Potential Risks to Human Health Caused by the Use of Pesticides in Soils of Three Municipalities Impacted by Localized Malaria in the Brazilian Amazon
Highlights
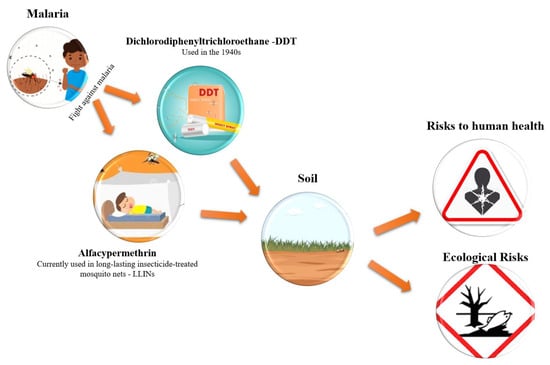
- The persistence of the pesticide DDT, which has been banned for decades, in regions affected by malaria.
- The predominance of DDT over its degradation products indicates slow degradation.
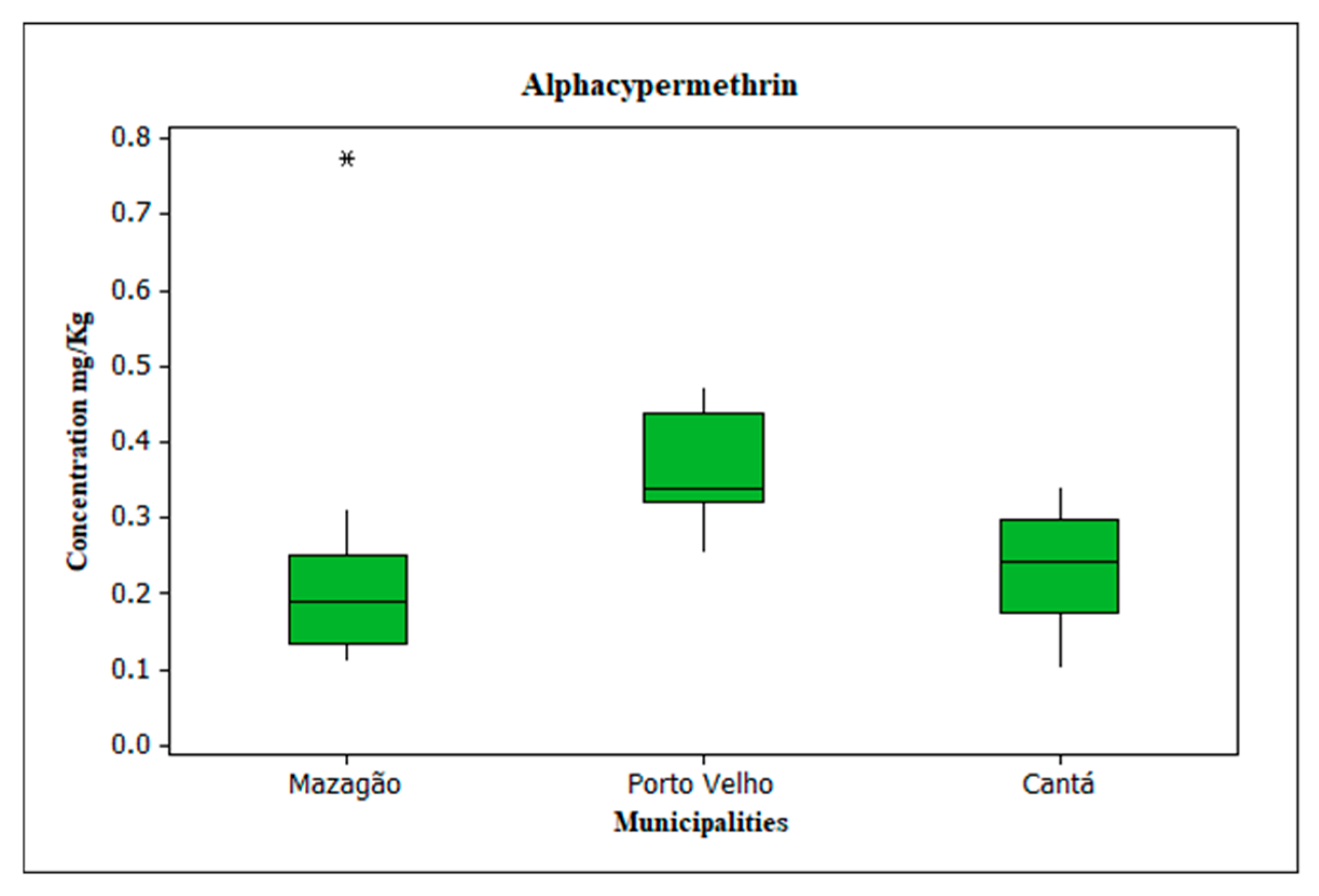
- The relevant concentrations of alfacipermethrin in the soil indicate inadequate management practices of MILDs.
- The ingestion of food contaminated with DDT and alfacipermethrin, especially by children, is the main route of exposure to carcinogenic and non-carcinogenic risks to human health.
