Abstract
α-Klotho is an anti-aging protein linked to various age-related diseases. Environmental metal exposure has been associated with oxidative stress and aging, but its effect on α-Klotho levels remains unclear. This study investigated the relationship between urinary metal concentrations and serum α-Klotho levels using data from the National Health and Nutrition Examination Survey (NHANES) 2007–2016 cycles. A total of 4071 adults aged 40 to 79 years were included in the analysis. After adjusting for potential confounders, positive associations were found between serum α-Klotho levels and barium (Ba), cesium (Cs), and molybdenum (Mo), while tungsten (W) and uranium (U) were negatively correlated with α-Klotho levels. The combined effects of multiple metals were further analyzed using the qgcomp model, which demonstrated a negative correlation between increased metal mixtures and serum α-Klotho levels. Specifically, U, total arsenic (t-As), W, cadmium (Cd), antimony (Sb), and lead (Pb) contributed to the reduction of α-Klotho levels, while Ba, Cs, dimethylarsinic acid (DMA), Mo, thallium (Tl), and cobalt (Co) were positively associated with α-Klotho levels. These findings suggest that exposure to certain metals, particularly in combination, may reduce serum α-Klotho levels, potentially accelerating aging processes. Further studies should investigate the underlying mechanisms responsible for these associations.
1. Introduction
The Klotho protein family, including α-Klotho and β-Klotho, plays a central role in regulating aging and longevity. Of particular interest is α-Klotho, a soluble form of the protein that has been shown to exert protective effects on various organ systems, such as the cardiovascular, renal, and nervous systems [1,2]. Animal studies have demonstrated that α-Klotho contributes to an extended lifespan and delayed onset of age-related diseases, such as kidney disease and neurodegeneration [1,3,4,5]. In humans, higher α-Klotho levels have been linked to reduced all-cause mortality, highlighting its potential as a therapeutic target for age-related conditions [6]. Recent research has further linked α-Klotho to the pathophysiology of chronic diseases, such as cardiovascular disease [7], cancer [8], and diabetes [9], highlighting its potential as both a biomarker for aging and a therapeutic target for various age-related diseases.
Environmental exposure to heavy metals is a widespread global health concern. These metals can enter the human body through inhalation, ingestion, and dermal absorption, leading to various adverse health outcomes, including neurodevelopmental disorders, cardiovascular diseases, and cancer [10]. Arsenic (As), lead (Pb), and cadmium (Cd) are well-documented toxic metals [11,12], while antimony (Sb), tungsten (W), and molybdenum (Mo) also pose potential health risks. Sb exposure has been associated with oxidative stress and immunotoxicity [13], W has been implicated in carcinogenesis and systemic toxicity [14], and Mo can be toxic at high levels, impairing enzymatic functions and metabolism [15]. Environmental metal exposure has also been linked to accelerated aging processes, indicating that metals contribute to increased oxidative stress, DNA damage, and impaired cellular function, all of which are hallmarks of aging [16]. Therefore, examining the relationship between metal exposure and aging biomarkers, such as α-Klotho, is critical for understanding how environmental pollutants influence aging and contribute to age-related diseases.
A growing body of research has explored the relationship between α-Klotho levels and environmental pollutants, including organic contaminants such as polycyclic aromatic hydrocarbons [17], perchlorates, nitrates, thiocyanates [18], and dichlorobenzene [19]. Emerging studies have elucidated the impact of heavy metal exposure on α-Klotho levels. A study using NHANES data found that higher Pb levels are associated with lower serum α-Klotho, further supporting the link between heavy metal exposure and aging-related processes [20]. Additionally, exposure to Cd and Pb has been linked to oxidative stress, with evidence indicating that α-Klotho homeostasis may be disrupted in individuals with kidney dysfunction, especially at more severe stages [21]. Another investigation demonstrated that α-Klotho partially mediated the association between blood Pb levels and estimated glomerular filtration rate (eGFR), suggesting a potential role of α-Klotho in Pb-induced renal dysfunction [22]. Furthermore, studies on three essential elements identified a negative correlation between serum α-Klotho and blood copper (Cu) levels [23]. These findings underscore the importance of investigating the effects of heavy metal exposure on serum α-Klotho levels, especially the potential synergistic effects of multiple metal exposures on health. Given the ubiquity and persistence of metal pollutants, it is essential to explore how combined metal exposure influences α-Klotho levels, particularly in the general population.
The goal of this study was to investigate the association between urinary metal concentrations and serum α-Klotho levels using data from the National Health and Nutrition Examination Survey (NHANES), which is a nationally representative survey that provides comprehensive health data on the U.S. population, making it an invaluable resource for environmental health studies [24,25]. We used multiple linear regression and quantile g-computation (qgcomp) models to evaluate the independent and combined effects of urinary metals on serum α-Klotho levels. By examining a wide range of urinary metals and their mixtures, this study aims to provide new insights into the relationship between environmental metal exposure and aging biomarkers.
