Human Skin Permeation of Ethoxy- and Propoxypropanol Commonly Found in Water-Based Products
Abstract
1. Introduction
- Characterize the human skin permeation of PGEE and PGPE when applied neat or in aqueous solutions providing the permeation rates (J), lag times (Tlag), and permeability coefficients (Kp) from human in vitro skin permeation experiments using a flow-through diffusion cells system;
- Characterize the influence of water in the aqueous PGEE and PGPE solutions on skin permeation using a static diffusion cells system;
- Explore the use of previously frozen human skin to screen these solvents for skin irritation.
2. Materials and Methods
2.1. Skin
2.2. Flow-Through Diffusion Cell Experiments
2.3. Static Diffusion Cell Experiments
2.4. Chemical Analysis
2.5. Statistical Analysis
2.6. Skin Irritation Analysis
2.7. Data Treatment
2.8. Mass Balance
3. Results
3.1. Flow-Through Diffusion Cell Experiments
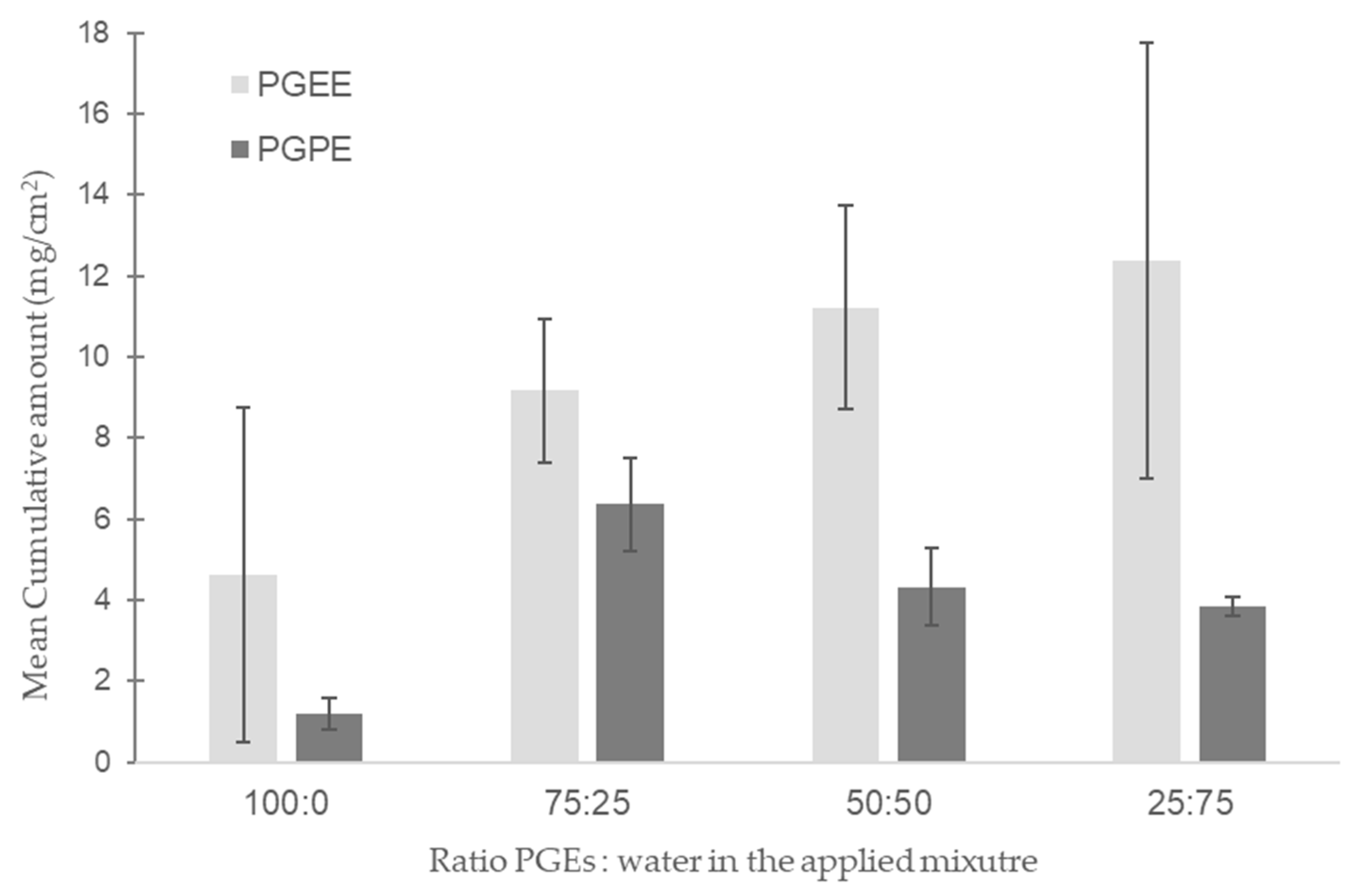
3.2. Static Diffusion Cell Experiments
3.3. Skin Irritation Analysis
3.4. Mass Balance
4. Discussion
4.1. Flow-Through In Vitro Skin Permeation
4.2. Mass Balance
4.3. Static In Vitro Human Skin Permeation
4.4. Effect of Water in Human Skin Permeation
4.5. Irritation
4.6. Workplace Scenario
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGE | Propylene glycol ether |
| EGE | Ethylene glycol ether |
| PGEE | Ethoxypropanol |
| PGPE | Propoxypropanol |
| EGBE | Ethylene glycol butyl ether |
| DPGME | Dipropylene glycol methyl ether |
| CLP | Classification, labeling and packaging of substances and mixtures |
| J | Permeability rate |