Abstract
1. Introduction
2. Materials and Methods
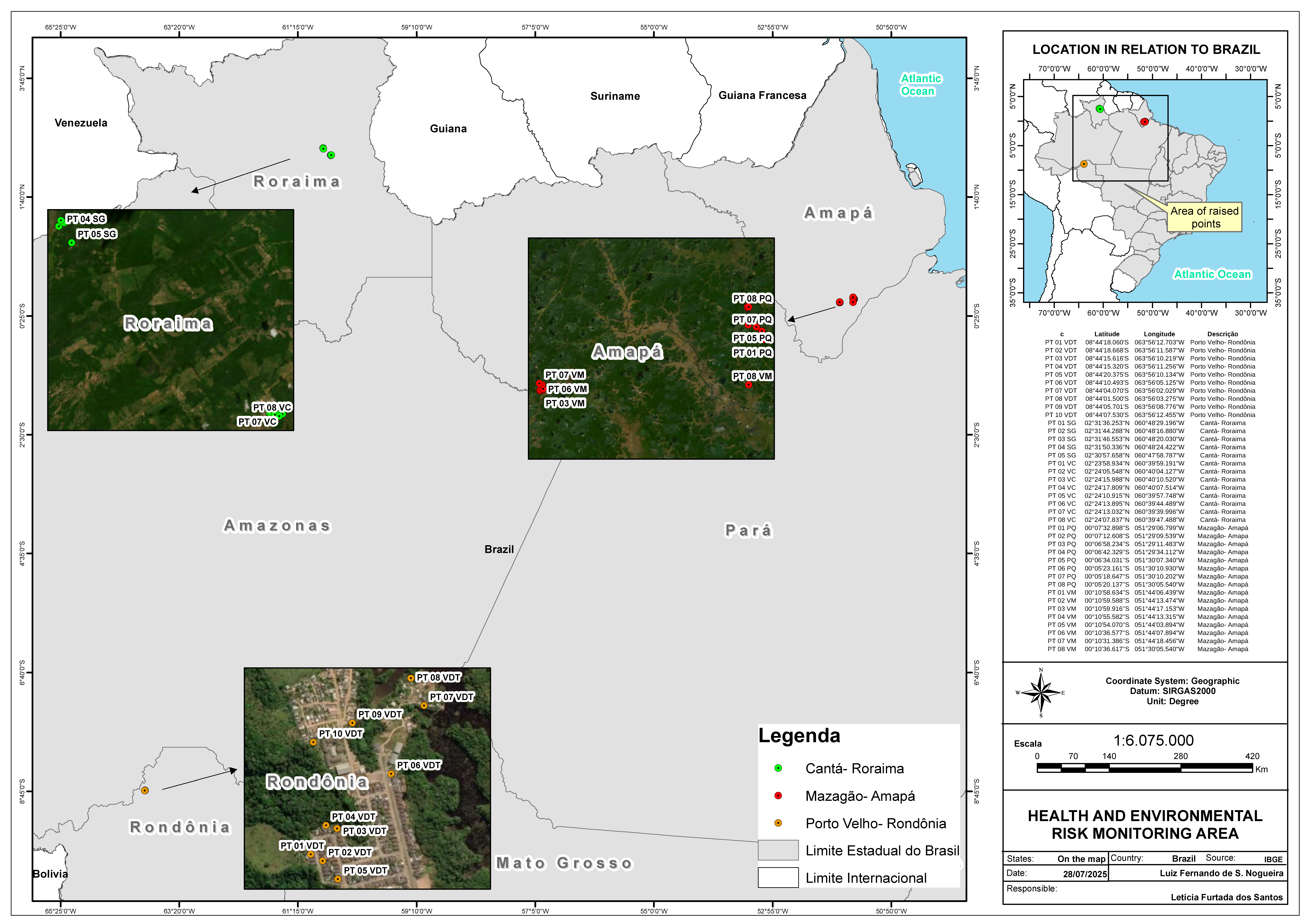
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Determination of pH
2.4. Organic Matter Analysis
2.5. Granulometric Analysis
2.6. Ecological Risk Analysis
2.7. Human Health Risk Assessment
2.8. Statistical Analysis
3. Results and Discussion
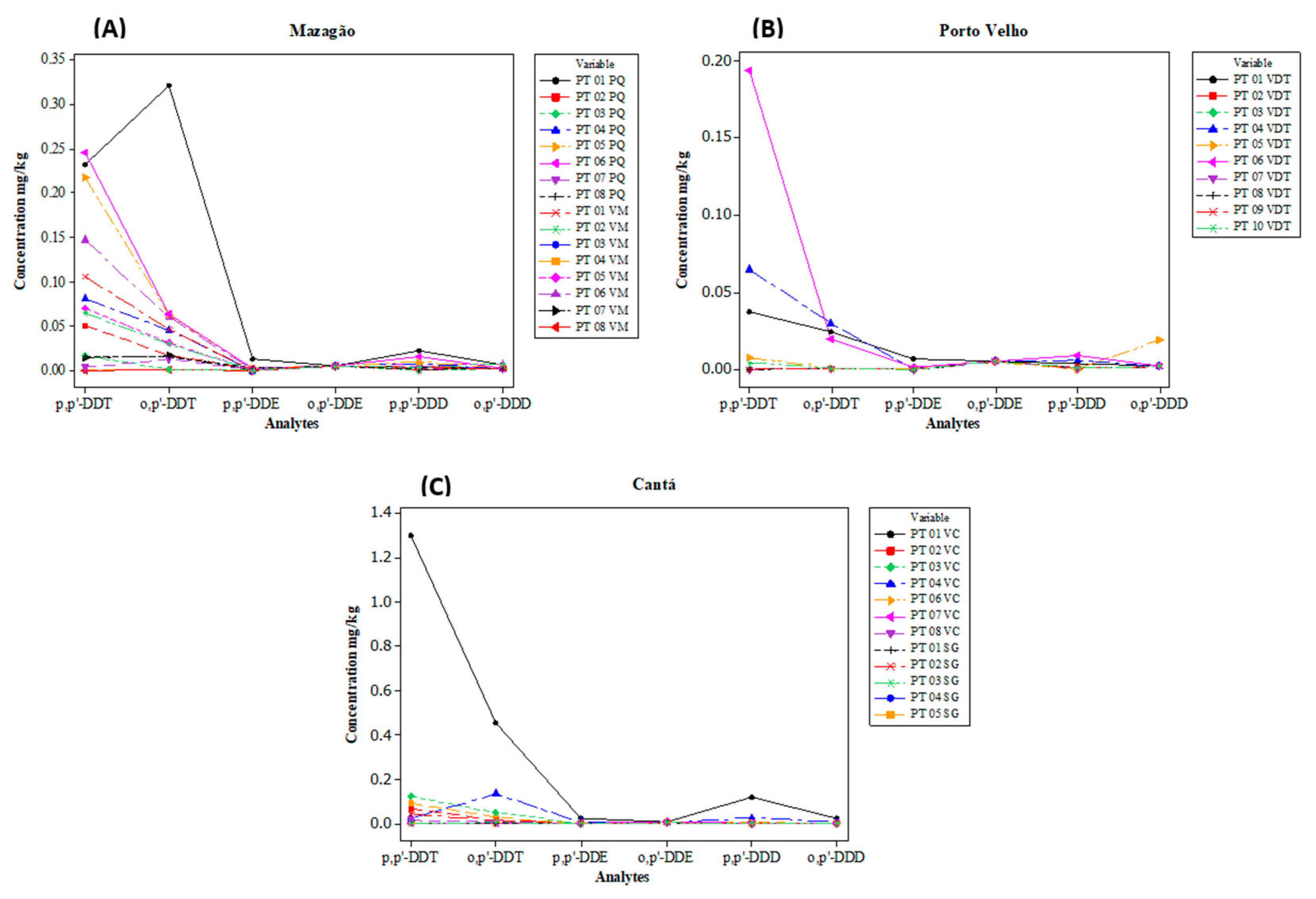
3.1. Evaluation of DDT and Its Metabolites
3.2. Analysis of Correlations Between DDT, pH, and OM
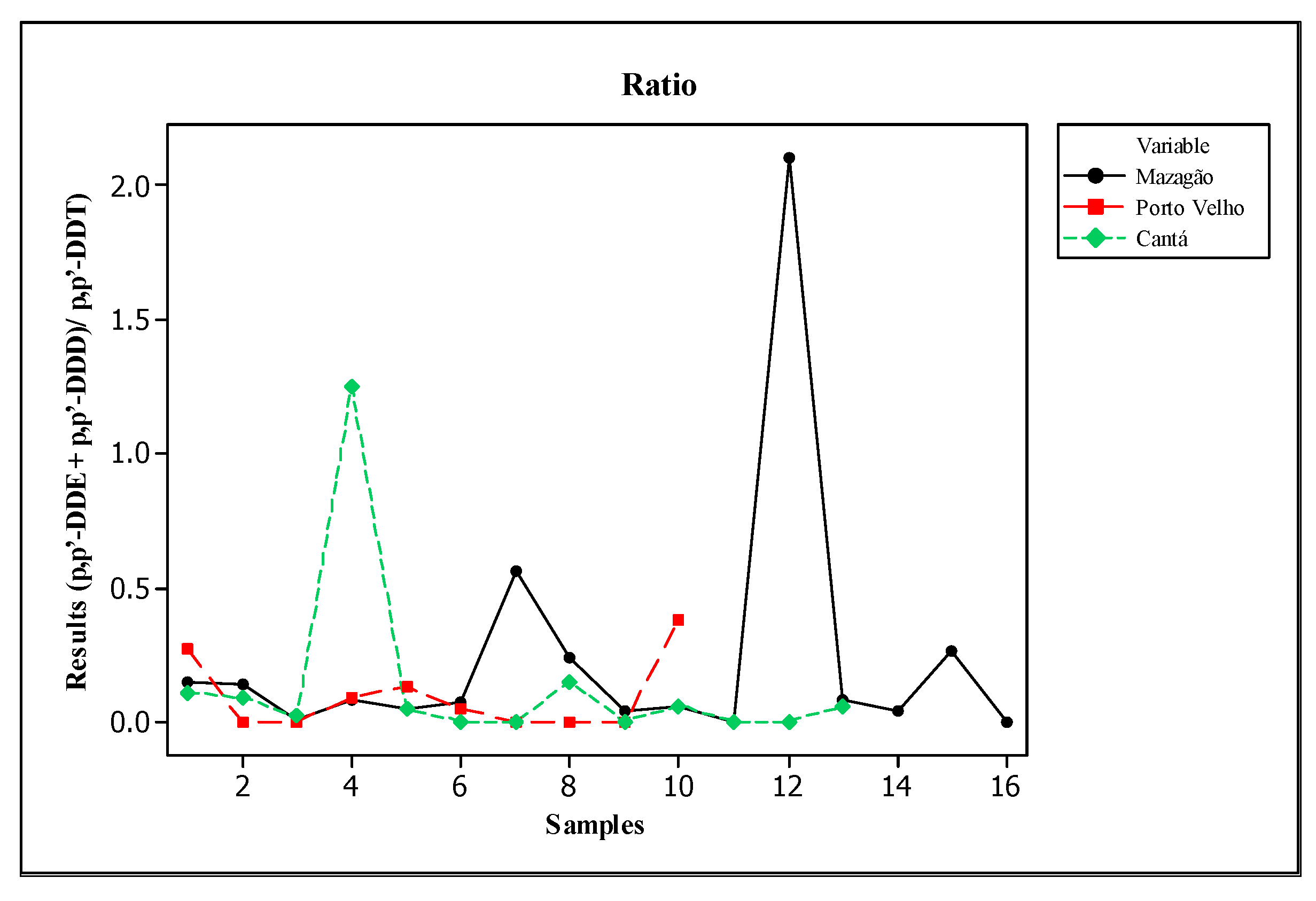
3.3. Degradation Conditions and Potential Sources of DDT
3.4. Evaluation of Alphacypermethrin
3.5. Results of Ecological Risk Analysis
3.6. Human Health Risk Analysis
3.6.1. Variations in Carcinogenic and Non-Carcinogenic Health Risks
3.6.2. Contribution of Individual Exposure Routes
3.6.3. Individual Contribution of DDTs to Health Risks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menezes, J.d.F.F.; Pena dos Santos, J.V.; Dutra, J.A.d.S.S.; Tavares, M.G.; Guimarães, H.A. Contaminação de água superficiais por agrotóxicos: Análise dos impactos causados na saúde humana e ambiental. Rev. Perspect. Online Biol. Saúde 2021, 11, 19–35. [Google Scholar] [CrossRef]
- WHO (World Health Organization). DDT and Its Derivatives—Environmental Aspects; World Health Organization: Geneva, Switzerland, 1989; Volume 83, pp. 1–73. [Google Scholar]