2. Materials and Methods
2.1. Study Design and Participants
This study analyzed data from five cycles of the NHANES conducted between 2007 and 2016 (2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016). NHANES emphasizes environmental exposures and their impacts on health outcomes. Participants were selected based on the availability of data on urinary metals and serum α-Klotho levels, as shown in Figure 1. Initially, 17,389 adults aged 40–79 years were included in the analysis, as serum α-Klotho levels were only assessed in individuals within this age group. After excluding participants with incomplete data on serum α-Klotho (n = 3625), urinary metals (n = 9420), or covariates (n = 273), the final study population consisted of 4071 participants. The NHANES study protocol was reviewed and approved by the Institutional Review Board of the National Center for Health Statistics (NCHS), and informed consent was obtained from all participants. No additional ethical approval was required for this secondary data analysis.
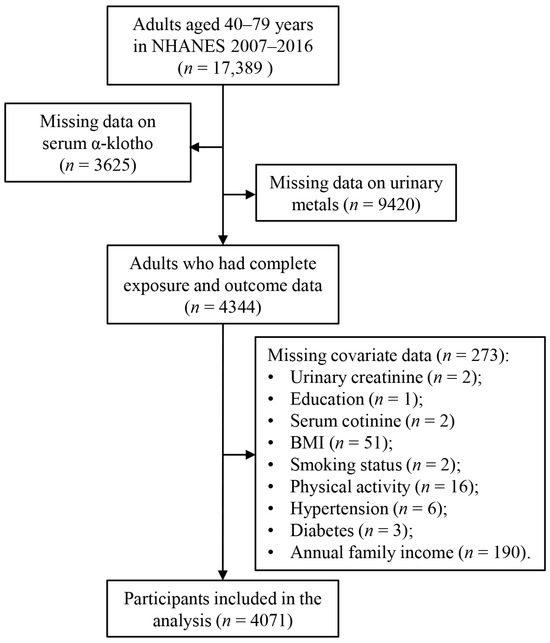
Figure 1.
Flow diagram of selection of study participants from NHANES 2007–2016.
2.2. Measurement of Urinary Metal Levels
Urine samples were collected, stored at −30 °C, and subsequently shipped to the National Center for Environmental Health (NCEH) for analysis. Urinary concentrations of total arsenic (t-As), barium (Ba), cobalt (Co), Mo, cesium (Cs), Cd, Pb, Sb, thallium (Tl), W, and uranium (U) were quantified using inductively coupled plasma mass spectrometry (ICP-MS). For As species, including dimethylarsinic acid (DMA), arsenobetaine, monomethylarsonic acid, arsenocholine, arsenous (III) acid, and arsenic (V) acid, high-performance liquid chromatography (HPLC) was used. However, only DMA had a detection rate exceeding 70%, while other arsenic species exhibited lower detection rates. For elements measured by ICP-MS and other techniques, values below the limit of detection (LOD) were imputed as LOD divided by the square root of two, following NHANES protocols. Detailed information on laboratory procedures for measuring urinary metals and arsenic species can be found on the NHANES website.
2.3. Measurement of Serum α-Klotho Levels
Serum samples were stored at −80 °C until further analysis. α-Klotho levels were measured using an ELISA kit from IBL International (Gunma, Japan). The assay was validated prior to the study, demonstrating excellent sensitivity (4.33 pg/mL, compared to the manufacturer’s claim of 6.15 pg/mL) and linearity (R2 = 0.998 and 0.997 for high and low α-Klotho concentrations). Intra-assay precision was 3.2–3.9% for recombinant samples and 2.3–3.3% for human samples, while inter-assay precision was 2.8–3.5% for recombinant samples and 3.4–3.8% for human samples. All samples were analyzed in duplicate, and results met the laboratory’s acceptance criteria. Additional methodological details can be found on the NHANES website (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2015/DataFiles/SSKL_I.htm) (accessed on 5 December 2024).
2.4. Covariates
Several covariates were selected based on their known associations with α-Klotho levels, as reported in previous literature [19,20,26,27], including age, sex, smoking status, physical activity, body mass index (BMI), diabetes mellitus, hypertension, race/ethnicity, education level, annual family income, marital status, serum cotinine, and NHANES cycle. Urinary creatinine levels were included to adjust for variability in urine concentration.
