Impact of PM2.5 Exposure from Wood Combustion on Reproductive Health: Implications for Fertility, Ovarian Function, and Fetal Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Air Pollution Exposure
2.2. Chambers and Filtration System
2.3. Air Analysis and Composition of PM2.5
2.4. Animals
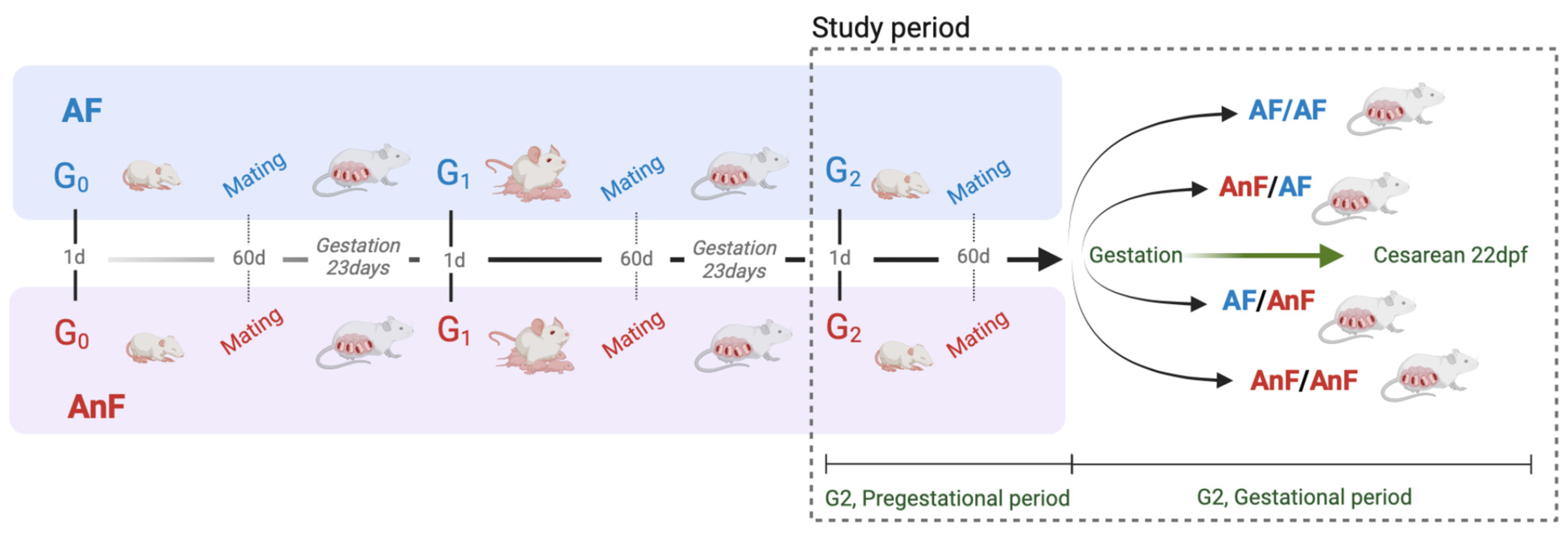
2.5. Experimental Design and Groups
2.6. Blood Collection and Hormone Measurements
2.7. Maternal, Fetal, and Reproductive Indices
2.8. Statistical Analysis
3. Results
3.1. Climatic Conditions at the Study Site
3.2. Air Pollution Exposure
3.3. Blood Collection and Measurement of Serum Hormone Levels
3.4. Maternal, Fetal, Reproductive, and Gestational Indices
3.5. Fetal Index
3.6. Reproductive Index
3.7. Gestational Index
4. Discussion
4.1. On Exposure and PM2.5
4.2. Endocrine Results in G2 Mother Rats
4.3. On the Body Weight of G2 Mother Rats Before and During Gestation
4.4. On the Fetuses
4.5. On Ovarian Cyclicity, Reproductive Capacity, and the Estrous Cycle
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villalobos, A.M.; Barraza, F.; Jorquera, H.; Schauer, J.J. Wood burning pollution in southern Chile: PM2.5 source apportionment using CMB and molecular markers. Environ. Pollut. 2017, 225, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Låg, M.; Øvrevik, J.; Refsnes, M.; Holme, J.A. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Respir. Res. 2020, 21, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.F.; Rokoff, L.B.; Garshick, E.; Grady, S.T.; Chipman, J.W.; Baker, E.R.; Koutrakis, P.; Karagas, M.R. Residential wood stove use and indoor exposure to PM2.5 and its components in Northern New England. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Rubilar, F.; Quinteros, M.E.; Cayupi, K.; Ayala, S.; Lu, S.; Jimenez, R.B.; Cárdenas, J.P.; Blazquez, C.A.; Delgado-Saborit, J.M.; et al. Spatial distribution of particulate matter on winter nights in Temuco, Chile: Studying the impact of residential wood-burning using mobile monitoring. Atmos. Environ. 2022, 286, 119255. [Google Scholar] [CrossRef]
