabditis elegans: Potential Involvement of Insulin/IGF Signaling and Collagen Gene Dysregulation. Toxics, 13(5), 384. https://doi.org/10.3390/toxics13050384