2.5. Statistical Analysis
Statistical analyses were performed using R software (version 4.2.1). Group differences in α-Klotho levels were evaluated using the Mann–Whitney U test for two-group comparisons and the Kruskal–Wallis test for multiple-group analyses. Urinary metal concentrations were analyzed both as continuous variables (after natural logarithmic transformation) and in quartiles. Multiple linear regression models were used to evaluate the association between serum α-Klotho levels and urinary metal concentrations, with results presented as the estimated percent changes in serum α-Klotho levels and their corresponding 95% confidence intervals (CIs) according to previous studies [28]. The p-value for trend was calculated by fitting urinary metal quartiles into linear models to assess dose–response relationships. All regression models were adjusted for age, sex, race/ethnicity, BMI, annual family income, smoking, education, marital status, hypertension, diabetes, NHANES cycle, physical activity, serum cotinine, and urinary creatinine.
In addition to analyzing single metal effects, the combined effects of multiple urinary metals on serum α-Klotho levels were assessed by adjusting for other metals. Finally, to investigate the impact of metal mixtures, the qgcomp model was implemented in the “qgcomp” R package. This model assigns positive or negative weights to urinary metals, estimating their combined exposure effect. The “qgcomp boot” function was used to assess the linearity of the exposure–response relationship and estimate the overall marginal effect.
3. Results
3.1. Characteristics of Study Participants and Serum α-Klotho Levels
A total of 4071 adults aged 40 to 79 years were included (Table 1), comprising 1999 males (49.1%) and 2072 females (50.9%). Serum α-Klotho levels varied significantly by age, sex, and race/ethnicity. Participants aged 40–49 years had the highest α-Klotho levels (883.5 ± 338.5 pg/mL), while those aged ≥ 70 years had the lowest levels (796.8 ± 264.3 pg/mL) (p < 0.001). Females had higher α-Klotho levels (882.4 ± 340.6 pg/mL) compared to males (819.5 ± 269.5 pg/mL) (p < 0.001), and non-Hispanic Black participants had the highest levels (905.5 ± 358.3 pg/mL) (p < 0.001). Serum α-Klotho levels were significantly higher in participants with hypertension (861.9 ± 309.9 pg/mL) compared to those without hypertension (839.3 ± 308.3 pg/mL) (p = 0.002). Underweight participants also had significantly higher α-Klotho (919.7 ± 331.3 pg/mL) than those in other BMI categories (p = 0.002). Significant variations in α-Klotho levels were also observed across NHANES cycles (p < 0.001), with the highest levels in the 2011–2012 cycle (897.5 ± 330.9 pg/mL) and the lowest in the 2015–2016 cycle (817.7 ± 329.3 pg/mL). Regarding marital status, participants who were never married had significantly higher serum α-Klotho levels (890.6 ± 405.3 pg/mL) compared to those who were married/cohabiting (849.1 ± 288.1 pg/mL) (p = 0.040). However, no significant differences were observed across education levels, family income, serum cotinine levels, physical activity, smoking status, or diabetes status.

Table 1.
Distribution of serum α-Klotho levels based on the characteristics of study participants in the NHANES 2007–2016.
3.2. Distribution of Urinary Metal Concentrations
The distribution of urinary metal concentrations among the study participants is presented in Table 2. The detection rates for the 12 urinary metals ranged from 70.4% (for Sb) to 100% (for Cs and Mo). The highest mean urinary concentrations were observed for Mo at 51.28 μg/L, followed by t-As at 19.86 μg/L and DMA at 5.72 μg/L. The lowest mean concentrations were found for uranium (U) at 0.0121 μg/L and Sb at 0.074 μg/L. The 95th percentile concentrations ranged from 0.0351 μg/L for U to 139.40 μg/L for Mo, indicating considerable variability in exposure levels across participants. Additionally, positive correlations were observed among the urinary metal concentrations, as shown in Figure S1, suggesting that participants with higher levels of one metal often had elevated levels of others. These inter-metal correlations highlighted the complexity of metal exposures in this population.

Table 2.
The distributions of urinary metals among study participants in the NHANES 2007–2016.
3.3. Relationships Between Urinary Metals and Serum α-Klotho Levels
The relationships between individual urinary metal concentrations and serum α-Klotho levels were assessed after adjusting for potential confounders (Table S1). After adjusting for age, sex, race/ethnicity, BMI, annual family income, education, marital status, hypertension, diabetes, NHANES cycle, physical activity, serum cotinine, and urinary creatinine, significant positive associations were observed for Ba (percent change = 1.96, 95% CI: 1.19–2.74), Cs (percent change = 2.67, 95% CI: 1.21–4.14), and Mo (percent change = 1.05, 95% CI: 0.05–2.05). Quartile-based analyses revealed that participants in higher quartiles (Q2–Q4) of urinary Ba, Q3–Q4 of Cs, and Q4 of Mo exhibited significantly elevated serum α-Klotho levels compared to the reference quartile (Q1). Conversely, inverse associations were identified for W (percent change = −0.90, 95% CI: −1.69 to −0.10) and U (percent change = −1.65, 95% CI: −2.43 to −0.86). Participants in Q4 of W and Q3–Q4 of U demonstrated significantly lower serum α-Klotho levels relative to Q1.