- Díaz-Robles, L.A.; Ortega, J.C.; Fu, J.S.; Reed, G.D.; Chow, J.C.; Watson, J.G.; Moncada-Herrera, J.A. A hybrid ARIMA and artificial neural networks model to forecast particulate matter in urban areas: The case of Temuco, Chile. Atmos. Environ. 2008, 42, 8331–8340. [Google Scholar] [CrossRef]
- Sanhueza, P.A.; Torreblanca, M.A.; Diaz-Robles, L.A.; Schiappacasse, L.N.; Silva, M.P.; Astete, T.D. Particulate air pollution and health effects for cardiovascular and respiratory causes in Temuco, Chile: A wood-smoke-polluted urban area. J. Air Waste Manag. Assoc. 2009, 59, 1481–1488. [Google Scholar] [CrossRef]
- Oyarzún, G.M.; Dussaubat, D.N.; Miller, A.M.E.; Labra, J.S.; González, B.S. Efectos proinflamatorios de la contaminación atmosférica. Rev. Chil. Enferm. Respir. 2011, 27, 183–190. [Google Scholar] [CrossRef]
- Díaz-Robles, L.; Cortes, S.; Ortega, J.C.; Vergara-Fernández, A. Short term health effects of particulate matter: A comparison between wood smoke and multi-source polluted urban areas in Chile. Aerosol. Air Qual. Res. 2015, 15, 306–318. [Google Scholar] [CrossRef]
- Boso, À.; Álvarez, B.; Oltra, C.; Hofflinger, Á.; Vallejos-Romero, A.; Garrido, J. Examining patterns of air quality perception: A cluster analysis for southern Chilean cities. SAGE Open 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Quinteros, M.E.; Blanco, E.; Sanabria, J.; Rosas-Diaz, F.; Blazquez, C.A.; Ayala, S.; Cárdenas, J.P.; Stone, E.A.; Sybesma, K.; Delgado-Saborit, J.M.; et al. Spatio-temporal distribution of particulate matter and wood-smoke tracers in Temuco, Chile: A city heavily impacted by residential wood-burning. Atmos Environ 2023, 294, 119529. [Google Scholar] [CrossRef]
- IQAir. World Air Quality Report. 2021. Available online: https://www.iqair.com/newsroom/WAQR_2021_PR (accessed on 4 July 2024).
- IQAir. First in Air Quality. 2022. Available online: https://www.iqair.com/world-air-quality-report (accessed on 2 June 2023).