- da Silva, G.R. Níveis de Agrotóxicos Organoclorados e Perfil Alimentar na Cidade dos Meninos Duque de Caxias, RJ, Brasil, Entre 2003 e 2004. Master’s Thesis, FIOCRUZ, Rio de Janeiro, Brazil, 2009. [Google Scholar]
- Alquisira, C.M.; Alarcón, A.; Cerrato, R.F. Lista de municípios pertencentes às áreas de risco ou endêmicas para malária Una revisión. TIP Rev. Espec. Cienc. Quím.-Biol. 2021, 24, 1–15. [Google Scholar]
- Wang, S.; Wang, Q.; Yuan, Z.; Wu, X. Organochlorine pesticides in riparian soils and sediments of the middle reach of the Huaihe River: A traditional agricultural area in China. Chemosphere 2022, 296, 134020. [Google Scholar] [CrossRef]
- Lima, B.I. The main measures adopted for vector and parasitological control of malaria: A systematic review. Int. Seven J. Health Res. 2023, 2, 1221–1248. [Google Scholar]
- de Souza, B.V. History of epidemiological aspects and analysis of effective public health interventions in malaria control in Brazil. Braz. J. Health Rev. 2021, 4, 1521–1533. [Google Scholar] [CrossRef]
- Ribeiro, H. Poluição, um veneno silencioso para a saúde humana. Rev. Ciênc. Elem. 2019, 7, 069. [Google Scholar] [CrossRef]