Subsequently, using mutually adjusted models, the combined effects of multiple urinary metals on serum α-Klotho levels were examined (Table 3). After adjusting for other covariates and metals, Ba, Cs, and Mo remained positively associated with serum α-Klotho levels, with percent change values of 2.10 (95% CI: 1.24, 2.96), 2.81 (95% CI: 0.86, 4.80), and 1.40 (95% CI: 0.22, 2.59), respectively. In contrast, W and U were negatively associated with serum α-Klotho levels (W: percent change = −0.97, 95% CI: −1.88, −0.05; U: percent change = −1.58, 95% CI: −2.42, −0.74). Participants with higher urinary levels of Ba and Cs (Q2, Q3, Q4) had significantly higher serum α-Klotho levels, while those with elevated levels of Sb and U (Q2, Q3, Q4) exhibited significantly lower serum α-Klotho levels.

Table 3.
Associations of multiple urinary metals with serum α-Klotho levels in the NHANES 2007–2016.
3.4. Association Between Metal Mixtures and Serum α-Klotho by the Qgcomp Model
To investigate the combined effects of multiple urinary metals on serum α-Klotho levels, the qgcomp model was employed (Figure 2). The regression index weights revealed that U, t-As, W, Cd, Sb, and Pb contributed negatively to serum α-Klotho levels, with U being the primary contributor to the overall negative association. In contrast, Ba, Cs, DMA, Mo, Tl, and Co showed positive associations with serum α-Klotho levels, with Ba having the greatest positive effect. Furthermore, the combined effect of the metal mixture was analyzed based on the quantile range (Figure 2B). The results demonstrated a negative correlation between the mixture of twelve urinary metals and serum α-Klotho levels. A linear decrease in serum α-Klotho levels was observed across increasing quartiles of the metal mixture, with a percent change of −0.61 (95% CI: −1.07, −0.14) (p = 0.002), suggesting that higher exposure to multiple metals is associated with reduced serum α-Klotho levels.
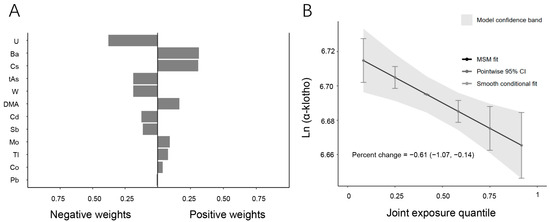
Figure 2.
Associations between metal mixtures and serum α-Klotho by the qgcomp model. (A) Direction and magnitude of the assigned weights for each metal in association with serum α-Klotho. (B) Combined effects of twelve urinary metals levels on serum α-Klotho (95% CI). The model was adjusted for age, sex, race/ethnicity, BMI, annual family income, smoking, education, marital status, hypertension, diabetes, NHANES cycle, physical activity, serum cotinine, and urinary creatinine.
4. Discussion
This study comprehensively analyzed the relationship between urinary metal exposures and serum α-Klotho levels using data from the NHANES 2007–2016 cycles [20,29]. While previous studies have explored the associations between α-Klotho and environmental pollutants, especially organic chemicals, the effects of mixed metal exposure on α-Klotho levels remain largely unexplored. Our study is the first to systematically examine both individual and combined metal exposures and their effects on α-Klotho levels in a large, nationally representative sample. This research filled a critical gap in the literature by addressing the previously unexplored relationship between mixed metal exposure and α-Klotho levels.
Although the relationship between metal exposure and α-Klotho remains under investigation, prior studies have demonstrated that heavy metals could impact α-Klotho levels, primarily through oxidative stress and kidney function impairment [10,30]. Previous research has shown that Pb exposure negatively correlates with α-Klotho levels and mediates its effect on kidney function, while Cd and Pb have been linked to oxidative-stress-related Klotho dysregulation, particularly in individuals with renal impairment [21,22]. Additionally, studies on trace elements found that a combined effect of selenium (Se), Cu, and zinc (Zn) decreased α-Klotho concentrations [23]. Our study extends these findings by assessing the effects of multiple metals on α-Klotho simultaneously, rather than focusing on single-metal exposures. We observed that urinary W and U were negatively associated with α-Klotho levels, and our quantile g-computation model revealed a dose-dependent decrease in α-Klotho with increasing metal mixture exposure. These findings suggest that exposure to multiple metals may have a cumulative or synergistic effect, amplifying their negative impact on aging biomarkers. Unlike previous studies that reported significant associations between Cd and Pb with α-Klotho [20], we did not observe statistically significant effects for these metals. This discrepancy may be attributed to differences in study design, population characteristics, or the influence of co-exposures in our multi-metal analysis, which could obscure individual metal effects.