- Frutos, V.; González-Comadrán, M.; Solà, I.; Jacquemin, B.; Carreras, R.; Vizcaíno, M.A.C. Impact of air pollution on fertility: A systematic review. Gynecol. Endocrinol. 2015, 31, 7–13. [Google Scholar] [CrossRef]
- Darbre, P.D. Overview of air pollution and endocrine disorders. Int. J. Gen. Med. 2018, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Conforti, A.; Mascia, M.; Cioffi, G.; De Angelis, C.; Coppola, G.; De Rosa, P.; Pivonello, R.; Alviggi, C.; De Placido, G. Air pollution and female fertility: A systematic review of literature. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Canipari, R.; De Santis, L.; Cecconi, S. Female fertility and environmental pollution. Int. J. Environ. Res. Public Health 2020, 17, 38802. [Google Scholar] [CrossRef]
- Lee, B.E.; Ha, E.H.; Park, H.S.; Kim, Y.J.; Hong, Y.C.; Kim, H.; Lee, J.T. Exposure to air pollution during different gestational phases contributes to risks of low birth weight. Hum. Reprod. 2003, 18, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Behlen, J.C.; Lau, C.H.; Li, Y.; Dhagat, P.; Stanley, J.A.; Rodrigues Hoffman, A.; Golding, M.C.; Zhang, R.; Johnson, N.M. Gestational exposure to ultrafine particles reveals sex- and dose-specific changes in offspring birth outcomes, placental morphology, and gene networks. Toxicol. Sci. 2021, 184, 204–213. [Google Scholar] [CrossRef]
- Ritz, B.; Wilhelm, M.; Hoggatt, K.J.; Ghosh, J.K.C. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am. J. Epidemiol. 2007, 166, 1045–1052. [Google Scholar] [CrossRef]
- Ananth, C.V.; Kioumourtzoglou, M.A.; Huang, Y.; Ross, Z.; Friedman, A.M.; Williams, M.A.; Wang, S.; Mittleman, M.A.; Schwartz, J. Exposures to air pollution and risk of acute-onset placental abruption: A case-crossover study. Epidemiology 2018, 29, 631–638. [Google Scholar] [CrossRef]
- González-Comadrán, M.; Jacquemin, B.; Cirach, M.; Lafuente, R.; Cole-Hunter, T.; Nieuwenhuijsen, M.; Brassesco, M.; Coroleu, B.; Checa, M.A. The effect of short term exposure to outdoor air pollution on fertility. Reprod. Biol. Endocrinol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Durham, T.; Guo, J.; Cowell, W.; Riley, K.W.; Wang, S.; Tang, D.; Perera, F.; Herbstman, J.B. Prenatal PM2.5 exposure in relation to maternal and newborn telomere length at delivery. Toxics 2022, 10, 13. [Google Scholar] [CrossRef]
- Villarroel, F.; Ponce, N.; Gómez, F.A.; Muñoz, C.; Ramírez, E.; Nualart, F.; Salinas, P. Exposure to fine particulate matter 2.5 from wood combustion smoke causes vascular changes in placenta and reduces fetal size. Reprod. Toxicol. 2024, 127, 108610. [Google Scholar] [CrossRef] [PubMed]
- Requia, W.; Kill, E.; Papatheodorou, S.; Koutrakis, P.; Schwartz, J. Prenatal exposure to wildfire-related air pollution and birth defects in Brazil. J. Exposure Sci. Environ. Epidemiol. 2021, 32, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Saenen, N.D.; Martens, D.S.; Neven, K.Y.; Alfano, R.; Bové, H.; Janssen, B.G.; Roels, H.A.; Plusquin, M.; Vrijens, K.; Nawrot, T.S. Air pollution-induced placental alterations: An interplay of oxidative stress, epigenetics, and the aging phenotype? Clin. Epigenet. 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.M.; Damaceno-Rodrigues, N.R.; Guimarães Silva, R.M.; Scoriza, J.N.; Saldiva, P.H.N.; Caldini, E.G.; Dolhnikoff, M. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ. Res. 2009, 109, 536–543. [Google Scholar] [CrossRef]
- Raja, S.; Chandrasekaran, S.R.; Lin, L.; Xia, X.; Hopke, P.K.; Valsaraj, K.T. Analysis of beta attenuation monitor filter rolls for particulate matter speciation. Aerosol. Air Qual. Res. 2017, 17, 14–23. [Google Scholar] [CrossRef]
- National Research Council (US); Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [CrossRef]
- Erken, H.A.; Erken, G.; Genç, O. Blood pressure measurement in freely moving rats by the tail cuff method. Clin. Exp. Hypertens. 2013, 35, 11–15. [Google Scholar] [CrossRef]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Chand, A.; Legge, M. Stereological assessment of developing mouse ovarian follicles in an in vitro culture system. Anat. Rec. 2011, 294, 379–383. [Google Scholar] [CrossRef]
- US EPA. Guidelines for Reproductive Toxicity Risk Assessment; US Environmental Protection Agency, Risk Assessment Forum: Washington, DC, USA, 1996. Available online: https://www.epa.gov/risk/guidelines-reproductive-toxicity-risk-assessment (accessed on 21 June 2024).