| Kp | Permeability coefficient |
| Tlag | Lag time |
| OECD | Organization for Economic Co-operation and Development |
| CHUV | Lausanne University Hospital |
| DAL | Department of Musculoskeletal Medicine |
| CER-VD | Commission cantonale d’éthique de la recherche sur l’être humain |
| TEWL | Trans epidermal water loss |
| GC-MS/MS | Gas-chromatography tandem mass spectrometry |
References
- Dentan, A.; Devanthéry, A.; de Peyer, J.E.; Droz, P.-O. Propylene glycol monomethyl ether (PGME) exposure. 2. Identification of products containing PGME, their importance and their use in Switzerland. Int. Arch. Occup. Environ. Health 2000, 73, 349–351. [Google Scholar] [CrossRef]
- European Centre for Ecotoxicology and Toxicology of Chemicals. The Toxicology of Glycol Ethers and Its Relevance to Man; ECETOC Aisbl: Brussels, Belgium, 1995. [Google Scholar]
- Welch, L.S.; Schrader, S.M.; Turner, T.W.; Cullen, M.R. Effects of exposure to ethylene glycol ethers on shipyard painters: II. Male reproduction. Am. J. Ind. Med. 1988, 14, 509–526. [Google Scholar] [CrossRef]
- European Chemical Industry Council. Glycol Ethers Charter. 2023. Available online: https://www.glycol-ethers.eu/glycol-ethers-charter/# (accessed on 20 June 2025).
- ECHA. Substance Infocard: 1-Ethoxypropan-2-Ol. 2022. Available online: https://echa.europa.eu/fr/substance-information/-/substanceinfo/100.014.886 (accessed on 20 June 2025).
- ECHA. Substance Infocard: 1-Propoxypropan-2-Ol. 2022. Available online: https://echa.europa.eu/fr/substance-information/-/substanceinfo/100.014.885 (accessed on 20 June 2025).