- Ma, Y.; Yun, X.; Ruan, Z.; Lu, C.; Shi, Y.; Qin, Q.; Men, Z.; Zou, D.; Du, X.; Xing, B.; et al. Review of hexachlorocyclohexane (HCH) and dichlorodiphenyltrichloroethane (DDT) contamination in Chinese soils. Sci. Total Environ. 2020, 749, 141212. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qu, C.; Li, M.; Shi, C.; Li, W.; Zhang, J.; Qi, S. Health risks of exposure to soil-borne dichlorodiphenyltrichloroethanes (DDTs): A preliminary probabilistic assessment and spatial visualization. Sci. Total Environ. 2021, 772, 144949. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Qiu, Y.; Yu, X.; Han, X.; Ma, Y. Integrating bioavailability and soil aging in the derivation of DDT criteria for agricultural soils using crop species sensitivity distributions. Ecotoxicol. Environ. Saf. 2018, 165, 527–532. [Google Scholar] [CrossRef]
- Ma, J.; Pan, L.; Yang, X.; Liu, X.; Tao, S.; Zhao, L.; Qin, X.; Sun, Z.; Hou, H.; Zhou, Y. DDT, DDD, and DDE in soil of Xiangfen County, China: Residues, sources, spatial distribution, and health risks. Chemosphere 2016, 163, 578–583. [Google Scholar] [CrossRef]
- Companhia Ambiental do Estado de São Paulo—CETESB. DDT e Derivados (DDE e DDD); Divisão de Toxicologia Humana e Saúde Ambiental: Curitiba, Brazil, 2020; pp. 1–4.
- Ministério do Meio Ambiente e da Mudança do Clima. Plano Nacional de Implementação do Brasil para a Convenção de Estocolmo sobre Poluentes Orgânicos Persistentes; Ministério do Meio Ambiente e da Mudança do Clima: Brasília, Brazil, 2023; pp. 1–249.