Given the central role of α-Klotho in aging and disease prevention, it is important to consider what constitutes a normal reference range for this biomarker. Currently, there is no universally established reference range for serum α-Klotho protein levels. Most studies on α-Klotho concentrations are based on data from the NHANES [19,20,31,32,33,34]. In our study, the quartile-based analysis of α-Klotho levels provides insight into how varying degrees of metal exposure may affect this anti-aging protein. While the specific health effects of small fluctuations in α-Klotho levels remain unclear, previous research suggests that reduced α-Klotho levels are associated with aging-related diseases. In this context, the observed associations between metal exposure and α-Klotho variations may have long-term implications for health and aging processes.
Our study also compared metal-associated effects on α-Klotho between different demographic groups. Notably, our analysis revealed that individuals who were never married exhibited higher serum α-Klotho levels than those who were married or cohabiting. This observation might reflect differences in psychosocial stress or lifestyle behaviors, which can influence oxidative stress [35] and, in turn, α-Klotho expression. Similarly, individuals with hypertension had significantly higher α-Klotho levels than those without hypertension. Previous studies have suggested that lower α-Klotho levels are associated with an increased risk of hypertension, identifying it as a potential risk factor for elevated blood pressure [4,36]. This discrepancy between our findings and prior research may be due to differences in study populations, measurement methods, or unmeasured confounders. The higher α-Klotho levels observed in hypertensive individuals in our study could represent a compensatory response to vascular stress or endothelial dysfunction. Additionally, potential residual confounding from factors such as medication use, kidney function, or inflammatory status may have influenced the observed association [6,37]. Moreover, α-Klotho levels varied significantly across NHANES cycles. This variation might reflect underlying population-level differences in environmental exposures, dietary patterns, or methodological factors related to sample collection and processing. Future studies should explore whether these variations are linked to temporal trends in metal exposure or other environmental stressors that may impact aging and disease susceptibility.
Interestingly, while most non-essential metals were associated with lower α-Klotho levels, we found that Ba and Mo were positively correlated with α-Klotho. Both metals are considered essential trace elements with antioxidative properties that may counteract oxidative-stress-induced α-Klotho depletion [15,38]. These metals may support the maintenance of α-Klotho levels, counteracting the oxidative damage caused by other environmental stressors. Furthermore, the potential therapeutic implications of these metals in maintaining α-Klotho levels could warrant further investigation into their possible use in anti-aging interventions. This contrasts with the negative effects observed for heavy metals, underscoring the complexity of metal interactions in biological systems. In addition to these compounding effects, it is plausible that antagonistic interactions may also occur between certain metals, either through functional interference or competition at receptor sites [39], which could modify the overall impact on α-Klotho levels. Further research is needed to determine the specific biological roles of different metals in modulating aging processes.
Moreover, our study emphasized the significance of considering metal mixtures rather than focusing solely on individual metal exposures. Using the quantile qgcomp model, we observed a clear dose–response relationship between increasing quartiles of metal mixtures and decreasing serum α-Klotho levels. This suggested that exposure to multiple metals in combination might have compounded or synergistic effects, amplifying their negative impact on aging biomarkers. This finding highlighted the necessity of studying metal mixtures, as they more accurately reflected real-world environmental exposures [40]. Previous research had predominantly focused on individual metal exposures [20,34], but our study demonstrated that the combined effect of multiple metals was likely stronger and more significant in influencing health outcomes, particularly in the context of aging.
The biological mechanisms through which metals influenced α-Klotho levels remain to be fully understood. However, oxidative stress appeared to be a central pathway. α-Klotho is a potent antioxidant and anti-inflammatory protein, and its deficiency is associated with increased ROS production and exacerbated oxidative stress [32]. Previous studies suggested that metals like Cd and Pb, known for their ability to induce oxidative damage, might reduce α-Klotho production, thereby promoting aging [20]. On the other hand, metals like Ba and Mo, which possess antioxidative properties [41,42], might mitigate oxidative damage and protected against age-related cellular dysfunction. These findings underscore the complexity of metal toxicity, as certain metals might have dual effects depending on exposure levels, the presence of other metals, and individual susceptibility.
Despite the valuable insights provided by our study, several limitations must be acknowledged. First, while urinary metals are widely used as biomarkers of exposure, they do not fully capture the total body burden of metals. Additionally, urinary metal concentrations might not reflect long-term accumulation, particularly for metals that accumulate in tissues over time [43]. Second, due to the nature of our cross-sectional study, establishing cause-and-effect relationships was not feasible. Longitudinal studies are needed to validate these associations and better understand the long-term effects of metal exposure on α-Klotho levels and aging. Furthermore, while we adjusted for several confounders, unmeasured factors, such as genetic predisposition, diet, and lifestyle, might also affect the relationship between metal exposure and α-Klotho levels. Moreover, we primarily examined the effects of individual metals and overall metal mixtures but did not explore specific interactions between chemically similar metals, such as Sb + As or Cd + Pb, which may exert unique combined effects. Future research should employ interaction models or machine learning algorithms to investigate whether certain metal pairs have synergistic or antagonistic effects on α-Klotho levels. Lastly, the potential impact of repeated freezing and thawing cycles on serum α-Klotho measurements cannot be ruled out, which might have introduced some variability in the results.