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Halekoh, U.; Højsgaard, S. A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models—The R package pbkrtest. J. Stat. Softw. 2014, 59, 1–30. [Google Scholar] [CrossRef]
- Díaz-Robles, L.A.; Fu, J.S.; Vergara-Fernández, A.; Etcharren, P.; Schiappacasse, L.N.; Reed, G.D.; Silva, M.P. Health risks caused by short term exposure to ultrafine particles generated by residential wood combustion: A case study of Temuco, Chile. Environ. Int. 2014, 66, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, H.; Barraza, F.; Heyer, J.; Valdivia, G.; Schiappacasse, L.N.; Montoya, L.D. Indoor PM2.5 in an urban zone with heavy wood smoke pollution: The case of Temuco, Chile. Environ. Pollut. 2018, 236, 477–487. [Google Scholar] [CrossRef]
- World Health Organization. Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. 2021. Available online: https://www.who.int/publications/i/item/9789240034228 (accessed on 14 May 2023).
- Ministerio del Medio Ambiente de Chile. Sistema Nacional de Información de Calidad del Aire. 2024. Available online: https://sinca.mma.gob.cl/index.php/estacion/index/id/186 (accessed on 2 July 2024).
- Suárez, L.; Mesías, S.; Iglesias, V.; Silva, C.; Cáceres, D.D.; Ruiz-Rudolph, P. Personal exposure to particulate matter in commuters using different transport modes (bus, bicycle, car and subway) in an assigned route in downtown Santiago, Chile. Environ. Sci. Process. Impacts 2014, 16, 1309–1317. [Google Scholar] [CrossRef]
- Kundu, S.; Stone, E.A. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ. Sci. Process. Impacts 2014, 16, 1360–1370. [Google Scholar] [CrossRef]
- Singh, D.; Tassew, D.D.; Nelson, J.; Chalbot, M.G.; Kavouras, I.G.; Demokritou, P.; Tesfaigzi, Y. Development of an integrated platform to assess the physicochemical and toxicological properties of wood combustion particulate matter. Chem. Res. Toxicol. 2022, 35, 1541–1557. [Google Scholar] [CrossRef]
- Cereceda-Balic, F.; Fadic, X.; Llanos, A.L.; Domínguez, A.M.; Guevara, J.L.; Vidal, V.; Díaz-Robles, L.A.; Schiappacasse, L.N.; Etcharren, P. Obtaining polycyclic aromatic hydrocarbon concentration ratios and molecular markers for residential wood combustion: Temuco, a case study. J. Air Waste Manag. Assoc. 2012, 62, 44–51. [Google Scholar] [CrossRef]
- Zeb, B.; Alam, K.; Sorooshian, A.; Blaschke, T.; Ahmad, I.; Shahid, I. On the morphology and composition of particulate matter in an urban environment. Aerosol. Air Qual. Res. 2018, 18, 1431–1447. [Google Scholar] [CrossRef]
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of heavy metals on human male fertility—An overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, X.; Qiu, J.; You, X.; Xu, J. Association between heavy metals exposure and infertility among American women aged 20–44 years: A cross-sectional analysis from 2013 to 2018 NHANES data. Front. Public Health 2023, 11, 1122183. [Google Scholar] [CrossRef]