- Rauch, S.; Bradman, A.; Coker, E.; Chevrier, J.; AN, S.; Bornman, R.; Eskenazi, B. Determinants of Exposure to Pyrethroid Insecticides in the VHEMBE Cohort, South Africa. Environ. Sci. Technol. 2018, 52, 12108–12121. [Google Scholar] [CrossRef]
- Kumar, A.; Jasrotia, S.; Dutta, J.; Kyzas, G.Z. Pyrethroids toxicity in vertebrates and invertebrates and amelioration by bioactive compounds: A review. Pestic. Biochem. Physiol. 2023, 196, 105615. [Google Scholar] [CrossRef]
- Nascimento, L.; Melnyk, A. A química dos pesticidas no meio ambiente e na saúde. Rev. Mangaio Acad. 2016, 1, 54–61. [Google Scholar]
- Saillenfait, A.M.; Ndiaye, D.; Sabaté, J.P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Environ. Health 2015, 2018, 281–292. [Google Scholar] [CrossRef]
- Gutiérrez-Araujo, M.K.; Alayo-Aguirre, E.P.; Burgos-Wilson, N.E.; Wilson-Krugg, J.H. Degradación del insecticida alfacipermetrina por Trichoderma harzianum. Véritas 2017, 16, 61–64. [Google Scholar]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef]
- Verma, V.; Rahi, M.; Divya, K.; Kamaraju, R. Laboratory evaluation of a new alphacypermethrin long-lasting insecticidal net against Anopheles culicifacies s.l. Parasitol. Res. 2022, 121, 2725–2731. [Google Scholar] [CrossRef]
- Lima, A.C.d.S.F.; Galardo, A.K.R.; Müller, J.N.; Corrêa, A.P.S.d.A.; Ribeiro, K.A.N.; Silveira, G.A.; Hijjar, A.V.; Bauzer, L.G.S.d.R.; Lima, J.B.P. Evaluation of Long-lasting insecticidal nets (LLINs) for malaria control in an endemic area in Brazil. Parasites Vectors 2023, 16, 162. [Google Scholar] [CrossRef]
- Matsuo, N. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde, Secretaria de Vigilância em Saúde e Ambiente. Caracterização da Malária em áreas especiais da região Amazônica. Bol. Epidemiol. 2024, 55, 14. [Google Scholar]
- Ministério da Saúde, Secretaria de Vigilância em Saúde e Ambiente. Dia da Malária nas Américas—Um panorama da malária no Brasil em 2022 e no primeiro semestre de 2023. Bol. Epidemiol. 2024, 55, 1. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística—IBGE. Available online: https://www.ibge.gov.br/cidades-e-estados/ap/mazagao.html (accessed on 3 July 2025).
- Climate-Data. Dados Climáticos para Cidades Mundiais. CLIMATE-DATA.ORG. 2023. Available online: https://pt.climate-data.org/america-do-sul/brasil/roraima/canta-43900/ (accessed on 30 June 2025).
- Ministério da Saúde. Lista de Municípios Pertencentes às Áreas de Risco ou Endêmicas para Malária. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/m/malaria/situacao-epidemiologica-da-malaria/indicadores/lista-de-municipios-pertencentes-as-areas-de-risco-04-11-2024 (accessed on 30 June 2025).
- Ministério da Saúde, Secretaria de Vigilância em Saúde. Malária 2020. Bol. Epidemiol. 2020, 1–118. [Google Scholar]
- Cidade Brasil. Available online: https://www.cidade-brasil.com.br/municipio-porto-velho.html (accessed on 2 July 2025).
- Instituto Brasileiro de Geografia e Estatística—IBGE. Cidades e Estados: Porto Velho. Available online: https://www.ibge.gov.br/cidades-e-estados/ro/porto-velho.html (accessed on 2 July 2025).
- Rondônia, Secretaria de Estado do Desenvolvimento Ambiental (SEDAM). Indicador de Anomalia da Precipitação Mensal: Índice “BMDI” no Estado de Rondônia, Período Chuvoso 2017–2018; Rondônia, Secretaria de Estado do Desenvolvimento Ambiental (SEDAM): Alta Floresta D’Oeste, Brazil, 2018.
- Instituto Brasileiro de Geografia e Estatística—IBGE. Cidades e Estados: Cantá. Available online: https://www.ibge.gov.br/cidades-e-estados/rr/canta.html (accessed on 3 July 2025).
- Barros, D.S.; Santos, C.S.V.; Melo, V.F.; Lopes, G.N. Mapeamento e Caracterização Ambiental das Áreas Apícolas dos Municípios de Mucajaí e Cantá do Estado de Roraima. Agro@mbiente 2008, 2, 77–87. [Google Scholar]
- Cunha, G.B.; Moura, J.F.L.; Naves, E.L.M.; Andrade, A.O.; Pereira, A.A.; Milagre, S.T. A utilização de uma rede neural artificial para previsão da incidência da malária no Município de Cantá, Estado de Roraima. Rev. Soc. Bras. Med. Trop. 2010, 43, 567–570. [Google Scholar] [CrossRef]
- Centro de Desenvolvimento da Tecnologia Nuclear—CDTN. DOQ—CGCRE-008, Revisão 09, Orientação Sobre Validação de Métodos Analíticos; Centro de Desenvolvimento da Tecnologia Nuclear: Belo Horizonte, Brazil, 2020; pp. 1–30.
- EMBRAPA. Manual de Métodos e Análise de Solo, 3rd ed.; EMBRAPA: Brasília, Brazil, 2017; pp. 199–202. [Google Scholar]
- Carmo, D.L.; Silva, C.A. Métodos de Quantificação de Carbono e Matéria Orgânica em Resíduos Orgânicos. Rev. Bras. Cienc. Solo 2012, 36, 1211–1220. [Google Scholar] [CrossRef]
- de Almeida, B.G.; Donagemma, G.K.; Ruiz, H.A.; Braida, J.A.; Viana, J.H.M.; Reichert, J.M.M.; Oliveira, L.B.; Ceddia, M.B.; Wadt, P.G.S.; Fernandes, R.B.A.; et al. Padronização de Métodos para Análise Granulométrica no Brasil. Comun. Técnico 2012, 66, 1–11. [Google Scholar]
- De Maria, I.C.; Coelho, R.M.; Cantarella, H.; Freitas, M.E.A.; Rodrigues, R.F.; Souza, A.R. Métodos de Análise Física de Solos do Instituto Agronômico de Campinas. Boletim Técnico de Análise Granulométrica; Secretaria da Agricultura e Abastecimento: Curitiba, Brazil, 2021; pp. 1–33. [Google Scholar]
- CCME (Canadian Council of Ministers of the Environment). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999.