- Miller, R.R.; Hermann, E.A.; Young, J.T.; Landry, T.D.; Calhoun, L.L. Ethylene glycol monomethyl ether and propylene glycol monomethyl ether: Metabolism, disposition, and subchronic inhalation toxicity studies. Environ. Health Perspect. 1984, 57, 233–239. [Google Scholar] [CrossRef]
- Spencer, P.J.; Crissman, J.W.; Stott, W.T.; Corley, R.A.; Cieszlak, F.S.; Schumann, A.M.; Hardisty, J.F. Propylene glycol monomethyl ether (PGME): Inhalation toxicity and carcinogenicity in Fischer 344 rats and B6C3F1 mice. Toxicol. Pathol. 2002, 30, 570–579. [Google Scholar] [CrossRef]
- Stewart, R.D.; Baretta, E.D.; Dodd, H.C.; Torkelson, T.R. Experimental human exposure to vapor of propylene glycol monomethyl ether. Arch. Environ. Health Int. J. 1970, 20, 218–223. [Google Scholar] [CrossRef]
- Keski-Säntti, P.; Kaukiainen, A.; Hyvärinen, H.-K.; Sainio, M. Occupational chronic solvent encephalopathy in Finland 1995-2007: Incidence and exposure. Int. Arch. Occup. Environ. Health 2010, 83, 703–712. [Google Scholar] [CrossRef]
- Triebig, G.; Barocka, A.; Erbguth, F.; Höll, R.; Lang, C.; Lehrl, S.; Rechlin, T.; Weidenhammme, W.; Weltle, D. Neurotoxicity of solvent mixtures in spray painters. II. Neurologic, psychiatric, psychological, and neuroradiologic findings. Int. Arch. Occup. Environ. Health 1992, 64, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Fiorito, A.; Adami, G.; Barbieri, P.; Coceani, N.; Bussani, R.; Reisenhofer, E. Skin absorption in vitro of glycol ethers. Int. Arch. Occup. Environ. Health 1999, 72, 480–484. [Google Scholar] [CrossRef]
- Venier, M.; Adami, G.; Larese, F.; Maina, G.; Renzi, N. Percutaneous absorption of 5 glycol ethers through human skin in vitro. Toxicol. Vitr. 2004, 18, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.C.; Williams, F.M. Effects of experimental conditions on absorption of glycol ethers through human skin in vitro. Int. Arch. Occup. Environ. Health 2002, 75, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Berthet, A.; Oltramare, C.; Spring, P.; Hechon, J.; Hopf, N.B. Human skin permeation rates ex vivo following exposures to mixtures of glycol ethers. Toxicol. Lett. 2020, 335, 1–10. [Google Scholar] [CrossRef]
- Miles, A.; Berthet, A.; Hopf, N.; Gilliet, M.; Raffoul, W.; Vernez, D.; Spring, P. A new alternative method for testing skin irritation using a human skin model: A pilot study. Toxicol. Vitr. 2014, 28, 240–247. [Google Scholar] [CrossRef]
- OFSP. List of Products Containing Glycol Ethers of Interest; OECD: Paris, France, 2022. [Google Scholar]
- Poet, T.; Ball, N.; Hays, S.M. Deriving Biomonitoring Equivalents for selected E- and P-series glycol ethers for public health risk assessment. Int. J. Hyg. Environ. Health 2016, 219, 88–100. [Google Scholar] [CrossRef]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; Organisation for Economic Co-operation and Development (OECD): Paris, France, 2004; Available online: https://www.oecd.org/en/publications/guidance-document-for-the-conduct-of-skin-absorption-studies_9789264078796-en.html (accessed on 20 June 2025).
- OECD. Test No. 428: Skin Absorption: In Vitro Method; Organisation for Economic Co-operation and Development (OECD): Paris, France, 2004; Available online: https://www.oecd.org/en/publications/2004/11/test-no-428-skin-absorption-in-vitro-method_g1gh4b52.html (accessed on 20 June 2025).
- OECD. Guidance Notes on Dermal Absorption; Organisation for Economic Co-operation and Development (OECD): Paris, France, 2011; Available online: https://one.oecd.org/document/env/jm/mono%282011%2936/rev1/en/pdf (accessed on 20 June 2025).