5. Conclusions
This study was the first to investigate the effects of twelve urinary metals on serum α-Klotho levels using the NHANES database. Our findings indicated that exposure to metals, especially in combination, was associated with decreased serum α-Klotho levels, potentially influencing aging processes. The identification of both positive and negative correlations underscored the complex biological effects of metals on aging. Further research is needed to clarify the mechanisms through which metals, individually and in mixtures, regulate α-Klotho and impact health. Longitudinal studies should explore causal relationships, while mechanistic investigations can elucidate metal-induced effects on α-Klotho regulation. Additionally, assessing the combined toxicity or protective effects of metal mixtures and the therapeutic potential of metals positively linked to α-Klotho may provide valuable insights into aging-related interventions. Despite its limitations, this study provided a foundation for future research on environmental metal exposure and its impact on aging biomarkers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics13040237/s1, Figure S1: Pairwise Pearson correlation matrix among ln-transformed urinary metals among study population in the NHANES 2007–2016; Table S1: Associations of single urinary metals with serum α-Klotho levels in the NHANES 2007–2016.
Author Contributions
Conceptualization, S.X., F.K. and H.X.; methodology, X.Z. and H.X.; validation, W.Z. and Z.J.; formal analysis, X.Z., C.D., Y.L. and M.F.; data curation, X.Z. and W.Z.; writing—original draft preparation, X.Z. and W.Z.; writing—review and editing, Z.J., M.F., F.K., S.X. and H.X.; visualization, X.Z.; supervision, H.X.; project administration, H.X.; funding acquisition, X.Z. and H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Innovation and Entrepreneurship Training Program for College Students of Zhejiang Province (S202413023126), the National Natural Science Foundation of China (82404231), and the Key Discipline of Zhejiang Province in Public Health and Preventive Medicine (First Class, Category A).
Institutional Review Board Statement
The NHANES was conducted following the Declaration of Helsinki and approved by the National Center for Health Statistics Institutional Review Board. NCHS IRB/ERB Protocol Number or Description: Continuation of Protocol #2005-06, Continuation of Protocol #2005-06, Protocol #2011-17, Continuation of Protocol #2011-17, and Continuation of Protocol #2011-17. Information regarding the specific approval codes and dates is available at: https://www.cdc.gov/nchs/nhanes/about/erb.html?CDC_AAref_Val=https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed on 10 February 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data utilized in this study are publicly accessible through the National Health and Nutrition Examination Survey at: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 10 November 2024).
Acknowledgments
The authors would like to thank the NHANES project for providing the publicly available data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Prud’homme, G.J.; Wang, Q. Anti-Inflammatory Role of the Klotho Protein and Relevance to Aging. Cells 2024, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.R.; Mullen, P.C.; Tucker-Zhou, T.; Chen, C.D.; Zeldich, E. Klotho Is a Neuroprotective and Cognition-Enhancing Protein. Vitam. Horm. 2016, 101, 215–238. [Google Scholar] [CrossRef]
- Charrin, E.; Dabaghie, D.; Sen, I.; Unnersjö-Jess, D.; Möller-Hackbarth, K.; Burmakin, M.; Mencke, R.; Zambrano, S.; Patrakka, J.; Olauson, H. Soluble Klotho Protects against Glomerular Injury through Regulation of ER Stress Response. Commun. Biol. 2023, 6, 208. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Kurt, M.; Wang, Q. Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations. Front. Aging 2022, 3, 931331. [Google Scholar] [CrossRef]