- USEPA. Exposure Factors Handbook 2011 Edition (Final Report); EPA/600/R-09/052F; USEPA: Washington, DC, USA, 2011.
- ASTM E2081-22; Standard Guide for Risk-Based Corrective Action. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2010.
- WHO; IFCS Intergovernmental Forum on Chemical Safety. Persistent Organic Pollutants. In Proceedings of the IFCS Expert Meeting on POPs 1996, Manila, Philippines, 17–19 June 1996. Prepared by WHO. Unpublished. [Google Scholar]
- Manirakiza, P.; Akinbamijo, O.; Covaci, A.; Pitonzo, R.; Schepens, P. Assessment of Organochlorine Pesticide Residues in West African City Farms: Banjul and Dakar Case Study. Arch. Environ. Contam. Toxicol. 2003, 44, 171–179. [Google Scholar] [CrossRef] [PubMed]
- USEPA (U.S. Environmental Protection Agency). Risk Assessment for Superfund: Volume III—Part A, Process for Conducting Probabilistic Risk Assessment; Office of Emergency and Remedial Response, USEPA: Washington, DC, USA, 2001.
- Kuang, H.; Sandanielo, V.L.M.; de Oliveira Junior, G.J. Principal Component Analysis: Theory, interpretations and applications. Eng. Sci. 2015, 1, 83–90. [Google Scholar]
- Downing, D.; Clark, J. Estatística Aplicada, 3rd ed.; Editora Saraiva: São Paulo, Brazil, 2003; pp. 1–398. [Google Scholar]
- Ministério do Meio Ambiente e Mudança Do Clima; Conselho Nacional do Meio Ambiente—CONAMA. Resolução Nº 420, de 28 de Dezembro de 2009; CONAMA: Brasília, Brazil, 2009; pp. 1–16.
- Mendez, A.; NG, C.A.; Torres, J.P.M.; Bastos, W.; Bogdal, C.; dos Reis, G.A.; Hungerbuehler, K. Modeling the dynamics of DDT in a remote tropical floodplain: Indications of post-ban use? Environ. Sci. Pollut. Res. 2015, 23, 10317–10334. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Salinas, R.I.; Díaz-Barriga, F.; Batres-Esquivel, L.E.; Pérez-Maldonado, I.N. Assessment of the Levels of DDT and its Metabolites in Soil and Dust Samples from Chiapas, Mexico. Bull. Environ. Contam. Toxicol. 2011, 86, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Torres-Dosal, A.; Martinez-Salinas, R.I.; Hernandez-Benavides, D.; Perez-Vazquez, F.J.; Ilizaliturri-Hernandez, C.; Perez-Maldonado, I.N. Assessment of the levels of DDT and DDE in soil and blood samples from Tabasco, Mexico. Environ. Monit. Assess. 2012, 184, 7551–7559. [Google Scholar] [CrossRef]
- Kumar, B.; MIshra, M.; Verma, V.K.; Kumar, S.; Sharma, C.S. Distribution of dichlorodiphenyltrichloroethane and hexachlorocyclohexane in urban soils and risk assessment. J. Xenobiot. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Rodrigues, A.O.; de Souza, L.C.; da Silva Rocha, C.C.; da Costa, A.C.G.; Mendes, R.d.A. Assessment of DDT and Metabolites in Soil and Sediment of Potentially Contaminated Areas of Belém, Amazon Region, Brazil. Bull. Environ. Contam. Toxicol. 2017, 99, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Filizola, H.F.; Pessoa, M.C.P.Y.; Gomes, M.A.F.; de Souza, M.D. Contaminação dos Solos em Áreas Agrícolas. Em Uso agrícola dos Solos Brasileiros, 1st ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2002; pp. 154–196. [Google Scholar]
- Nascimento, R.Z. Degradação de Cloririfós em Neossolo e Cambissolo de Santa Catarinathesis; Universidade do Estado de Santa Catarina: Lages, Brazil, 2021. [Google Scholar]
- Krohn, C.; Jin, J.; Ryan, J.; Fabijański, P.; Franks, A.E.; Tang, C. Composition of soil organic matter drives total loss of dieldrin. Sci. Total Environ. 2019, 691, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zaffani, A.G.; Figueiredo, C.M.; Forhat, A.; Wendland, E.C.; Crestana, S. Escoamento do herbicida atrazina em região de Cerrado com solo arenoso. In Proceedings of the 16º Simpósio do Programa de Pós-Graduação em Ciências da Engenharia Ambiental, São Carlos, Brazil, 29–31 October 2018. [Google Scholar]
- Silva, P.L.F.; de Oliveira, F.P.; Martins, A.F.; Perreira, W.E.; Santos, T.E.D.; do Amaral, A.J. Caracterização físico-hídrica de solos arenosos através da curva de retenção de água, índice S e distribuição de poros por tamanho. Agrarian 2020, 13, 478–492. [Google Scholar] [CrossRef]
- Perez, L.L. Dinâmica da Mistura Formulada de Sulfentrazone com Diuron e o Herbicida Diclosulam no Solo e os Efeitos Residuais em Milho, Sorgo e Feijão. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2023. [Google Scholar]
- Torres, J.P.M.; Pfeiffer, W.C.; Markowitz, S.; Pause, R.; Malm, O.; Japenga, J. Dichlorodiphenyltrichloroethane in Soil, River Sediment, and Fish in the Amazon in Brazil. Environ. Res. 2002, 88, 134–139. [Google Scholar] [CrossRef]