- Reale, E.; Berthet, A.; Wild, P.; Vernez, D.; Hopf, N.B. Influence of experimental parameters on in vitro human skin permeation of Bisphenol A. Toxicol. Vitr. 2021, 73, 105129. [Google Scholar] [CrossRef] [PubMed]
- Bronaugh, R.L. In vitro diffusion cell studies in dermal absorption models in toxicology and pharmacology. In Dermal Absorption Models in Toxicology and Pharmacology; CRC PRESS: Boca Raton, FL, USA, 2005; Volume 2. [Google Scholar]
- Pinnagoda, J.; Tupker, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1990, 22, 164–178. [Google Scholar] [CrossRef] [PubMed]
- RStudioTeam. RStudio: Integrated Development Environment for R; RStudio Desktop 1.4.1717; Posit Software; PBC: Boston, MA, USA, 2021; Available online: https://www.posit.co (accessed on 20 July 2021).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem Compound Summary for CID 6993458, Propylene Glycol Ethyl Ether, (S)-. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Propylene-glycol-ethyl-ether_-_S (accessed on 22 April 2024).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem Compound Summary for CID 15286, 1-Propoxy-2-Propanol. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1-Propoxy-2-propanol (accessed on 22 April 2024).
- Tikuisis, P.; Meunier, P.; Jubenville, C. Human body surface area: Measurement and prediction using three dimensional body scans. Eur. J. Appl. Physiol. 2001, 85, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, S. Physiology, Blood Volume; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schenk, L.; Rauma, M.; Fransson, M.N.; Johanson, G. Percutaneous absorption of thirty-eight organic solvents in vitro using pig skin. PLoS ONE 2018, 13, e0205458. [Google Scholar] [CrossRef]
- Gerster, F.M.; Vernez, D.; Wild, P.P.; Hopf, N.B. Hazardous substances in frequently used professional cleaning products. Int. J. Occup. Environ. Health 2014, 20, 46–60. [Google Scholar] [CrossRef]
- Kaukiainen, A.; Riala, R.; Martikainen, R.; Akila, R.; Reijula, K.; Sainio, M. Solvent-related health effects among construction painters with decreasing exposure. Am. J. Ind. Med. 2004, 46, 627–636. [Google Scholar] [CrossRef]
- Lundberg, I.; Michelsen, H.; Nise, G.; Hogstedt, C.; Hogberg, M.; Alfredsson, L.; Almkvist, O.; Gustavsson, A.; Hagman, M.; Herlofson, J.; et al. Neuropsychiatric function of housepainters with previous long-term heavy exposure to organic solvents. Scand. J. Work Environ. Health 1995, 21 (Suppl. S1), 1–44. [Google Scholar]
- Zhai, H.; Maibach, H.I. Effects of occlusion: Percutaneous absorption. In Percutaneous Absorption; CRC PRESS: Boca Raton, FL, USA, 2005; pp. 271–282. [Google Scholar]
- Cheung, C.; Smith, C.K.; Hoog, J.-O.; Hotchkiss, S.A.M. Expression and Localization of Human Alcohol and Aldehyde Dehydrogenase Enzymes in Skin. Biochem. Biophys. Res. Commun. 1999, 261, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lockley, D.J.; Howes, D.; Williams, F.M. Cutaneous metabolism of glycol ethers. Arch. Toxicol. 2005, 79, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Traynor, M.J.; Wilkinson, S.C.; Williams, F.M. Metabolism of butoxyethanol in excised human skin in vitro. Toxicol. Lett. 2008, 177, 151–155. [Google Scholar] [CrossRef]