- Gupta, S.; Moreno, A.J.; Wang, D.; Leon, J.; Chen, C.; Hahn, O.; Poon, Y.; Greenberg, K.; David, N.; Wyss-Coray, T.; et al. KL1 Domain of Longevity Factor Klotho Mimics the Metabolome of Cognitive Stimulation and Enhances Cognition in Young and Aging Mice. J. Neurosci. 2022, 42, 4016–4025. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J. Association between Serum Klotho Concentration and All-Cause and Cardiovascular Mortality among American Individuals with Hypertension. Front. Cardiovasc. Med. 2022, 9, 1013747. [Google Scholar] [CrossRef]
- Akhiyat, N.; Ozcan, I.; Gulati, R.; Prasad, A.; Tchkonia, T.; Kirkland, J.L.; Lewis, B.; Lerman, L.O.; Lerman, A. Patients With Coronary Microvascular Dysfunction Have Less Circulating α-Klotho. J. Am. Heart Assoc. 2024, 13, e031972. [Google Scholar] [CrossRef]
- Ligumsky, H.; Merenbakh-Lamin, K.; Keren-Khadmy, N.; Wolf, I.; Rubinek, T. The Role of α-Klotho in Human Cancer: Molecular and Clinical Aspects. Oncogene 2022, 41, 4487–4497. [Google Scholar] [CrossRef]
- Qiu, S.; Li, C.; Zhu, J.; Guo, Z. Associations between the TyG Index and the α-Klotho Protein in Middle-Aged and Older Population Relevant to Diabetes Mellitus in NHANES 2007–2016. Lipids Health Dis. 2024, 23, 188. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of Exposure of Heavy Metals and Their Impact on Health Consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhou, W.; Lin, J.; Sun, W.; Qin, Y.; Li, X.; Xu, H. Independent and Combined Associations of Blood Manganese, Cadmium and Lead Exposures with the Systemic Immune-Inflammation Index in Adults. Toxics 2023, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Caruntu, A.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef]
- Bolt, A.M.; Mann, K.K. Tungsten: An Emerging Toxicant, Alone or in Combination. Curr. Environ. Health Rep. 2016, 3, 405–415. [Google Scholar] [CrossRef]
- Schwarz, G.; Belaidi, A.A. Molybdenum in Human Health and Disease. Met. Ions Life Sci. 2013, 13, 415–450. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Chen, W.-L. The Relationship between Polycyclic Aromatic Hydrocarbons Exposure and Serum Klotho among Adult Population. BMC Geriatr. 2022, 22, 198. [Google Scholar] [CrossRef]
- Guo, X.; Wu, B.; Hu, W.; Wang, X.; Su, W.; Meng, J.; Lowe, S.; Zhao, D.; Huang, C.; Liang, M.; et al. Associations of Perchlorate, Nitrate, and Thiocyanate with Metabolic Syndrome and Its Components among US Adults: A Cross-Sectional Study from NHANES. Sci. Total Environ. 2023, 879, 163083. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, Y. Exposure to P-Dichlorobenzene and Serum α-Klotho Levels among US Participants in Their Middle and Late Adulthood. Sci. Total Environ. 2023, 858, 159768. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Choi, J.-Y.; Lee, J.; Lee, H.-J.; Min, J.-Y.; Min, K.-B. Association of α-Klotho and Lead and Cadmium: A Cross-Sectional Study. Sci. Total Environ. 2022, 843, 156938. [Google Scholar] [CrossRef]
- Jain, R.B. Serum Klotho and Its Associations with Blood and Urine Cadmium and Lead across Various Stages of Glomerular Function: Data for US Adults Aged 40–79 Years. Environ. Sci. Pollut. Res. 2022, 29, 57412–57420. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, T.; Zhong, X.; Cai, Y.; Yang, W.; Zhang, J. Serum Protein α-Klotho Mediates the Association between Lead, Mercury, and Kidney Function in Middle-Aged and Elderly Populations. Environ. Health Prev. Med. 2025, 30, 24–00296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.; Ding, X.; Liu, L.; Xu, P.; Ma, Y.; Xing, H.; Keerman, M.; Niu, Q. Elevated Serum Copper, Zinc, Selenium, and Lowered α-Klotho Associations: Findings from NHANES 2011–2016 Dataset. Biol. Trace Elem. Res. 2025, 203, 1395–1404. [Google Scholar] [CrossRef]
- Briefel, R.R. Assessment of the US Diet in National Nutrition Surveys: National Collaborative Efforts and NHANES. Am. J. Clin. Nutr. 1994, 59, 164S–167S. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, Z.; Shen, J.; Wu, Y.; Fang, L.; Xu, S.; Ma, Y.; Zhao, H.; Pan, F. Associations of Exposure to Blood and Urinary Heavy Metal Mixtures with Psoriasis Risk among U.S. Adults: A Cross-Sectional Study. Sci. Total Environ. 2023, 887, 164133. [Google Scholar] [CrossRef] [PubMed]