- Saldanha, G.C.; Bastos, W.R.; Torres, J.P.M.; Malm, O. DDT in Fishes and Soils of Lakes from Brazilian Amazon: Case Study of Puruzinho Lake (Amazon, Brazil). J. Braz. Chem. Soc. 2010, 21, 306–311. [Google Scholar] [CrossRef]
- Ben Mukiibi, S.B.; Nyanzi, S.A.; Kwetegyeka, J.; Olisah, C.; Taiwo, A.M.; Mubiru, E.; Tebandeke, E.; Matovu, H.; Odongo, S.; Abayi, J.J.M.; et al. Organochlorine pesticide residues in Uganda’s honey as a bioindicator of environmental contamination and reproductive health implications to consumers. Ecotoxicol. Environ. Saf. 2021, 214, 112094. [Google Scholar] [CrossRef]
- Li, C.; Xu, S.; Guan, D.; Chen, X.; He, H. Comparison of in vitro strategies for predicting Dichlorodiphenyltrichloroethane (DDT) and its metabolites bioavailability from soils. Ecotoxicol. Environ. Saf. 2023, 256, 114885. [Google Scholar] [CrossRef]
- Fernandes, C.L.F.; Ramires, P.F.; de Moura, R.R.; da Souza Pohren, R.; Volcão, L.M.; da Silva Júnior, F.M.R. Which pesticides are contaminating a Brazilian soils? Res. Soc. Dev. 2020, 9, e114932569. [Google Scholar] [CrossRef]
- Gereslassie, T.; Workineh, A.; Atieno, O.J.; Wang, J. Determination of Occurrences, Distribution, Health Impacts of Organochlorine Pesticides in Soils of Central China. Int. J. Environ. Res. Public Health 2019, 16, 146. [Google Scholar] [CrossRef]
- Kengara, F.O.; Doerfler, U.; Welzl, G.; Munch, J.C.; Schroll, R. Evidence of non-DDD pathway in the anaerobic degradation of DDT in tropical soil. Environ. Sci. Pollut. Res. 2019, 26, 8779–8788. [Google Scholar] [CrossRef]
- Laskowski, D.A. Physical and chemical properties of pyrethroids. Rev. Environ. Contam. Toxicol. 2002, 174, 49–170. [Google Scholar]
- Rafique, N.; Tariq, S.R. Photodegradation of α-cypermethrin in soil in the presence of trace metals (Cu2+, Cd2+, Fe2+ and Zn2+). Environ. Sci. Process. Impacts 2015, 17, 166–176. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Xiong, Z.P.; Zhou, S.P.; Xie, S.D.; Li, H.J.; Li, Q.S.; Yang, G.B. Analysis of reproductive damage in earthworms (Amynthas corticis) exposed to cypermethrin. Ecotoxicol. Environ. Saf. 2022, 244, 114038. [Google Scholar] [CrossRef]
- Del Prado-Lu, J.L. Insecticide Residues in Soil, Water, and Eggplant Fruits and Farmers’ Health Effects Due to Exposure to Pesticides. Environ. Health Prev. Med. 2015, 20, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Jindal, R. Assessment of cypermethrin induced hepatic toxicity in Catla catla: A multiple biomarker approach. Environ. Res. 2020, 184, 109359. [Google Scholar] [CrossRef] [PubMed]
- Korucu, M.K.; Elibol, P.S.; Isleyen, M. An environmental risk assessment for a DDX-contaminated agricultural area in Turkey: Soil vs. plant or human vs. Animal. Environ. Sci. Pollut. Res. 2021, 28, 50127–50140. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y. Coming ecological risks of organochlorine pesticides and novel brominated flame retardants in the Yellow River Basin. Sci. Total Environ. 2023, 857, 159296. [Google Scholar] [CrossRef]
- Hage, R.S.; Soares, A.T.; Bohm, B.C.; Bruhn, F.R.P. Incidência da malária no estado de Roraima no período de 2007 a 2023. In Proceedings of the XXVI ENPÓS—Encontro de Pós Graduação, Pelotas, Brazil, 25–29 November 2024. [Google Scholar]
- Eskenazi, B.; An, S.; Rauch, S.A.; Coker, E.S.; Maphula, A.; Obida, M.; Crause, M.; Kogut, K.R.; Bornman, R.; Chevrier, J. Prenatal Exposure to DDT and Pyrethroids for Malaria Control and Child Neurodevelopment: The VHEMBE Cohort, South Africa. Environ. Health Perspect. 2018, 126, 047004-1–047004-11. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.K.D.; Barber, L.M.; Schleier, J.J. Net Risk: A Risk Assessment of Long-Lasting Insecticide Bed Nets Used for Malaria Management. Am. J. Trop. Med. Hyg. 2011, 84, 951–956. [Google Scholar] [CrossRef]
- Rincón-Rubio, R.; Mérida-Ortega, A.; Ugalde-Resano, R.; Gamboa-Loira, B.; Rothenberg, S.J.; González, F.B.; Cebrián, M.E.; López-Carrillo, L. Carcinogenic, non-carcinogenic risk, and attributable cases to organochlorine pesticide exposure in women from Northern Mexico. Environ. Monit. Assess. 2024, 196, 421. [Google Scholar] [CrossRef]
- Bornman, R.; Acerini, C.L.; Chevrier, J.; Rauch, S.; Crause, M.; Obida, M.; Eskenazi, B. Maternal exposure to DDT, DDE, and pyrethroid insecticides for malaria vector control and hypospadias in the VHEMBE birth cohort study, Limpopo, South Africa. Sci. Total Environ. 2022, 845, 157084. [Google Scholar] [CrossRef]
- USEPA (U.S. Environmental Protection Agency). Human Health Risk Assessment; USEPA: Washington, DC, USA, 2002.