- Radwan, P.; Wielgomas, B.; Radwan, M.; Krasiński, R.; Bujak-Pietrek, S.; Polańska, K.; Kilanowicz, A.; Jurewicz, J. Urinary concentration of selected nonpersistent endocrine disrupting chemicals—Reproductive outcomes among women from a fertility clinic. Environ. Sci. Pollut. Res. 2023, 30, 45088–45096. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Ledoux, F.; Seigneur, M.; Oikonomou, K.; Sciare, J.; Courcot, D.; Afif, C. Chemical profiles of PM2.5 emitted from various anthropogenic sources of the Eastern Mediterranean: Cooking, wood burning, and diesel generators. Environ. Res. 2022, 211, 113032. [Google Scholar] [CrossRef] [PubMed]
- Colicino, E.; Cowell, W.; Foppa Pedretti, N.; Joshi, A.; Youssef, O.; Just, A.C.; Kloog, I.; Petrick, L.; Niedzwiecki, M.; Wright, R.O.; et al. Maternal steroids during pregnancy and their associations with ambient air pollution and temperature during preconception and early gestational periods. Environ. Int. 2022, 165, 107320. [Google Scholar] [CrossRef]
- Kaur, K.; Lesseur, C.; Deyssenroth, M.A.; Kloog, I.; Schwartz, J.D.; Marsit, C.J.; Chen, J. PM2.5 exposure during pregnancy is associated with altered placental expression of lipid metabolic genes in a US birth cohort. Environ. Res. 2022, 211, 113066. [Google Scholar] [CrossRef]
- Forchhammer, L.; Møller, P.; Riddervold, I.S.; Bønløkke, J.; Massling, A.; Sigsgaard, T.; Loft, S. Controlled human wood smoke exposure: Oxidative stress, inflammation and microvascular function. Part. Fibre Toxicol. 2012, 9, 7. [Google Scholar] [CrossRef]
- Wang, L.; Luo, D.; Liu, X.; Zhu, J.; Wang, F.; Li, B.; Li, L. Effects of PM2.5 exposure on reproductive system and its mechanisms. Chemosphere 2021, 264, 128436. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, P.; Chen, M. Research on the impact of PM2.5 on human reproductive health in recent years: A review. JSM Environ. Sci. Ecol. 2022, 10, 1078. [Google Scholar]
- Shao, X.; Cheng, H.; Zhou, J.; Zhang, J.; Zhu, Y.; Yang, C.; Di Narzo, A.F.; Yu, J.; Shen, Y.; Li, Y.; et al. Prenatal exposure to ambient air multi-pollutants significantly impairs intrauterine fetal development trajectory. Ecotoxicol. Environ. Saf. 2020, 201, 110726. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Weisskopf, M.G.; Laden, F.; Coull, B.A.; Modest, A.M.; Hacker, M.R.; Wylie, B.J.; Wei, Y.; Schwartz, J.; Papatheodorou, S. Exposure to PM2.5 during pregnancy and fetal growth in Eastern Massachusetts, USA. Environ. Health Perspect. 2022, 130, 17004. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Ji, X.; Li, G.; Hu, M.; Sang, N. Maternal exposure to PM2.5 affects fetal lung development at sensitive windows. Environ. Sci. Technol. 2020, 54, 316–324. [Google Scholar] [CrossRef]
- Li, R.; Peng, J.; Zhang, W.; Wu, Y.; Hu, R.; Chen, R.; Gu, W.; Zhang, L.; Qin, L.; Zhong, M.; et al. Ambient fine particulate matter exposure disrupts placental autophagy and fetal development in gestational mice. Ecotoxicol. Environ. Saf. 2022, 239, 113680. [Google Scholar] [CrossRef]
- Beyerlein, A.; Schiessl, B.; Lack, N.; von Kries, R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: A novel approach. Am. J. Clin. Nutr. 2009, 90, 1552–1558. [Google Scholar] [CrossRef]
- Boamah-Kaali, E.; Jack, D.W.; Ae-Ngibise, K.A.; Quinn, A.; Kaali, S.; Dubowski, K.; Oppong, F.B.; Wylie, B.J.; Mujtaba, M.N.; Gould, C.F.; et al. Prenatal and postnatal household air pollution exposure and infant growth trajectories: Evidence from a rural Ghanaian pregnancy cohort. Environ. Health Perspect. 2021, 129, 117009. [Google Scholar] [CrossRef]