- Kamizono, Y.; Shiga, Y.; Suematsu, Y.; Imaizumi, S.; Tsukahara, H.; Noda, K.; Kuwano, T.; Fujimi, K.; Saku, K.; Miura, S. Impact of Cigarette Smoking Cessation on Plasma α-Klotho Levels. Medicine 2018, 97, e11947. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mao, Y.; Hu, Y.; Xu, B. Association between Exposure to Polyfluoroalkyl Chemicals and Increased Fractional Exhaled Nitric Oxide in Adults. Environ. Res. 2021, 198, 110450. [Google Scholar] [CrossRef]
- Liang, M.; Guo, X.; Ding, X.; Song, Q.; Wang, H.; Li, N.; Su, W.; Liang, Q.; Sun, Y. Combined Effects of Multiple Metals on Hearing Loss: A Bayesian Kernel Machine Regression Approach. Ecotoxicol. Environ. Saf. 2022, 247, 114279. [Google Scholar] [CrossRef]
- Chen, R.; Yin, H.; Cole, I.S.; Shen, S.; Zhou, X.; Wang, Y.; Tang, S. Exposure, Assessment and Health Hazards of Particulate Matter in Metal Additive Manufacturing: A Review. Chemosphere 2020, 259, 127452. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, X.; Yin, T.; Zhu, Q.; Shi, S.; Cheang, I.; Yue, X.; Tang, Y.; Liao, S.; Zhou, Y.; et al. Renal Function Mediates the Association Between Klotho and Congestive Heart Failure Among Middle-Aged and Older Individuals. Front. Cardiovasc. Med. 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Jena, S.; Sarangi, P.; Das, U.K.; Lamare, A.A.; Rattan, R. Serum α-Klotho Protein Can Be an Independent Predictive Marker of Oxidative Stress (OS) and Declining Glomerular Function Rate in Chronic Kidney Disease (CKD) Patients. Cureus 2022, 14, e25759. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Ma, L.; Wu, C. Association between Serum Klotho and Physical Frailty in Middle-Aged and Older Adults: Finding From the National Health and Nutrition Examination Survey. J. Am. Med. Dir. Assoc. 2023, 24, 1173–1178.e2. [Google Scholar] [CrossRef]
- Guan, G.; Cai, J.; Zheng, S.; Xiang, Y.; Xia, S.; Zhang, Y.; Shi, J.; Wang, J. Association between Serum Manganese and Serum Klotho in a 40–80-Year-Old American Population from NHANES 2011–2016. Front. Aging 2023, 4, 1–8. [Google Scholar] [CrossRef]
- Picard, M. Pathways to Aging: The Mitochondrion at the Intersection of Biological and Psychosocial Sciences. J. Aging Res. 2011, 2011, 814096. [Google Scholar] [CrossRef]
- Yu, L.; Kang, L.; Ren, X.Z.; Diao, Z.L.; Liu, W.H. Circulating α-Klotho Levels in Hemodialysis Patients and Their Relationship to Atherosclerosis. Kidney Blood Press. Res. 2018, 43, 1174–1182. [Google Scholar]
- Chung, C.-P.; Chang, Y.-C.; Ding, Y.; Lim, K.; Liu, Q.; Zhu, L.; Zhang, W.; Lu, T.-S.; Molostvov, G.; Zehnder, D.; et al. α-Klotho Expression Determines Nitric Oxide Synthesis in Response to FGF-23 in Human Aortic Endothelial Cells. PLoS ONE 2017, 12, e0176817. [Google Scholar] [CrossRef]
- Cannas, D.; Loi, E.; Serra, M.; Firinu, D.; Valera, P.; Zavattari, P. Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients 2020, 12, 2074. [Google Scholar] [CrossRef]
- Yu, X.; Tian, X.; Wang, Y.; Zhu, C. Metal-Metal Interaction and Metal Toxicity: A Comparison between Mammalian and D. Melanogaster. Xenobiotica 2021, 51, 842–851. [Google Scholar] [CrossRef]
- Renaud, M.; El Morabet, H.; Reis, F.; da Silva, P.M.; Siciliano, S.D.; Sousa, J.P.; Natal-da-Luz, T. Are Structural and Functional Endpoints of Soil Communities Similarly Affected by Metal Mixtures?—A Terrestrial Model Ecosystem Approach. Sci. Total Environ. 2021, 795, 148909. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Rouhi-Parkouhi, M.; Church, S.J.; Lechanteur, Y.T.; Lorés-Motta, L.; Kouvatsos, N.; Clark, S.J.; Bishop, P.N.; Hoyng, C.B.; den Hollander, A.I.; et al. Association of Plasma Trace Element Levels with Neovascular Age-Related Macular Degeneration. Exp. Eye Res. 2020, 201, 108324. [Google Scholar] [CrossRef] [PubMed]
- Adamus, J.P.; Ruszczyńska, A.; Wyczałkowska-Tomasik, A. Molybdenum’s Role as an Essential Element in Enzymes Catabolizing Redox Reactions: A Review. Biomolecules 2024, 14, 869. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morata, I.; Sobel, M.; Tellez-Plaza, M.; Navas-Acien, A.; Howe, C.G.; Sanchez, T.R. A State-of-the-Science Review on Metal Biomarkers. Curr. Environ. Health Rep. 2023, 10, 215–249. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).