- Ingber, S.Z.; Buser, M.C.; Pohl, H.R.; Abadin, H.G.; Murray, H.E.; Scinicariello, F. DDT/DDE and breast cancer: A meta-analysis. Regul. Toxicol. Pharmacol. 2013, 67, 421–433. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Krigbaum, N.Y.; Cirillo, P.M.; Cohn, B.A. Association between maternal exposure to the pesticide dichlorodiphenyltrichloroethane (DDT) and risk of obesity in middle age. Int. J. Obes. 2020, 44, 1723–1732. [Google Scholar] [CrossRef]
- Kalinina, T.; Kononchuk, V.; Klyushova, L.; Gulyaeva, L. Effects of Endocrine Disruptors o,p′-Dichlorodiphenyltrichloroethane, p,p′-Dichlorodiphenyltrichloroethane, and Endosulfan on the Expression of Estradiol-, Progesterone-, and Testosterone-Responsive MicroRNAs and Their Target Genes in MCF-7 Cells. Toxics 2022, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Teeny, S.; Jarrell, Z.R.; Krigbaum, N.Y.; Cirillo, P.M.; Go, Y.-M.; Cohn, B.A.; Jones, D.P. Environmental basis for early onset breast cancer. Reprod. Toxicol. 2025, 133, 108866. [Google Scholar] [CrossRef] [PubMed]
- Caldas, E.D.; Jardim, A.N.O. Exposure to toxic chemicals in the diet: Is the Brazilian population at risk? J. Expo. Sci. Environ. Epidemiol. 2012, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khatun, P.; Islam, A.; Sachi, S.; Islam, M.Z.; Islam, P. Pesticides in vegetable production in Bangladesh: A systemic review of contamination levels and associated health risks in the last decade. Toxicol. Rep. 2023, 11, 199–211. [Google Scholar] [CrossRef]


| Municipalities | p,p′-DDT X a ± S b (min–max) c | o,p′-DDT X ± S (min–max) | p,p′-DDE X ± S (min–max) | o,p′-DDE X ± S (min–max) | p,p′-DDD X ± S (min–max) | o,p′-DDD X ± S (min–max) | ∑DDT X ± S (min–max) |
|---|---|---|---|---|---|---|---|
| Mazagão | 0.079 ± 0.087 (<LD d − 0.245) | 0.045 ± 0.077 (<LD − 0.320) | 0.002 ± 0.003 (<LD − 0.013) | <LD | 0.005 ± 0.006 (<LD − 0.022) | 0.003 ± 0.002 (<LD − 0.007) | 2.219 0.139 ± 0.159 (<LD − 0.598) |
| Porto Velho | 0.031 ± 0.061 (<LD − 0.193) | 0.008 ± 0.012 (<LD − 0.030) | 0.001 ± 0.002 (<LD − 0.007) | <LD | 0.003 ± 0.003 (<LD − 0.009) | 0.004 ± 0.005 (<LD − 0.019) | 0.510 0.051 ± 0.072 (<LD − 0.230) |
| Cantá | 0.128 ± 0.354 (<LD − 1.297) | 0.055 ± 0.125 (<LD − 0.452) | 0.002 ± 0.007 (<LD − 0.024) | <LD | 0.013 ± 0.032 (<LD − 0.119) | 0.004 ± 0.006 (<LD − 0.023) | 2.694 0.207 ± 0.519 (<LD − 1.919) |
| Conama No. 420/2009 | 2 mg/kg | 1 mg/kg | 3 mg/kg | - | |||
| p,p′-DDT | o,p′-DDT | p,p′-DDE | p,p′-DDD | o,p′-DDD | pH | |
|---|---|---|---|---|---|---|
| o,p′-DDT | 0.864 | |||||
| p,p′-DDE | 0.866 | 0.947 | ||||
| p,p′-DDD | 0.965 | 0.894 | 0.910 | |||
| o,p′-DDD | 0.683 | 0.672 | 0.694 | 0.728 | ||
| pH | -* | - | - | - | - | |
| OM | - | - | - | - | - | 0.416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.F.d.; Deus, R.J.A.d.; Sucupira, I.M.C.; Brasil, D.d.S.B.; Mendes, R.d.A. Potential Risks to Human Health Caused by the Use of Pesticides in Soils of Three Municipalities Impacted by Localized Malaria in the Brazilian Amazon. Toxics 2025, 13, 900. https://doi.org/10.3390/toxics13100900
Santos LFd, Deus RJAd, Sucupira IMC, Brasil DdSB, Mendes RdA. Potential Risks to Human Health Caused by the Use of Pesticides in Soils of Three Municipalities Impacted by Localized Malaria in the Brazilian Amazon. Toxics. 2025; 13(10):900. https://doi.org/10.3390/toxics13100900
Chicago/Turabian StyleSantos, Letícia Furtado dos, Ricardo Jorge Amorim de Deus, Izis Mônica Carvalho Sucupira, Davi do Socorro Barros Brasil, and Rosivaldo de Alcântara Mendes. 2025. "Potential Risks to Human Health Caused by the Use of Pesticides in Soils of Three Municipalities Impacted by Localized Malaria in the Brazilian Amazon" Toxics 13, no. 10: 900. https://doi.org/10.3390/toxics13100900
APA StyleSantos, L. F. d., Deus, R. J. A. d., Sucupira, I. M. C., Brasil, D. d. S. B., & Mendes, R. d. A. (2025). Potential Risks to Human Health Caused by the Use of Pesticides in Soils of Three Municipalities Impacted by Localized Malaria in the Brazilian Amazon. Toxics, 13(10), 900. https://doi.org/10.3390/toxics13100900