- Lapillonne, A.; Bocquet, A.; Briend, A.; Chouraqui, J.P.; Darmaun, D.; Feillet, F.; Frelut, M.L.; Guimber, D.; Hankard, R.; Peretti, N.; et al. Pollutants in breast milk: A public health perspective—A commentary of the Nutrition Committee of the French Society of Pediatrics. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 343–346. [Google Scholar] [CrossRef]
- Sundram, T.K.M.; Tan, E.S.S.; Lim, H.S.; Amini, F.; Bustami, N.A.; Tan, P.Y.; Rehman, N.; Ho, Y.B.; Tan, C.K. Effects of ambient particulate matter (PM2.5) exposure on calorie intake and appetite of outdoor workers. Nutrients 2022, 14, 4858. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Moreno-Macias, H.; London, S.J. Gene by environment interaction and ambient air pollution. Proc. Am. Thorac. Soc. 2010, 7, 116–122. [Google Scholar] [CrossRef]
- Pryor, J.T.; Cowley, L.O.; Simonds, S.E. The physiological effects of air pollution: Particulate matter, physiology and disease. Front. Public Health 2022, 10, 882569. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 153, 362–367. [Google Scholar] [CrossRef] [PubMed]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does air pollution play a role in infertility? A systematic review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Giorgis-Allemand, L.; Thalabard, J.C.; Rosetta, L.; Siroux, V.; Bouyer, J.; Slama, R. Can atmospheric pollutants influence menstrual cycle function? Environ. Pollut. 2020, 257, 113605. [Google Scholar] [CrossRef]
- Plunk, E.C.; Richards, S.M. Endocrine-disrupting air pollutants and their effects on the hypothalamus-pituitary-gonadal axis. Int. J. Mol. Sci. 2020, 21, 9191. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Li, G.; Zhang, Y.; Li, J.; Chen, H. Fluorescent reconstitution on deposition of PM2.5 in lung and extrapulmonary organs. Proc. Natl. Acad. Sci. USA 2019, 116, 2488–2493. [Google Scholar] [CrossRef]



| Study Period | Annually | |
|---|---|---|
| PM2.5 [ug/m3] | 48.8 ± 36.1 (CV: 74.0%) | 26.2 ± 30.6 (CV: 117%) |
| MP10 [ug/m3] | 56.9 ± 38.3 (CV: 67.3%) | 36.6 ± 30.8 (CV: 84.2%) |
| CO [ppm] | 0.78 ± 0.49 (CV: 61.5%) | 0.44 ±0.43 (CV: 97.7%) |
| Mean | SD | Min. | Max. | |
|---|---|---|---|---|
| O | 44.03 | 6.89 | 39.08 | 54.23 |
| C | 24.00 | 7.35 | 16.93 | 32.06 |
| Si | 14.15 | 7.89 | 5.51 | 24.16 |
| Al | 5.25 | 2.97 | 1.44 | 7.62 |
| Na | 2.50 | 1.31 | 0.92 | 4.11 |
| Cl | 1.95 | 1.50 | 0.60 | 4.07 |
| Fe | 3.25 | 1.76 | 0.90 | 5.15 |
| Zn | 1.60 | 1.00 | 0.57 | 2.67 |
| Ca | 0.83 | 0.75 | 0.00 | 1.81 |
| K | 1.32 | 1.00 | 0.00 | 2.43 |
| Mg | 0.44 | 0.53 | 0.00 | 1.04 |
| S | 0.26 | 0.27 | 0.04 | 0.66 |
| Ti | 0.32 | 0.36 | 0.00 | 0.64 |
| Pb | 0.21 | 0.31 | 0.00 | 0.67 |
| Rotated Component Matrix | ||
|---|---|---|
| PC | ||
| 1 | 2 | |
| O | −0.614 | −0.743 |
| C | 0.959 | 0.085 |
| Si | −0.883 | −0.431 |
| Al | 0.029 | 0.773 |
| Na | 0.799 | 0.583 |
| Cl | 0.852 | 0.389 |
| Fe | 0.073 | 0.962 |
| Zn | 0.515 | 0.739 |
| Ca | 0.826 | 0.519 |
| K | 0.076 | 0.977 |
| Mg | 0.756 | −0.065 |
| S | 0.950 | −0.090 |
| Ti | 0.285 | 0.798 |
| Pb | −0.656 | 0.730 |
| Estimate | Std. Error | t-Value | Pr(>|t|) | |
|---|---|---|---|---|
| FA/FA | 279.393 | 9.299 | 30.046 | 6.07 × 10−10 *** |
| FA/NFA | −23.615 | 2.772 | −8.519 | 3.35 × 10−14 *** |