- Chilcott, R.; Price, S. Principles and Practice of Skin Toxicology; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Lane, M.E.; Santos, P.; Watkinson, A.C.; Hadgraft, J. Passive Skin Permeation Enhancement; Wiley: New York, NY, USA, 2012; pp. 23–42. [Google Scholar]
- Navia, M.; Chaturvedi, P.R. Design principles for orally bioavailable drugs. Drug Discov. Today 1996, 1, 179–189. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Riviere, J. Comparative studies on the effects of water, ethanol and water/ethanol mixtures on chemical partitioning into porcine stratum corneum and silastic membrane. Toxicol. Vitr. 2005, 19, 69–77. [Google Scholar] [CrossRef]
- Bunge, A.L.; Persichetti, J.M.; Payan, J.P. Explaining skin permeation of 2-butoxyethanol from neat and aqueous solutions. Int. J. Pharm. 2012, 435, 50–62. [Google Scholar] [CrossRef]
- Traynor, M.J.; Wilkinson, S.C.; Williams, F.M. The influence of water mixtures on the dermal absorption of glycol ethers. Toxicol. Appl. Pharmacol. 2007, 218, 128–134. [Google Scholar] [CrossRef]
- Tapfumaneyi, P.; Imran, M.; Alavi, S.E.; Mohammed, Y. Science of, and insights into, thermodynamic principles for dermal formulations. Drug Discov. Today 2023, 28, 103521. [Google Scholar] [CrossRef]
- Lynde, C.B.; Obadia, M.; Liss, G.M.; Ribeiro, M.; Holness, D.L.; Tarlo, S.M. Cutaneous and respiratory symptoms among professional cleaners. Occup. Med. 2009, 59, 249–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snow, J.E. Occupational exposure to glycol ethers: Implications for occupational health nurses. AAOHN J. 1994, 42, 413–419. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2021. [Google Scholar]
- Hughson, G.W.; Aitken, R.J. Determination of Dermal Exposures During Mixing, Spraying and Wiping Activities. Ann. Occup. Hyg. 2004, 48, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Borgatta, M.; Hechon, J.; Wild, P.; Hopf, N.B. Influence of collection and storage materials on glycol ether concentrations in urine and blood. Sci. Total Environ. 2021, 792, 148196. [Google Scholar] [CrossRef] [PubMed]

| Tested Chemical | Skin Thickness (mm) | Skin State (Frozen/Fresh) | Receptor Fluid (mL) | Cell Size (cm2) | Temperature (°C) | Applied Dose (mg/cm2) | Duration (h) | Kp ± SD (10−3 cm/h) | J ± SD (µg/cm2/h) | Tlag ± SD (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| Berthet et al., 2020 [15] | ||||||||||
| PGME | Dermatomed (0.8) | Frozen | NaCl 0.9% (12) | 1.77 | 32 | 109.6 | 24 | 0.18 ± 0.06 | 174 ± 62.2 | 0.88 ± 0.05 |
| PGME | Dermatomed (0.8) | Fresh | NaCl 0.9% (12) | 1.77 | 32 | 109.6 | 24 | 0.04 ± 0.04 | 40.4 ± 37.7 | 1.18 ± 0.16 |
| PGBE | Dermatomed (0.8) | Frozen | NaCl 0.9% (12) | 1.77 | 32 | 100.0 | 24 | 0.05 ± 0.02 | 44.1 ± 16.2 | 0.86 ± 0.23 |