| NFA/FA | −15.143 | 2.906 | −5.211 | 7.13 × 10−7 *** |
| NFA/NFA | −6.714 | 2.762 | −2.431 | 0.0164 * |
| Estimate | Std. Error | df | t Ratio | p-Value | |
|---|---|---|---|---|---|
| FA/FA—FA/NFA | 23.61 | 2.77 | 131 | 8.519 | <0.0001 |
| FA/FA—NFA/FA | 15.14 | 2.91 | 131 | 5.211 | <0.0001 |
| FA/FA—NFA/NFA | 6.71 | 2.76 | 131 | 2.431 | 0.0764 |
| FA/NFA—NFA/FA | −8.47 | 2.72 | 130 | −3.117 | 0.0119 |
| FA/NFA—NFA/NFA | −16.9 | 2.56 | 130 | −6.595 | <0.0001 |
| NFA/FA—NFA/NFA | −8.43 | 2.71 | 130 | −3.112 | 0.0121 |
| FA | NFA | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | CV (%) | n | Mean (SD) | CV (%) | |||
| Ovary cyclicity | N° of cycles per study period | 12 | 2.0 (0.6) | 30.2 | 12 | 1.42 (0.5) | 36.3% | 0.0432 |
| Estrous cycle length (days) | 12 | 20.7 (1.97) | 9.53% | 12 | 23.7 (1.23) | 5.2% | 0.0001 | |
| Estrus phase length (days) | 12 | 4.0 (0.70) | 17.7% | 12 | 5 (0.76) | 15.4% | 0.0057 | |
| Days of estrus/cycle (%) | 12 | 19.5 (3.92) | 20.1% | 12 | 21.2 (3.4) | 16.1 | 0.1724 | |
| Follicles | Small | 6 | 314 (96.8) | 30.9 | 6 | 333 (83.8) | 25.1 | 0.5176 |
| Growing | 6 | 144 (81.3) | 56.7 | 6 | 97.4 (33.9) | 33.9 | 0.0317 | |
| Antral | 6 | 120 (25.7) | 21.4 | 6 | 87.4 (32.5) | 31.7 | 0.0020 | |
| Corpus luteum | 6 | 93.8 (36.4) | 38.8 | 6 | 79.8 (28.3) | 35.5 | 0.2048 | |
| Reproductive capacity | Mating rate (%) | 12 | 100 | 12 | 100 | ns | ||
| Mating time (days) | 12 | 4.67 (2.27) | 48.6% | 12 | 3.58 (1.51) | 42.0% | 0.1456 | |
| Fertility rate (%) | 12 | 91.7 | 12 | 83 | 0.540 | |||
| Pregnancy rate (%) | 12 | 100 | 12 | 100 | ns | |||
| FA/FA | FA/NFA | NFA/FA | NFA/NFA | p-Value | |
|---|---|---|---|---|---|
| N° of live fetuses per litter | 13.5 (2.17) | 11.2 (3.70) | 10.2 (1.92) | 7.8 (1.79) | 0.0125 |
| N° of dead fetuses per litter | 0 (0) | 0.6 (0.89) | 0.2 (0.44) | 0.4 (0.54) | 0.3485 |
| implantation rate (%) | 14 (2.37) | 11.8 (4.21) | 8.4 (3.44) | 8.2 (1.64) | 0.0149 |
| pre-implantation loss (%) | 4.33 (3.39) | 6 (6.04) | 4.2 (6.02) | 13 (9.41) | 0.1323 |
| post-implantation loss (%) | 0 (0) | 4.2 (6.02) | 1.6 (3.58) | 5.4 (7.80) | 0.3203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, P.; Ponce, N.; del Sol, M.; Vásquez, B. Impact of PM2.5 Exposure from Wood Combustion on Reproductive Health: Implications for Fertility, Ovarian Function, and Fetal Development. Toxics 2025, 13, 238. https://doi.org/10.3390/toxics13040238
Salinas P, Ponce N, del Sol M, Vásquez B. Impact of PM2.5 Exposure from Wood Combustion on Reproductive Health: Implications for Fertility, Ovarian Function, and Fetal Development. Toxics. 2025; 13(4):238. https://doi.org/10.3390/toxics13040238
Chicago/Turabian StyleSalinas, Paulo, Nikol Ponce, Mariano del Sol, and Bélgica Vásquez. 2025. "Impact of PM2.5 Exposure from Wood Combustion on Reproductive Health: Implications for Fertility, Ovarian Function, and Fetal Development" Toxics 13, no. 4: 238. https://doi.org/10.3390/toxics13040238
APA StyleSalinas, P., Ponce, N., del Sol, M., & Vásquez, B. (2025). Impact of PM2.5 Exposure from Wood Combustion on Reproductive Health: Implications for Fertility, Ovarian Function, and Fetal Development. Toxics, 13(4), 238. https://doi.org/10.3390/toxics13040238