| PGBE | Dermatomed (0.8) | Fresh | NaCl 0.9% (12) | 1.77 | 32 | 100.0 | 24 | 0.009 ± 0.002 | 7.93 ± 1.57 | 0.89 ± 0.13 |
| Venier et al., 2004 [13] | ||||||||||
| DPGME | Full thickness (1) | Frozen | NaCl 0.9% (14) | 3.29 | 32 | 57.8 | 8 | 0.11 ± 0.04 | 106.3 ± 37.6 | 1.51 ± 0.48 |
| Wilkinson and Williams 2002 [14] | ||||||||||
| PGME | Dermatomed (0.5) | Frozen | * SHC/G (0.4) | 0.64 | 32 | 286.3 | 5 | na | 4.3 ± 0.5 | 0.68 ± 0.09 |
| Larese et al., 1999 [12] | ||||||||||
| PGME | Full thickness | Frozen | NaCl 0.9% (15) | 3.14 | 32 | 58.3 | 4 | 0.51 ± 0.13 | 472 ± 120 | 0.55 ± 0.05 |
| PGMEac | Full thickness | Frozen | NaCl 0.9% (15) | 3.14 | 32 | 61.7 | 4 | 0.06 ± 0.006 | 59 ± 44 | 0.5 ± 0.05 |
| PGBE | Full thickness | Frozen | NaCl 0.9% (15) | 3.14 | 32 | 56.1 | 4 | 0.02 ± 0.005 | 17 ± 5 | 0.62 ± 0.03 |
| Substance | Diffusion Cell Type | Flow Rate (µL/min) | Applied Substance Concentration (%) | Volume (µL) | Amount (mg) |
|---|---|---|---|---|---|
| Ethoxypropanol (PGEE) | |||||
| Neat | Flow-through | 200 (0–3 h); 40 (3–24 h) | 100 | 200 | 179.2 |
| Neat | Static | - | 100 | 200 | 179.2 |
| Aqueous | Static | - | 75 | 500 | 336 |
| Aqueous | Flow-through | 200 (0–3 h); 40 (3–24 h) | 50 | 500 | 224 |
| Aqueous | Static | - | 50 | 500 | 224 |
| Aqueous | Static | - | 25 | 500 | 112 |
| Propoxypropanol (PGPE) | |||||
| Neat | Flow-through | 200 (0–3 h); 40 (3–24 h) | 100 | 200 | 177 |
| Neat | Static | - | 100 | 200 | 177 |
| Aqueous | Static | - | 75 | 500 | 331.9 |
| Aqueous | Flow-through | 200 (0–3 h); 40 (3–24 h) | 50 | 500 | 221.3 |
| Aqueous | Static | - | 50 | 500 | 221.3 |
| Aqueous | Static | - | 25 | 500 | 110.6 |
| Conditions | Donor Chamber (%) | Receptor Chamber (%) | Receptor Chamber over 24 h * (%) | Recovery (%) |
|---|---|---|---|---|
| PGEE neat | 0.0003 | 0.5 | 16.8 | 17.3 |
| PGEE 50:50 (aq) | 0.02 | 1.1 | 44.0 | 45.1 |
| PGPE neat | 0.18 | 0.3 | 9.4 | 9.9 |
| PGPE 50:50 (aq) | 0.05 | 0.4 | 24.1 | 24.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, H.P.; Pache, J.; Spring, P.; Berthet, A.; Hopf, N.B. Human Skin Permeation of Ethoxy- and Propoxypropanol Commonly Found in Water-Based Products. Toxics 2025, 13, 675. https://doi.org/10.3390/toxics13080675
De Luca HP, Pache J, Spring P, Berthet A, Hopf NB. Human Skin Permeation of Ethoxy- and Propoxypropanol Commonly Found in Water-Based Products. Toxics. 2025; 13(8):675. https://doi.org/10.3390/toxics13080675
Chicago/Turabian StyleDe Luca, Hélène P., Jennifer Pache, Philipp Spring, Aurélie Berthet, and Nancy B. Hopf. 2025. "Human Skin Permeation of Ethoxy- and Propoxypropanol Commonly Found in Water-Based Products" Toxics 13, no. 8: 675. https://doi.org/10.3390/toxics13080675
APA StyleDe Luca, H. P., Pache, J., Spring, P., Berthet, A., & Hopf, N. B. (2025). Human Skin Permeation of Ethoxy- and Propoxypropanol Commonly Found in Water-Based Products. Toxics, 13(8), 675. https://doi.org/10.3390/toxics13080675







